Abstract
An efficient method of studying skeletal adaptation to mechanical loading is to assess side-to-side differences (i.e. asymmetry) within individuals who unilaterally exercise one side of the body. Within-subject controlled study designs have been used to explore skeletal mechanoadaptation at upper extremity sites; however, there is no established model in the lower extremities. The current study assessed tibial diaphysis and distal tibia asymmetry in collegiate-level jumping athletes (N = 12). To account for normal crossed asymmetry, data in jumping athletes were compared to asymmetry in a cohort of athletic controls not routinely exposed to elevated unilateral lower extremity loading (N = 11). Jumpers exhibited side-to-side differences between their jump and lead legs at both the tibial diaphysis and distal tibia, with differences at the former site persisting following comparison to dominant-to-nondominant leg differences in controls. In particular, jump-to-lead leg differences for cortical area and thickness at the tibial diaphysis in jumpers were 3.6% (95% Cl =0.5% to 6.8%) and 3.5% (95% Cl = 0.4% to 6.6%) greater than dominant-to-nondominant differences in controls, respectively (all p < 0.05). Similarly, jump-to-lead leg differences in jumpers for tibial diaphysis maximum second moment of area and polar moment of inertia were 7.2% (95% Cl, 1.2–13.2%) and 5.7% (95% Cl, 1.7–9.8%) greater than dominant-to-nondominant differences in controls, respectively (all p < 0.05). Assessment of region-specific differences of the tibial diaphysis in jumpers indicated that the jump leg had greater pericortical radii on the medial and posterior sides and greater radial cortical thickness posteromedially when compared to the lead leg. These data suggest that athletes who perform repetitive and forceful unilateral jumping may be a useful and efficient within-subject controlled model for studying lower extremity skeletal mechanoadaptation.
Keywords: adaptation, bone, exercise, mechanical loading, physical activity, tibia
INTRODUCTION
Exercise is promoted as a means of improving bone health as the skeleton responds and adapts to mechanical forces. Randomized controlled trials (RCTs) have provided strong evidence of the skeletal benefit of mechanical loading [1,2]; however, RCTs are costly in terms of both time and money. An efficient alternative is to cross-sectionally compare bone health between former athletes and controls. However, this approach does not control for inherited and other systemic influences on bone properties. To cross-sectionally explore skeletal mechanoadaptation while controlling for the influence of inherited/systemic factors, a within-subject controlled study design can be used.
Within-subject controlled study designs involve assessing side-to-side differences (i.e. asymmetry) within individuals who unilaterally exercise one side of the body. By comparing skeletal properties on the exercised side to the contralateral non-exercised side, the influence of inherited/systemic factors can be controlled. Inherited/systemic influences may modulate bone responses to exercise; however, these effects on asymmetry are considered small relative to the overall loading effect.
Within-subject controlled study designs have almost exclusively been used to address questions regarding skeletal mechanoadaptation at upper extremity sites. For instance, by comparing dominant-to-nondominant arm differences in racquet sport players, the relative benefits and surface-specific effects of exercise at different times during the pubertal growth period have been identified [3,4]. Similarly, the lifelong benefits of exercise completed when young were observed by comparing throwing-to-nonthrowing arm bone properties in former baseball players [5,6].
In contrast to the upper extremity, there is no established model to explore exercise-induced side-to-side skeletal differences in the lower extremities. Model involving the lower extremity are required as: 1) fractures associated with age-related bone loss cause the greatest morbidity when they occur in the lower extremity, and; 2) upper and lower extremity sites potentially respond differently to stimuli, such as mechanical loading [7]. Individual studies have demonstrated lower extremity skeletal asymmetry in some athletic populations, including rhythmic gymnasts (takeoff > landing leg) [8], ten-pin bowlers (slide > trail leg) [9], soccer players (stance > kicking leg) [10], and fencers (lunging > trail leg) [11]. However, findings have yet to be replicated and a number of the studies did not compare lower extremity skeletal asymmetry in their athletes to that measured in controls. Humans exhibit crossed asymmetry [12,13] whereby the lower extremity opposite the dominant arm possesses enhanced bone properties [14,15]. By comparing asymmetry in athletes to that measured in controls, within-subject exercise benefits can be corrected for crossed asymmetry effects.
We hypothesized athletes who compete in unilateral jumping activities (such as high and long jump) possess greater lower extremity asymmetry than controls and, thereby, represent a possible population wherein lower extremity skeletal mechanoadaptation can be explored using a within-subject controlled study design. Jumping athletes repetitively and forcibly jump off one leg, with the jump leg being exposed to an active peak vertical ground reaction force during take-off that is more than double that experienced during maximal sprinting [16–19]. RCTs have demonstrated the osteogenic potential of jumping exercises [20–23], and previous cross-sectional studies have suggested that jumping athletes have substantially enhanced bone properties [24,25]. Also, a unilateral hopping exercise program was prospectively shown to be beneficial to proximal femur properties in older men [26,27]. To our knowledge, only one previous study has explored lower extremity asymmetry in jumping athletes, with asymmetry limited to tibial trabecular bone mineral content when compared to asymmetry in non-jumpers [28].
The aim of the current within-subject controlled study was to assess tibial diaphysis and distal tibia asymmetry in collegiate-level jumping athletes. While the tibia is not a common site of osteoporotic fracture, its accessibility and loading during activities such as jumping make it a suitable site to preliminarily explore the within-subject lower extremity benefits of exercise. To account for crossed asymmetry, data in jumping athletes were compared to asymmetry measured in a cohort of athletic controls not routinely exposed to elevated unilateral lower extremity loading.
MATERIALS AND METHODS
Participants
Male collegiate-level jumping (jumpers; n = 12) and cross-country running (controls; n = 11) athletes aged 18–30 years were recruited via convenience sampling from local universities and colleges. Jumpers were included if they were currently competing or practicing in collegiate-level long and/or high jump and had been continuously participating in competitive jumping for at least 3 years. Participants in both groups were excluded if they had: 1) participated >2 times per month for >6 months within the previous 3 years in an athletic activity (including basketball, triple jump, volleyball) that may preferentially load one lower extremity (except high or long jump in jumpers), or; 2) been exposed to lower extremity surgery or lower extremity immobilization for >2 weeks within the past 2 years. The take-off leg was defined as the jump leg in jumpers. The dominant leg in controls was defined as the leg on the opposite side of the body to their dominant arm (to account for crossed asymmetry). The contralateral leg was defined as the lead and nondominant leg in jumpers and controls, respectively. The study was approved by the Institutional Review Board of Indiana University and all participants provided written informed consent.
Demographic and anthropometric characteristics
Jumpers completed a questionnaire to document their participation (including age started competitive jumping, and estimated jump training time and jumps per week) and best performance in jumping endeavours. Height (to nearest 0.1 cm) and weight (to nearest 0.1 kg) were measured using a wall mounted digital stadiometer and electronic balance scale, respectively. Body mass index (BMI, kg/m2) was calculated as mass divided by height squared. Tibial length (to nearest 1 mm) was measured using a sliding anthropometer as the distance between the medial tibial plateau and distal tip of the medial malleolus. Whole-body, spine and stride/nondominant total hip areal bone mineral density (aBMD; g/cm2), and whole-body lean (kg) and fat mass (%) were assessed in all subjects via dual-energy X-ray absorptiometry (DXA) using the manufacturer’s standard protocols on a Hologic Discovery-W machine equipped with Apex v4.0 software (Hologic, Inc., Waltham, MA, USA). Subregional analyses of whole body scans were performed to obtain whole leg lean mass (kg) and bone mineral content (g), with the neck of femur being the landmark for the division of the lower extremities from the pelvis.
Jumping performance
To determine whether unilateral jump training engendered side-to-side differences in jump performance, jump height and force for each leg were assessed during single-leg counter-movement jumps with freely moving arms. Subjects took a single step and jumped as high as possible off of the leg being tested. Jump height was measured using a Vertec vertical jump meter with moveable vanes every one-half inch (1.27 cm) (Sports Imports, Columbus, OH). Single-hand vertical reach was measured from a flat-foot standing position before subjects performed a single-leg countermovement jump to displace the highest vane possible. Vertical jump height (cm) was measured as the distance difference between standing and jump reach. Subjects were permitted to perform 3 jumps on each leg separated by ≥1 min, with the best jump on each leg recorded as jump height.
Jump force was measured as per jump height, but with subjects performing jump movements on an AMTI force platform (OR6-7-1000 with Gen5 digital amplifiers; Advanced Mechanical Technologies, Inc., Watertown, MA). Subjects stood quietly on the force platform to first measure the force exerted by body mass. Subjects subsequently performed single-leg countermovement jumps on each leg during which force within the acceleration phase of the jump was collected at 100 Hz using Vicon Nexus software (version 1.8.5; Vicon, Oxford, UK). Subjects were permitted to perform 3 jumps on each leg separated by ≥1 min, with the highest force on each leg recorded as the jump force. Jump force data for each leg was subsequently converted into units of body weight (BW) by dividing by body mass force.
Peripheral quantitative computed tomography (pQCT)
pQCT was performed using a Stratec XCT 3000 machine equipped with software version 6.20C (Stratec Medizintechnik GmbH, Germany). Subjects were positioned supine with the test leg centred within the machine’s gantry and anchored by stretchable straps to limit movement during testing. A scout scan was performed to localize the talocrural joint and a reference line was placed at the distal tibial plateau, bisecting the region of highest density at the lateral side of the distal tibia. Tomographic slices (thickness = 2.3 mm; voxel size = 400 μm; scan speed = 20 mm/s) were taken at 66% (tibial diaphysis) and 4% (distal tibia) of tibial length proximal from the reference line, with tibial length measured earlier using a sliding anthropometer. The procedure was repeated on contralateral side to obtain bilateral measures.
Analysis of the tibial diaphysis slice was restricted to cortical bone parameters as trabecular bone is not present at this site. Cortical mode 1 (threshold, 710 mg/cm3) was used to obtain total area (Tt.Ar, cm2), and cortical volumetric bone mineral density (Ct.vBMD, mg/cm3), bone mineral content (Ct.BMC, mg/mm), and area (Ct.Ar, cm2). Medullary area (Me.Ar, mm2) was derived as Tt.Ar minus Ct.Ar. Average cortical thickness (Ct.Th, mm) was obtained using a circular ring model by analyzing the slices using contour mode 1 (threshold, 710 mg/cm3) to define the outer bone edge and peel mode 2 (threshold, 400 mg/cm3) to separate the cortical and subcortical/medullary compartments. As some previous studies have suggested benefits of exercise on the fibula [29,30], exploratory analyses of the fibula were performed at the level of the tibial diaphysis slice using the same analysis protocol.
Bone strength of the tibial diaphysis was estimated by the density-weighted minimum (IMIN, cm4) and maximum (IMAX, cm4) second moments of area, and polar moment of inertia (IP, mm4) obtained using cortical mode 2 (threshold = 400 mg/cm3). IMIN and IMAX were estimated according to Gere and Timoshenko [31], and represent the distribution of bone material about the orthogonal planes of least and most bending resistance, respectively. IP was calculated as the sum of IMIN and IMAX, and was used to estimate the ability of the bone structure to resist torsion [32]. The ratio of IMAX to IMIN was derived to provide an indication of the directionality of diaphyseal adaptation.
Each tomographic slice at the level of the tibial diaphysis was also analysed to obtain lower leg lean cross-sectional area (CSA, mm2). Contour mode 3 (threshold, 100 mg/cm3) was used to locate the skin surface and peel mode 2 (threshold, 40 mg/cm3) used to locate the subcutaneous fat muscle boundary. A F03F05 filter was used to remove noise. Short-term precision for the pQCT scanning procedure on 30 healthy individuals scanned six times with interim repositioning showed root mean square coefficients of variation (RMS-CVs) of <1% for bone density, mass, structure, and estimated strength measures, and <1.5% for lean CSA [33].
To determine site-specificity of bone geometry adaptive responses associated with jumping, polar pericortical and endocortical radii at the tibial diaphysis were obtained for the jump and lead legs in jumpers. Stratec pQCT image files and data were opened in ImageJ (v1.45s; National Institutes of Health) and analyzed using the BoneJ plugin [34], as previously described [35]. Images were rotated to align the bones according to the IMAX and IMIN axes, and right-sided images were flipped to superimpose left-side images. Using a threshold value of 350 mg/cm3 to locate bone tissue, the distance of the endocortical and pericortical surfaces from the centroid of the medullary cavity were measured in 10° polar sectors. Ct.Th within each sector was calculated as the pericortical minus endocortical radius.
Analyses of the distal tibia slice included total, cortical and trabecular measures, and were achieved using contour mode 1 (threshold, 300 mg/cm3) to define the outer bone edge and peel mode 2 (threshold, 600 mg/cm3) to separate the cortical/subcortical and trabecular compartments. Properties recorded included total vBMD (Tt.vBMD, mg/cm3) and area (Tt.Ar, cm2), and total (Tt.BMC, mg/mm), Ct.BMC, and trabecular (Tb.BMC, mg/mm) BMC. Strength of the distal tibia to resist compressive forces was estimated by the Bone Strength Index (BSI, mg2/mm4). BSI was calculated as the product of Tt.Ar and squared Tt.vBMD, and is predictive of compressive failure load [36].
Statistical analyses
Two-tailed analyses with a level of significance set at 0.05 were performed with IBM SPSS Statistics (v21; SPSS Inc., Chicago, IL). Demographic and anthropometric characteristics (including whole-body, spine and total hip DXA data) were compared between jumpers and controls using independent sample t-tests. Jump versus lead leg differences for tibial properties in jumpers were assessed by calculating mean percent differences ([jump leg lead leg] / lead leg × 100%) and their 95% confidence intervals (CIs). Similarly, mean percent differences ([dominant leg nondominant leg] / dominant leg × 100%) and their 95% confidence intervals (CIs) were calculated to determine crossed asymmetry effects in controls. 95% CIs not crossing 0% were considered statistically significant, as determined by single sample t-tests on the mean percent differences with a population mean of 0%. Bivariate correlations were performed in jumpers to assess whether side-to-side differences in bone properties were related to side-to-side differences in jump performance (height and force).
To establish the effect of jumping on bone properties independent of crossed asymmetry effects, jump-to-lead leg differences in jumpers were compared to dominant-to-nondominant differences in controls using independent sample t-tests. To explore the regional-specificity of bone geometry adaptive responses associated with jumping, tibial diaphysis polar pericortical and endocortical radii and polar Ct.Th data were assessed using two-way repeated measures ANOVA, with leg (jump vs. lead leg) and sector (1 through 36) as within-subject variables. In the presence of a significant leg x sector interaction, post-hoc paired t-tests were used to compare jump vs. lead leg differences within each individual sector. No adjustments were applied on the post-hoc comparisons because of the small sample size and preliminary nature of the regional analyses.
RESULTS
Jumpers and controls had similar age and height (all p = 0.30 to 0.31); however, jumpers had greater whole-body total and lean mass, and greater spine aBMD than controls (all p < 0.01) (Table 1). Ten jumpers matched the concept of crossed asymmetry and used the leg opposite their dominant arm as their jump leg. One left-hand and one right-hand dominant jumper used the leg on the ipsilateral side as their jump leg. All controls were right handed and, thus, their left leg was categorized as being dominant. There were no side-to-side differences in lean measures in either jumpers or controls, or dominant-to-nondominant leg differences in jump performance in controls (all p = 0.68 to 0.97) (Table 2). In contrast, jumpers jumped 13.1% (95% CI = 3.4% to 22.9%) higher and generated 10.4% (95% CI = 2.6% to 18.2%) more force when jumping off their jump leg compared to their lead leg (all p < 0.01) (Table 2).
Table 1.
Demographic and anthropometric characteristics of jumpers and controlsa
| Controls | Jumpers | ||
|---|---|---|---|
| Demographics | |||
| Age (yr) | 22.3 ± 3.0 | 21.8 ± 2.1 | |
| Dominant arm (R:L) | 11:0 | 9:3 | |
| Dominant/jump leg (R:L) | 0:11 | 3:9 | |
| Age starting competitive jumping (yr) | — | 14.2 ± 1.8 | |
| Years competing (yr) | — | 6.2 ± 2.9 | |
| Jumping sport (long:high jump) | — | 12:6b | |
| Jump training per week (min) | — | 193 ± 107 | |
| Jumps per week (n) | — | 71 ± 58 | |
| Personal best: long jump (m) | — | 7.11 ± 0.49 | |
| Personal best: high jump (m) | — | 1.97 ± 0.18 | |
| Whole-body anthropometry | |||
| Height (m) | 1.79 ± 0.07 | 1.82 ± 0.07 | |
| Mass (kg) | 67.7 ± 8.7 | 78.5 ± 5.9** | |
| BMI (kg/m2) | 21.1 ± 1.9 | 23.7 ± 1.8** | |
| aBMD (g/cm2)c,d | 1.23 ± 0.10 | 1.35 ± 0.10 | |
| Lean mass (kg)c | 49.0 ± 5.7 | 58.6 ± 4.5** | |
| Fat mass (%)c | 16.3 ± 4.4 | 14.0 ± 1.3 | |
| Regional anthropometry | |||
| Spine aBMD (g/cm2)c,d | 1.02 ± 0.09 | 1.23 ± 0.13** | |
| Total hip aBMD (g/cm2)c,d,e | 1.13 ± 0.16 | 1.30 ± 0.14 | |
Data indicate mean ± SD (except for frequencies)
6 jumpers participated in both long and high jump
Obtained via dual-energy x-ray absorptiometry
Corrected for whole-body lean mass
Of the nondominant and stride legs in controls and jumpers, respectively
p < 0.01 (independent sample t-test: controls vs. jumpers)
Table 2.
Bilateral leg lean measures, jump performance and bone mass in jumpers and controls
| Controls | Jumpers | |||||
|---|---|---|---|---|---|---|
| Nondominanta | Dominanta | % diff. (95% CI)b | Leada | Jumpa | % diff. (95% CI)b | |
| Lean measures | ||||||
| Whole leg lean mass (kg)c | 9.03 ± 1.1 | 9.01 ± 1.2 | -0.2% (-2.1, 1.6%) | 10.8 ± 0.92 | 10.8 ± 0.94 | -0.1% (-2.2, 2.1%) |
| Lower leg lean CSA (cm2)d | 59.5 ± 4.7 | 59.6 ± 4.8 | -0.3% (-1.4, 2.1%) | 65.1± 5.9 | 65.1± 6.0 | -0.0% (-1.7, 1.7%) |
| Jump performance | ||||||
| Height (cm) | 44.9 ± 10.9 | 46.3 ± 10.1 | 4.2% (-2.8, 11.3%) | 60.3 ± 19.7 | 68.1 ± 23.0 | 13.1% (3.4, 22.9%)** |
| Force (BW) | 2.80 ± 0.44 | 2.74 ± 0.28 | -1.2% (-7.0, 4.7%) | 3.04 ± 0.36 | 3.35 ± 0.46 | 10.4% (2.6, 18.2%)** |
| Bone mass | ||||||
| Whole leg BMC (g)c | 566.2 ± 90.1 | 574.4 ± 83.1 | 1.7% (-1.1, 4.7%) | 703.8 ± 80.6 | 715.4 ± 75.4 | 1.8% (-0.5, 4.2%) |
Data are mean ± SD.
Mean percent differences between take-off and lead leg (controls:dominant and nondominant) were assessed using single sample t-tests with a population mean of 0. Significance is indicated by: *p<0.05, **p<0.01.
Assessed using DXA.
Assessed using pQCT.
There were no dominant-to-nondominant leg differences in controls at either the tibial diaphysis or distal tibia (all p = 0.06 to 0.97) (Table 3). In contrast, the jump leg in jumpers had greater Ct.BMC, Ct.Ar, Ct.Th, IMAX, IP and IMAX/IMIN ratio at the tibial diaphysis and greater Tt.BMC, Ct.BMC and BSI at the distal tibia than in the lead leg (all p < 0.05) (Table 3). There were no jump-to-lead leg differences in jumpers for Ct.vBMD, Tt.Ar, Me.Ar or IMIN at the tibial diaphysis or Tt.vBMD, Tt.Ar, or Tb.BMC at the distal tibia (all p = 0.07 to 0.92) (Table 3). There was a significant correlation at the tibial diaphysis in jumpers between jump-to-lead leg difference in jump height and IMAX (R2 = 0.34; p < 0.05) and significance was approached for a correlation between jump-to-lead leg difference in jump height and IP (R2 = 0.26; p = 0.09). There were no side-to-side differences at the fibula for any parameter in either jumpers or controls (all p = 0.15 to 0.98) or differences in side-to-side differences between jumpers and controls (all p = 0.33 to 0.87).
Table 3.
Bilateral bone density, mass, structure and estimated strength at the tibial diaphysis and distal tibia in the legs of jumpers and controls
| Controls | Jumpers | |||||
|---|---|---|---|---|---|---|
| Nondominanta | Dominanta | % diff. (95% CI)b | Leada | Jumpa | % diff. (95% CI)b | |
| Tibial diaphysis | ||||||
| Ct.vBMD (mg/cm3) | 1142 ± 12 | 1147 ± 16 | 0.5% (-0.1, 1.1%) | 1153 ± 18 | 1152 ± 20 | -0.1% (-0.6, 0.4%) |
| Ct.BMC (mg/mm) | 449 ± 50 | 451 ± 51 | 0.5% (-1.2, 2.3%) | 513 ± 58 | 531 ± 67 | 3.6% (0.6, 6.5%)* |
| Tt.Ar (cm2) | 6.81 ± 0.82 | 6.81 ± 0.72 | 0.2% (-1.5, 1.8%) | 7.76 ± 0.88 | 7.87 ± 0.84 | 1.5% (-0.5, 3.5%) |
| Ct.Ar (cm2) | 3.94 ± 0.45 | 3.94 ± 0.46 | 0.1% (-1.8,1.9%) | 4.45 ± 0.48 | 4.61 ± 0.56 | 3.7% (1.0, 6.3%)** |
| Me.Ar (cm2) | 2.87 ± 0.66 | 2.87 ± 0.62 | 0.3% (-3.1, 3.8%) | 3.32 ± 0.81 | 3.26 ± 0.75 | -1.2% (-4.2, 1.8%) |
| Ct.Th (mm) | 5.19 ± 0.58 | 5.19 ±0.63 | -0.1% (-2.2, 2.1%) | 5.49 ± 0.72 | 5.68 ± 0.78 | 3.5% (0.9, 6.0%)** |
| IP (cm4) | 7.38 ± 1.62 | 7.36 ± 1.42 | 0.2% (-2.9, 3.4%) | 9.02 ± 1.59 | 9.52 ± 1.70 | 5.7% (1.0, 10.3%)* |
| IMIN (cm4) | 1.87 ± 0.43 | 1.87 ± 0.41 | 0.3% (-2.5, 3.1%) | 2.62 ± 0.60 | 2.64 ± 0.60 | 1.1% (-4.3, 6.4%) |
| IMAX (cm4) | 5.51 ± 1.29 | 5.48 ± 1.11 | 0.3% (-3.6, 4.2%) | 6.41 ± 1.04 | 6.88 ± 1.19 | 7.5% (2.5, 12.5%)** |
| IMAX/IMIN | 2.97 ± 0.52 | 2.95 ± 0.50 | -0.3% (-3.9, 3.4%) | 2.49 ± 0.28 | 2.66 ± 0.39 | 6.8% (1.2, 12.3%)* |
| Distal tibia | ||||||
| Tt.vBMD (mg/cm3) | 394 ± 38 | 406 ± 37 | 3.0% (-0.3, 6.3%) | 403 ± 48 | 411 ± 38 | 2.3% (-0.2, 4.8%) |
| Tt.BMC (mg/mm) | 438 ± 45 | 442 ± 50 | 1.1% (-3.5, 5.6%) | 504 ± 62 | 515 ± 62 | 2.3% (0.5, 4.1%)* |
| Tt.Ar (cm2) | 11.2 ± 1.3 | 11.0 ± 1.8 | -1.6% (-7.8, 4.7%) | 12.6 ± 1.6 | 12.6 ± 1.5 | 0.1% (-2.0, 2.1%) |
| Ct.BMC (mg/mm) | 110 ± 23 | 120 ± 25 | 9.6% (-0.1, 19.3%) | 138 ± 38 | 150 ± 35 | 10.8% (2.4,19.1%)* |
| Tb.BMC (mg/mm) | 327 ± 34 | 322 ± 47 | -1.9% (-6.9, 3.1%) | 367 ± 20 | 366 ± 46 | -0.2% (-2.7, 2.2%) |
| BSI (mg2/cm4) | 173 ± 29 | 179 ± 23 | 4.0% (-0.6, 8.5%) | 205 ± 45 | 213 ± 43 | 4.7% (0.8, 8.5%)* |
Data are mean ± SD.
Mean percent differences between take-off and lead leg (controls:dominant and nondominant) were assessed using single sample t-tests with a population mean of 0. Significance is indicated by: *p<0.05, **p<0.01.
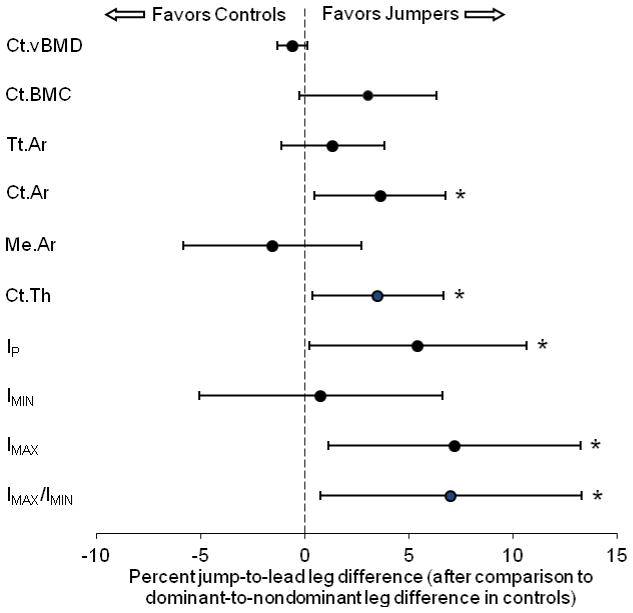
The jump-to-lead leg difference in tibial diaphysis Ct.BMC in jumpers did not differ from dominant-to-nondominant differences in controls (p = 0.07). However, jump-to-lead leg differences for tibial diaphysis Ct.Ar and Ct.Th in jumpers were 3.6% (95% Cl =0.5% to 6.8%) and 3.5% (95% Cl = 0.4% to 6.6%) greater than dominant-to-nondominant differences in controls, respectively (all p < 0.05) (Fig. 1). Similarly, jump-to-lead leg differences in jumpers for IMAX, IP and IMAX/IMIN ratio were 7.2% (95% Cl, 1.2–13.2%), 5.7% (95% Cl, 1.7–9.8%) and 7.0% (95% Cl, 0.8–13.3%) greater than dominant-to-nondominant differences in controls, respectively (all p < 0.05) (Fig. 1).
Fig 1.
Effect of jumping on tibial diaphysis bone density, mass, structure and estimated strength. Jumpers had greater jump-to-lead leg differences for cortical area (Ct.Ar), cortical thickness (Ct.Th), maximum second moment of area (IMAX), and polar moment of inertia (IP) than dominant-to- nondominant leg differences in controls. There were no group differences for cortical volumetric bone mineral density (Ct.vBMD), cortical bone mineral content (Ct.BMC), total area (Tt.Ar), medullary area (Me.Ar), or minimum second moment of area (IMIN). Data represent the difference between mean percent jump-to-lead leg differences in jumpers and mean percent dominant-to-nondominant leg differences in controls, with error bars indicating 95% confidence intervals. Confidence intervals greater than 0% indicate greater jump-to-lead leg differences in jumpers compared to dominant-to-nondominant leg differences in controls (*p < 0.05, unpaired t-test).
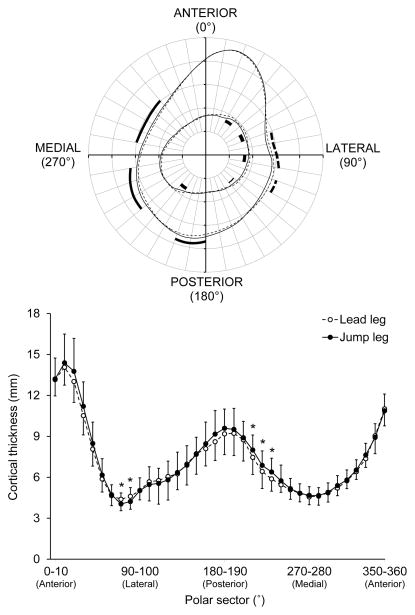
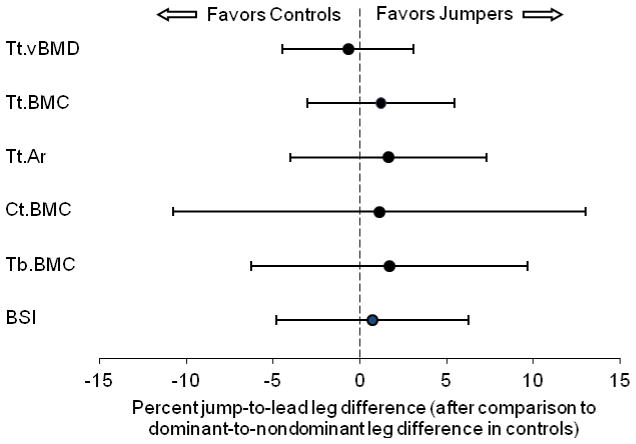
There were significant leg x sector interactions for polar pericortical and endocortical radii, and polar Ct.Th (all p < 0.05). The jump leg in jumpers had greater pericortical radii on the medial and posterior sides and smaller pericortical and endocortical radii on the lateral side of the tibial diaphysis compared to the lead leg (all p < 0.05) (Fig. 2A). The jump leg had greater Ct.Th in posteromedial polar sectors and less Ct.Th in lateral sectors compared to the lead leg (all p < 0.05) (Fig. 2B). There were no jump-to-lead leg differences detected at the distal tibia when compared to dominant-to-nondominant differences in controls (all p = 0.13 to 0.90) (Fig. 3).
Fig 2.
A) Map of average pericortical and endocortical radii and B) average ± SD cortical thickness in 10 polar sectors at the tibial diaphysis in the jump (solid line) and lead (broken line) legs of jumpers. Sectors in A wherein jump leg pericortical radii were significantly (p < 0.05) greater and less than corresponding radii in lead legs are indicated by thick solid and broken lines, respectively. Sectors in A wherein jump leg endocortical radii were significantly (p < 0.05) less than corresponding radii in lead legs are indicated by thick solid lines. Sectors in B wherein cortical thickness significantly (p < 0.05) differed between jump and lead legs are indicated by *.
Fig 3.
Effect of jumping on distal tibia bone density, mass, structure and estimated strength. There were no differences between jump-to-lead leg differences in jumpers and dominant-to-nondominant leg differences in controls for total volumetric bone mineral density (Tt.vBMD), total (Tt.BMC), cortical (Ct.BMC) or trabecular (Tb.BMC) bone mineral content, total area (Tt.Ar), or Bone Strength Index (BSI). Data represent the difference between mean percent jump-to-lead leg differences in jumpers and mean percent dominant-to-nondominant leg differences in controls, with error bars indicating 95% confidence intervals.
DISCUSSION
The current data suggest that athletes competing in activities involving repetitive and forceful unilateral jumping may be a useful within-subject controlled model for studying lower extremity mechanoadaptation. Jumping athletes possessed superior spine aBMD than controls, and group differences for whole-body and hip aBMD approached significance (both p = 0.07), consistent with previous studies demonstrating enhanced bone health in power (i.e. jumping/sprinting) versus endurance (i.e. cross-country running) athletes [37–39]. In addition to having an enhanced background skeletal phenotype, jumpers exhibited side-to-side differences between their jump and lead legs at both the tibial diaphysis and distal tibia, with differences at the former site being greater than dominant-to-nondominant leg differences in cross-country runners. By using the lead leg as an internal control site and assessing the within-subject skeletal effects of jumping, the skeletal benefits of jumping were isolated from the potential influence of selection bias, which limit the findings of cross-sectional studies comparing bone properties between subjects. We also isolated the skeletal benefits of jumping from any crossed asymmetry not attributable to unilateral jumping activities by comparing side-to-side differences in jumpers to those in a group who historically have not participated in an athletic endeavour introducing unilateral lower extremity loading.
The current findings extend those of Ireland et al. [28] who compared side-to-side differences in Master-level jumping athletes (pole vaulters and long jumpers) to sprinters and unconditioned jumpers who did not engage or competed, but did not train, in jump events, respectively. In jumping athletes, Ireland et al. [28] reported jump-to-lead leg differences for tibial diaphysis Ct.BMC and IP of ~2.5% and ~7%, respectively. These side-to-side differences are comparable with the jump-to-lead leg differences of 3.6% and 5.7% for Ct.BMC and IP observed in jumpers in the current study, respectively. However, in contrast to Ireland et al. [28], a number of the side-to-side differences in jumpers in the current study persisted when compared to dominant-to-nondominant leg differences in controls. Thus, we observed jumping had skeletal benefits over-and-above any crossed asymmetry that is not attributable to participating in unilateral jumping activities. The reasons why side-to-side differences in jumpers were greater than observed in controls in the current study are not clear, but may be due to less data variability and/or the study of less comparative groups (jumpers vs. controls). With regards to the latter, Ireland et al. [28] compared side-to-side differences in four groups of athletes which required the use of an analysis of variance and relatively stringent post-hoc comparisons (Tukey’s HSD) to identify differences between individual groups.
There was no benefit of jumping on jump-to-lead leg differences in bone mass when compared to dominant-to-nondominant leg differences in controls, although significance was approached (p = 0.07). In contrast, jumpers exhibited greater jump-to-lead leg differences in tibial diaphyseal Ct.Ar, Ct.Th, IMAX and IP than dominant-to-nondominant leg differences in controls. These data suggest jumping led to a redistribution of bone mass to sites where needed most. There was no benefit of jumping on overall bone size (i.e. Tt.Ar), which may have been mediated by the fact that most of our jumpers begun unilateral jumping activities in adolescence (i.e. after puberty) when loading-induced periosteal bone accrual appears to slow [3,40]. However, assessment of pericortical radii and Ct.Th in polar sections of the tibia indicated directionally specific increased bone size in the posterior and medial directions. Increasing bone size and distributing bone material further from bending axes in the posterior and medial directions is functionally important as it increases resistance to bending in these directions, which may decrease stress fracture risk to the injury prone posteromedial region of the tibia.
Increased polar pericortical radii in the medial direction were offset by reduced radii in the lateral direction. As the mediolateral plane in the tibial diaphyseal cross-section approximates the plane of least bending resistance (i.e. IMIN), summation of the mediolateral changes meant that IMIN was not altered in the jump leg of jumpers. This does not necessarily mean that resistance to bending in the mediolateral direction was not enhanced as the cumulative changes in medial and lateral pericortical radii suggest a modeling drift whereby the tibial diaphyseal cross-sectional as a whole had shifted medially. Such a shift would theoretically reduce the moment arm of the force that causes the tibia to bend medially during weight bearing activities [41].
In contrast to IMIN, IMAX was greater at the tibial diaphysis in the jump leg of jumpers. The anteroposterior plane approximates the plane of greatest bending resistance (i.e. IMAX) and the tibial diaphysis had greater pericortical radii and Ct.Th in the posterior direction that was not offset by jump-to-lead leg differences in the anterior direction. The greater IMAX contributed to the jump leg having greater torsional rigidity, as IP is derived as the sum of IMAX and IMIN. The greater IMAX also contributed to the jump leg having a greater IMAX/IMIN ratio, which further highlights the directional adaptation of the tibia to jumping activities. The pattern of tibial adaptation in the IMAX plane associated with jumping is likely an adaptive response to stiffen the bone to the predominantly posterior bending of the tibia during weight bearing activities [41], and supports the pattern of tibial adaptation to jumping and other weight bearing activities prospectively observed in boys [42] and observed in between-subject cross-sectional studies [25,43,44].
Surprisingly, no skeletal benefits of jumping were observed at the distal tibia site in the current study. This is surprising considering that the elevated unilateral forces associated with jumping activities generated differences at the tibial diaphysis and that the distal tibia would presumably also be exposed to elevated unilateral loading. Jumpers did have jump-to-lead leg differences in estimated compressive strength (i.e. BSI) at the distal tibia as a result of elevated BMC in the jump leg; however, these differences were not statistically greater than dominant-to-nondominant leg differences observed in controls. That is, the side-to-side differences observed in jumpers could be explained by normal crossed asymmetry, as opposed to unilateral jumping activities. Possible explanations for the lack of an effect of jumping at the distal tibia include a lesser effect size of jumping activities and greater variability in data at this site resulting in reduced power to detect statistical differences.
The magnitudes of side-to-side differences observed at the tibial diaphysis in the current study are much smaller than those observed in within-subject controlled studies of the upper extremity. In the upper extremity, chronically exercised arms have been reported to have nearly twice the estimated strength of contralateral nonexercised arms [6,45]. The most likely explanation for the reduced asymmetry in the lower extremity is the fact that both the exercised and contralateral ‘nonexercised’ legs are habitually exposed to elevated mechanical loading during weight bearing activities, which include running and sprinting in jumping athletes. Chronic elevated loading of the contralateral leg alters its phenotype changing the magnitude of the denominator in asymmetry calculations, whereas chronic habitual loading and adaptation of the exercised leg reduces its sensitivity to superimposed heightened mechanical loads, such as those associated with jumping, due to cellular accomodation [46]. It should also be noted that jumping athletes often perform plyometric and other exercises whereby the contralateral leg serves as the landing leg and, thus, is exposed to its own unilateral loading which would reduce within-subject asymmetry in jumpers. Ultimately, the relatively small side-to-side differences observed in jumpers in the current study, despite their relatively prolonged and high level of participation in jumping activities, reduces the usefulness of this athlete population as a within-subject controlled model for exploring deeper questions regarding mechanoadaptation in the lower extremity.
Our study has a number of strengths, including the comparison of tibial properties within-subject to control for selection bias, the inclusion of an athletic comparative group not exposed to unilaterally elevated loads to account for any normal crossed asymmetry, and the use of polar analyses to assess regional-specificity of bone geometry adaptive responses associated with jumping. However, the study also possesses a number of limitations that warrant consideration when interpreting the data. These include the study of a relatively small sample size and males only, the inclusion of some long jump athletes who also competed in high jump, and limitations associated with the region specific analyses. The small sample size may have reduced our power to detect subtle group differences in some measures and contributed to our region specific analyses being preliminary in nature with at heightened risk of a Type-I error due to multiple comparisons. Our male only data may not be representative of tibial adaptation to jumping activities in females, while the inclusion of some long jump athletes who also competed in high jump may have contributed to variability in the data, with long and high jump requiring differing biomechanics that potentially introduce heterogeneous load magnitudes and distributions. With regard to the region specific analyses, these involved measuring the distance of the endocortical and pericortical surfaces from the center of the medullary cavity in polar sectors. Apposition and/or removal of bone from the endocortical surface may move the centre of the medullary cavity and artefactually alter radii length.
In summary, the current data demonstrate that collegiate-level jumping athletes have enhanced tibial bone properties in their jump leg compared to their contralateral lead (non-jump) leg. The jump-to-lead leg differences in jumpers a the tibial diaphysis were greater than dominant-to-nondominant leg differences in controls indicating that the enhanced tibial properties in the jump leg of jumpers was due to jumping activities, as opposed to any normally occurring cross asymmetry. Overall, these data suggest that athletes who perform repetitive and forceful unilateral jumping may be a useful and efficient within-subject controlled model for studying lower extremity skeletal mechanoadaptation. However, additional studies are required to more fully establish the model’s utility and its ability to address deeper questions regarding mechanoadaptation in the lower extremity.
Acknowledgments
This work was made possible by supported from the National Institutes of Health (R01 AR057740 to S.J.W.). The authors declare that they have no conflict of interest.
References
- 1.Behringer M, Gruetzner S, McCourt M, Mester J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J Bone Miner Res. 2014;29:467–478. doi: 10.1002/jbmr.2036. [DOI] [PubMed] [Google Scholar]
- 2.Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, McKay HA. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29:2161–81. doi: 10.1002/jbmr.2254. [DOI] [PubMed] [Google Scholar]
- 3.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–80. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 4.Kannus P, Haapasalo H, Sankelo M, Sievänen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Warden SJ, Mantila Roosa SM. Physical activity completed when young has residual bone benefits at 94 years of age: a within-subject controlled case study. J Musculoskelet Neuronal Interact. 2014;14:239–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Warden SJ, Mantila Roosa SM, Kersh ME, Hurd AL, Fleisig GS, Pandy MG, Fuchs RK. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci USA. 2014;111:5337–42. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikander R, Sievanen H, Uusi-Rasi K, Heinonen A, Kannus P. Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity: a pQCT study of adult female athletes. Bone. 2006;39:886–94. doi: 10.1016/j.bone.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Ishizaki S, Kato Y, Kuroda Y, Fukashiro S. The side-to-side differences of bone mass at proximal femur in female rhythmic sports gymnasts. J Bone Miner Res. 1998;13:900–6. doi: 10.1359/jbmr.1998.13.5.900. [DOI] [PubMed] [Google Scholar]
- 9.Young KC, Sherk VD, Bemben DA. Inter-limb musculoskeletal differences in competitive ten-pin bowlers: a preliminary analysis. J Musculoskelet Neuronal Interact. 2011;11:21–6. [PubMed] [Google Scholar]
- 10.Anliker E, Sonderegger A, Toigo M. Side-to-side differences in the lower leg muscle-bone unit in male soccer players. Med Sci Sports Exerc. 2013;45:1545–52. doi: 10.1249/MSS.0b013e31828cb712. [DOI] [PubMed] [Google Scholar]
- 11.Chang G, Regatte RR, Schweitzer ME. Olympic fencers: adaptations in cortical and trabecular bone determined by quantitative computed tomography. Osteoporos Int. 2009;20:779–85. doi: 10.1007/s00198-008-0730-z. [DOI] [PubMed] [Google Scholar]
- 12.Auerbach BM, Ruff CB. Limb bone bilateral asymmetry: variability and commonality among modern humans. J Hum Evol. 2006;50:203–18. doi: 10.1016/j.jhevol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Latimer HB, Lowrance EW. Bilateral asymmetry in weight and in length of human bones. Anat Rec. 1965;152:217–24. doi: 10.1002/ar.1091520213. [DOI] [PubMed] [Google Scholar]
- 14.Dane S, Akar S, Hacibeyoglu I, Varoglu E. Differences between right- and left-femoral bone mineral densities in right- and left-handed men and women. Int J Neurosci. 2001;111:187–92. doi: 10.3109/00207450108994230. [DOI] [PubMed] [Google Scholar]
- 15.Gumustekin K, Akar S, Dane S, Yildirim M, Seven B, Varoglu E. Handedness and bilateral femoral bone densities in men and women. Int J Neurosci. 2004;114:1533–47. doi: 10.1080/00207450490509186. [DOI] [PubMed] [Google Scholar]
- 16.Coh M, Supej M. Biomechanical model of the take-off action in the high jump: a case study. New Stud Athlet. 2008;23:63–73. [Google Scholar]
- 17.Luhtanen P, Komi PV. Mechanical power and segmental contribution to force impulses in long jump take-off. Eur J Appl Physiol Occup Physiol. 1979;41:267–274. doi: 10.1007/BF00429743. [DOI] [PubMed] [Google Scholar]
- 18.Mero A, Komi PV. Force-, EMG-, and elasticity-velocity relationships at submaximal, maximal and supramaximal running speeds in sprinters. Eur J Appl Physiol Occup Physiol. 1986;55:553–61. doi: 10.1007/BF00421652. [DOI] [PubMed] [Google Scholar]
- 19.Muraki Y, Ae M, Koyama H, Yokozawa T. Joint torque and power of the takeoff leg in the long jump. Int J Sport Health Sci. 2008;6:21–32. [Google Scholar]
- 20.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen A, Kannus P, Sievanen H, Oja P, Pasanen M, Rinne M, Uusi-Rasi K, Vuori I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet. 1996;348:1343–7. doi: 10.1016/S0140-6736(96)04214-6. [DOI] [PubMed] [Google Scholar]
- 22.McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: A randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. 2000;136:156–162. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 23.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen A, Sievanen H, Kyrolainen H, Perttunen J, Kannus P. Mineral mass, size, and estimated mechanical strength of triple jumpers' lower limb. Bone. 2001;29:279–285. doi: 10.1016/s8756-3282(01)00574-9. [DOI] [PubMed] [Google Scholar]
- 25.Nikander R, Kannus P, Rantalainen T, Uusi-Rasi K, Heinonen A, Sievanen H. Cross-sectional geometry of weight-bearing tibia in female athletes subjected to different exercise loadings. Osteoporos Int. 2010;21:1687–94. doi: 10.1007/s00198-009-1101-0. [DOI] [PubMed] [Google Scholar]
- 26.Allison SJ, Folland JP, Rennie WJ, Summers GD, Brooke-Wavell K. High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone. 2013;53(2):321–8. doi: 10.1016/j.bone.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Allison SJ, Poole KE, Treece GM, Gee AH, Tonkin C, Rennie WJ, Folland JP, Summers GD, Brooke-Wavell K. The influence of high impact exercise on cortical and trabecular bone mineral content and 3D distribution across the proximal femur in older men: a randomised controlled unilateral intervention. J Bone Miner Res. 2015;30:1709–16. doi: 10.1002/jbmr.2499. [DOI] [PubMed] [Google Scholar]
- 28.Ireland A, Korhonen M, Heinonen A, Suominen H, Baur C, Stevens S, Degens H, Rittweger J. Side-to-side differences in bone strength in master jumpers and sprinters. J Musculoskelet Neuronal Inter. 2011;11:298–305. [PubMed] [Google Scholar]
- 29.Marchi D, Shaw CN. Variation in fibular robusticity reflects variation in mobility patterns. J Hum Evol. 2011;61:609–16. doi: 10.1016/j.jhevol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Rantalainen T, Nikander R, Heinonen A, Suominen H, Sievanen H. Direction-specific diaphyseal geometry and mineral mass distribution of tibia and fibula: a pQCT study of female athletes representing different exercise loading types. Calcif Tissue Int. 2010;86:447–4. doi: 10.1007/s00223-010-9358-z. [DOI] [PubMed] [Google Scholar]
- 31.Gere J, Timoshenko S. Mechanics of Materials. 2. Boston (MA): PWS-Kent; 1984. p. 492. [Google Scholar]
- 32.Weatherholt AM, Avin KG, Hurd AL, Cox JL, Marberry ST, Santoni BG, Warden SJ. Peripheral quantitative computed tomography predicts humeral diaphysis torsional mechanical properties with good short-term precision. J Clin Densitom. 2015 doi: 10.1016/j.jocd.2014.10.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swinford RR, Warden SJ. Factors affecting short-term precision of musculoskeletal measures using peripheral quantitative computed tomography (pQCT) Osteoporos Int. 2010;21:1863–70. doi: 10.1007/s00198-009-1151-3. [DOI] [PubMed] [Google Scholar]
- 34.Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ. BoneJ: free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–9. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rantalainen T, Nikander R, Heinonen A, Daly R, Sievänen H. An open source approach for regional cortical bone mineral density analysis. J Musculoskelet Neuronal Inter. 2011;11:243–8. [PubMed] [Google Scholar]
- 36.Kontulainen S, Johnston J, Liu D, Leung C, Oxland T, McKay H. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8:401–9. [PubMed] [Google Scholar]
- 37.Nowak A, Straburzynska-Lupa A, Kusy K, Zielinski J, Felsenberg D, Rittweger J, Karolkiewicz J, Straburzynska-Migaj E, Pilaczynska-Szczesniak L. Bone mineral density and bone turnover in male masters athletes aged 40–64. Aging Male. 2010;13:133–41. doi: 10.3109/13685531003657776. [DOI] [PubMed] [Google Scholar]
- 38.Weidauer LA, Eilers MM, Binkley TL, Vukovich MD, Specker BL. Effect of different collegiate sports on cortical bone in the tibia. J Musculoskelet Neuronal Inter. 2012;12:68–73. [PubMed] [Google Scholar]
- 39.Wilks DC, Winwood K, Gilliver SF, Kwiet A, Chatfield M, Michaelis I, Sun LW, Ferretti JL, Sargeant AJ, Felsenberg D, Rittweger J. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone. 2009;45:91–7. doi: 10.1016/j.bone.2009.03.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ducher G, Daly R, Bass S. The effects of repetitive loading on bone mass and geometry in young male tennis players: a quantitative study using magnetic resonance imaging. J Bone Miner Res. 2009;24:1686–92. doi: 10.1359/jbmr.090415. [DOI] [PubMed] [Google Scholar]
- 41.Yang PF, Sanno M, Ganse B, Koy T, Bruggemann GP, Muller LP, Rittweger J. Torsion and antero-posterior bending in the in vivo human tibia loading regimes during walking and running. PLoS One. 2014;9:e94525. doi: 10.1371/journal.pone.0094525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald HM, Cooper DM, McKay HA. Anterior-posterior bending strength at the tibial shaft increases with physical activity in boys: evidence for non-uniform geometric adaptation. Osteoporos Int. 2009;20:61–70. doi: 10.1007/s00198-008-0636-9. [DOI] [PubMed] [Google Scholar]
- 43.Ma H, Leskinen T, Alen M, Cheng S, Sipila S, Heinonen A, Kaprio J, Suominen H, Kujala UM. Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res. 2009;24:1427–33. doi: 10.1359/jbmr.090309. [DOI] [PubMed] [Google Scholar]
- 44.Rantalainen T, Weeks BK, Nogueira RC, Beck BR. Effects of bone-specific physical activity, gender and maturity on tibial cross-sectional bone material distribution: a cross-sectional pQCT comparison of children and young adults aged 5–29 years. Bone. 2015;72:101–8. doi: 10.1016/j.bone.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Warden SJ. Extreme skeletal adaptation to mechanical loading. J Orthop Sports Phys Ther. 2010;40:188. doi: 10.2519/jospt.2010.0404. [DOI] [PubMed] [Google Scholar]
- 46.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accomodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]