Abstract
Creative thinking is central to the arts, sciences, and everyday life. How does the brain produce creative thought? A series of recently published papers has begun to provide insight into this question, reporting a strikingly similar pattern of brain activity and connectivity across a range of creative tasks and domains, from divergent thinking to poetry composition to musical improvisation. This research suggests that creative thought involves dynamic interactions of large-scale brain systems, with the most compelling finding being that the default and executive control networks, which can show an antagonistic relationship, actually cooperate during creative cognition and artistic performance. These findings have implications for understanding how brain networks interact to support complex cognitive processes, particularly those involving goal-directed, self-generated thought.
Keywords: creativity, expertise, improvisation, imagination, networks, connectivity
Creativity, Cognitive Control, and Self-Generated Thought
In this article, we highlight recent developments in the neuroscience of creative cognition, with a focus on understanding the roles of cognitive control and self-generated thought. Creativity is a broadly defined construct, but it is generally assumed to involve the generation of some product that is both novel and useful [1–3]. Creative cognition can thus be understood as a set of mental processes that support the generation of novel and useful ideas. Here, we focus primarily on creative thought processes related to the production and evaluation of self-generated ideas in a range of creative domains. Self-generated thoughts arise from internally-focused mental activity that is largely independent of external input [4]. Although self-generated thoughts can occur spontaneously in mind, they also have been shown to benefit from goal-directed processing and cognitive control [5]. We suggest that creative cognition may also involve such goal-directed, self-generated thought processes, particularly when cognition must be constrained to meet specific task demands.
A growing number of studies have used functional magnetic resonance imaging (fMRI) and functional connectivity analysis of fMRI data to assess dynamic interactions between large-scale brain systems, such as the default and executive control networks, during creative cognition and artistic performance. We consider neuroimaging evidence related to both domain-general (e.g., divergent thinking) and domain-specific (e.g., musical improvisation) creative thought. Notably, we do not address research on creative insight, but we refer readers interested in the neuroscience of insight to recent articles [6–7] for thorough reviews on the topic. This paper proposes a framework to account for the dynamic interplay of the default and control networks supporting creative idea generation and evaluation. The model is discussed in the context of research that has provided evidence for related default-control network interactions in studies of mind-wandering [8–9], autobiographical planning [10–11], and other modes of self-generated thought [5].
Default and Executive Control Network Dynamics
Resting-state and task-based fMRI have identified several large-scale brain networks that underlie core cognitive and attentional processes. Two of the most widely studied networks are the default network and the executive control network. The default network consists of midline and posterior inferior parietal regions that show increased metabolic activity in the absence of most externally-presented cognitive tasks [12]. Default network activity is associated with spontaneous and self-generated thought, including mind-wandering, mental simulation, social cognition, autobiographical retrieval, and episodic future thinking [5, 13–14]. The control network consists of lateral prefrontal and anterior inferior parietal regions, and its activity is associated with cognitive processes that require externally-directed attention, including working memory, relational integration, and task-set switching [15]. The default and control networks can exhibit an antagonistic relationship at rest and during many cognitive tasks. During working memory tasks, for example, the control network typically shows increased activation while the default network deactivates, presumably reflecting the suppression of task-unrelated thoughts during cognitive control [16].
Importantly, the default and control networks have also shown cooperation during several cognitive processes [8, 17–21]. Such processes typically involve the top-down modulation of self-generated information [5]. Although the default network has previously been associated with generative processes such as imagining future experiences [22–23], its coupling with the control network has recently been shown to be important for more goal-directed cognitive processes. For example, the default and control networks show cooperation during autobiographical future planning—constructing a detailed and sequential mental representation about a future goal state [10, 19, 24]. Goal-directed, self-generated thought thus appears to involve both the generative functions of the default network and the strategic functions of the control network.
We propose that certain aspects of creative thought, particularly creative idea generation and evaluation, may also involve goal-directed, self-generated cognition. Our research group and others have previously noted that creative thought may benefit from dynamic interactions of the default and control networks [25–29]. We suggest that the default network contributes to the generation of candidate ideas—potentially useful information derived from long-term memory—in light of its role in self-generated cognition (e.g., episodic memory; [5]). Yet the control network is often required to evaluate the efficacy of candidate ideas and modify them to meet the constraints of task-specific goals. Our model builds on a seminal theory [28] that suggested that the default and control networks may contribute to idea generation and selection, respectively, by means of the evolutionary processes of blind variation and selective retention (cf. [30–31]). Notably, however, our framework does not incorporate such evolutionary mechanisms. Below, we discuss this model in the context of recent research on both domain-general [26, 32–33] and domain-specific [27, 34–35] creative thought processes.
Brain Network Interaction During Domain-General Creative Cognition
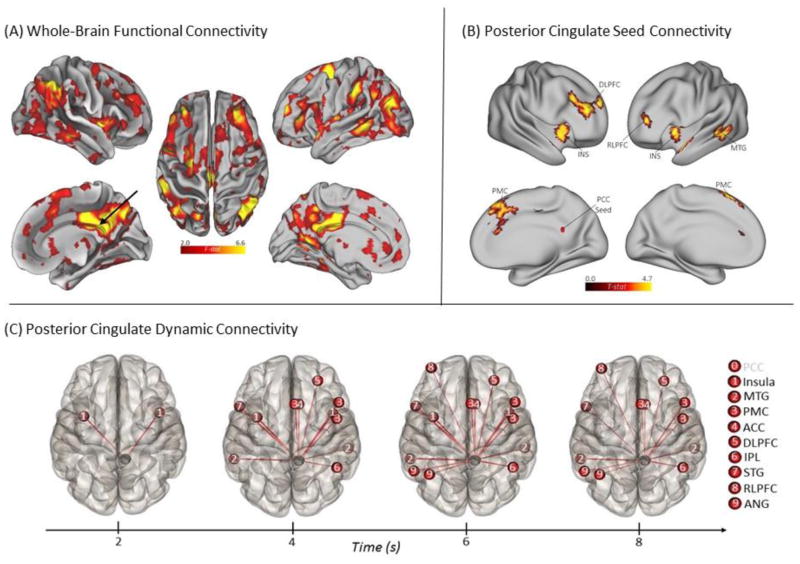
One of the most widely used assessments of domain-general creative cognition is the alternate uses divergent thinking task [36]. In contrast to convergent thinking tasks, which involve discovering a single solution to a creative problem (e.g., insight; [6–7]), divergent thinking involves generating several possible solutions to an open-ended problem, such as inventing creative uses for common objects. A recent study assessed dynamic interactions between brain regions during an alternate uses divergent thinking task [26]. Whole-brain functional connectivity analysis was used to identify a network of brain regions associated with divergent thinking, which included several regions of the default, control, and salience networks (see Figure 1A). Seed-based analyses revealed direct functional connections between these network hubs.
Figure 1. Functional Connectivity Associated with Divergent Thinking.
(A) Whole-brain multivariate pattern analysis contrasting alternate uses divergent thinking with object characteristic generation. Brain maps show differential functional connectivity patterns during divergent thinking. (B) The posterior cingulate cortex (PCC) shows increased connectivity with regions of the control (DLPFC) and salience (insula) networks during divergent thinking. (C) The PCC (black sphere) shows early coupling with salience network regions (bilateral insulae) and later coupling with control network regions (DLPFC). Regions labeled in black on the right show positive connectivity with the source ROI; regions labeled in gray were not significant. Adapted from [26].
As depicted in Figure 1B, the posterior cingulate cortex (PCC) showed increased coupling with regions of the control network (i.e., DLPFC) and salience network (i.e., bilateral insula and anterior cingulate). Moreover, dynamic functional connectivity analysis showed differential coupling of network hubs across the task duration; for example, the PCC showed early coupling with the right anterior insula and later coupling with the right DLPFC, among other regions (see Figure 1C). In light of the right anterior insula’s role as a node of the salience network, a network involved in switching between the default and control networks [18], early coupling between default and salience networks was interpreted as an intermediate switching mechanism that facilitated later coupling between default and control networks.
Cooperation of the default and control networks has been reported in other neuroimaging investigations of domain-general creative cognition. Using a divergent thinking task paradigm similar to the one described above [26], a recent study found that the creative quality of divergent thinking responses, assessed via trained raters, predicted increased functional coupling of the ventral ACC and the left angular gyrus, regions involved in cognitive control and self-generated thought, respectively [32]. In a similar vein, another study employed a verb generation task requiring the production of semantically distant verbs in response to a series of presented nouns [33]. Generating semantically-distant verbs—responses that were remotely associated with nouns, assessed via Latent Semantic Analysis—was associated with activation of the medial prefrontal cortex (MPFC), a hub of the default network; moreover, as the semantic distance between the noun and verb increased, the MPFC showed greater coupling with the ventral ACC.
Such findings are also consistent with a recent study that examined resting-state network patterns in people with high divergent thinking ability [25]. Highly creative participants showed increased coupling of default network regions with the left inferior frontal gyrus, a region associated with cognitive control that is widely implicated in studies of divergent thinking [37]. Taken together, these findings indicate that creative thought may benefit from the cooperation of default and control network regions.
Brain Network Interaction During Artistic Performance
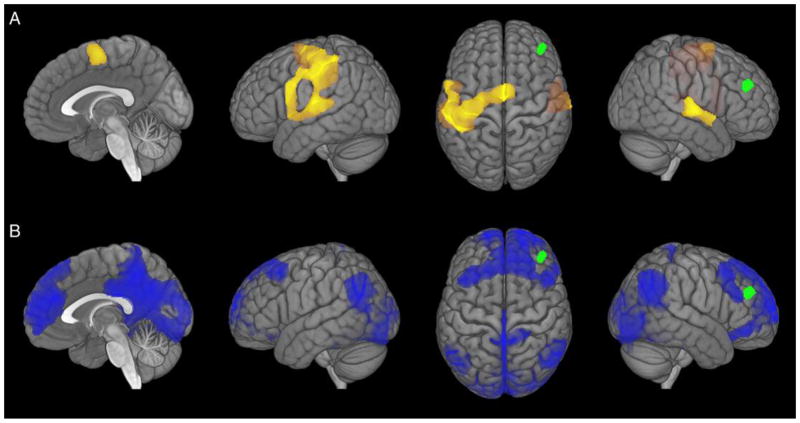
The neuroscience of artistic performance has largely centered on musical improvisation [35, 38–44], although other domains have increasingly been explored, including visual art [27, 45], creative writing [34, 46–47], and lyrical improvisation [48]. Recently, [35] sought to address the role of control network regions in musical improvisation using functional connectivity analysis. The authors asked pianists to improvise following one of two different cognitive strategies: using specific sets of piano keys (“pitch sets”) or expressing specific emotions. Constraining performance to specific pitch sets revealed increased coupling of the DLPFC and several regions associated with cognitive and motor control, including the dorsal pre-motor area and the pre-supplementary motor area (see Figure 2A). Expressing emotion, in contrast, was associated with increased functional connectivity between the DLPFC and the default network (see Figure 2B). In this context, the DLPFC may exert top-down influence over generative processes stemming from the default network during the strategic expression of emotionally-based improvisation.
Figure 2. Dorsolateral Prefrontal Cortex Connectivity During Musical Improvisation.
The right DLPFC (green) shows differential connectivity as a function of task goals during musical improvisation in professional pianists. (A) Functional connectivity associated with the goal of using specific sets of piano keys; brain maps show increased coupling between the right DLPFC and motor regions (e.g., dorsal pre-motor area and the pre-supplementary motor area). (B) Functional connectivity associated with the goal of expressing specific emotions; brain maps show increased coupling between the right DLPFC and default network regions (e.g., MPFC, PCC, and bilateral IPL). Adapted from [35].
Creativity researchers have long speculated that creative thought involves a two-stage process of idea generation and idea evaluation [49]. Idea generation is often conceived as a bottom-up process associated with diffuse attention, whereas idea evaluation is thought to involve focused attention and cognitive control [28]. The neural networks that support idea generation and evaluation, however, have only recently been examined using brain imaging techniques.
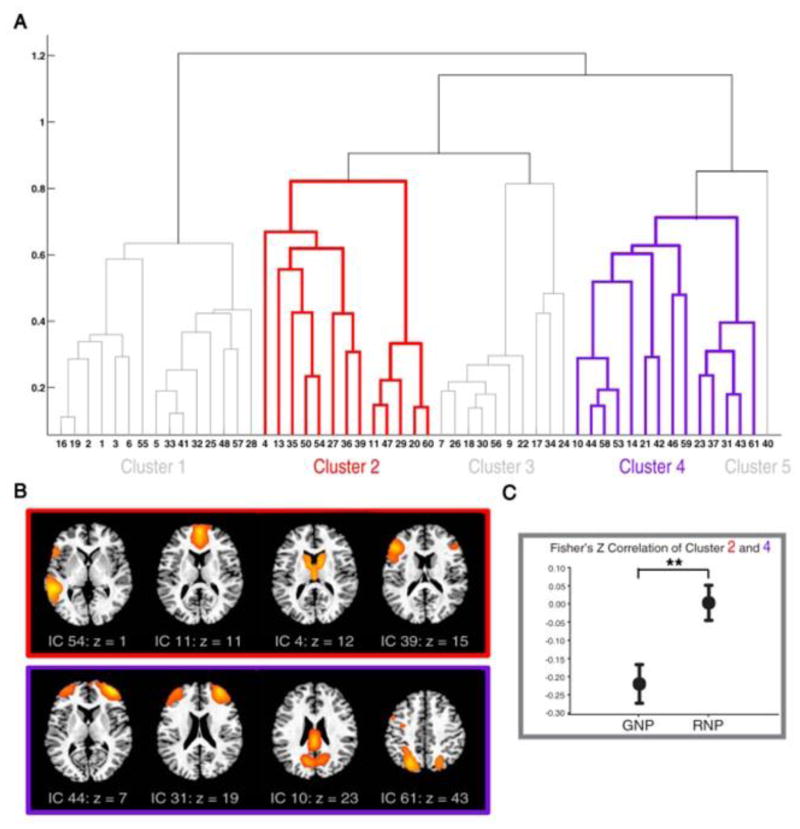
A recent study of professional poets contrasted brain networks involved in poetry composition [34]. During fMRI, poets were asked to spontaneously generate new poetry in one condition, and to revise their self-generated poems in another. Using Independent Component Analysis (ICA), the authors identified several components associated with poetry composition that formed five hierarchical clusters (see Figure 3A). One cluster included default network regions (e.g., MPFC) and another included control network regions (e.g., DLPFC & IPS; see Figure 3B). During idea generation, the default and control network clusters were negatively correlated (see Figure 3C). During idea revision, however, the correlation between the networks increased, suggesting that evaluating and revising self-generated poetry involves relatively increased cooperation of the default and control systems.
Figure 3. Brain Network Connections Associated with Poetry Generation and Revision.
(A) Independent Component Analysis (ICA) identified 53 functional networks associated with poetry composition in professional poets; these networks formed five hierarchical clusters. (B) Hierarchical clusters 2 (red box) and 4 (purple box) are depicted, along with examples of their respective components (functional networks). Note that cluster 2 included default network regions (MPFC) and cluster 4 included control network regions (DLPFC and IPS). (C) Clusters 2 and 4 were negatively correlated during the generation of new poetry (GNP); this correlation increased significantly during the revision of new poetry (RNP). Adapted from [34].
The involvement of the default and control networks during idea generation and evaluation was further explored in a study of visual artists [27]. Using an MRI-compatible drawing tablet system, art students were asked to sketch ideas for a book cover and then evaluate their ideas. At the univariate level of analysis, idea generation was associated with widespread activity of default regions, whereas idea evaluation was associated with both default (e.g., MPFC and PCC) and control network activity (e.g., DLPFC and ACC). Moreover, a functional connectivity analysis revealed increased coupling of default and control network regions during idea evaluation, consistent with the poetry composition study described above [34]. Both experiments showed that idea evaluation involves increased functional coupling of the default and control networks. These studies suggest that generating new ideas in poetry and visual art may benefit from self-generated thoughts originating in the default network, but that such ideas may require top-down modulation during evaluation, thus reflected in default-control network coupling.
Brain Networks and Creative Cognition: An Integrative Framework
The research described above suggests that creative cognition involves dynamic interaction of the default and control networks. This pattern has been reported in studies of both domain-general creativity [26, 32–33] and various domains of artistic performance, such as music [35], literature [34], and visual art [27]. Here, we describe a framework to account for the interplay of the default and control networks underlying creative thought processes. We propose that creative thought involves similar cognitive and neural mechanisms as other forms of goal-directed, self-generated cognition (e.g., autobiographical future planning; [10, 19, 24]). In general, we contend that the default network influences the generation of candidate ideas, but that the control network can constrain and direct this process to meet task-specific goals via top-down monitoring and executive control.
As noted above, a primary function of the default network is episodic memory retrieval [22]. Recent research points to an important role of the default network and episodic memory in creative cognition ([50–51]; see Box 1). We suspect that memory systems may play a key role in the generation of candidate ideas across domain-general and domain-specific contexts (cf. [52]). Although memory retrieval appears to play an important role in idea generation, cognitive control systems can also be recruited to evaluate and modify candidate ideas to meet specific goals, in line with behavioral [53–59] and neuroimaging [37, 60–64] evidence showing consistent involvement of executive mechanisms in creative thought. Thus, the control and default networks may cooperate to leverage both top-down (executive) and bottom-up (generative) processes during creative cognition. Default-control network coupling is not ubiquitous, however, as the networks have shown both cooperation and competition during creative thinking tasks.
Box 1. The Default Network and Episodic Memory: Links to Creative Cognition.
The default network has shown robust activation when people imagine future experiences [14, 22–23]. More specifically, a subset of default network regions referred to as the core network [22] is similarly engaged when people are asked to remember past experiences (i.e., episodic memory) and imagine future experiences (i.e., episodic simulation; for a recent meta-analysis, see [68]). Given the engagement of the default network regions during creative cognition, a natural question concerns possible links between creative cognition on the one hand and episodic memory and episodic simulation on the other. Several recent studies have provided evidence for such links. Amnesic patients with hippocampal damage, who have severe episodic memory deficits, also perform poorly on the widely used Torrance Tests of Creative Thinking [69]. It has also been reported that healthy young adults sometimes draw on episodic memories when performing the alternate uses divergent thinking task mentioned earlier, although retrieval of episodic memories during performance of this task occurred infrequently [70]. Moreover, a study of healthy young and older adults reported that performance on the alternate uses task was positively correlated with the number of episodic details that participants reported when they imagined future personal experiences [71]. This correlation with divergent thinking, however, was specific to imagined future events and was not observed for recalled or imagined past events.
Even stronger evidence linking performance on the alternates uses task with episodic memory and simulation comes from a recent study in which participants received an episodic specificity induction—brief training in recollecting specific details of a recent experience—prior to performing the alternate uses divergent thinking task [72]. Previous work had shown that the specificity induction selectively boosts the number of episodic details that participants provide on subsequent tasks that require remembering past experiences and imagining future experiences, while having no effect on the number of semantic details that participants provide on such tasks ([73]; for review, see [74]). Critically, administering the specificity induction increased the number of appropriate uses that participants generated on a subsequent alternate uses task, and also increased episodic details on a future simulation task [72]. These findings suggest that further research investigating the relations among episodic memory, episodic simulation, and divergent thinking, and how they are underpinned by the default network, should be extremely informative.
One possible contributing factor to default-control network coupling is the degree of goal-directedness of a given creative task. Creative thought can be considered “goal-directed” when it is constrained to meet task-specific goals (e.g., conveying an abstract concept; [35]) or when explicit top-down processes are required (e.g., evaluating the efficacy of self-generated ideas; [27]). It has previously been suggested that the default and control networks will cooperate to generate and maintain an “internal train of thought” [65] or during the extended evaluation of internal information [5]. In this context, the control network may modify and direct self-generated thoughts to meet the demands of task-specific goals [10–11, 20].
Recent research on musical improvisation has provided support for this notion [35]. Here, default and control networks showed cooperation when musicians were asked to improvise based on a specific emotion. Thus, the task goal of expressing a specific emotion may recruit the strategic and top-down mechanisms of the control network, which may oversee the spontaneous generation of candidate ideas originating in the default network. In the absence of such control, musicians may rely solely on spontaneously generated melodic sequences that do not necessarily adhere to the task goal of expressing a specific emotion. The control network may therefore maintain an “internal train of thought” by keeping the task goal activated, inhibiting aesthetically undesirable or goal-incongruent melodic sequences, and strategically searching memory for goal-congruent sequences.
Studies of artistic performance have also reported default-control network coupling during creative idea evaluation. A study of visual artists [27] found increased functional connectivity between the PCC and DLPFC when artists were asked to evaluate their previously generated ideas. The authors of this study suggest that idea evaluation may invoke a unique mode of analytic processing characterized by both deliberate (top-down) and spontaneous (bottom-up) forms of evaluative thought [27]. In this context, the default network may provide bottom-up evaluations via spontaneously-generated and self-referential mechanisms; the control network, in turn, may compare this information to the task goal and modify it via cognitive control mechanisms such as inhibition and selection.
Yet the default and control networks may also show less cooperation in some situations, such as in the absence of a clear task goal or when top-down constraints are relaxed. In the study of study of poetry composition [34], for example, poets were simply asked to spontaneously generate novel poetry—not to engage a generative strategy, as in the study of musical improvisation (e.g., [35])—so the top-down functions of the control network were not required. Indeed, the authors reported increased activation of default regions during poetry generation, and a functional connectivity analysis revealed a negative correlation between default and control network regions during generation. But this negative correlation was markedly attenuated during idea evaluation, possibly due to increased task demands and top-down control required. Default-control network coupling may therefore depend on the extent to which creative cognition relies on goal-directed processing.
Concluding Remarks
Creativity researchers have long questioned whether creative thought involves more or less cognitive control [66–67]. The research described above highlights the benefits of a nuanced approach to addressing this question, using experimental manipulations that differentiate between sub-processes of creative cognition (e.g., idea generation and evaluation) and neuroimaging data analysis methods that assess interactions between brain regions. This emerging literature suggests that creative thought involves cooperation of the default and control networks—similar to other forms of goal-directed, self-generated thought—and that the extent of control network involvement may depend on the extent to which creative thought is constrained to meet specific task goals. Future research should continue to explore the brain network dynamics underlying creative cognition and artistic performance, with a focus on understanding how and when creative thought benefits from cognitive control (see Box 2).
Acknowledgments
R.E.B. was supported by grant RFP-15-12 from the Imagination Institute, funded by the John Templeton Foundation. D.L.S was supported by National Institute of Mental Health R01 MH060941 and National Institute on Aging RO1 AG08441.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barron F. The disposition toward originality. J Abnorm Soc Psych. 1955;51:478–485. doi: 10.1037/h0048073. [DOI] [PubMed] [Google Scholar]
- 2.Runco MA, Jaeger GJ. The standard definition of creativity. Creat Res J. 24:92–96. [Google Scholar]
- 3.Diedrich J, et al. Are creative ideas novel and useful? Psychol Aesthet Crea Arts. 2015;9:35–40. [Google Scholar]
- 4.Smallwood J. Distinguishes how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychol Bull. 2014;139:519–535. doi: 10.1037/a0030010. [DOI] [PubMed] [Google Scholar]
- 5.Andrews-Hanna JR, et al. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann NY Acad Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden EM, et al. New approaches to demystifying insight. Trends Cogn Sci. 2005;9:322–328. doi: 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Kounios J, Beeman M. The cognitive neuroscience of insight. Ann Rev Psych. 2014;65:71–93. doi: 10.1146/annurev-psych-010213-115154. [DOI] [PubMed] [Google Scholar]
- 8.Christoff K, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox KC, et al. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Spreng RN, et al. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spreng RN, et al. Autobiographical planning and the brain: Activation and its modulation by qualitative features. J Cogn Neurosci. 2015 doi: 10.1162/jocn_a_00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner RL, et al. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 15.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anticevic A, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhle JT, et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocchi L, et al. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci. 2013;17:494–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach KD, et al. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spreng RN, et al. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 2014;34:14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi M. Shaped by the past: The default mode network supports cognition that is independent of immediate perceptual input. PLoS ONE. 2015;10:e0132209. doi: 10.1371/journal.pone.0132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schacter DL, et al. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 23.Schacter DK, et al. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerlach KD, et al. Future planning: Default network couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc Cogn Affect Neurosci. 2014;9:1942–1951. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaty RE, et al. Creativity and the default mode network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia. 2014;64:92–98. doi: 10.1016/j.neuropsychologia.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaty RE, et al. Default and executive network coupling supports creative idea production. Sci Rep. 2015;5:10964. doi: 10.1038/srep10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellamil M, et al. Evaluative and generative modes of thought during the creative process. NeuroImage. 2012;59:1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Jung RE, et al. The structure of creative cognition in the human brain. Front Hum Neurosci. 2013;7:330. doi: 10.3389/fnhum.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan RL, et al. Ode to positive constructive daydreaming. Front Psych. 2013;4:626. doi: 10.3389/fpsyg.2013.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell DT. Blind variation and selective retentions in creative thought as in other knowledge processes. Psych Rev. 1960;67:380–400. doi: 10.1037/h0040373. [DOI] [PubMed] [Google Scholar]
- 31.Simonton DK. Creativity as blind variation and selective retention: Is the creative process Darwinian? Psych Inq. 1999;10:309–328. [Google Scholar]
- 32.Mayseless N, et al. Generating original ideas: The neural underpinnings of originality. NeuroImage. 2015;116:232–239. doi: 10.1016/j.neuroimage.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Green AE, et al. Frontopolar activity and connectivity support dynamic conscious augmentation of creative state. Hum Brain Map. 2015;36:923–934. doi: 10.1002/hbm.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, et al. Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Hum Brain Map. 2015;36:3551–3372. doi: 10.1002/hbm.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinho AL, et al. Addressing a paradox: Dual strategies for creative performance in introspective and extrospective networks. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv130. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman JC, Plucker JA, Baer J. Essentials of creativity assessment. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 37.Gonen-Yaacovi G, et al. Rostral and caudal prefrontal contributions to creativity: A meta-analysis of functional imaging data. Front Hum Neurosci. 2013;7:465. doi: 10.3389/fnhum.2013.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkowitz AL, Ansari D. Generation of novel motor sequences: The neural correlates of musical improvisation. NeuroImage. 2008;41:535–543. doi: 10.1016/j.neuroimage.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Berkowitz AL, Ansari D. Expertise-related deactivation of the right temporoparietal junction during musical improvisation. NeuroImage. 2010;49:712–719. doi: 10.1016/j.neuroimage.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 40.de Manzano Ö, Ullén F. Activation and connectivity patterns of the presupplementary and dorsal premotor areas during free improvisation of melodies and rhythms. NeuroImage. 2012a;63:272–280. doi: 10.1016/j.neuroimage.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 41.de Manzano Ö, Ullén F. Goal-independent mechanisms for free response generation: Creative and pseudo-random performance share neural substrates. NeuroImage. 2012b;59:1783–1794. doi: 10.1016/j.neuroimage.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Donnay GF, et al. Neural substrates of interactive musical improvisation: An fMRI study of ‘trading fours’ in jazz. PLoS One. 2014;3:e88665. doi: 10.1371/journal.pone.0088665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinho A, et al. Connecting to create: Expertise in musical improvisation is associated with increased functional connectivity between premotor and prefrontal areas. J Neurosci. 2014;34:6156–6163. doi: 10.1523/JNEUROSCI.4769-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limb CL, Braun AR. Neural substrates of spontaneous musical performance. An fMRI study of jazz improvisation. PLoS One. 2008;3:e1679. doi: 10.1371/journal.pone.0001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlegel A, et al. The artist emerges: Visual art learning alters neural structure and function. NeuroImage. 2015;105:440–451. doi: 10.1016/j.neuroimage.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Erhard K, et al. Professional training in creative writing is associated with enhanced fronto-striatal activity in a literary text continuation task. NeuroImage. 2014;100:15–23. doi: 10.1016/j.neuroimage.2014.05.076. [DOI] [PubMed] [Google Scholar]
- 47.Shah C, et al. Neural correlates of creative writing: An fMRI study. Hum Brain Map. 2013;34:1088–1101. doi: 10.1002/hbm.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, et al. Neural correlates of lyrical improvisation: An fMRI study of freestyle rap. Sci Rep. 2012;2:834. doi: 10.1038/srep00834. doi:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finke RA, et al. Creative cognition: Theory, research, and applications. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 50.Benedek M, et al. To create or to recall? Neural mechanisms underlying the generation of creative new ideas. NeuroImage. 2014b;88:125–133. doi: 10.1016/j.neuroimage.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedek M, et al. Creating metaphors: The neural basis of figurative language production. NeuroImage. 2014a;90:99–106. doi: 10.1016/j.neuroimage.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenett YN, et al. Investigating the structure of semantic networks in low and high creative persons. Front Hum Neurosci. 2014;8:407. doi: 10.3389/fnhum.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaty RE, Silvia PJ. Metaphorically speaking: Cognitive abilities and the production of figurative language. Mem Cogn. 2013;41:255–267. doi: 10.3758/s13421-012-0258-5. [DOI] [PubMed] [Google Scholar]
- 54.Benedek M, et al. Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence. 2014c;46:73–83. doi: 10.1016/j.intell.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaty, et al. The roles of associative and executive processes in creative cognition. Mem Cogn. 2014;41:255–267. doi: 10.3758/s13421-014-0428-8. [DOI] [PubMed] [Google Scholar]
- 56.Barr, et al. Reasoned connections: A dual-process perspective on creative thought. Think Reason. 2015;21:61–75. [Google Scholar]
- 57.Nusbaum EC, Silvia PJ. Are intelligence and creativity really so different? Fluid intelligence, executive processes, and strategy use in divergent thinking. Intelligence. 2011;39:36–45. [Google Scholar]
- 58.Silvia, et al. Verbal fluency and creativity: General and specific contributions of broad retrieval ability (Gr) factors to divergent thinking. Intelligence. 2013;41:323–340. [Google Scholar]
- 59.Zabelina DL, Robinson MD. Creativity as flexible cognitive control. Psychol Aesthet Crea Arts. 2010;4:136–143. [Google Scholar]
- 60.Wu X, et al. A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Hum Brain Map. 2015;36:2703–2718. doi: 10.1002/hbm.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beaty RE. The neuroscience of musical improvisation. Neurosci Biobehav Rev. 2015;51:108–117. doi: 10.1016/j.neubiorev.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Vartanian O, et al. The effects of a single night of sleep deprivation on fluency and prefrontal cortex function during divergent thinking. Front Hum Neurosci. 2014;8:214. doi: 10.3389/fnhum.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleibeuker SW, et al. Prefrontal cortex involvement in creative problem solving in middle adolescence and adulthood. Devel Cogn Neurosci. 2013;5:197–206. doi: 10.1016/j.dcn.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q, et al. Association of creative achievement with cognitive flexibility by combined voxel-based morphometry and resting-state functional connectivity study. NeuroImage. 2014;102:474–483. doi: 10.1016/j.neuroimage.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Smallwood J, et al. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 66.Abraham A. Creative thinking as orchestrated by semantic processing vs. cognitive control brain networks. Front Hum Neurosci. 2014;8:95. doi: 10.3389/fnhum.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psych Bull. 2010;136:822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- 68.Benoit RG, Schacter DL. Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia. 2015;75:450–457. doi: 10.1016/j.neuropsychologia.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duff MC, et al. Hippocampal amnesia disrupts creative thinking. Hippocampus. 2013;23:1143–1149. doi: 10.1002/hipo.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilhooly KJ, et al. Divergent thinking: Strategies and executive involvement in generating novel uses for familiar objects. Brit J Psych. 2007;98:611–625. doi: 10.1111/j.2044-8295.2007.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 71.Addis DR, et al. Divergent thinking and constructing episodic simulations. Mem. 2014 doi: 10.1080/09658211.2014.985591. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madore KP, et al. Creativity and memory: Effects of an episodic specificity induction on divergent thinking. Psych Sci. 2015;26:1461–1468. doi: 10.1177/0956797615591863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madore KP, et al. Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. J Exp Psych: Learn Mem Cogn. 2014;40:609–622. doi: 10.1037/a0034885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schacter DL, Madore KP. Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Mem Studies. doi: 10.1177/1750698016645230. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]