Abstract
Background
Individuals who smoke more cigarettes per day are at greater risk for developing smoking-related illness and have more difficulty quitting. Withdrawal-related negative mood is one factor thought to motivate drug use. However, heavy smokers are generally more sensitive to negative affect, not just negative emotion stemming from withdrawal. One possibility is that individual differences in how the brain processes negative stimuli may impact smoking use. Given the wealth of data implicating the insula in nicotine dependence and affective processing we hypothesize that the number of cigarettes an individual smokes per day will relate to insula reactivity to negative stimuli.
Methods
A functional magnetic resonance imaging (fMRI) emotional processing task collected by the Human Connectome Project was assessed in 21 daily tobacco smokers who reported smoking between 5–20 cigarettes per day. The number of cigarettes smoked per day was correlated with right and left anterior insula reactivity to faces expressing a negative emotion relative to a control. This anterior insula region of interest has been associated with treatment outcome and smoking cue-reactivity in our prior work.
Results
Those who smoked more daily cigarettes showed greater right insula reactivity to negative stimuli (r = 0.564, p = 0.008). Left insula reactivity was not associated with cigarettes smoked per day.
Conclusion
Smokers who use more cigarettes per day have greater insula reactivity to negative stimuli, furthering the field’s understanding of the insula’s involvement in nicotine use. This preliminary work also suggests a mechanism contributing to higher rates of daily smoking.
Keywords: Cigarette smoking, Nicotine, Negative stimuli, Insulal Connectome
1. INTRODUCTION
Over 36 million adults in the United States smoke cigarettes daily (CDC, 2010), yet the number of cigarettes smoked/day varies across individuals (Shiffman, 2009; Burns et al., 1997). Understanding factors influencing smoking rates is essential, as greater smoking rates are associated with increased risk of smoking related illness (Bjartveit and Tverdal, 2005; Law and Wald, 2003) and with poorer quit outcomes (Harris et al., 2004; Hymowitz et al., 1997).
One factor possibly mediating daily cigarette use is reactivity to negative affect, as smoking is often maintained to alleviate negative mood states (Copeland et al., 1995; Brandon, 1994; Baker et al., 2004). Smokers self-administer greater numbers of cigarettes/day when faced with exogenous stressors (Aronson et al., 2008) and more rapid relapse is associated with decreased distress tolerance (Brown et al., 2002), suggesting that smoking may be related to how an individual processes negative affect. To test the hypothesis that daily smoking rates are associated with how negative affect is processed, we evaluated the relationship between the number of cigarettes an individual smokes/day and insula reactivity to negative stimuli.
Insula reactivity to negative stimuli in smokers is of particular interest, because this brain region is implicated in affective processing (Critchley, 2002; Critchley et al., 2004; Damasio et al., 2000; Phan et al., 2002; Craig, 2002, 2010) and smoking (Janes et al., 2010a, 2010b, 2015, 2015; Naqvi et al., 2007; Gray and Critchley, 2007). The insula is engaged during the processing of basic affective states, including the interpretation of external stimuli such as emotional faces (Carr et al., 2003) and internal awareness of one’s emotions (Critchley, 2002, 2004; Damasio et al., 2000; Craig, 2002, 2010). Additionally, the insula facilitates nicotine seeking-behavior (Naqvi et al., 2007; Forget et al., 2010; Scott and Hiroi, 2011; Pushparaj et al., 2013) and is related to factors that impact daily smoking such as cigarette craving (Brody et al., 2002; Wang et al., 2007) and nicotine dependence severity (Claus et al., 2013). Thus, our analysis focused on a previously identified anterior insula region of interest (ROI) that is associated with smoking (Janes et al., 2015).
To identify an association between daily cigarette use and insula reactivity to negative stimuli, we investigated data collected via the Human Connectome Project (HCP; Van Essen et al., 2013). Specifically, we assessed whether the number of cigarettes smoked/day was correlated with insula activity collected during an emotional processing task where individuals were presented with faces expressing negative emotion compared to a neutral control. Given the insula’s involvement in both emotional processing and nicotine dependence, we hypothesize that insula reactivity to negative stimuli will be associated with greater daily nicotine use.
2. METHODS
2.1 Participants
Data analyzed were obtained from the Human Connectome Project (HCP). A description of the recruitment criteria for the HCP is provided by Van Essen et al. (2013). Briefly, individuals were excluded by the HCP if they reported a significant history of psychiatric disorder, substance abuse, neurological disorder, or medical disorder known to influence brain function. Participants in the current study included all those reporting regular cigarette use who did not have a positive urine sample indicating any illicit drug use or a history of alcohol or marijuana dependence. Twenty-one tobacco smokers (16 women, 2 left-handed) between the ages of 28 and 36 (mean = 30.3 ± 3.3 years) with an average of 13 years of education (± 4.4) were assessed. All subjects reported smoking an average of 9.7 cigarettes/day (± 4.4; range: 5 – 20), had an average nicotine dependence level of 3.14 ± 1.8 as measured by the Fagerstrom Test for Nicotine Dependence (FTND, Heatherton et al., 1991), and were abstinent for at least 1 hour prior to the fMRI task.
2.2 fMRI data acquisition
All imaging data were acquired with a 32-channel head coil on a modified 3T Siemens Skyra. Ugurbil et al. (2013) provides a detailed description of the human connectome project (HCP) fMRI acquisition protocols, which is summarized by Barch et al. (2013).
2.3 fMRI data preprocessing
The “minimally preprocessed” Quarter 3 release of the HCP data was used for this study (40). We further preprocessed these data using tools from FSL 5.0.6 (FMRIB Software Library; Analysis Group, FMRIB, Oxford, UK; http://www.fmrib.ox.ac.uk/fsl). Data were spatially smoothed to 4 mm full width half max and high pass temporal filter was applied (Gaussian-weighted least squares straight line fitting, with sigma = 100 s).
2.4 Experimental task design
The emotional processing task used by the HCP was adapted from a well-validated task developed by Hariri et al. (2012). Two 2 min and 5 s runs of the task were acquired. Each of the two runs began with an 8 s fixation, followed by 6 alternating blocks: three blocks presented face stimuli (angry or fearful faces) and the other three blocks presented shape stimuli (wide or tall circles). Prior to each block, a cue indicating the type of block (shape or face) was shown for 3 s. Each block lasted 18 s, in which six 2 s trials were followed by a 1 s inter-trial-interval. There was a bug in the E-prime script for this task, such that the task stopped short of the last three trials of the last block (face) in each run. As described on the HCP website (http://www.humanconnectome.org/documentation/Q1/task-fMRI-protocol-details.html), the last block of each run lasted only 9 seconds, which limited trial presentation of that face block to 3 face trials.
2.5 fMRI data analysis
At the first level, two regressors, one corresponding to emotional faces block and the other to shapes block, were included in the general linear model. These regressors were constructed using boxcar functions of length equal to the duration of the block and convolved with the double gamma hemodynamic response function. Motion was modeled using the six motion regressors (x, y, z, translation and rotation). First-level results were combined across runs followed by a group-level analysis using the mixed model (FLAME) with automatic outlier detection (Woolrich, 2008). A Pearson’s correlation coefficient was calculated between the number of cigarettes smoked per day and right and left insula reactivity to negative faces > shapes. Anterior insula regions of interest were comprised of 5-mm spheres located at MNI coordinates (±34, 26, 2; x, y, z; Figure 1), which are identical to the ROIs we used previously (Janes et al., 2015). To test the specificity of the anterior insula’s involvement, we evaluated the relationship between cigarettes/day and the right and left mid (±8, 2, 2) and posterior insula (±38, −14, 8); ROIs, also taken from our prior work (Janes et al., 2015). To ensure that findings were not unduly affected by possible outliers, correlations were confirmed using Spearman’s nonparametric test.
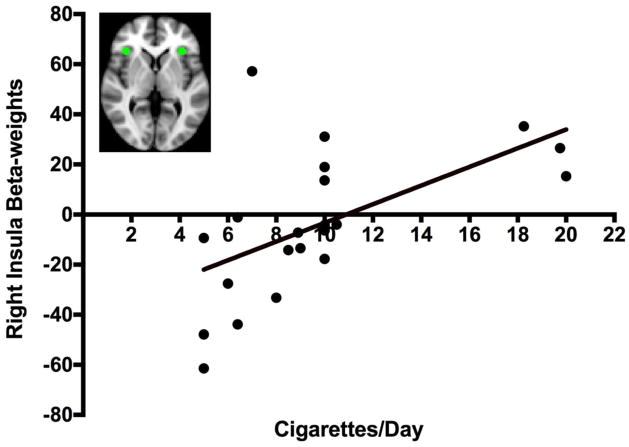
Figure 1. Right Insula Activation: Negative Faces > Shapes.
There is a positive relationship between right insula beta-weights for the negative faces > shapes contrast and the average number of cigarettes smoked per day (Pearson’s r = 0.564, p = 0.008). The brain image on the top left shows the right and left insula ROIs in green.
3. RESULTS
3.1 Association between Insula reactivity and cigarettes/day
There was a positive association between cigarettes smoked/day and reactivity to negative faces > shapes in the right (Pearson’s r = 0.564, p = 0.008; Figure 1), but not left anterior insula (Pearson’s r = 0.253, p = 0.268). This finding was supported by non-parametric testing using Spearman’s rho (right anterior insula r = 0.653, p = 0.001). While there was a marginal association between cigarettes smoked/day and beta weights extracted from the right posterior insula (Pearson’s r = 0.446, p = 0.43), this association was not significant when testing with Spearman’s rho suggesting outliers were impacting this finding. No relationship was found between cigarettes smoked/day and any other ROI. No relationship was found between age and any ROI.
4. DISCUSSION
Negative affect is associated with nicotine use (Kassel et al., 2003; Hughes et al., 1994), as ameliorating withdrawal-related negative affect is one of the most cited reasons for precipitating relapse (Copeland et al., 1995; Brandon, 1994; Baker et al., 2004). The current finding expands our understanding of the link between negative affect and daily cigarette use by demonstrating that right anterior insula reactivity to negative stimuli is positively correlated with cigarettes smoked/day.
While the bilateral (Naqvi et al., 2007; Janes et al., 2010b) and left insula (Englemann et al., 2012, Janes et al., 2013) have been implicated in aspects of nicotine dependence such as reactivity to and memory for smoking cues, our finding that right insula activity is related to cigarettes/day is in line with emotion literature that demonstrates that the right insula is uniquely responsible for processing negative affect (Craig, 2010). Given the insula’s role in internal, or interoceptive, awareness (Craig, 2002), the current findings suggest that individuals who smoke more cigarettes per day may have greater interoceptive awareness of negative emotional states. Prior work supports this concept as greater insula reactivity to emotional stimuli indicates heightened sensitivity to negative emotion (Iaria et al., 2008; Stein et al., 2007). Baker and colleagues (2004) hypothesized that smokers learn to quickly detect interoceptive cues that indicate very early stages of withdrawal such as a rise in negative affect. However, smokers may be vulnerable to misinterpreting the source of interoceptive changes associated with negative affect leading to nicotine use even when negative affect is not due to withdrawal. For instance, heavy smokers, relative to lighter smokers, are particularly poor at differentiating between various negative emotions and may use nicotine as an emotion regulation strategy (Sheets et al., 2015). The idea that negative affect, irrespective of origin, can influence smoking is suggested by the current work given that a relationship was found between daily cigarette use and insula reactivity to negative stimuli.
4.1 Limitations
There are several limitations to the present study. First, we are unable to confirm whether insula reactivity to negative affect causally enhances cigarette use or vice versa. There is a growing body of translational research supporting the idea that the insula mediates nicotine use (Naqvi et al., 2007; Forget et al., 2010; Scott and Hiroi, 2011; Pushparaj et al., 2013), suggesting that individual variability in smoking behavior may be mediated by insula function. However, the relationship between tobacco use and negative affect may be dynamic as others report that emotional distress can initially lead to tobacco use, but overtime tobacco use enhances emotional distress (Orlando et al., 2001). Irrespective of causal direction, providing treatment that enhances emotional regulation may help break the cycle leading to more successful cessation.
Given the modest sample size of twenty-one subjects from the HCP database we were unable to determine whether other factors such as sex interact with insula reactivity and smoking behavior. Additionally, while it is plausible that a larger sample size would show a relationship between cigarettes smoked/day and the posterior insula, the current work supports the strong relationship between cigarettes smoked/day and the anterior insula.
Future studies would benefit from taking into account other aspects of nicotine use beyond number of cigarettes per day. Our ability to evaluate nicotine use more broadly was limited by the variables provided by the HCP. For instance, expired carbon monoxide was not measured and the exact number of years an individual had been smoking was not provided by the HCP. While all participants were abstinent for at least 1 hour prior to the scan, the HCP did not measure the exact duration of abstinence nor were withdrawal symptoms quantified making it impossible to determine the impact of withdrawal on the current findings. Despite these limitations, this data set adequately captures variability in quantity of nicotine use across the group via the number of cigarettes smoked/day for a week prior to the study visit. Finally, the emotional processing task only included faces with a negative valence and we were not able to conclude whether cigarettes/day is related to insula reactivity to negative affect specifically or emotional processing more generally.
4.2 Conclusion
Given the association between cigarettes/day and anterior insula reactivity during the emotional processing task, the current results suggest that individuals who smoke more cigarettes/day are more reactive to negative emotional stimuli. These findings further our understanding of the insula’s involvement in nicotine use. While our prior work showed that insula reactivity to smoking cues impacts relapse vulnerability (Janes et al., 2010b), the current work suggests that insula reactivity to affective stimuli, unrelated to smoking, also is associated with smoking.
Highlights.
Prior work shows the insula’s role in nicotine dependence and affective processing.
We evaluated the link between insula reactivity to negative affect and cigarette use.
Smokers using more cigarettes/day have greater insula reactivity to negative stimuli.
Individual variability in affective processing is associated with cigarette use.
Acknowledgments
Role of Funding
This study was supported by NIH funded grants: K01 DA029645 (PI Amy Janes) and T32 DA015036 (Supported author ND; PI Scott Lukas). Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Footnotes
Contributors
AJ defined the question and guided data analysis and manuscript preparation, ND and AP contributed to data analysis and wrote the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
No conflict declared for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, Block RC, van Wijngaarden E. Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction. 2015;110:1994–2003. doi: 10.1111/add.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson KR, Almeida DM, Stawski RS, Klein LC, Kozlowski LT. Smoking is associated with worse mood on stressful days: results from a national diary study. Ann Behav Med. 2008;36:259–269. doi: 10.1007/s12160-008-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annu Rev Psychol. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, et al. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartveit K, Tverdal A. Health consequences of smoking 1–4 cigarettes per day. Tob Control. 2005;14:315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Curr Dir Psychol Sci. 1994;3:33–37. [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111:180–185. [PubMed] [Google Scholar]
- Burns D, Lee L, Shen L, Gilpin E, Tolley HD, Vaughn J, et al. Smoking And Tobacco Control Monograph No 8. National Cancer Institute; Bethesda, MD: 1997. Cigarette Smoking Behavior In The United States: Changes In Cigarette-Related Disease Risks And Their Implication For Prevention And Control; pp. 13–112. [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥ 18 years. MMWR. 2010;59:1135–1140. [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:1–11. [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Critchley H. Volitional control of autonomic arousal: a functional magnetic resonance study. NeuroImage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2012;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, Ahluwalia JS. Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Prev Med. 2004;38:498–502. doi: 10.1016/j.ypmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6:S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Committeri G, Pastorelli C, Pizzamiglio L, Watkins KE, Carota A. Neural activity of the anterior insula in emotional processing depends on the individuals’ emotional susceptibility. Hum Brain Mapp. 2008;29:363–373. doi: 10.1002/hbm.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, de Frederick BB, Lukas SE. Insula–dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Jensen JE, Farmer SL, de Frederick BB, Pizzagalli DA, Lukas SE. GABA levels in the dorsal anterior cingulate cortex associated with difficulty ignoring smoking-related cues in tobacco-dependent volunteers. Neuropsychopharmacology. 2013;38:1113–1120. doi: 10.1038/npp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de Frederick BB, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ. Neural substrates of attentional bias for smoking-related cues: an fMRI study. Neuropsychopharmacology. 2010a;35:2339–2345. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de Frederick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010b;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Law MR, Wald NJ. Environmental tobacco smoke and ischemic heart disease. Prog Cardiovasc Dis. 2003;46:31–38. doi: 10.1016/s0033-0620(03)00078-1. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando M, Ellickson PL, Jinnett K. The temporal relationship between emotional distress and cigarette smoking during adolescence and young adulthood. J Consult Clin Psychol. 2001;69:959–970. doi: 10.1037//0022-006x.69.6.959. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, Le Foll B. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38:690–698. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Hiroi N. Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry. 2011;69:1052–1059. doi: 10.1016/j.biopsych.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets ES, Bujarski S, Leventhal AM, Ray LA. Emotion differentiation and intensity during acute tobacco abstinence: a comparison of heavy and light smokers. Addict Behav. 2015;47:70–73. doi: 10.1016/j.addbeh.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, time-line follow-back, and ecological momentary assessment. Health Psychol. 2009;28:519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage. 2013;80:80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]