Abstract
Background
Pharmacotherapeutic agents that could facilitate extinction of cocaine cues would be useful in the treatment of cocaine addiction. We tested whether SR 21502, selective dopamine (DA) D3 receptor antagonist, can facilitate extinction of cocaine conditioned place preference (CPP) in rats.
Methods
In experiment 1, cocaine (10 mg/kg) CPP was first established and then extinguished. During the extinction phase the rats were injected with SR 21502 and placed in the previously cocaine-paired compartment for four sessions and vehicle in the other compartment on four alternating sessions. The rats were then tested again for cocaine CPP. In experiment 2, different groups of rats were trained to associate SR 21502 to one compartment and saline to the other.
Results
In Experiment 1, the animals spent significantly more time in the cocainepaired compartment after cocaine conditioning than they did before conditioning. Subsequently, the animals treated with SR 21502 during the extinction phase spent significantly less time in the cocaine-paired compartment than the vehicle group. In experiment 2, animals conditioned with SR 21502 preferred neither side of the CPP apparatus, indicating that SR 21502 produced no effects of its own.
Conclusions
These findings suggest that treatment with SR 21502, a DA D3 receptor antagonist, in the presence of cocaine cues can facilitate extinction of cocaine CPP and further suggest that this compound might be an effective cocaine addiction treatment.
Keywords: cocaine, D3 receptor antagonist, conditioned place preference, addiction, reward, extinction
1. INTRODUCTION
In cocaine addicts cocaine cues (e.g., paraphernalia) may trigger craving and relapse (Childress et al., 1993; Ehrman et al., 1992). In animals, they can maintain drug seeking for prolonged periods (Arroyo et al., 1998), retard extinction of cocaine seeking (Ciccocioppo et al., 2004; Ranaldi and Roberts, 1996) and reinstate cocaine seeking (Arroyo et al., 1998; Meil and See, 1996). Such cues can also produce a conditioned place preference (CPP; Bardo et al., 1986; Spyraki et al., 1982) or hyperactivity and stereotypy (Barr et al., 1983).
The mesolimbic dopamine (DA) system is strongly implicated in the rewarding effects of cocaine and cocaine-related stimuli (de Wit and Wise, 1977; Wise and Rompré, 1989). Cocaine’s rewarding effects are directly related to its ability to enhance dopaminergic neurotransmission in terminal regions of the mesocorticolimbic system (Di Chiara and Imperato, 1988; Pettit et al., 1990). The same cortical regions and limbic structures can also be activated by cocaine cues, as shown by functional imaging in addicts (Childress et al., 1999; Grant et al., 1996), electrochemical measurements (Di Ciano et al., 1998; Duvauchelle et al., 2000) and cfos expression (Brown and Fibiger, 1992; Le Foll et al., 2002).
DA D3 receptors are involved in cocaine reward and conditioned reward. DA D3 receptor antagonists and partial agonists significantly reduce intravenous cocaine self administration (Galaj et al., 2014; Song et al., 2011; Xi and Gardner, 2007; Xi et al., 2005), inhibit cue-, stress- and drug-induced reinstatement of cocaine seeking (Galaj et al., 2014; Gilbert et al., 2005; Song et al., 2014; Xi et al., 2004) and block the expression of cocaine CPP (Duarte et al., 2003; Hachimine et al., 2014; Song et al., 2013; Vorel et al., 2002). Conditioned cues can drive cocaine-related behavior and its underlying neuronal mechanisms; both of which require DA D3 receptors (Le Foll et al., 2002).
This is interesting because it suggests that DA D3 antagonists might blunt the rewarding effect of cocaine stimuli. Given this, it is possible that repeated exposure to cocaine stimuli under DA D3 receptor antagonist treatment might reduce the rewarding effects of the stimuli so that they are less effective in eliciting cocaine conditioned responses; in other words they might facilitate extinction of cocaine cues.
We have recently focused on the novel DA D3 receptor antagonist, SR 21502, whose anti-cocaine behavioral effects have already been mentioned above. In the present study we investigated whether SR 21502 could facilitate extinction of an established cocaine CPP.
2. METHODS AND RESULTS
2.1 Subjects
Subjects were male Long Evans rats (Charles River, Kingston, NY), housed individually in standard cages with free access to food (Lab Diet Chow) and water and maintained on a reverse 12 h light:12 h dark cycle (lights on at 8:00 pm). The sessions were conducted during the animals’ active period and home cage injections were administered during the inactive (light) period.
2.2 Experiment 1. Extinction of cocaine conditioned place preference
The experiments were conducted in CPP chambers consisting of two distinct compartments separated by a guillotine door. Experiment 1 consisted of: pre-exposure, cocaine conditioning, initial preference test, extinction of cocaine CPP with SR 21502 pretreatment and post-extinction preference test.
During the pre-exposure session, all animals (n= 44) freely explored the CPP chambers for 15 min. During conditioning rats were injected intraperitoneally with cocaine (10 mg/kg; a gift from NIDA) every other day for 4 days and saline on 4 alternate days and immediately placed for 30 min in one of the two compartments with doorways closed. For half of the rats cocaine was paired with the preferred compartment (determined as the compartment in which the rat spent the most time in the pre-exposure session) and for the other half with the non-preferred compartment. After the last conditioning session, all rats were tested for initial cocaine CPP having free access to both compartments for 15 min.
During the extinction phase rats were injected with vehicle, 3.75, 7.5 or 15 mg/kg of SR 21502 (dissolved in distilled water; a gift from Southern Research Institute, Birmingham, AL) in a volume of 1 ml/kg prior to placement in the previously cocaine-paired compartment or with vehicle prior to placement in the previously saline-paired compartment, receiving a total of 4 SR 21502 injections and 4 vehicle injections. Assignment to SR 21502 dose groups was based on ranked magnitude of side preference determined during pre-exposure. Eight hours after each extinction session all rats received home-cage injections. The experimental groups (rats treated with 3.75, 7.5 or 15 mg/kg of SR 21502 in the CPP chambers) received vehicle injections in their home cages (8 injections in total). The vehicle group (rats treated with vehicle prior to all extinction sessions) received SR 21502 home-cage injections every other day and vehicle injections on the alternate days (4 injections of 15 mg/kg SR 21502 and 4 injections of distilled water in total). This protocol ensured that all groups received equal pharmacological exposure to SR 21502 prior to the post-extinction preference test. The day after the eighth extinction session all rats were placed directly in the open doorway of the CPP chambers and allowed free exploration of both compartments for 15 min.
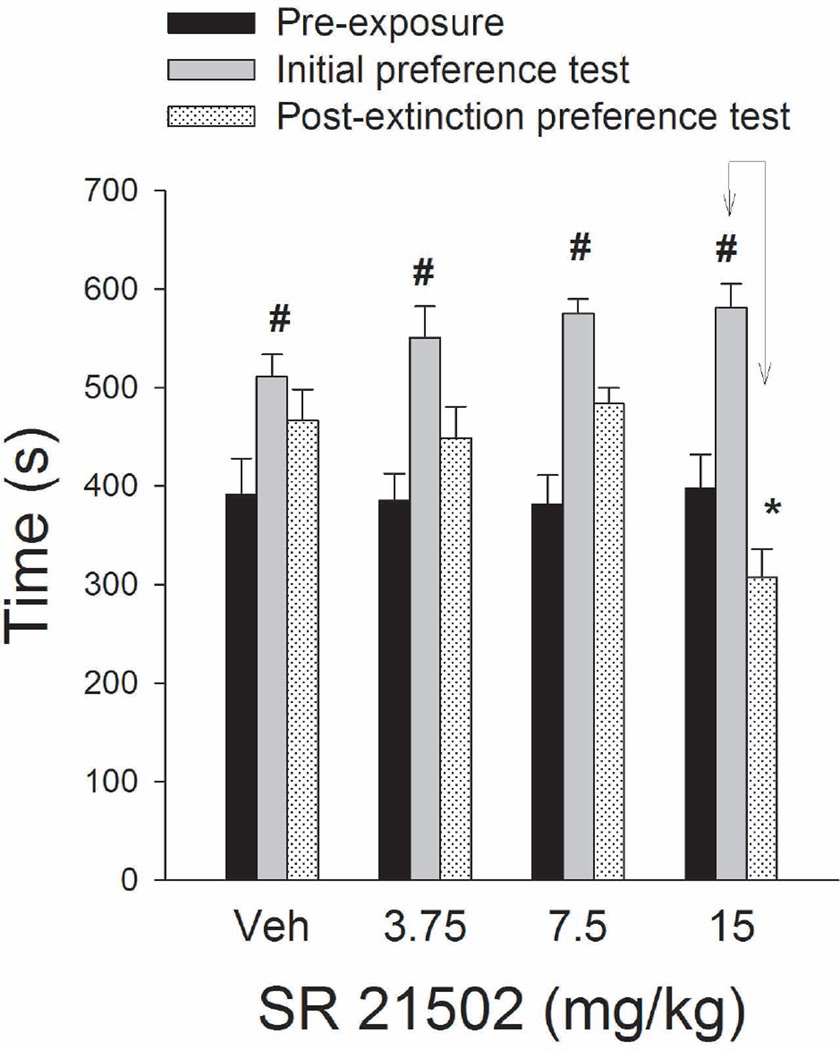
The number of seconds spent in the cocaine compartment during the pre-exposure, initial preference and post-extinction preference tests was recorded. All groups that would later be treated with SR 21502 spent more time in the cocaine-paired compartment during the initial preference test than during the pre-exposure session (see Figure 1). After the extinction phase the groups treated with vehicle, 3.75 or 7.5 mg/kg of SR 21502 spent the same amount of time in the cocaine-paired environment during the initial preference test and post-extinction preference test. However, the 15 mg group spent less time in the cocaine-paired environment in the post-extinction preference test than they did in the initial preference test and as compared to the vehicle group (Figure 1). A 4 × 3 (dose × phase) ANOVA revealed a significant dose by phase interaction [F3,80= 5.28, p < .001]. Interaction comparisons showed a significant phase effect at the pre-extinction level [F1,80= 126.37, p < .001] but no phase by dose interaction at this level, indicating that the dose groups showed similar cocaine CPP. The interaction comparisons also showed a significant phase by dose interaction [F3,80= 8.12, p < .001] at the post-extinction level. Subsequent tests of simple effect of SR 21502 dose at each level of phase (initial preference test vs. post-extinction preference test) revealed a significant dose effect on the post-extinction preference test [F3,80 =12.48, p < .001]. Dunnet’s tests showed that the 15mg/kg group spent significantly less time in the cocaine-paired environment after extinction than the vehicle group, p < .05. Groups treated with vehicle, 3.75 or 7.5 mg/kg of SR 21502 did not differ on the post-extinction test.
Figure 1.
Mean (± SEM) time spent in the cocaine-paired compartment of the CPP apparatus during pre-exposure, initial preference test and post-extinction preference test in rats treated with SR 21502 prior to extinction sessions. # represents significantly more time spent in the cocaine-paired environment after the conditioning compared to preexposure. * represents a significant reduction in time spent in the cocaine-paired compartment after SR 21502 extinction treatment compared to the initial preference test.
2.3 Experiment 2. Aversion conditioning test
To rule out the possibility that this facilitation of extinction effect was caused by aversive conditioning with SR 21502 we conducted an additional experiment. Experiment 2 consisted of 3 phases as follows: pre-exposure, 8 days of 30-min conditioning with SR21502 and test. During the conditioning phase the rats (n=12) were injected with SR 21502 (vehicle or 15 mg/kg, IP) every other day for four days and with vehicle on four alternative days. The two distinctive compartments were paired repeatedly, one with the SR 21502 injections and the other with the vehicle injections. On the test day the rats were allowed to freely explore both compartments of the CPP chambers for 15 min. Place preference was determined based on the time spent in the conditioning compartment before and after conditioning.
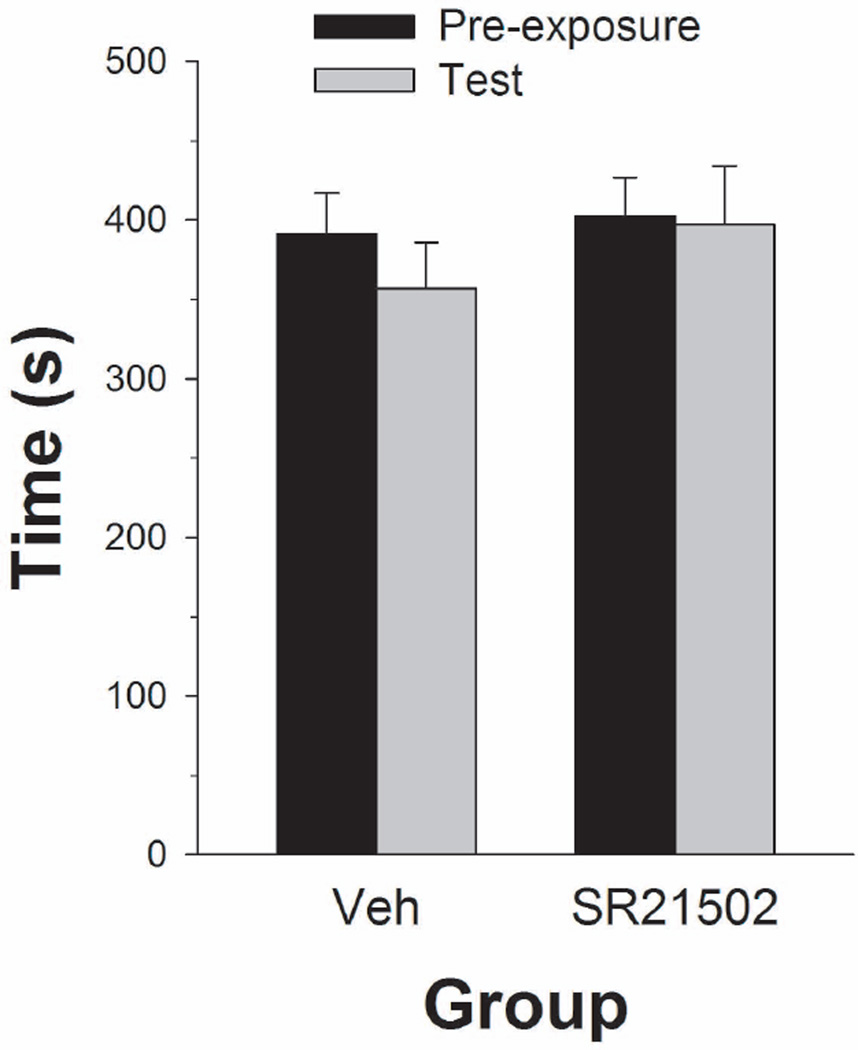
The vehicle and 15 mg/kg SR 21502 groups spent the same amount of time in the conditioning compartment during the test session as during the pre-exposure session (see Figure 2). A two-way (group × phase) ANOVA on these data revealed no significant effects.
Figure 2.
Mean (± SEM) time spent in the SR 21502-paired compartment of the CPP apparatus during pre-exposure and test sessions.
3. DISCUSSION
We investigated whether treatment with SR 21502, a selective DA D3 receptor antagonist, in the presence of cocaine cues reduces an established cocaine CPP. In experiment 1, rats that showed cocaine CPP and later were treated repeatedly with SR 21502 in the cocaine environment showed significantly reduced cocaine CPP whereas the vehicle group, which was treated with the same amount of SR 21502 but in the home cage, maintained their established cocaine CPP when tested in a drug-free state. Therefore, the data from Experiment 1 suggest that repeated exposure to a cocaine environment under treatment with SR 21502 can reduce previously established cocaine CPP but only when DA D3 antagonism occurs simultaneously with cocaine cue exposure. The reduction in cocaine CPP was not likely caused by possible aversive effects of SR 21502 since rats conditioned with SR 21502, but never exposed to cocaine (Experiment 2), did not show a conditioned place aversion.
The data support our hypothesis that repeated treatment with SR 21502 in the presence of cocaine conditioned cues can facilitate extinction of cocaine CPP. These findings correspond with a recent report that treatment with SB-277011A prior to each exposure to the cocaine chamber during extinction sessions resulted in animals losing their preference for the cocaine side at a faster rate than animals treated with vehicle (Ashby et al., 2015). That study consisted of 8 extinction sessions/tests, each with only exposure to the cocaine-paired side during SB-277011A treatment and preference tests conducted immediately after each extinction session. The progressive decrease in time spent in the cocaine-paired side could be due to several factors: (1) SB-277011A buildup and (2) SB-277011A-induced aversion to the cocaine side. Because the authors did not conduct home-cage treatment control or aversion experiments – as were done here – these factors cannot be ruled out.
One explanation for the extinction-facilitating effects seen here with SR 21502 is that repeated blockade of DA D3 receptors in the presence of cocaine conditioned cues reduces the reward value of the conditioned cues and this reward devaluation results in reduction of the established cocaine CPP. Selective DA D3 receptor antagonists reduce the expression of cocaine CPP established with cocaine (Cervo et al., 2005; Hachimine et al., 2014; Song et al., 2013; Vorel et al., 2002), opiates (Ashby et al., 2003; Hu et al., 2013) or nicotine (Pak et al., 2006) and reduce conditioned hyperlocomotion to drug cues (Le Foll et al., 2002). This suggests that DA D3 receptor antagonism may attenuate the rewarding and other stimulant effects of drug conditioned cues reducing their capacities to elicit approach and other responses.
Results from self administration studies (Galaj et al., 2014; Song et al., 2011; Xi and Gardner, 2007) also suggest that DA D3 receptors are involved in the rewarding effects of conditioned stimuli on behaviors more so than in the effects of unconditioned stimuli (primary rewards). In the progressive ratio and second order schedules of reinforcement, responding relies heavily on conditioned cues. DA D3 receptor antagonists and partial agonists also reduce drug cue-induced reinstatement of drug seeking (Cervo et al., 2007; Galaj et al., 2014; Gilbert et al., 2005). Thus, it appears that blockade of DA D3 receptors can devalue cocaine cues, rendering them less effective in their capacities to elicit conditioned responses. This could result in the facilitation of extinction of cocaine CPP observed in the present study with SR 21502.
In summary, we found that treatment with the DA D3 receptor antagonist, SR 21502, facilitated extinction of cocaine CPP. These results suggest that exposure to cocaine cues under the influence of a highly selective DA D3 receptor antagonist can render them less effective at eliciting cocaine-related behavior. The capacity of cocaine cues to induce relapse is significant and constitutes a major problem in the treatment of cocaine addiction. Effective pharmacological treatment of cocaine addiction would be able to block the relapse-inducing effects of drug cues while the medication is on board. But it would also be beneficial if the diminished effects of cocaine cues remain diminished after medication treatment is stopped. Previous research indicates that SR 21502 can achieve the former (Galaj et al., 2014) and the current research indicates it might also achieve the latter.
Highlights.
The selective dopamine D3 receptor antagonist, SR 21502, facilitates extinction of cocaine conditioned place preference
The selective dopamine D3 receptor antagonist, SR 21502, fails to establish a conditioned place aversion
SR 21502 may have potential as an anti-cocaine addiction treatment
Acknowledgments
Role of Funding: This research was supported in part by NIH Grant R01DA024675 (to S.A.) from the National Institute on Drug Abuse (NIDA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Ewa Galaj participated in the design and analysis of the study, was the principle collector of the data and wrote the complete first draft of the manuscript and all revisions. Joseph Haynes and Rudolf Nisanov participated in data collection and assisted in editing the manuscript. Subramaniam Ananthan designed and synthesized the test compound (SR 21502) and assisted in writing the manuscript. Robert Ranaldi served as principle investigator, participated in the design and analysis of the study and assisted in writing all drafts of the manuscript. All authors have approved the final version of this manuscript.
Conflict of Interest: No conflict declared.
REFERENCES
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition,maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats:effects of conditioned cues and continuous access to cocaine. Psychopharmacology. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Rice OV, Heidbreder CA, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A significantly accelerates extinction to environmental cues associated with cocaine-induced place preference in male sprague-dawley rats. Synapse. 2015;69:512–514. doi: 10.1002/syn.21839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Miller JS. Repeated testing attenuates conditioned place preference with cocaine. Psychopharmacology. 1986;89:239–243. doi: 10.1007/BF00310636. [DOI] [PubMed] [Google Scholar]
- Barr GA, Sharpless NS, Cooper S, Schiff SR, Paredes W, Bridger WH. Classical conditioning, decay and extinction of cocaine-induced hyperactivity and stereotypy. Life Sci. 1983;33:1341–1351. doi: 10.1016/0024-3205(83)90817-2. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience. 1992;3:621–629. doi: 10.1016/0306-4522(92)90406-r. [DOI] [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol. Biochem. Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int. J. Neuropsychopharmacol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat. Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- de Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with noradrenergic blockers phentolamine and phenoxybenzamine. Can. J. Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur. J. Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot M-H. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castañeda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav. Neurosci. 2000;114:1156–1166. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Galaj E, Ananthan S, Saliba M, Ranaldi R. The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology. 2014;231:501–510. doi: 10.1007/s00213-013-3254-y. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng X-Q, Xi Z-X. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12040–12050. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachimine P, Seepersad N, Ananthan S, Ranaldi R. The novel dopamine D3 receptor antagonist, SR 21502, reduces cocaine conditioned place preference in rats. Neurosci. Lett. 2014;569:137–141. doi: 10.1016/j.neulet.2014.03.055. [DOI] [PubMed] [Google Scholar]
- Hu R, Song R, Yang R, Su R, Li J. The dopamine D3 receptor antagonist YQA14 that inhibits the expression and drug-primed reactivation of morphineinduced conditioned place preference in rats. Eur. J. Pharmacol. 2013;720:212–217. doi: 10.1016/j.ejphar.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Francès H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur. J. Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav. Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi Z-X, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Pan H-T, Parsons LH, Justice JB., Jr Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J. Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Roberts DCS. Initiation, maintenance and extinction of cocaine self-administration with and without conditioned reward. Psychopharmacology. 1996;128:89–96. doi: 10.1007/s002130050114. [DOI] [PubMed] [Google Scholar]
- Song R, Bi G-H, Zhang H-Y, Yang R-F, Gardner EL, Li J, Xi Z-X. Blockade of D3 receptors by YQA14 inhibits cocaine's rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology. 2014;77:398–405. doi: 10.1016/j.neuropharm.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Yang R-F, Wu N, Su R-B, Li J, Peng X-Q, Li X, Gaál J, Xi Z-X, Gardner EL. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptorknockout mice. Addict. Biol. 2011;17:259–273. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang H-Y, Peng X-Q, Su R-B, Yang R-F, Li J, Xi Z-X, Gardner EL. Dopamine D3 receptor deletion or blockade attenuates cocaineinduced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253:195–203. doi: 10.1016/0006-8993(82)90686-2. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J. Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompré P-P. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur. J. Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]