Abstract
Early mother-infant relationships play important roles in infants’ optimal development. New mothers undergo neurobiological changes that support developing mother-infant relationships regardless of great individual differences in those relationships. In this article, we review the neural plasticity in human mothers’ brains based on functional magnetic resonance imaging (fMRI) studies. First, we review the neural circuits that are involved in establishing and maintaining mother-infant relationships. Second, we discuss early postpartum factors (e.g., birth and feeding methods, hormones, and parental sensitivity) that are associated with individual differences in maternal brain neuroplasticity. Third, we discuss abnormal changes in the maternal brain related to psychopathology (i.e., postpartum depression, posttraumatic stress disorder, substance abuse) and potential brain remodeling associated with interventions. Last, we highlight potentially important future research directions to better understand normative changes in the maternal brain and risks for abnormal changes that may disrupt early mother-infant relationships.
Keywords: brain imaging, fMRI, neural plasticity, attachment, parenting, caregiving, oxytocin, maternal, postpartum psychopathology, intervention
1. Introduction
Maternal caregiving plays a critical role for infant survival and optimal development. After birth, the brain and body of mothers undergo dynamic changes to support the establishment and maintenance of maternal caregiving behaviors. In this review, we focus on neuroimaging studies that revealed neural plasticity among human parents using functional magnetic resonance imaging (fMRI). While animal literature elegantly demonstrate neural plasticity due to pregnancy, parturition, and caregiving (Cohen and Mizrahi, 2015; Fleming et al., 2002; Leuner et al., 2010), our review will be mostly limited to neural plasticity across the postpartum period beyond birth based on available literature in human parents. This review aims to contribute to the current literature by providing an overview of individual differences in brain plasticity and multi-dimensional factors that are associated with such individual differences. We will first review several important sets of maternal brain networks that support sensitive responses to infants. Auditory and visual signals from infants, such as infant cry sounds and infant images, activate brain regions from emotion response and regulation circuits to executive function and attention cortical circuits that function in parental thoughts, empathy and sensitive behavior. Second, we will review environmental, behavioral and hormonal factors that may relate to variations in maternal brain responses to infants. We will also discuss psychopathology in mothers – that may be considered as maladaptive plasticity, including postpartum depression (PPD), posttraumatic stress disorder (PTSD) and substance abuse. Identifying problems with maternal brain plasticity may ultimately offer hope for effective treatments for concerned parents with the development of brain-based targeting of interventions.
2. Neurocognitive mechanisms underlying parental sensitivity
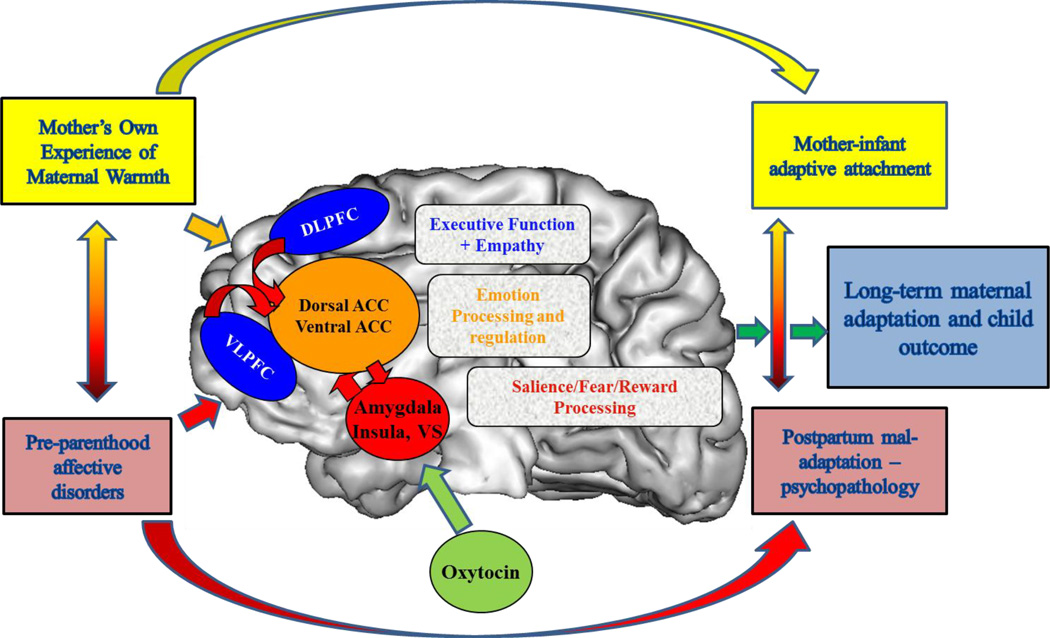
Sensitive caregiver responses to infant’s cues involve an array of complex thoughts and behaviors contingent on infant cues, including recognition and acknowledgment of the child’s signals, attribution of salience to the child’s cues, maintenance of visual contact, expression of positive affect, appropriate empathy mirroring and vocal quality, resourcefulness in handling the child’s distress or expanding the interaction, consistency of style, and display of an affective range that matches the infant’s readiness to interact. Such behaviors are likely the result of complex and highly plastic neural networks involved in generating and organizing emotional responses (Kober et al., 2008), as well as dissociable and volitional attention and executive function, reward and motivation, and sensorimotor circuits (Buckner et al., 2008; Seeley et al., 2007; Sripada et al., 2014). At this point, we propose a model for maternal brain function based on task-based imaging studies (Swain et al., 2014b; Swain and Lorberbaum, 2008) (Figure 1) and highlight brain processes that are mechanistically related to certain cortico-limbic circuits and are relevant for healthy parental sensitivity. The studies we reviewed largely use block design of infant stimuli and include analyses focusing on specific regions rather than connectivity. Thus, we would like to acknowledge that our model is necessarily over-simplistic and will require much more work to describe the physiology of key circuit elements, plus the connectivity between them which may itself also be plastic or flexible according to the demands of different stages of parenting and circumstances of psychopathology.
Figure 1.
Plasticity in the maternal brain - Early life factors, such as experience of parental warmth and previous mental health affect plastic brain circuits that ultimately regulate maternal affective regulation capacity and caregiving outcomes. Plastic or adaptable circuits, some of which are overlappping, include those for Emotion Response and Processing [Amygdala, Dorsal Anterior Cingulate Cortex (ACC), Ventral ACC] and Salience/Fear/Motivation Processing [Amygdala, Insula, Ventral Striatum (VS)] working with cortical executive function [Ventrolateral Prefrontal Cortex (VLPFC), Dorsolateral Prefrontal Cortex (DLPFC)] and empathy [Medial Prefrontal Cortex (MPFC), Precuneus, Superior Temporal Sulcus) circuits (adapted from Moses-Kolko et al., 2014).
2.1. Reward/Motivation
Both animal and human research (Numan and Woodside, 2010; Strathearn et al., 2009a) suggests that responses to infants form a model motivational system using dopamine (DA) and oxytocin (OT)-rich pathways. DA contributes importantly to reward-motivated behaviors, reinforcement learning, and drug addiction. Dopaminergic neurons, which originate in the brainstem’s ventral tegmental area and substantia nigra, project to the ventral and dorsal portions of the striatum, as well as to the medial prefrontal cortex (PFC), via mesocorticolimbic and nigrostriatal pathways. Natural reward-related stimuli, including food, sex, and faces of one’s sexual partner or child, activate the brain’s “reward” system (Aharon et al., 2001; Delgado et al., 2000; Melis and Argiolas, 1995; Stoeckel et al., 2008; Strathearn et al., 2008). In mothers, the initial experiences of pleasure and activity in these brain circuits when exposed to their own infants’ cues may increase the salience of their infants’ stimuli and promote greater attention and bond-formation to ensure continuous engagement in sensitive caregiving (Strathearn et al., 2009a; Strathearn et al., 2008). Such reward pathways may be relevant very early in the postpartum period, as a mother’s positive feelings towards her unborn fetus, as well as her perception of her fetus, have been associated with greater maternal sensitivity to the infant’s signals and more affectionate vocalizations and touch (Keller et al., 2003; Keren et al., 2003).
The amygdala also interacts with the reward circuit to motivate maternal behaviors. OT receptors are also abundantly present in the amygdala (Viviani et al., 2011), In rodents, during the postpartum period, the increased basolateral amygdala activation provides sensory inputs to the reward circuit including the nucleus accumbens (NAcc) and ventral pallidum (Numan, 2014; Numan and Woodside, 2010). In response to infant stimuli, infant cry and smiles activate the amygdala (Barrett et al., 2012; Seifritz et al., 2003; Swain et al., 2008), which has often been interpreted as a sign of emotional salience (Seifritz et al., 2003; Strathearn and Kim, 2013) or positive emotion associated with attachment (Leibenluft et al., 2004). On the other hand, in virgin rats, activation in the medial nucleus of the amygdala was associated with reduced maternal behaviors (Morgan et al., 1999; Oxley and Fleming, 2000). Thus, while increased activation of the amygdala to infant stimuli is interpreted as a more negative response to infants among typical adults (Riem et al., 2011), in mothers, it can be associated with more positive responses to one’s own infant (Barrett et al., 2012).
Another perspective on maternal motivations and rewards involve preoccupations that may be part of healthy maternal responses to their infants that draw them close in order to meet the infant’s physical and psychological needs (Bowlby, 1969; Winnicott, 1956). This suggests the importance of “checking and worrying” brain circuits overlapping with those hyperactive in obsessional anxiety (Leckman et al., 2004). Indeed, parental anxiety peaks immediately after childbirth and then begins to diminish during the first three to four months postpartum (Feldman et al., 1999; Kim et al., 2013; Leckman et al., 1999). This matches apparent increased responses to baby cry in postpartum anxiety circuits including the basal ganglia and orbitofrontal cortex that diminish over the first 4 months postpartum (Swain et al., 2014b; Swain et al., 2015). This plasticity in anxiety circuits may be part of a healthy range of threat detection and harm avoidance (Feygin et al., 2006), yet also perhaps an opportunity for problems to occur with such adaptations – in which abnormally reduced or excessive worry may be part of postpartum psychopathology.
2.2. Emotion Regulation
During interactions with an infant, particularly a distressed infant, it is critical for mothers to perceive the distressed cues of infants appropriately, and manage their own distress in response to their infants’ negative emotions. A mother’s sensitivity to distress has been a better predictor of the child’s outcome than her sensitivity to non-distress cues (Joosen et al., 2012; Leerkes, 2011; Leerkes et al., 2009; McElwain and Booth-Laforce, 2006).
The medial and lateral PFC and anterior cingulate cortex (ACC) are involved in emotion regulation including suppression of the amygdalar responses to negative emotional information (Ochsner et al., 2012). Activation of the medial and lateral PFC and ACC are consistently observed among new mothers in response to an infant’s cry (Laurent and Ablow, 2012; Lorberbaum et al., 2002), to videos of a distressed infant (Noriuchi et al., 2008), and to one’s own child greater than to familiar/unknown faces (Leibenluft et al., 2004) were observed among new mothers.
2.3. Parental Empathy
Parental empathy (the appropriate perception, experience, and response to another’s emotion) may be especially relevant for pre-verbal infants. Experiencing pain of a loved one activates relatively focused anterior area of the dorsal ACC – perhaps a subset of a broader circuit for experiencing personal pain that also includes brainstem, cerebellum, and sensorimotor cortex. Such partial overlap of representations of empathy for others with self-related processing in cortical structures such as in the anterior insula are postulated as necessary for a theory of mind (Saxe, 2006), namely our ability to understand the thoughts, beliefs, and intentions of others in relation to ourselves (Frith and Frith, 2003; Hein and Singer, 2008). Humans may utilize separate circuitry to ‘decouple’ representations of external vs. internal information in order to understand many social exchanges. Indeed, considerable brain imaging research on empathy, largely related to the imitation of others’ emotions using stimuli such as emotional faces, images of others in pain or crying sounds (Fan et al., 2011), has highlighted the functional importance of medial PFC, precuneus/posterior cingulate cortex, temporoparietal junction, and posterior superior temporal sulcus (Frith and Frith, 2006; Mitchell, 2009). Additional regions including the anterior insula, orbitofrontal cortex and amygdala may be important for emotion information processing but also for both sharing and explicitly considering affectively charged states (Decety, 2015; Zaki and Ochsner, 2012).
Such ability to share and understand others’ emotional state is of importance for parental caring responses to own baby-cry (Swain et al., 2004) and other stimuli. For example, a complex set of brain responses was reported in a recent study of mothers responding to child visual feedback after a caring decision (Ho et al., 2014). Responses that correlated with dimensions of empathy included the aforementioned amygdala and ventrolateral PFC. Experiments using a parenting-empathy task will shed light on maternal brain function when actually asked to empathize with their infant. In sum, preliminary brain-based experiments suggest that parenting may be one instance of altruistic functions that apply to different social situations (Preston, 2013; Swain et al., 2012).
2.4. Executive Function
Executive functions, including attention and inhibitory control, working memory, and flexible task-switching, are likely to be important to parents’ sensitivity (Lovic and Fleming, in press). The dorsolateral and ventrolateral PFC are involved in cognitive flexibility (Mitchell et al., 2008), and the striatum is involved in inhibition control (Vink et al., 2005). Deficits in cognitive flexibility and spatial working memory have been linked with poor maternal sensitivity to non-distress infants’ cues (Gonzalez et al., 2012). Mothers with a classification of disorganized attachment responded more slowly to negative attachment words (e.g. abandon, reject) during the stroop task; these findings suggest that negative associations with attachment stimuli may contribute to ongoing cognitive difficulties during mother-infant interactions (Atkinson et al., 2009). Increased attention bias to infants’ distress cues in late pregnancy also has been associated with higher scores on a questionnaire about parental bonding (Pearson et al., 2010) and on a continuum with healthy postpartum preoccupations. Attention bias to infants’ distress has also been compared between breastfeeding and formula-feeding mothers of infants three to six months old and observed to be greater in breastfeeding mothers (Pearson et al., 2011).
2.5. Summary
Maternal adaptation to the role of motherhood and performance of associated behaviors for sensitive moment-to-moment responses to infant cues can be expected to depend on certain brain systems that need to be plastic to specific circumstances, and adapt to environmental cues. These included circuits that respond to and interpret emotional stimuli, provide reward and motivation for behavior, connect with more sophisticated executive function to process thoughts and attention and allow a mother to experience empathy and sensitively care for their child.
3. Postpartum Factors of Maternal Brain Plasticity
3.1. Anatomical changes in maternal brain
Drastic changes in hormones, neurochemistry, and experience can lead to neural plasticity in the maternal brain. In rodents, changes in the neural structure and function during the early postpartum period have been observed consistently to support maternal motivation and to enhance processing of sensory information that is specialized to detect and respond to infants’ calls (Fleming et al., 2002; Numan, 2007). Similar changes have been found in human mothers. Structural images of mothers’ brains from the first and third to fourth months postpartum were examined for longitudinal anatomical changes (Kim et al., 2010a). Several brain regions involved in maternal motivation and reward processing, including the striatum, amygdala, hypothalamus, and the substantia nigra, exhibited structural growth from the first time point to the next. Structural growth was also observed in areas involved in processing sensory information and empathy, including the superior temporal gyrus, thalamus, insula, and pre- and post-central gyri. Finally, regions associated with regulating emotions, such as the inferior and medial frontal gyri and the ACC, also showed structural increases. Interestingly, no neural regions showed reduction in grey matter during this time period. The evidence suggests that neural plasticity, particularly growth, occurs in a wide range of brain regions, each serving important aspects of child caregiving in human mothers during the first few months postpartum. Furthermore, the greater the observed structural growth in the midbrain region (involved in reward and motivation), the stronger the positive emotions a mother reported having about her baby in the third and fourth months postpartum. This finding further supports a bidirectional association between parenting experience and neural plasticity during the first few months postpartum.
3.2. Birth and feeding methods
To explain individual differences in the human maternal brain, studies have examined the maternal brain according to birth and feeding methods. Vaginal delivery provides critical sensory stimulation that increases release of OT and helps to establish maternal behaviors (Morgan et al., 1992). Such sensory stimulation is absent in cesarean section delivery. Although the sample size was relatively small, a fMRI study revealed significant associations between birth method and neural responses to cry sounds from their own babies (Swain et al., 2008). During the first month postpartum, mothers from the vaginal delivery group exhibited greater neural responses to their own babies’ cries (vs. control babies’ cries) compared to mothers who delivered via cesarean section. The vaginal delivery was associated with greater responses in areas important for the motivation region (striatum, hypothalamus, amygdala), sensory information processing (the superior and middle temporal gyri, fusiform gyrus, and thalamus), and cognitive and emotional control regions (superior frontal gyrus), compared to the cesarean section. Interestingly, further plasticity among healthy mothers is exhibited by the disappearance of significant functional difference between vaginal and cesarean delivering mothers brains in response to baby-cry by three to four months postpartum (Swain, 2011).
During the first month postpartum, feeding methods may also be significantly associated with maternal neural responses to cries of one’s own baby. Breastfeeding can increase the release of OT and in some studies is associated with higher maternal sensitivity and reduced levels of child neglect (Britton et al., 2006; Strathearn et al., 2009b). In an fMRI study, exclusive breastfeeding was associated with greater neural responses to cries of one’s own baby (vs. unrelated baby’s cry sounds), compared to exclusive formula-feeding (Kim et al., 2011). Interestingly, brain regions associated with breastfeeding vs. formula feeding largely overlapped those associated with vaginal vs. cesarean delivery. The neural regions showing greater activation among breastfeeding mothers included those related to maternal motivation (striatum, amygdala), sensory information processing (superior and middle temporal gyri), and cognitive and emotional control (superior frontal gyrus). Furthermore, greater neural responses in the striatum/amygdala and superior frontal gyrus to one’s own infant’s cries at the first month postpartum were positively associated with maternal sensitivity, observed during mother-infant interactions at three to four months postpartum. Thus, both delivery and feeding methods, which are potentially associated with maternal hormones such as OT, may enhance neural sensitivity to cries of one’s own baby’s in brain regions involved in maternal motivation, sensory information processing, and emotion regulation.
3.3. Hormones
Exciting recent discoveries about the impact of OT on neural responses to infant stimuli have been made using administration of OT. Among non-parent women, administration of OT was associated with reduced responses in the amygdala to negative emotional images that were stressful (Rupp et al., 2012), as well as stimuli that depict infants either crying (Riem et al., 2011) or laughing (Riem et al., 2013). In fact, in these studies, administration of OT was associated with increased activations in areas related to regulation of emotion and empathy, including the ACC, the OFC, and insula (Riem et al., 2011; Riem et al., 2013). Increased insula responses to infants’ cries were found among non-parent women when testosterone was administrated (Bos et al., 2010). This finding is not surprising given that testosterone is metabolized into estradiol, which can subsequently increase OT levels.
In addition to direct administration of hormones, genetic variations in hormonal receptor expressions have been associated with neural responses to child-related stimuli among mothers. In one study (Michalska et al., 2014), mothers’ parenting quality was assessed based on observations of mother-child interactions when children were aged 4 to 6 years. Years later, genetic samples were collected from mothers to allow the examination of single nucleotide polymorphisms in the gene encoding the OT receptor. They also viewed images of their own children aged 4 to 6 years, as well as unrelated children, during a neuroimaging session. Supporting the importance of OT in the central brain for parenting, mothers with the AA genotype, which has been associated with greater receptor expressions, showed significantly higher levels of positive parenting as well as greater neural responses to images of their own children compared to mothers with the GA and GG genotypes (Michalska et al., 2014). The OT gene polymorphism was also associated with increased activations in the OFC, ACC and hippocampus, particularly those regions related to emotion and stress regulation.
Another study of a similar sample of mothers examined genetic variations in the estrogen receptor gene (Lahey et al., 2012). Estrogen is important in priming maternal motivation regions of the brain: in animals, mothers with higher levels of estrogen show less hostile responses and increased caring behaviors toward their own pups. In this study of a human sample, a genetic variation associated with lower expressions of the estrogen receptor gene was related to harsh parenting practices. The associations were mediated by neural responses in the inferior frontal gyrus in response to one’s own child versus an unrelated child. Taken together, these studies suggest that individual differences in hormonal levels, including testosterone, estrogen, and OT, may influence maternal neural responses to infants and, in turn, behavioral sensitivity to infants.
3.4. Exposure to Stress and Childhood Adversity
Whereas OT has been shown to increase neural and behavioral sensitivity to infants, stress has been shown to diminish it. For example, current levels of parenting-related stress in human mothers were associated with individual differences in neural responses to infants (Barrett et al., 2012). Mothers at three months postpartum viewed images of their own versus an unrelated infant. Lower levels of parental distress and more positive attachment-related feelings about their own infants were associated with increased responses in the amygdala to their own infants’ versus unrelated infants’ positive images (e.g. smiling).
Early life stress also shapes later brain function (McGowan et al., 2009). Exposure to stress activates the hypothalamic–pituitary–adrenal (HPA) axis and increases cortisol levels, which may reduce neural responses of mothers to an infant’s cry, in part due to difficulties in regulation of emotions. Mothers with infants aged 18 months old had their cortisol levels measured following a strange situation, a task that can elicit stress reactivity in the HPA axis to mother-child separation (Laurent et al., 2011). Mothers then participated in a neuroimaging session while listening to their own infant’s cry versus an unrelated infant’s cry sounds. Reduced reactivity to stress in the HPA axis was associated with greater activation in the midbrain and striatum, areas involved in maternal motivation; the insula and OFC, areas involved in negative-emotion processing; and the dorsolateral PFC and ACC, areas involved in emotion regulation. Thus, poor physiological stress regulation can contribute to diminished neural responses to infants’ cry sounds among mothers.
In addition to current stress levels, early adversity may have long-term effects on stress regulation and maternal motivation (Champagne et al., 2003; Fleming et al., 2002). Mothers with infants aged one month old were divided into two groups based on retrospective self-reported quality of maternal care received during childhood (Kim et al., 2010b). Mothers with high vs. low scores on maternal care were compared on neural structure and functional responses to infants’ cries. High quality of maternal care in childhood was associated with increased grey matter volumes in regions involved in regulation of emotions and social and sensory information processing, including the superior frontal gyrus, the OFC, the superior and middle temporal gyri, and the fusiform gyrus. Furthermore, higher levels of neural responses to infants’ cry sounds were found in these same regions. The only region that was more active in mothers who reported low quality of maternal care in childhood was the hippocampus. The hippocampus is particularly rich in the glucocorticoid receptors, and critically involved in stress regulation (McEwen, 2001). Increased hippocampal activations has been observed in response to both acute and chronic exposure to stress in human and animals (Tottenham and Sheridan, 2009; van Hasselt et al., 2012). Thus, the increased activation in the hippocampus in response to infants’ cries among mothers with lower quality childhood experiences may reflect greater stress responses to the cries. Therefore, the findings suggest that differences in maternal neural responses to infants are influenced by mothers’ own childhood experiences, which contribute to baseline differences in terms of neural structure and functions.
Although OT plays a role in stimulating the onset and maintenance of maternal behavior, exposure to childhood adversity may result in abnormal development of the OT system in the offspring, as well as lead to poorer quality of parenting behavior later in adulthood (Champagne and Meaney, 2001; Champagne, 2011; Champagne and Meaney, 2007). In rhesus monkeys, for example, non-maternal (or nursery) rearing was associated with reduced levels of cerebrospinal fluid OT for the first three years of life (Winslow et al., 2003). Similarly, cerebrospinal fluid OT levels were reduced in women who reported childhood maltreatment, and they were inversely correlated with scores of emotional abuse (Heim et al., 2009). Furthermore, in first-time mothers, the amygdala responds to infants’ affect cues as a salience signal, responding more to one’s own infant faces than to unknown infants (Strathearn and Kim, 2013)
Differences in classifications of adult attachments may be associated with the quality of childhood caregiving experience. Previous studies have shown that mothers with an insecure/dismissing type of adult attachment show a diminished OT response to mother-infant interaction, which is associated with reduced activation in the hypothalamus and the ventral striatum. Mothers from this attachment group also show reduced activation of the mesocorticolimbic DA system, including the ventral striatum and ventromedial PFC, when viewing images of their own versus unknown infants’ faces (Strathearn et al., 2009a). Furthermore, this pattern of attachment is associated with differences in maternal behavior, including less attuned mother-to-infant vocalization at seven months postpartum (Kim et al., 2014), and with higher rates of insecure child attachment at 14 months, based on the Strange Situation Procedure (Shah et al., 2010).
3.5. Maternal Brain – Behavior Associations
Since fMRI techniques were first used to examine maternal neural activation for parenting, researchers have looked for specific associations between neural and behavioral sensitivity to infants, particularly during the early postpartum period when mother-infant relationships are first established. Several exciting studies have now demonstrated that individual differences in parenting behaviors may be based on variations in neural responses to infant stimuli. In one of these studies, mothers at four to six months postpartum were divided into two groups: mothers with high synchronous scores and low intrusiveness scores (synchronous mothers) and mothers with low synchronous scores and high intrusiveness scores (intrusive mothers) (Atzil et al., 2011). Synchronous maternal behaviors, including coordination of gaze, touch, and vocalizations with infants, are interpreted as more sensitive parenting behaviors and are associated with positive infant outcomes. Contrariwise, intrusive maternal behaviors include lack of coordination and more directedness with the infant, and they tend to be associated with maternal anxiety and the HPA and stress responses (Feldman, 2007). During a neuroimaging session, mothers were presented with video clips of their own infants and an unfamiliar infant. The main contrast between responses to their own versus the unfamiliar infant was greater activation in the NAcc, a key reward/motivation region, and the amygdala, a key stress and negative emotion processing area. When intrusive and synchronous mothers were compared, intrusive mothers showed greater responses in the amygdala to their own babies, whereas synchronous mothers showed greater activation in the NAcc. Furthermore, functional connectivity in the whole brain using the NAcc and the amygdala as seed regions was examined, and the intrusive and synchronous mothers were compared. In synchronous mothers, activity in the NAcc was correlated with activity in attention and social information processing regions, including the inferior frontal gyrus, the medial frontal gyrus, visual and motor areas, and the parietal cortex. Contrariwise, intrusive mothers showed greater connectivity between the amygdala and the OFC, which is characteristic of elevated anxiety. Thus, reward-related neural responses to one’s own infant were associated with enhanced neural connectivity for attention and social information processing, which may further support synchronous mother-infant interactions. Anxiety-related neural responses to one’s own infant were associated with more disrupted and intrusive mother-infant interactions.
In another study, the same group of researchers demonstrated that among mothers, plasma OT levels correlated positively with activations in the ventral ACC, the left NAcc, the inferior parietal lobule, and the temporal and frontal gyri in response to videos of their own infants (Atzil et al., 2012). When mothers watched videos of various mother-infant interactions, they exhibited greater neural responses to synchronous interactions compared to abnormal interactions (such as between depressed or anxious mothers and their infants), particularly in the dorsal ACC (Atzil et al., 2013). Additionally, greater activation in the dorsal ACC in response to synchronous interactions was positively associated with mothers’ own synchronous scores. The dorsal ACC is involved in integrating affective and social processes as well as regulating social pain such as social rejection. Thus, greater activation in the dorsal ACC may contribute to more sensitive processing of social cues, which may be further associated with highly synchronous behaviors among mothers interacting with their own infants.
Other researchers have found associations between parenting behaviors and greater responses to infant stimuli in neural regions important for regulating emotions and processing social information. First-time mothers and their infants at 18 months participated in structured mother-infant play interactions (free play and cleanup segments) during which maternal sensitivity and intrusiveness were assessed (Musser et al., 2012). During a neuroimaging session, mothers listened to their own infants’ cry sounds as well as those of an unfamiliar infant. When neural responses to their own versus a control infant’s cry were examined, the observed maternal sensitivity was associated with prefrontal activations (superior and inferior frontal gyri), regions involved in regulation of emotions. Mother-infant harmony was associated with activations in the hippocampus and the parahippocampus, as well as the precuneus, which may indicate better stress management. In another study of mothers with infants aged four to ten months, the observation of positive mother-infant interactions was associated with increased response to videos of their own infants (vs. control infants) in the putamen and the inferior and middle frontal gyri, areas involved in maternal motivation and regulation of emotions (Wan et al., 2014). Self-reported maternal warmth was associated with greater neural responses to videos of their own infants in the precuneus, visual areas, the insula, and the medial frontal gyrus. The findings suggest that positive parenting behaviors are associated with greater responses to mothers’ own infants compared to unfamiliar infants in brain areas involved in social and sensory information processing.
3.6. Summary
Taken together, these findings demonstrate that during the early postpartum period, the human maternal brain exhibits great neural plasticity, both in structure and function, which supports the mother’s new role. Research on variations in birth and feeding methods, exposure to stress, hormone levels, and gene expressions can significantly enhance the understanding of individual differences in the neural structure and functioning that support parenting. Furthermore, neural activations in regions involved in increased maternal motivation and regulation of emotions are particularly important for predicting sensitive parenting behaviors among human mothers. Although these activations were largely related to a normal range of individual differences, psychopathology may be associated with abnormal maternal neural and behavioral responses to infants. In the following section, we will review differences between healthy moms and those affected by psychopathology that demonstrates maladaptive plasticity, particularly in relation to the brain-OT associations.
4. Maternal Psychopathology and Neural Plasticity
4.1. Postpartum Depression (PPD)
PPD is a major public health concern, affecting 15 to 20% of pregnancies (Gavin et al., 2005; Gaynes et al., 2005). Risk factors for developing PPD include high-stress circumstances, minority status, younger age, low-socioeconomic status, and reduced experience of sensitive parenting from one’s own parents (Gotlib et al., 1991; Murray and Cooper, 1997a; Stowe and Nemeroff, 1995; Weisman et al., 2010). High-risk status, which includes previous major depressive episodes and abnormal stress hormone regulation, specifically cortisol and adrenocorticotropic (Marcus et al., 2011; Sexton et al., 2011; Vazquez et al., 2015), confers alarming rates of PPD as high as 50–80%, depending on the measures, postpartum timing, and definitions used (Deal and Holt, 1998; Gee and Rhodes, 2003; Hipwell and Kumar, 1996; Hipwell et al., 2005; Logsdon et al., 2005). For most women with PPD (∼89%), diagnosis is made between two weeks and four months postpartum (Stowe et al., 2005) and can last for a year or more, resulting in a heightened risk for future lifetime episodes of depressive illness (Evans et al., 2001; Gotlib et al., 1989; Stowe et al., 2005; Warner et al., 1996).
Many studies show that the infant also suffers when the mother is affected by PPD, even when the mother’s symptoms do not reach symptomatic severity of a major depressive disorder (McLearn et al., 2006; Murray and Cooper, 1997b; Righetti-Veltema et al., 2003). Long-term adverse effects on the child’s development, emotions, and regulation of behavior (Grace et al., 2003; Halligan et al., 2007; Hay et al., 2008) cross generations through impairments in maternal care-giving sensitivity (Feldman et al., 2009; Field et al., 1988; Madigan et al., 2015; Murray and Cooper, 1997a; Stanley et al., 2004; Weissman et al., 2004) – in part via OT effects on neural circuits (Apter-Levy, et al. 2013; Feldman et al., 2010). Despite the enormous personal, familial, and societal costs of these mental health disorders, progress in the study of underlying neurohormonal mechanisms that could improve identification and inform strategies for personally tailored intervention has been slow.
Existing literature points to dampened neural responses in the emotion regulation circuits among mothers with PPD. Two existing studies (Moses-Kolko et al., 2010; Silverman et al., 2007) of mothers attending to non-infant negative emotional stimuli found that PPD versus healthy status and greater PPD severity was associated with reduced activity in the amygdala to fearful and threatening faces or negatively valenced words. This finding is in apparent opposition to the increased activity of the amygdala found in non-postpartum depression (Groenewold et al., 2012). Perhaps PPD, which reduces maternal sensitivity, (Feldman et al., 2009) is associated with an amygdala shut-down not seen in other forms of depression, and plasticity in amygdala function may be a therapeutic target (see section 4.4). A small study (n=8) revealed reduced activity in the posterior OFC in response to negative words in women with PPD compared to healthy postpartum women (Silverman et al., 2007). Further, Laurent and colleagues (Laurent and Ablow, 2012) reported, for own vs. control sound, that depressed vs. non-depressed mothers had less activity in the medial thalamus, caudate and NA; and that the higher depressive symptoms (Center for Epidemiologic Studies Depression Scale, CESD) were associated with decreased activity in caudate, putamen, NA-medial thalamus and posterior OFC. These studies suggest reduced neural responses across an array of limbic and cortical structures to negative emotional stimuli and infant distress cues, which may be associated with increased risks for mood dysregulation and maternal insensitivity.
Little is known about within-circuit connectivity of cortical brain regions that can directly modulate limbic reactivity to infants and other emotional cues in postpartum women. In a single such study, women with PPD demonstrated an absence or disengagement of dorsomedial PFC- amygdala connectivity while viewing fearful and threatening faces, whereas healthy mothers had intact dorsomedial PFC-amygdala connectivity to fearful and threatening faces. (Moses-Kolko et al., 2010). The available data, therefore, suggest that women with PPD disengage critical cortico-limbic neurocircuitry - that is involved in emotional salience and processing a threat - during exposure to infants’ and negative affective stimuli. This finding requires further examination with techniques for analyzing within-circuit regional connectivity.
An interesting exception, however, was the finding that heightened activity in the lenticular nucleus and left medial PFC to the cry of one’s own baby was associated with more depressive symptoms and more anxious intrusive thoughts in a group of 12 mothers scanned on average three weeks postpartum (Swain et al., 2008). Swain and colleagues argue for a potentially adaptive function subserved by these apparent anxious/depressive symptoms in non-depressed mothers in the early postpartum phase, which are different to findings in mothers who are three or more months postpartum. Swain and colleagues, in other publications (Swain et al., 2015; Swain and Lorberbaum, 2008), postulate that maternal neural responses to infants’ stimuli change with time since delivery, with diminished anxiety-related subcortical and increased regulatory cortical responses to their infants’ cries, among healthy mothers. Indeed, both human (Kim et al., 2010a) and rodent (Kinsley and Meyer, 2010), maternal brain research reveal postpartum structural brain growth in orbitofrontal and temporal cortices, which may reflect social learning in parents in preparation for changing baby signals over time in addition to the increasingly complex array of possible interactional scenarios. This also opens windows into domains of dysfunction when these adaptations fail to occur, leaving new parents underprepared to respond to increasingly complex situations. Therefore, postpartum duration-dependent analysis of fear/salience network activity and PFC modulatory networks is an important area in need of future investigation.
4.2. Posttraumatic stress disorder (PTSD)
PTSD is debilitating condition characterized by flashbacks, hyperarousal, hypervigilance, emotional numbing, mood liability, insomnia, and avoidance of potentially triggering situations (DSM-IV; American Psychiatric Association, 1994). It has also been associated with mild to moderate cognitive impairment, most notably, reduced concentration and difficulties associated with learning and memory (Vasterling et al., 2002). In the general population, lifetime prevalence of PTSD across the Western world ranges from 1.9% to 6.8% (Kessler et al., 2005; Wittchen et al., 2011). Neurobiological correlates in non-postpartum samples include increased insula and amygdala activity while listening to traumatic audio clips in the scanner (Hopper et al., 2007).
While PDD was associated with dampened neural response, PTSD among mothers is associated with heightened neural responses to infants in the emotion regulation circuits. Similar to non-postpartum populations, but in contrast to anxious and depressed postpartum mothers,12–48 months postpartum mothers with PTSD secondary to adult interpersonal violence-related PTSD (IPV-PTSD) had heightened subcortical responses in the fear circuitry (Schechter et al., 2012). In this study of mothers viewing child separation vs. free play videos, IPV-PTSD mothers activated an “unrestrained” fear-circuitry that was associated with higher subjective ratings of stress. The circuitry described included heightened activity in emotion regulation and empathy regions -- bilateral anterior entorhinal cortex, left caudate, left insula, and reduced frontocortical activity (superior frontal gyrus and bilateral superior parietal lobes) in women with IPV-PTSD compared to healthy mothers.
PTSD symptoms were inversely correlated with superior frontal gyrus activity and positively associated with caudate activity. Based upon behavioral data in this same population (Schechter et al., 2010), it was suggested that hyperactivity within the fear circuitry associated with IPV-PTSD might mediate reduced maternal emotional availability for coordinated joint attention during play. A further analysis with the same dataset demonstrated that dissociative symptoms, when present with or without PTSD, were associated with increased emotion regulation cortical regions (right dorsolateral PFC) and decreased limbic activity (perirhinal cortex, hippocampus, insula) to separation versus play videos (Moser et al., 2013). This is in accord with a report in other PTSD-dissociative-subtype populations viewing traumatic and nonspecific negative emotional stimuli (Lanius et al., 2007). This finding is also consistent with the dampened amygdala response seen in mothers with unresolved attachment trauma, after viewing their own (but not unknown) infant’s crying faces (Kim et al., 2014a). These findings highlight the importance of the emotion regulation region and limbic activation for dissociation, PTSD symptoms and other domains of cognitive function that affect maternal sensitivity and child outcome.
4.3. Substance Abuse
Drug addiction continues to be a substantial and growing public health problem in the US. Substance abuse in mothers is particularly problematic because of the negative long-term impact it may have on their children. Many women rapidly resume substance use after childbirth, with resultant adverse effects on their parenting capacity and their children’s development. Addiction problems in mothers are associated with a range of major parenting difficulties, often resulting in child abuse and neglect (Mayes et al., 1997; Strathearn and Mayes, 2010).
Unlike many mothers who find engaging with their own children to be a uniquely rewarding experience, mothers with addictions may be less able to respond appropriately to their children’s cues, finding them less intrinsically rewarding or salient and more stress-provoking (Rutherford et al., 2011). Current research is focusing on the ways in which drug addiction involves altered brain responses that underlie maternal behavior (Strathearn and Mayes, 2010), having previously demonstrated that infants’ cues activate dopaminergically innervated brain reward circuits similar to those associated with drugs of abuse (Strathearn et al., 2008). This finding raises the possibility that drugs of abuse may co-opt the brain circuitry critical for maternal caregiving.
Likewise, drugs of abuse such as cocaine stimulate DA pathways in similar brain regions (Kufahl et al., 2005). As with other biological systems, the development of the dopaminergic system appears to be regulated by patterns of stimulation over time. For example, chronic stimulation of DA systems by drugs of abuse tends to up-regulate and desensitize DA receptors, which may lead to escalating drug use and withdrawal symptoms during early abstinence.
The pathways leading to addiction are complex and multidimensional, and they include differences in molecular and genetic expression, altered brain sensitivities to reward- and stress-related cues, and behavioral patterns that include risk-taking, social isolation, and/or trauma symptomatology. Epidemiological studies have linked addiction in adulthood to prior adverse early childhood experiences (Anda et al., 2006; Dube et al., 2003b), with increased risk/severity related to the number of adverse factors experienced, such as childhood abuse, domestic violence, and parental addiction. Early exposure to low quality maternal care may also alter the development of dopaminergic pathways associated with reward-related behaviors, and response to drugs. For example, rodents who were reared in isolation showed changes in distribution of DA receptors (Gill et al., 2013), displayed more inattentive and impulsive behaviors (Lovic et al., 2011; Ouchi et al., 2013), and had an exaggerated DA response in the ventral striatum to systemic amphetamine (Hall et al., 1998). Similarly, humans with low self-reported “parental bonding” on the Parental Bonding Instrument show altered ventral striatal DA responses to psychological stress (Pruessner et al., 2004). Whereas striatal DA responses to natural rewards, such as infants’ cues, may be blunted in animals exposed to lower levels of maternal caregiving (Champagne et al., 2004; Strathearn, 2011), responses to stress and stimulant drugs appear to be increased.
Current research has shown that mothers in treatment for drug addiction have almost universally experienced severe childhood trauma that remains unresolved into adulthood (98% of an addiction cohort [n=44] vs. 67% of a control group [n=18]) (Kim et al., 2014b). Animal models have helped to elucidate developmental pathways by which stress experienced in early life may impact neuroendocrine development and translate into behavioral patterns that increase the susceptibility to addiction. Through epigenetic mechanisms, experiences in early life appear to modify gene and receptor expression in neuroendocrine systems, leading to changes in adult behavior, including drug seeking and addiction (Caldji et al., 2011; Champagne, 2011; Meaney et al., 2002; Sng and Meaney, 2009; Weaver et al., 2004).
Parenting difficulties that result from addiction can lead to the perpetuation of childhood abuse and neglect (Dube et al., 2003a), thus continuing the cycle of childhood adversity and addiction. In fact, addicted mothers are far more likely to lose custody of their children as a result of child neglect or abuse (Minnes et al., 2008). Furthermore, growing up in a negative home environment and with childhood neglect is significantly correlated with higher levels of parenting stress in adulthood and an increased display of problematic parenting behaviors (Harmer et al., 1999). Taken together, there appears to be increasing evidence of intergenerational effects, whereby mothers who abuse drugs have often experienced grossly inadequate caregiving environments during their own childhood, perhaps further accentuating neglectful or abusive care of their children that is associated with substance abuse..
4.4. How Neuroimaging Studies Inform Parenting Intervention
Our understanding on neural regions involved in parenting is still limited to inform the development of specific intervention strategies, but as the first step, the field is interested in evaluating neural changes following parenting interventions. Given the apparent plasticity of the maternal brain in adaptive mental health and psychopathological maladaptation, brain changes are likely occurring when parenting interventions are applied. The importance of such interventions is underlined by a growing body of research demonstrating that infants of mothers with mental health problems are at increased risk for developmental delays, cognitive and functional impairments, physical symptoms and injuries, as well as behavioral and emotional problems in the affected pre-school and school age children (Bagner et al., 2010; Bureau et al., 2009; Cornish et al., 2005; Feldman et al., 2009; Grace et al., 2003; Halligan et al., 2007; Hay et al., 2008; Sohr-Preston and Scaramella, 2006). Currently, mental health interventions are thus being implemented for real-world primary care treatment settings where mothers seek treatment for difficulties with parenting due to attachment, mood and anxiety disorders, and promotion of attachment security in the child. One recent study revealed that mothers with unresolved trauma, but whose attachment pattern was reorganizing toward security, were more likely to have children with secure attachment (Iyengar et al., 2014).
Specific intervention programs including the Circle of Security (Hoffman et al., 2006; Powell et al., 2014), Triple P (Positive Parenting Program) (Sanders et al., 2014), Video Interaction for Promoting Positive Parenting Programme (Van Zeijl et al., 2006), and Mom Power (Muzik et al., 2015), have been validated according to randomized clinical trial approaches. Preliminary functional imaging findings on Mom Power parenting intervention for mothers of children aged two to seven years at mental health risk due to history of psychopathology involving at least one trauma (Swain et al., 2014a) suggest brain correlates of the intervention. There were significant increases in brain-activity as a function of parenting treatment (n=14), controlling for time and treatment-as-usual (n=15), in response to stimuli from one’s own vs. other infant, in the amygdala, precuneus, dorsal anterior cingulate cortex and dorsolateral prefrontal cortex (DLPFC) (p < 0.001, uncorrected). Brain regions with increased response to “your-baby-cry” versus “just-listen” included emotion regulation regions -- the amygdala, the precuneus, the dorsal ACC, and the dorsolateral PFC. Brain activity for an own-child empathy task was also increased in the dorsolateral PFC and insula. Furthermore, brain activity was inversely related to parenting stress (p < 0.001, uncorrected) for the own baby-cry task, in empathy regions: precuneus, medial PFC and temporoparietal junction. The findings provide preliminary evidence for neural mechanisms for parenting intervention in plastic empathy circuits.
4.5. Summary
We reviewed abnormal neural regulation of parenting among mothers with psychopathology. Mothers with syndromal level depression have blunted amygdala and insula activity to negative emotional stimuli and infant distress stimuli, which is the opposite of that described in non-postpartum depressed samples. Heightened intrusive/anxious responses that accompany subsyndromal affective symptoms in very early (3 weeks) postpartum mothers, associated with medial PFC and lenticular nucleus activity to infant cry, may change over time and serve an adaptive role that fails in the development of syndromal depression. Reward-related neural responses to positive words and monetary gain are noted to be reduced in depressed mothers. Mothers, more than one year postpartum, who have PTSD, have a neurobiology which resembles that in non-postpartum populations, characterized by hyperactivity within the fear circuitry (amygdala and anterior insula) and alternatively decreased or increased (dissociative-type) voluntary ventromedial PFC/ventral ACC regulation of subcortical activity. Circuits that support social cognition/mentalization/empathy in healthy mothers are hyporesponsive to infant stimuli in mothers with insecure attachment and substance use disorders. This broad domain confers potential as a marker of impaired maternal caregiving that might benefit from greater specificity and standardization in the future. Detecting neural endophenotypes of disrupted maternal care may guide earlier inventions and improve the mental and physical health of mother and offspring.
5. Future directions of the field
There has been significant improvement in our understanding of the human maternal brain based on a growing number of fMRI studies with human mothers. However, important questions remain unanswered. First, although most of the studies with human mothers have focused on understanding neural functions, little work has been done regarding structural changes. Although human evidence suggests structural growth occurs in the maternal brain (Kim et al., 2010a), animal evidence suggests mixed findings on reduced neurogenesis in the hippocampus but increased synaptic density in the prefrontal cortex during the postpartum period (Leuner et al., 2010). Furthermore, current literature suggests mixed evidence in the direction of the anatomical and functional correlations. While training-induced increased grey matter volumes have been associated with increased activation in the hippocampus (Hamzei et al., 2012), decreased grey matter volumes were associated with increased activation in the amygdala among trauma-exposed individuals (Ganzel et al., 2008). Therefore, it would be important to clarify hormone-related and experienced-based anatomical changes and how they interact with neural functions among human mothers during the early postpartum period, which will provide deeper understanding of the neural plasticity of the maternal brain.
Second, prospective and longitudinal studies across important transition periods for parenting are important to map the temporal processes of neural changes in the human maternal brain. Existing findings of the human maternal brain are based primarily on studies with women during the postpartum period or the first few years of a child’s life. However, measures in these studies, such as maternal mood, hormones, neural activation, and parenting behaviors, are measured cross-sectionally, providing only correlative associations that must be interpreted with caution. Thus, causal or temporal conclusions cannot be drawn on how these factors are related to each other. Therefore, prospective studies, particularly studying women during pregnancy and/or even before conception with follow-up until the postpartum periods, may help determine if hormonal changes during the pregnancy prime and enhance neural activation in response to infants during the early postpartum period, which will be further associated with more sensitive maternal behavioral responses to infants during later postpartum periods.
Third, negative environments such as living in poverty, being a single or teenage mother, and high marital conflicts are significant risk factors for maternal insensitivity toward infants and for psychopathology (Magnuson and Duncan, 2002; Sturge-Apple et al., 2006). However, little is known about whether such negative environments can increase risk for negative maternal outcomes through changes in the neurobiological processes of parenting and mood regulation (Kim and Bianco, 2014). Therefore, future research may recruit mothers in at-risk environments and examine the effects of the environmental factors on neural responses to infant stimuli.
Fourth, we have discussed findings in mothers with defined psychopathology in the previous section, including postpartum depression and substance abuse. Larger and more targeted samples may help to identify specific neural mechanisms that are most affected in specific psychopathologies. For instance, dysfunctions in the regulation of the emotion network may be associated with postpartum depression, whereas the reward/motivation network may be more associated with substance abuse. Alternately, as proposed in the National Institute of Mental Health’s Research Domain Criteria (RDoC) (Cuthbert, 2014) continuous symptom spectra, which may overlap across defined psychopathologies, may better align with neurobiological systems. Such specificity can be critical for developing targeted interventions and treatments that are more effective in preventing psychopathology and improving symptoms of psychopathology among new mothers.
Fifth, the field will benefit from continuing to move toward combining well-established paradigms known to probe certain aspects of brain function, such as executive functions and emotion response/regulation with naturalistic and personally salient infant information. At the same time, the field also lacks studies examining specific links between neural regions/networks and behaviors throughout pregnancy and the postpartum period. For example, verbal recall memory declines during pregnancy and the postpartum period (Glynn, 2010). The next step may include examining whether activation in specific neural regions/circuits such as the hippocampus and precuneus/posterior cingulate cortex, which are parts of the neural memory circuits, change over time across pregnancy and the postpartum period, and moreover, whether the neural changes in the memory circuit are associated with memory performance using standardized episodic memory tasks.
Furthermore, the field has pointed out the need to incorporate more naturalistic and personally relevant stimuli. The child may be included in forms of neuroimaging that allow for some natural movement, such as with functional near-infrared spectroscopy (fNIRS) and electroencephalography (EEG). This may yield brain-based models that reflect real-life parental planning, responding and decision-making, and perhaps avoid neuroimaging problems in other fields that have typically been difficult to replicate or relate to, perhaps because of not using personally tailored stimuli.
Finally, alternate neuroimaging methods will also be needed to incorporate brain structure, resting state and functional neural activation, and parenting behaviors. Such multimodal approaches that use machine learning methods promise diagnostic and prognostic models for healthy maternal adaptation vs. psychopathology (Orru et al., 2012) that may not be possible with any one method. Perhaps in the future, a routine brain-scan - with advanced postprocessing - will provide biomarkers for earlier assessment and correction of parenting problems toward breaking trans-generational mental health problems.
Highlights.
Human maternal caregiving is governed by plastic brain networks.
The maternal brain networks integrate emotion and reward with cognitive control.
The human maternal brain adapts for mother-infant bonding.
The human maternal brain changes with psychopathology.
Parenting interventions may affect the plasticity of the human maternal brain.
Acknowledgements
The authors of this paper are supported by grants from National Institute of Child Health and Human Development (R21 HD078797 [PK], R01 HD065819 [LS]), the National Institute on Drug Abuse (R01 DA026437 [LS]), the National Center for Advancing Translational Sciences via the University of Michigan Institute for Clinical Health Research UL1 TR000433 (JES), Centers for Disease Control & Prevention Award Number via the University of Michigan Injury Center U49/CE002099 (JES), Brain & Behavior Research Foundation (JES) and Klingenstein Third Generation Foundation (JES). The authors also thank Dr. B. Lee Ligon, Center for Research, Innovation and Scholarship (CRIS), Department of Pediatrics, Baylor College of Medicine, in addition to Ilinca Caluser, Zainab Mahmood and Madalyn Meldrim at the University of Michigan for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson L, Leung E, Goldberg S, Benoit D, Poulton L, Myhal N, Blokland K, Kerr S. Attachment and selective attention: disorganization and emotional Stroop reaction time. Dev. Psychopathol. 2009;21:99–126. doi: 10.1017/S0954579409000078. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacol. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. The brain basis of social synchrony. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Pettit JW, Lewinsohn PM, Seeley JR. Effect of maternal depression on child behavior: a sensitive period? J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:699–707. doi: 10.1016/j.jaac.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc. Neurosci. 2012;7:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocr. 2010;35:114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. 2nd ed. New York: Basic Books; 1969. [Google Scholar]
- Britton JR, Britton HL, Gronwaldt V. Breastfeeding, sensitivity, and attachment. Pediatr. 2006;118:e1436–e1443. doi: 10.1542/peds.2005-2916. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bureau JF, Easterbrooks MA, Lyons-Ruth K. Maternal depressive symptoms in infancy: unique contribution to children’s depressive symptoms in childhood and adolescence? Dev. Psychopathol. 2009;21:519–537. doi: 10.1017/S0954579409000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Hellstrom IC, Zhang T-Y, Diorio J, Meaney MJ. Environmental regulation of the neural epigenome. FEBS Letters. 2011;585:2049–2058. doi: 10.1016/j.febslet.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog. Brain. Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Maternal imprints and the origins of variation. Horm. Behav. 2011;60:4–11. doi: 10.1016/j.yhbeh.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behav. in the rat. J. Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinol. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Cohen L, Mizrahi A. Plasticity during Motherhood: Changes in Excitatory and Inhibitory Layer 2/3 Neurons in Auditory Cortex. J. Neurosci. 2015;35:1806–1815. doi: 10.1523/JNEUROSCI.1786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish AM, McMahon CA, Ungerer JA, Barnett B, Kowalenko N, Tennant C. Postnatal depression and infant cognitive and motor development in the second postnatal year: The impact of depression chronicity and infant gender. Infant Behav. Dev. 2005;28:407–417. [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal LW, Holt VL. Young maternal age and depressive symptoms: results from the 1988 National Maternal and Infant Health Survey. Am. J. Public Health. 1998;88:266–270. doi: 10.2105/ajph.88.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J. The neural pathways, development and functions of empathy. Curr. Opin. Behav. Sci. 2015;3:1–6. [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the Hemodynamic Responses to Reward and Punishment in the Striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood Abuse, Neglect, and Household Dysfunction and the Risk of Illicit Drug Use: The Adverse Childhood Experiences Study. Pediatr. 2003a;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatr. 2003b;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. Bmj. 2001;323:257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011;35:903–911. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:919–927. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Leckman JF, Kuint J, Eidelman AI. The nature of the mother’s tie to her infant: maternal bonding under conditions of proximity, separation, and potential loss. J Child Psychol. Psychiatry. 1999;40:929–939. [PubMed] [Google Scholar]
- Feygin DL, Swain JE, Leckman JF. The normalcy of neurosis: evolutionary origins of obsessive-compulsive disorder and related behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:854–864. doi: 10.1016/j.pnpbp.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Field T, Healy B, Goldstein S, Perry S, Bendell D, Schanberg S, Zimmerman EA, Kuhn C. Infants of depressed mothers show “depressed” behavior even with nondepressed adults. Child. Dev. 1988;59:1569–1579. doi: 10.1111/j.1467-8624.1988.tb03684.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol. Biochem. Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ.) 2005:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CB, Rhodes JE. Adolescent mothers’ relationship with their children’s biological fathers: social support, social strain, and relationship continuity. J. Fam. Psychol. 2003;17:370–383. doi: 10.1037/0893-3200.17.3.370. [DOI] [PubMed] [Google Scholar]
- Gill KE, Beveridge TJR, Smith HR, Porrino LJ. The effects of rearing environment and chronic methylphenidate administration on behavior and dopamine receptors in adolescent rats. Brain Res. 2013;1527:67–78. doi: 10.1016/j.brainres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM. Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148–1155. doi: 10.1016/j.psyneuen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. Maternal early life experiences and parenting: the mediating role of cortisol and executive function. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:673–682. doi: 10.1016/j.jaac.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J. Consult. Clin. Psychol. 1989;57:269–274. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Wallace PM, Mount JH. Prospective investigation of postpartum depression: factors involved in onset and recovery. J. Abnorm. Psychol. 1991;100:122–132. doi: 10.1037//0021-843x.100.2.122. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch. Womens Ment. Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2012;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation Rearing in Rats: Pre- and Postsynaptic Changes in Striatal Dopaminergic Systems. Pharmacol. Biochem. Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J. Affect. Disord. 2007;97:145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Glauche V, Schwarzwald R, May A. Dynamic gray matter changes within cortex and striatum after short motor skill training are associated with their increased functional interaction. Neuroimage. 2012;59:3364–3372. doi: 10.1016/j.neuroimage.2011.10.089. [DOI] [PubMed] [Google Scholar]
- Harmer ALM, Sanderson J, Mertin P. Influence of negative childhood experiences on psychological funtioning, support, and parenting for mothers recovering from addiction. Child Abuse Negl. 1999;23:421–433. doi: 10.1016/s0145-2134(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol. Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Sharp D. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J. Child Psychol. Psychiatry. 2008;49:1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr. Opin. Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Kumar R. Maternal psychopathology and prediction of outcome based on mother-infant interaction ratings (BMIS) Br. J. Psychiatry. 1996;169:655–661. doi: 10.1192/bjp.169.5.655. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Murray L, Ducournau P, Stein A. The effects of maternal depression and parental conflict on children’s peer play. Child Care Health Dev. 2005;31:11–23. doi: 10.1111/j.1365-2214.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- Ho SS, Konrath S, Brown S, Swain JE. Empathy and stress related neural responses in maternal decision making. Front. Neurosci. 2014;8:152. doi: 10.3389/fnins.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KT, Marvin RS, Cooper G, Powell B. Changing toddlers’ and preschoolers’ attachment classifications: the Circle of Security intervention. J. Consult. Clin. Psychol. 2006;74:1017–1026. doi: 10.1037/0022-006X.74.6.1017. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. trauma. stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Maternal sensitivity to infants in various settings predicts harsh discipline in toddlerhood. Attach. Hum. Dev. 2012;14:101–117. doi: 10.1080/14616734.2012.661217. [DOI] [PubMed] [Google Scholar]
- Keller H, Lohaus A, Völker S, Elben C, Ball J. Warmth and contingency and their relationship to maternal attitudes toward parenting. J. Genet. Psychol. 2003;164:275–292. doi: 10.1080/00221320309597984. [DOI] [PubMed] [Google Scholar]
- Keren M, Feldman R, Eidelman AI, Sirota L, Lester B. Clinical interview ofr high-risk parents of premature infants (CLIP): relations to mother-infant interaction. Infant Ment. Health J. 2003;24:93–110. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim P, Bianco H. How Motherhood and Poverty Change the Brain. Zero to Three. 2014;34:29–36. [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J. Child Psychol. Psychiatry. 2011b;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosc. 2010a;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman M-A, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev. Sci. 2010b;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Mayes L, Feldman R, Leckman JF, Swain JE. Early Postpartum Parental Preoccupation and Positive Parenting Thoughts: Relationship with ParentInfant Interaction. Infant Ment. Health J. 2013;34:104–116. doi: 10.1002/imhj.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Martinez SR, Iyengar U, Strathearn L. Mothers who are securely attached during pregnancy show more attuned infant mirroring at 7 months postpartum. Infant Behav. & Dev. 2014;37:491–504. doi: 10.1016/j.infbeh.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Strathearn L. Mothers’ unresolved trauma blunts amygdala response to infant distress. Soc. Neurosci. 2014a;9:352–363. doi: 10.1080/17470919.2014.896287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Iyengar U, Mayes L, Potenza MN, Rutherford HJ, Strathearn L. Society for Neuroscience. Washington DC: 2014b. Mothers with addictions show reduced reward response to infant cues: Can oxytocin reverse this pattern?, Society for Neuroscience. p. Poster#: 56.05/X59. [Google Scholar]
- Kinsley CH, Meyer EA. The construction of the maternal brain: theoretical comment on Kim et al. (2010) Behav. Neurosci. 2010;124:710–714. doi: 10.1037/a0021057. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Michalska KJ, Liu C, Chen Q, Hipwell AE, Chronis-Tuscano A, Waldman ID, Decety J. Preliminary genetic imaging study of the association between estrogen receptor-alpha gene polymorphisms and harsh human maternal parenting. Neurosci. Lett. 2012;525:17–22. doi: 10.1016/j.neulet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RWJ, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc. cogn. affect. neurosci. 2012;7:125–134. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic-pituitary-adrenal regulation of mothers with their infants. Biol. Psychiatry. 2011;70:826–832. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J. Neural Transm. 2004;111:753–771. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive-compulsive disorder. Child Adolesc. Psychiatr. Clin. N. Am. 1999;8:635–665. [PubMed] [Google Scholar]
- Leerkes EM. Maternal sensitivity during distressing tasks: a unique predictor of attachment security. Infant Behav. Dev. 2011;34:443–446. doi: 10.1016/j.infbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Nayena Blankson A, O’Brien M. Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Dev. 2009;80:762–775. doi: 10.1111/j.1467-8624.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in reponse to pictures of their children and other children. Biol. Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Parenting and plasticity. Trends Neurosci. 2010;33:465–473. doi: 10.1016/j.tins.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon MC, Birkimer JC, Simpson T, Looney S. Postpartum depression and social support in adolescents. J. Obstet. Gynecol. Neonatal. Nurs. 2005;34:46–54. doi: 10.1177/0884217504272802. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hammer MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol. Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Propagation of maternal behavior across generations is associated with changes is non-maternal cognitive and behavioral processes. Behavioural processes. doi: 10.1016/j.beproc.2015.02.016. in press. [DOI] [PubMed] [Google Scholar]
- Lovic V, Keen D, Fletcher PJ, Fleming AS. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav. Neurosci. 2011;125:481–491. doi: 10.1037/a0024367. [DOI] [PubMed] [Google Scholar]
- Madigan S, Wade M, Plamondon A, Jenkins J. Maternal abuse history, postpartum depression, and parenting: links with preschoolers’ internalizing problems. Infant Ment. Health J. 2015;36:146–155. doi: 10.1002/imhj.21496. [DOI] [PubMed] [Google Scholar]
- Magnuson KA, Duncan GJ. Parents in poverty. In: Bronstein MH, editor. Handbook of Parenting. 2nd ed. Erlbaum, Mahwah, NJ: 2002. pp. 195–121. [Google Scholar]
- Marcus S, Lopez JF, McDonough S, Mackenzie MJ, Flynn H, Neal CR, Jr, Gahagan S, Volling B, Kaciroti N, Vazquez DM. Depressive symptoms during pregnancy: impact on neuroendocrine and neonatal outcomes. Infant Behav. Dev. 2011;34:26–34. doi: 10.1016/j.infbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC, Feldman R, Granger R. The effects of polydrug use with and without cocaine on mother infant interaction at 3 and 6 months. Infant Behav. Dev. 1997;20:489–502. [Google Scholar]
- McElwain NL, Booth-Laforce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. J. Fam. Psychol. 2006;20:247–255. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McLearn KT, Minkovitz CS, Strobino DM, Marks E, Hou W. Maternal depressive symptoms at 2 to 4 months post partum and early parenting practices. Arch. Pediatr. Adolesc. Med. 2006;160:279–284. doi: 10.1001/archpedi.160.3.279. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinol. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, Hipwell AE, Lee SS, Chronis-Tuscano A, Waldman ID, Lahey BB. Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Front.behav. neurosci. 2014;8:21. doi: 10.3389/fnbeh.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Humphrey-Wall R, Satayathum S. Psychosocial and behavioral factors related to the post-partum placements of infants born to cocaine-using women. Child Abuse Negl. 2008;32:353–366. doi: 10.1016/j.chiabu.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Rhodes RA, Pine DS, Blair RJ. The contribution of ventrolateral and dorsolateral prefrontal cortex to response reversal. Behav. Brain Res. 2008;187:80–87. doi: 10.1016/j.bbr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philos Trans R Soc Lond B Biol Sci. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Fleming AS, Stern JM. Somatosensory control of the onset and retention of maternal responsiveness in primiparous Sprague-Dawley rats. Physiol. Behav. 1992;51:549–555. doi: 10.1016/0031-9384(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long lasting effects of electrical simulation of the medial preoptic area and medial amygdala on maternal behavior in female rats. Behav. Brain Res. 1999;99:61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Moser DA, Aue T, Wang Z, Rusconi Serpa S, Favez N, Peterson BS, Schechter DS. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress. 2013;16:493–502. doi: 10.3109/10253890.2013.816280. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J. Neuroendocrinol. 2014;26:665–684. doi: 10.1111/jne.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am. J. Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper P. Effects of postnatal depression on infant development. Arch. Dis. Child. 1997a;77:99–101. doi: 10.1136/adc.77.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper PJ. Postpartum Depression and Child Development. New York: Guilford Press; 1997b. [DOI] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: An fMRI study. Dev. cogn. neurosci. 2012;2:428–436. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Rosenblum KL, Alfafara EA, Schuster MM, Miller NM, Waddell RM, Kohler ES. Mom Power: preliminary outcomes of a group intervention to improve mental health and parenting among high-risk mothers. Arch. Womens Ment. Health. 2015 doi: 10.1007/s00737-014-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol. Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Numan M. Neurobiology of Social Behavior: Toward an Understanding of the Prosocial and Antisocial Brain. 1st ed. Academic Press; 2014. [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav. Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci. Biobehav. Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Ouchi H, Ono K, Murakami Y, Matsumoto K. Social isolation induces deficit of latent learning performance in mice: a putative animal model of attention deficit/hyperactivity disorder. Behav.l Brain Res. 2013;238:146–153. doi: 10.1016/j.bbr.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Oxley G, Fleming AS. The effects of medial preoptic area and amygdala lesions on maternal behavior in the juvenile rat. Dev. Psychobiol. 2000;37:253–265. [PubMed] [Google Scholar]
- Pearson RM, Cooper RM, Penton-Voak IS, Lightman SL, Evans J. Depressive symptoms in early pregnancy disrupt attentional processing of infant emotion. Psychol. Med. 2010;40:621–631. doi: 10.1017/S0033291709990961. [DOI] [PubMed] [Google Scholar]
- Pearson RM, Lightman SL, Evans J. The impact of breastfeeding on mothers’ attentional sensitivity towards infant distress. Infant Behav. Dev. 2011;34:200–205. doi: 10.1016/j.infbeh.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Powell B, Cooper G, Hoffman K, Marvin B. The Circle of Security Intervention: Enhancing Attachment in Early Parent-Child Relationships. Guilford Press; 2014. [Google Scholar]
- Preston SD. The origins of altruism in offspring care. Psychol. Bull. 2013;139:1305–1341. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van IJzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol. Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur. Neuropsychopharmacol. 2013;23:1288–1295. doi: 10.1016/j.euroneuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M, Bousquet A, Manzano J. Impact of postpartum depressive symptoms on mother and her 18-month-old infant. Eur. Child Adolesc. Psychiatry. 2003;12:75–83. doi: 10.1007/s00787-003-0311-9. [DOI] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Amygdala response to negative images in postpartum vs nulliparous women and intranasal oxytocin. Soc. Cogn. Affect Neurosci. 2012 doi: 10.1093/scan/nss100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford H, Williams S, Moy S, Mayes L, Johns J. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Front. Psychiatry. 2011:2. doi: 10.3389/fpsyt.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MR, Kirby JN, Tellegen CL, Day JJ. The Triple P-Positive Parenting Program: a systematic review and meta-analysis of a multi-level system of parenting support. Clin. Psychol. Rev. 2014;34:337–357. doi: 10.1016/j.cpr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Saxe R. Why and how to study Theory of Mind with fMRI. Brain Res. 2006;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc. Cogn. Affect. Neurosci. 2012;7:969–979. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Willheim E, Hinojosa C, Scholfield-Kleinman K, Turner JB, McCaw J, Zeanah CH, Myers MM. Subjective and objective measures of parent-child relationship dysfunction, child separation distress, and joint attention. Psychiatry. 2010;73:130–144. doi: 10.1521/psyc.2010.73.2.130. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential Sex-Independent Amygdala Response to Infant Crying and Laughing in Parents versus Nonparents. Biol. Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Sexton MB, Flynn HA, Lancaster C, Marcus SM, McDonough SC, Volling BL, Lopez JF, Kaciroti N, Vazquez DM. Predictors of recovery from prenatal depressive symptoms from pregnancy through postpartum. J. Womens Health (Larchmt) 2011;21:43–49. doi: 10.1089/jwh.2010.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PE, Fonagy P, Strathearn L. Is Attachment Transmitted Across Generations? The Plot Thickens. Clin. Child Psychol. Psychiatry. 2010;15:329–346. doi: 10.1177/1359104510365449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- Sng J, Meaney MJ. Environmental regulation of the neural epigenome. Epigenomics. 2009;1:131–151. doi: 10.2217/epi.09.21. [DOI] [PubMed] [Google Scholar]
- Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin. Child Fam. Psychol. Rev. 2006;9:65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, Welsh RC, Kim P, Swain JE. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2014;89:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C, Murray L, Stein A. The effect of postnatal depression on mother-infant interaction, infant response to the Still-face perturbation, and performance on an Instrumental Learning task. Dev. Psychopathol. 2004;16:1–18. doi: 10.1017/s0954579404044384. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Am. J. Obstet. Gynecol. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. Am. J. Obstet. Gynecol. 1995;173:639–645. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal Neglect: Oxytocin, Dopamine and the Neurobiology of Attachment. J. Neuroendocrinol. 2011;23:1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacol. 2009a;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Front.Neurosci. 2013;7:1–10. doi: 10.3389/fnins.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatr. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mamun AA, Najman JM, O’Callaghan MJ. Does breastfeeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatr. 2009b;123:483–493. doi: 10.1542/peds.2007-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann. N.Y. Acad. Sci. 2010;1187:1–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cummings EM. Impact of hostility and withdrawal in interparental conflict on parental emotional unavailability and children’s adjustment difficulties. Child Dev. 2006;77:1623–1641. doi: 10.1111/j.1467-8624.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Swain JE. Brain Imaging of Human Parent-Infant Relationships. Arch. Womens Ment. Health. 2011;14:s93–s94. [Google Scholar]
- Swain JE, Ho SS, Dayton CJ, Rosenblum KL, Muzik M. Brain Activity in Empathy and Approach-Motivation Domains for High-risk Parents is Increased by Intervention and Inversely Related to Parenting Stress. Neuropsychopharmacol. 2014a;39:S523–S524. [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Res. 2014b;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Konrath S, Brown SL, Finegood ED, Akce LB, Dayton CJ, Ho SS. Parenting and Beyond: Common Neurocircuits Underlying Parental and Altruistic Caregiving. Parent Sci. Pract. 2012;12:115–123. doi: 10.1080/15295192.2012.680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Hoyt E, Kang H, Kim P, David D, Nguyen S, Constable RT, Schultz RT. Functional Brain Activations of Parents Listening to Their Own Baby-Cry Change over the Early Postpartum. Dev. Psychobiol. under review. 2015 [Google Scholar]
- Swain JE, Lorberbaum JP. Imaging the Human Parental Brain. Neurobiol. Parent. Brain. 2008:83–100. [Google Scholar]
- Swain JE, Mayes LC, Leckman JF. The development of parent-infant attachment through dynamic and interactive signaling loops of care and cry. Behav. Brain. Res. 2004;27:472–473. [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J. Child Psychol. Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hasselt FN, Cornelisse S, Yuan Zhang T, Meaney MJ, Velzing EH, Krugers HJ, Joëls M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- Van Zeijl J, Mesman J, van IJzendoorn MH, Bakermans-Kranenburg MJ, Juffer F, Stolk MN, Koot HM, Alink LRA. Attachment-based intervention for enhancing sensitive discipline in mothers of 1-to 3-year-old children at risk for externalizing behavior problems: A randomized controlled trial. J. Consult. Clin. Psychol. 2006;74:994–1005. doi: 10.1037/0022-006X.74.6.994. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychol. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, McDonough S, Marcus S, Flynn H, Neal CR, Jr, Volling B, Kaciroti N, Lopez JF. Women at Risk for Depression: Prenatal Depressive Symptoms and Afternoon ACTH Levels Predict Post-Partum Depression. Arch. Gen. Psychiatry in submission. 2015 [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum. Brain Mapp. 2005;25:336–344. doi: 10.1002/hbm.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin Selectively Gates Fear Responses Through Distinct Outputs from the Central Amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Wan MW, Downey D, Strachan H, Elliott R, Williams SR, Abel KM. The neural basis of maternal bonding. PLoS One. 2014;9:e88436. doi: 10.1371/journal.pone.0088436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner R, Appleby L, Whitton A, Faragher B. Demographic and obstetric risk factors for postnatal psychiatric morbidity. Br. J. Psychiatry. 1996;168:607–611. doi: 10.1192/bjp.168.5.607. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weisman O, Granat A, Gilboa-Schechtman E, Singer M, Gordon I, Azulay H, Kuint J, Feldman R. The experience of labor, maternal perception of the infant, and the mother’s postpartum mood in a low-risk community cohort. Arch. Womens Ment. Health. 2010;13:505–513. doi: 10.1007/s00737-010-0169-z. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Feder A, Pilowsky DJ, Olfson M, Fuentes M, Blanco C, Lantigua R, Gameroff MJ, Shea S. Depressed mothers coming to primary care: maternal reports of problems with their children. J. Affect. Disord. 2004;78:93–100. doi: 10.1016/s0165-0327(02)00301-4. [DOI] [PubMed] [Google Scholar]
- Winnicott DW. Primary Maternal Preoccupation. In: Winnicott DW, editor. Through Paediatrics to Psycho-analysis. London: Hogarth; 1956. [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacol. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, Olesen J, Allgulander C, Alonso J, Faravelli C, Fratiglioni L, Jennum P, Lieb R, Maercker A, van Os J, Preisig M, Salvador-Carulla L, Simon R, Steinhausen HC. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]