Abstract
The hedonic value of a sweet food reward, or how much a taste is ‘liked’, has been suggested to be encoded by neuronal firing in the posterior ventral pallidum (VP). Hedonic impact can be altered by psychological manipulations, such as taste aversion conditioning, which can make an initially pleasant sweet taste become perceived as disgusting. Pairing nausea-inducing LiCl injection as a Pavlovian unconditioned stimulus (UCS) with a novel taste that is normally palatable as the predictive conditioned stimulus (CS+) suffices to induce a learned taste aversion that changes orofacial ‘liking’ responses to that sweet taste (e.g., lateral tongue protrusions) to ‘disgust’ reactions (e.g., gapes) in rats. We used two different sweet tastes of similar initial palatability (a sucrose solution and a polycose/saccharin solution, CS± assignment was counterbalanced across groups) to produce a discriminative conditioned aversion. Only one of those tastes (arbitrarily assigned and designated as CS+) was associatively paired with LiCl injections as UCS to form a conditioned aversion. The other taste (CS−) was paired with mere vehicle injections to remain relatively palatable as a control sweet taste. We recorded the neural activity in VP in response to each taste, before and after aversion training. We found that the safe and positively hedonic taste always elicited excitatory increases in firing rate of VP neurons. By contrast, aversion learning reversed the VP response to the ‘disgusting’ CS+ taste from initial excitation into a conditioned decrease in neuronal firing rate after training. Such neuronal coding of hedonic impact by VP circuitry may contribute both to normal pleasure and disgust, and disruptions of VP coding could result in affective disorders, addictions and eating disorders.
Keywords: taste aversion, hedonic value, ventral pallidum
1 Introduction
The learning of a taste aversion for a novel food (Pavlovian conditioned stimulus or CS+) that has consequences such as nausea (an unconditioned stimulus or UCS), transforms a palatable taste into a disgusting one, and is an evolved function shared by many species, from humans to rats (Pelchat et al. 1983; Garcia et al. 1974). Many species have been shown to have experimentally-induced taste aversion learning: mammals, birds, fish, and reptiles. Pavlovian taste aversion is an extremely robust form of learning and can occur to a wide range of foods and liquids across all taste categories (Garcia et al. 1974).
In rats, a novel sweet taste paired with visceral illness induced by LiCl becomes not only avoided and no longer ingested, but also if tasted again elicits ‘disgust’ or aversive affective orofacial reactions, such as gapes, headshakes, and forelimb flails (Parker, 2013; Grill and Norgren, 1978; Berridge et al. 1981). This conditioned ‘disgust’ is best measured by the taste reactivity test, in which a flavored solution is infused into the rat's mouth via a previously-implanted oral cannula, to control stimulus exposure while affective reactions are video recorded (Grill and Norgen 1978). The taste reactivity test allows full assessment of conditioned ‘disgust’ reactions because the experimenter can control exposure to the taste, whereas conditioned flavor avoidance makes it unlikely that an animal would approach and voluntarily consume a taste that had been paired with illness, making taste reactions difficult to gauge. The taste reactivity test exploits affective orofacial expressions elicited by sweet versus bitter tastes and shared by many mammals, including human infants, old and new world primates, and rodents (Grill and Norgren 1978, Grill and Berridge 1985, Berridge 2000).
While affective reactions to taste do not need to be learned, they are at the same time not purely reflexive. Affective reactions to a particular taste are altered by preference learning as well as aversion learning, and by relevant physiological states of caloric hunger or satiety (for sweetness), and specific appetites (for saltiness) (Berridge 1996; Berridge et al. 1984, Schulkin 1991). Additionally, several brain manipulations of hedonic substrates in forebrain and brainstem are able to manipulate palatability to alter taste reactivity patterns (Peciña et al. 2006, Grill and Norgren 1978, Cromwell and Berridge 1993, Pfaffmann et al. 1977, Berridge 2000; Smith et al., 2011; Ho & Berridge, 2014). Therefore affective taste reactivity patterns reflect more than mere sensory properties. Rather, the affective reactions reflect the palatability of tastes, i.e. whether the taste is ‘liked’ or ‘disliked’.
The ventral pallidum (VP) is the chief target of the nucleus accumbens and integrates and processes reward information flowing through the mesocorticolimbic system (Kelley et al. 2005, Napier and Mickiewicz 2010, Richard et al. 2012, Smith et al. 2009, Thompson and Swanson 2010, Zahm et al. 2013). VP projections additionally target preoptic regions of the lateral hypothalamus, the mediodorsal nucleus of the thalamus, and in turn connect to larger limbic cortico-striatopallidal-thalamocortical loops, and to additional basal ganglia and brainstem nuclei such as the subthalamic nucleus, substantia nigra, and pedunculopontine nucleus (Churchill et al. 1996, Groenewegen et al. 1993, Grove 1988 a and b, Mogenson et al. 1980). Given its extensive connections, the VP has been proposed to be a region that can mediate various aspects of reward (Mahler et al. 2014; Napier & Mickiewicz, 2010; Richard et al., 2012; Smith et al., 2009) and to be involved in translating motivational signals into action (Mogenson et al., 1980; Mogenson & Yang, 1991).
In particular, the posterior half of the VP in rats contains an approximately cubic-millimeter ‘hedonic hotspot’, in which opioid or orexin agonist microinjections can double the number of positive ‘liking’ orofacial expressions elicited by sucrose taste (Ho and Berridge 2013, Smith and Berridge 2005). Further evidence suggests that the VP is necessary for normal levels of ‘liking’, as excitotoxin lesions that destroy neurons in posterior VP, or temporary inactivations of posterior VP induced by pharmacological microinjections, eliminate positive orofacial expressions to sweet tastes, and replace them with ‘disgust reactions’ (Crowell and Berridge 1993; Ho and Berridge 2014; Shimura et al., 2006).
The firing rates and population responses of neurons in the posterior VP track the hedonic impact of UCS tastes in electrophysiological recording experiments, as well as the incentive motivation value of learned CS predictors (Tindell et al., 2004; Tindell et al., 2005; Tindell et al., 2006; Tindell et al., 2009; Smith et al., 2011). For example, a previous study found that VP neurons fired in robust excitatory patterns response to an orally infused sucrose taste that was ‘liked’, but did not respond to an intensely salty taste (3X seawater concentration) that elicited ‘disgust’ reactions (Tindell et al., 2006). However, after animals were pharmacologically put into a physiological state of sodium deficiency, the hedonic value of the intensely salty taste changed from negative ‘disgust’ to positively ‘liked’, as assessed by affective orofacial expressions, and the VP neurons now responded to the salty taste with a large firing rate increase that became equal to the sucrose-elicited elevation in firing. In other words, the VP neuronal response represented the hedonic value of the taste. Further, the intensity of VP excitatory firing tracks the degree of ‘liking’ for a sweet taste, when both are quantitatively enhanced by opioid stimulation in nucleus accumbens (Smith et al., 2011). Similarly, human neuroimaging studies have reported that VP activity was correlated with the subject's rating of the inferred pleasantness for appetizing food images, especially in posterior VP, whereas anterior VP activity may correlate more with disgust ratings for images such as rotten foods (Calder et al. 2007, Simmons et al. 2013). In sum, the VP is in an anatomical position to be a principal player for reward functions, and the intensity of excitatory activation patterns of posterior VP neurons appears especially to code the degree of ‘liking’ for a taste's hedonic impact.
The overall goal of this study was to further investigate how the neuronal activity in ventral pallidum tracks in the hedonic value of a sweet taste from positive ‘liked’ to negative ‘disgust’ induced by a learned taste aversion.
Here we discriminatively paired nausea-inducing LiCl injections (Pavlovian UCS) with a particular sweet taste that is normally palatable, while simultaneously recording facial reactions and neural activity in the VP. The discriminative training procedures created a conditioned aversion selectively for just one (CS+) of two tastes. We used two distinctly different sweet, caloric tastes in this experiment (counterbalanced between groups): a sucrose solution versus a polycose/saccharin combination solution. Both tastes elicited ‘liking’ taste reactivity patterns prior to conditioned aversion, but are sufficiently different in sensory details that rats can tell them apart. One taste was arbitrarily designated that rat's CS+, to be subsequently paired associatively with several nausea-inducing LiCl injections (the UCS) to induce a conditioned aversion. The other was arbitrarily designated the CS− or safe taste for a particular rat, which was familiarized with that taste by allowing several days of free access to the CS− in the home cage (to induce Pavlovian latent inhibition, making CS− less likely to be included in the subsequently learned aversion).
We used latent inhibition to help preserve the ‘safe’ status of CS− (Berridge et al., 1981; Yamamoto and Ueji, 2011), because other studies that did not use pre-exposure reported significant generalization of aversion between one sweet CS+ taste paired with LiCl and other sweet tastes (even though they had not been paired) (Best and Batson, 1977; Pfaffmann et al., 1979; Nowlis et al., 1980). CS− pre-exposure may facilitate discriminative recognition of sensory differences between CS− and CS+, as well as inducing Pavlovian latent inhibition specifically for the CS− that protects from aversion generalization between the two sweet tastes. Altogether, this pre-exposure helps make the CS+ aversion more discriminatively specific to that single taste, and allows the CS− to remain relatively palatable after conditioning (Berridge et al., 1981).
We recorded neural activity in the VP, as well as behavioral taste reactivity, in response to each taste at the beginning and end of the experiment. We hypothesized that the shift of the hedonic value of CS+ taste from ‘liked’ to ‘disgusting’ would be reflected in a reduction of neural activity in the ventral pallidum. We also hypothesized that the VP activity to the CS− taste would be less altered.
2 Material and Methods
2.1 Subjects
Ten adult male Sprague-Dawley rats weighing 300 g – 400 g were used in this experiment. Rats were housed individually in tub cages on a 9:30 AM to 7:30 PM reversed light/dark schedule. Experiments were conducted during late morning to afternoon hours, coinciding with the rats’ active (dark) period after acclimating to housing conditions for 1-2 days. Food and water were available ad libitum throughout testing, except when in the recording chamber.
2.2 Apparatus
All training and testing was conducted while rats were placed in a clear plastic test cylinder of diameter 25 cm which was placed inside of a 28 cm × 35 cm × 60 cm clear plastic chamber with a glass floor. The chamber was illuminated with white light from below. The use of white light provided better illumination of the rat's mouth and tongue which was necessary for taste reactivity video scoring (see detailed description of behavioral analysis below). The top of the cylinder and chamber was open, allowing for plastic tubing connections from the oral cannulae to the syringe pump that delivered the tastes and also connections from the electrode to the commutator via a headstage cable.
Delivery of tastes and oral stimuli were controlled by a custom software program, MTASK. Neural activity was recorded during the testing sessions using a custom LabVIEW (National Instruments, Austin, TX) program, DataTask. Neural activity was amplified at a gain of 5000 and bandpass-filtered between 300 Hz and 6 kHz. Sessions were recorded at 30 frames a second via a video camera placed underneath the glass floor. Timestamp clocks for the taste delivery program, video recording, and neural recording were all synchronized to enable subsequent analysis of neural activity related to task events, stimulation, and behavioral events obtained from video analysis or recorded in Mtask.
2.3 Pre-exposure to the ‘safe’ CS− solution and habituation
A pre-exposure or Pavlovian latent inhibition paradigm was used to establish a specific sweet solution as a familiar and safe taste of CS−. For four days leading up to surgery, rats were given free access in their home cage to a sipper tube containing 20 ml of the CS− solution daily: either 17.4% w/v sucrose solution or 16% polycose/0.2% saccharine w/v solution (the CS+ versus CS− assignment was counterbalanced across rats). Previous pilot observations indicated that these two tastes were similarly consumed and elicited similar positive patterns of taste reactivity. Any rat that did not voluntarily consume 20 ml of the CS− within 24 hrs on the last day of pre-exposure was excluded from the experiment. In addition, for two days just before surgery, rats were also introduced to the test chamber. They were placed in the chamber for 5 minutes to acclimatize to the experimental set-up.
2.4 Surgical procedures
Rats were weighed, pretreated with penicillin, and anesthetized with ketamine (100mg/kg) and xylazine (10mg/kg) injected intraperitoneally (i.p.). On each side of the mouth, an intraoral cannula was inserted in the mouth lateral to the first molar and exited the head near the skull screws. A stainless steel 19 G guide cannula was then attached to each intraoral cannula where it exited at the top of the head. This implantation procedure allows for precise taste delivery via a computer controlled pump.
In the same surgery, rats were implanted with bilateral posterior VP-targeting electrodes (AP −0.8mm, ML ±2.8mm, DV 7-8.5mm). Each electrode consisted of two bundles of four 50 μm tungsten wires. Each bundle could be lowered or raised independently. Electrodes were implanted just dorsal to target and lowered to find cells as needed on recording days. Both the electrode and the metal connecters to the oral cannulas were anchored to the skull with bone screws and acrylic cement. After surgery, rats received 3-5 ml of isotonic saline via subcutaneous (s.c.) injection to maintain hydration. For up to 4 days post-surgery rats received daily injections of penicillin G benzathine to prevent infection (15,000 units/daily, s.c.) and flunixin meglumine (2.5 mg/kg, s.c.) to manage pain. Rats had access to normal chow and water at all times, and were also supplemented with free access to a moistened mash of commercial infant cereal (Gerber's) for the first 2-4 days after surgery. Animals were allowed to recover for at least 7 days while being monitored for good health. Oral cannulae were kept clean and clear from blockage throughout the experiment and did not disrupt normal eating once the animal had recovered.
2.5 Re-exposure to ‘safe’ CS− and habituation
After recovery, on the first two consecutive days each rat was placed in the experimental chamber for ten minutes, in order habituate to the test chamber. In their home cages the rats also received free access to 20 ml of their designated ‘safe’ CS− solution, replaced daily on those 2 days to help develop latent habituation that would prevent subsequent aversion conditioning from generalizing to the CS−.
2.6 Training
Pavlovian CS-UCS training took place on each of 6 consecutive days. All training days were comprised of a block of 10 stimuli, 1 taste per day. Stimuli consisted of single 0.1 ml taste infusions delivered by a computer-controlled pump over 1 sec, via intra-oral cannulae (variable 1 min ITI), over a roughly 30 min period. The first day consisted of ten ‘safe’ taste (CS−) presentations while neural and behavioral data were collected. An injection of isotonic saline (drug control; 10 ml/kg, i.p.) was given at the end of the training session after all ten ‘safe’ CS− taste stimuli were delivered, prior to the animal being returned to its home cage.
The second day of training was the first time the rats ever encountered the novel CS+ taste that would be followed by a lithium chloride (LiCl) injection. On the CS+ day, 10 presentations of the ‘novel’ taste were delivered into the rat's mouth over a roughly 30 min period, while neural data were recorded and behavioral orofacial reactions were videotaped from beneath the transparent floor. Lithium chloride (64 mg/10 ml/kg, i.p., 0.15 M isotonic solution), which induces moderate nausea, was injected at the end of the CS+ session, prior to the rats being returned to their home cage. Therefore the CS+ taste did not begin to elicit devalued hedonic reactions until it was next encountered (on training day 4), allowing us to use the data from day 2, prior to the first LiCl injection, as an initial hedonic impact condition. CS− and CS+ tastes were alternated on the subsequent days 3 to 6 so that rats were exposed to each taste and injection pairing three times.
2.7 Testing
A test of both tastes followed on day 7, when the rats were exposed to 2 blocks of stimuli: first the ‘safe’ CS− and next the now-devalued CS+. The first block of 10 stimuli was always the ‘safe’ CS−. The rationale for this design was based on previous studies which indicate that hedonic reactions are more vulnerable to state-induced changes (such as hunger and satiety) whereas aversion is generally unaffected (Berridge, 1991), indicating that responses to the CS− might be vulnerable to lingering negative affect (i.e. conditioned nausea) if the CS+ was presented before the CS− on test days. Time-stamped clocks were synchronized for taste infusions, neural recordings, and videotape recordings.
2.8 Behavioral analysis: taste reactivity
For all training and testing sessions, digital video of the stimulus duration and the subsequent 10 seconds of each trial was analyzed off-line in slow-motion (1/30 s frame-by-frame to 1/10th actual speed) using established procedures developed to assess hedonic, aversive and neutral taste reactivity patterns during liquid taste infusions (Grill and Berridge, 1985; Berridge, 2000) and the Datarat scoring program (developed by the Aldridge lab). Hedonic responses included rhythmic midline tongue protrusions, lateral tongue protrusions, and paw licks. Aversive responses included gapes, head-shakes, face washes, forelimb flails, and chin rubs. Neutral responses included passive dripping of solution out of the mouth and rhythmic mouth movements (see Berridge and Grill, 1981, for technical definitions of these behavioral reactions). Individual reaction totals were calculated by adding all response scores within an affective category per rat (hedonic, aversive, or neutral) (Berridge, 2000). These were statistically examined for CS− versus CS+ and Training versus Test Day effects using ANOVAs.
2.9 Ventral Pallidum Neuronal Activation Spike discrimination
Single neurons (N=121) were identified using principle components or peak-width analysis of waveforms using Offline Sorter (Plexon Inc., Dallas TX). Neurons were verified by distinct spike waveforms (whose shapes remained consistent throughout the whole recording). Units with more than 2% of spikes within a 1 ms refractory period window in an autocorrelation histogram were excluded. A cross-correlation analysis was also performed to ensure that neurons were counted only once. (NeuroExplorer, Nex Techologies, Littleton MA).
2.10 Firing rate analyses
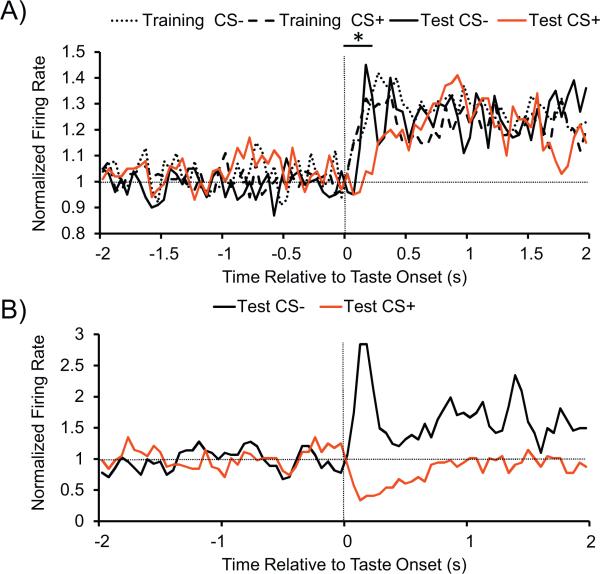
The first 250 ms after taste infusion onset (0-250 ms) was the epoch that best captured the difference in hedonic value between the CS+ and CS− tastes (Figure 2A). Therefore results discussed in this paper are based on this period. The difference was also apparent to a lesser degree during the 250-500 ms period after taste onset. From 0.5-1.7 s after taste onset, all four taste conditions showed similar sustained responses. While the average firing rate response to the CS+ taste after conditioning appeared to start to return towards baseline sooner than the CS− taste (and CS+ prior to conditioning), there were no statistically significant rate differences for epochs extending beyond the first 250 ms after taste onset. Our baseline epoch was a 10 sec period prior to stimulus onset (−11 to −1 s). Normalized firing response to a stimulus event for each neuron was obtained by dividing a neuron's absolute firing response in epochs of interest by its baseline (1 would represent the baseline rate, > 1 hz an increase in rate, and ░<1 hz decrease in rate).
Figure 2.
A) The average normalized firing rates (horizontal line indicates baseline rate) up to 2 s before and after taste onset, which is centered at time 0 and indicated by the vertical line. There were significant rate difference between the CS+ after aversion conditioning and the three ‘liked’ tastes (CS− before and after conditioning, CS+ before conditioning) in the 0-250 ms epoch after taste onset (*p < 0.05). There were no statistically significant rate differences between tastes beyond the first 250 ms after taste onset. B) Example perievent time histogram for a unit which showed opposing responses to the ‘liked’ sweet CS− taste and ‘disliked’ CS+ sweet taste. Plots show unit activity up to 2 s before and after taste onset, which is centered at time 0 and indicated by the vertical line. Gaussian Smoothed Histograms (bin width = 50 ms) show the average firing rate across all trials.
2.11 Responsive Populations
A neuron was considered ‘responsive’ if the epoch of interest contained a significant increase in rate, decrease in rate, or a mixed response. For each unit, raster plots and perievent time histograms (PETHs) with 50 ms bins were aligned to the onset of taste delivery. The criteria for an increase in rate were either one 50 ms bin being 3 SD greater than the baseline epoch, or two consecutive bins 2 SD above baseline. Decreases in rate were defined by one bin 2 SD below baseline or two bins 1 SD below baseline. Mixed responses were defined as any combination of an increase in rate and decrease in rate as defined above. Visual inspections of spike rasters were used to eliminate any false positives due to a single trial being the source of the rate change.
2.12 Histology
Anatomical localization of electrode sites was assessed after completion of testing. Rats were euthanized with FatalPlus at the end of the experiment. Brains were removed, flash-frozen in an isopentane and isopropyl alcohol solution, sectioned coronally into 40 μm sections on a CM 1850 cryostat (Leica Microsystems, Buffalo Grove, IL), and digitally photographed while wet using a bottom-lit microscope. The electrode placement was confirmed by observing the brain slices under a light microscope and a map illustrating electrode recording sites (supplementary Figure S2) was constructed.
3 Results
3.1 Behavioral Results
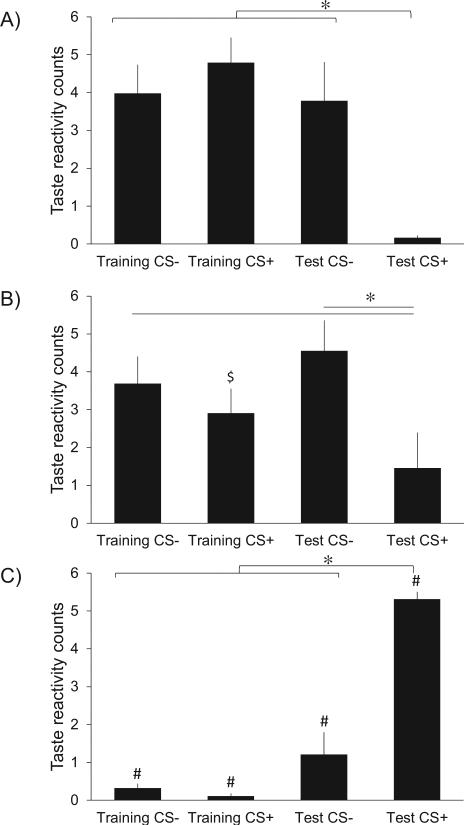
A discriminative taste aversion was evident for the CS+ taste by the test day, after three pairings of CS+ with injections of LiCl as UCS (Figure 1; 2-way ANOVA, reaction category p=0.002, test day NS, interaction p<0.001). Before being paired with LiCl, the novel sweet taste (CS+ on training day) elicited robust positive hedonic reactions, and virtually no negative aversive reactions, producing a ratio of over 40 times more hedonic reactions than aversive taste reactions (Student-Newman-Keuls post-hock, p<0.001). But after the CS+ was associatively paired with LiCl injections as UCS, positive hedonic reactions to the CS+ taste were nearly abolished, and replaced by robust ‘disgust’ reactions: gapes, headshakes, forelimb flails, chin rubs, and face washes. Consequently, the devalued CS+ taste elicited more aversive taste reactions than either hedonic (Student-Newman-Keuls post-hock, p<0.001) or neutral (Student-Newman-Keuls post-hock, p<0.001) reactions on the test day.
Figure 1.
A) ‘Liking’ taste reactions. B) Neutral taste reactions. C) ‘Disliking’ taste reactions. Prior to any aversion learning, both sweet tastes elicited ‘liking’ reactions and almost no aversive reactions. After the CS+ taste was repeatedly paired with LiCl injections, it became strongly aversive and produced almost no hedonic reactions. Error bars represent standard error. *Denotes a significant difference between the aversive taste and the ‘liked’ tastes (p<0.05). #Denotes a significant difference between the aversive taste reactivity counts and both the hedonic and neutral counts (p<0.05). $Denotes a significant difference between the hedonic taste reactivity counts and neutral counts (p<0.05).
By contrast, the CS− taste that was never paired with LiCl always elicited predominantly postive and neutral taste reactions and very few aversive reactions, and did not change significantly between training and test (Student-Newman-Keuls post-hocks, all NS). On test day the CS− elicited a modest (NS) increase in aversive reactions compared to training, indicating a slight degree of generalization between sweet tastes of CS+ and CS− had occurred despite the latent inibition pre-treatment. However, the CS+ taste still elicited more than 400% of the number of aversive reactions as the CS− taste on the test day, indicating that a strong discriminative association had been formed as intended.
3.2 Ventral Pallidum Neuronal Activation
3.2.1 Sites and rate coding of tastes
Overview of sites within ventral pallidum
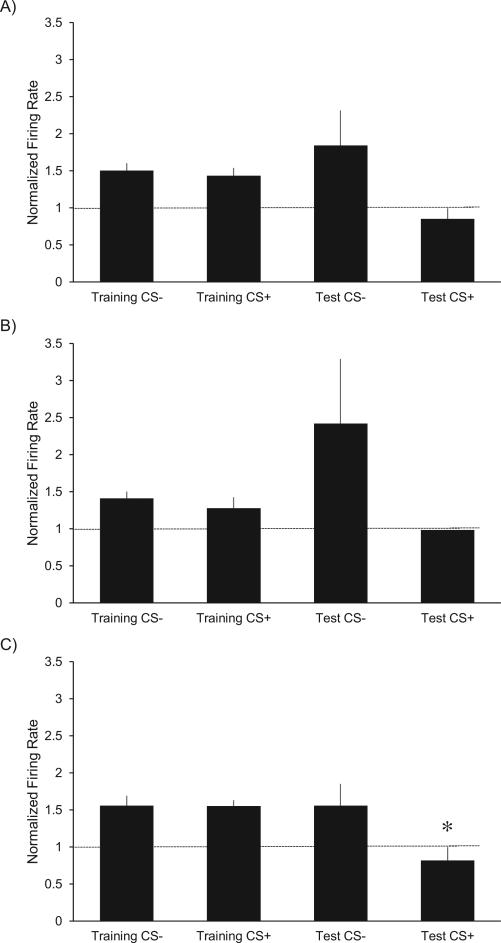
Most electrode sites were contained in the posterior half of ventral pallidum (located posterior to −0.4 mm relative to bregma). Posterior VP neurons responded with comparable excitations to initial CS− and CS+ tastes before aversion conditioning (average firing rates normalized to baseline: 1.5597 Hz and 1.5520 Hz, respectively). After the discriminative aversion had been conditioned to CS+ taste by pairing with LiCl illness, posterior VP firing became suppressed to CS+ taste (0.8185 Hz normalized rate). By comparison, VP firing remained high to safe CS− taste even after conditioning (1.5589 Hz normalized rate). In other words, posterior VP neurons appeared to faithfully code hedonic palatability, and to discriminate most clearly between CS+ and CS− after learning (i.e., did not generalize between tastes) (Figure 3C, one-way ANOVA, F3,22=4.533, p = 0.015, Student-Newman-Keuls post-hock comparison: Training CS− vs. Test CS+, p = 0.02; Training CS+ vs. Test CS+, p = 0.004; Test CS− vs. Test CS+, p = 0.025; all other comparisons NS).
Figure 3.
The average normalized firing rate 0-250 ms after each taste is depicted. A normalized rate of 1, indicated by the horizontal line, represents the average basal firing rate 10-0 sec before taste delivery. Error bars represent standard error. A) All VP units: The average normalized firing rate of units responding to the ‘liked’ tastes was slightly higher than the aversive taste, but there were no statistically significant differences. B) Anterior VP units: Similar to all units, there were no significant differences in normalized firing rates between tastes C) Posterior VP Units: All three ‘liked’ tastes resulted in a higher normalized firing rate than the aversive taste after conditioning (*p < 0.05).
Within the posterior half of VP, the palatability coding effects described above were mostly driven by neurons located in the furthest posterior one-third of VP (between −0.72 to −1.20 mm relative to bregma). That far-posterior anatomical region corresponds closely to the hedonic hotspot identified previously, in which mu opioid or orexin stimulation causes enhancement of positive hedonic patterns of taste reactivity elicited by sucrose, and where lesions or temporary inactivations produce excessive aversive reactions to sweetness (Smith & Berridge 2005; Ho & Berridge 2014). By comparison, the most anterior group of VP neurons, located at roughly the AP midpoint of VP (−0.48 to −0.60 mm relative to bregma), showed enormous variability in firing rates after aversion conditioning especially for the CS− taste after conditioning. The higher variability of anterior VP neurons tended to obscure the distinction between CS+ and CS− tastes after conditioning. Thus anterior VP neurons did not as reliably show the hedonic pattern of CS+/CS− coding displayed by neurons located more posteriorly in VP (Figure 3B, One Way ANOVA: failed equal variance test, Kruskal-Wallis One Way Analysis of Variance on Ranks: NS). The high variability of the anterior VP rates lead to similar effects in the rate data from all VP neurons combined (Figure 3A, one-way ANOVA: failed normality test, Kruskal-Wallis One Way Analysis of Variance on Ranks: H[3]=8.186, p= 0.042, Dunn's Method pairwise multiple comparison procedures: all NS).
3.2.2 Population response types
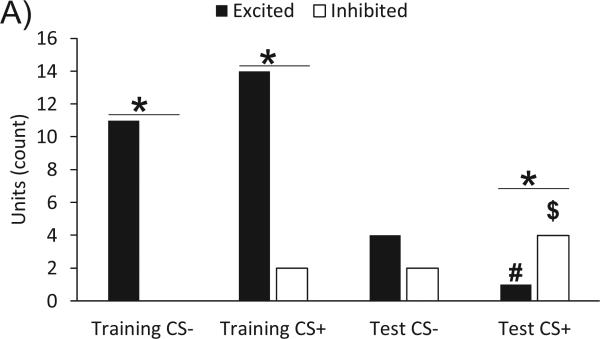
The rate coding of the hedonic value of sweet tastes by VP neurons described above was primarily driven by differences in the response types (units that showed an excitation response vs. units that were inhibited by the taste), rather than by differences in the strength of the individual responses (measured by individual changes in firing rate). As a population, VP neurons tracked the hedonic valence of the sweet CS+ taste, showing predominantly increases in firing rate in response to CS− and CS+ ‘liked’ tastes before conditioning, but shifting selectively to predominantly decrease firing rate to CS+ when that sweet taste became aversive, while the CS− taste that was still relatively safe triggered both excitations and inhibitions (Figure 4A).
Figure 4.
A) For each taste condition (the safe taste on the first day of training, the first exposure to the novel sweet taste prior to any aversion learning, the safe taste on the test day, and the now aversive taste on the test day) the number of units that showed an increase in firing rate (black bars) or decrease in firing rate (white bars) within the first 250 ms after taste onset are graphed. *Denotes a significant difference in the number of units that responded with an increase in firing rate than units that responded with a decrease in rate (p<0.05). #Denotes there were fewer units (p<0.05) that were excited in response to the aversive taste (Test CS+) than either of the ‘liked’ tastes on the training days (Training CS− and Training CS+). $Denotes there were more units (p<0.05) inhibited by the aversive taste (Test CS+) than the safe taste on the training day (Training CS−).
Prior to aversion conditioning, VP population responses to the CS− and CS+ on training days contained significantly more excitations than inhibitions (both X2 p<0.05). After taste aversion learning, VP units yielded significantly fewer excitations in response to the aversive taste than either of the ‘liked’ tastes on the training days (X2 p<0.05). The VP data also showed more inhibitions in response to the aversive taste (CS+ post-training) than the CS− on training day (X2 p<0.05).
There were no inhibitory responses to the CS− prior to training, when the hedonic values were the highest. After training, there were still more excitatory than inhibitory responses to the CS− in the VP units, but the modest (non-significant) decrease in excitations and slight (non-significant) increase in inhibitions compared to pre-training likely reflected the slightly diminished ‘liking’ of the safe sweet taste as discussed in section 3.1.
Overall, we found fewer responsive units post-aversion (27 vs. 11, X2 p<0.02). Likewise, there were more CS+ responsive cells pre-training vs post-training (16 vs. 5, X2 p<0.02). However, there was no significant difference in the number of CS− responsive cells pre-training vs post-training (11 vs. 6, X2 NS).
4 Discussion
4.1 Taste aversion learning
By associatively pairing a particular sweet taste as a Pavlovian CS+ with LiCl-illness as UCS, we induced a learned ‘disgust’ reaction that was displayed mostly to that CS+ taste, while leaving the reaction to another sweet but discriminable CS− taste relatively positive and ‘liked’. Taste reactivity patterns shifted from hedonic to aversive for the CS+, but remained mostly hedonic for the CS−. Induction of a discriminative Pavlovian taste aversion is consistent with previous studies (Experiment 5 of Berridge et al., 1981; Pelchat, 1983; Parker and Jenson 1992; Parker, 2013; Carelli and West 2014).
The firing rate of VP neurons essentially tracked the hedonic status of CS+ and CS− tastes across the course of Pavlovian training and testing. Hedonic coding was especially observed for neurons located in the ‘hedonic hotspot’ of posterior VP. VP neurons did not appear to distinguish a novel sweet taste (CS+) from a familiar sweet taste (CS−) on the first day when both tastes were palatable. The equally robust response to both tastes prior to taste aversion conditioning indicated that the shared unconditioned hedonic impact of sweetness, rather than taste novelty, was the primary determinant of VP responsiveness prior to aversion conditioning. Before aversion training, both sweet tastes were positively ‘liked’, and both tastes evoked a robust phasic increase in VP neuronal firing. That is, both CS+ and CS− tastes initially before learning, evoked an average increase in firing rate of 56-58% in posterior VP neurons during the first 250 ms above pre-taste baseline levels. By contrast, after aversion conditioning, the CS+ taste elicited ‘disgust’ reactions and an 18% reduction in firing below baseline in posterior VP neurons during the same epoch. Thus the shift from VP firing increase to firing suppression corresponded to the loss of ‘liking’ and induction of negative ‘disgust’ for the CS+ taste (decrease in rate from baseline). At the same time, the CS− taste still evoked mostly positive hedonic orofacial reactions after conditioning, and still elicited an increase in firing rate equivalent to its original excitatory rise elicited before aversion conditioning.
The hedonic-coding pattern appeared to be expressed most robustly by neurons anatomically located in the posterior one-third of VP, which has previously been identified as the center of a ‘hedonic hotspot’ where opioid and orexin neurochemical stimulations via agonist microinjections induce enhancement in ‘liking’ reactions elicited by sweet tastes. The anatomical site overlaps with VP regions found previously to exhibit neuronal coding for changes in palatability and dynamic changes in incentive value (Tindell et al 2006; Tindell et al. 2009). By comparison, in more anterior neurons located in mid-VP, corresponding to the anterior edge of the hedonic hotspot, the shift in hedonic coding after aversion conditioning was not as apparent due to a failure to observe consistent differences in firing between the two tastes after conditioning. For example, the CS− taste evoked a highly variable response at anterior recording sites in mid-VP after learning (41-142% average increase in firing rate in the first 250 ms). That post-training CS− response was much more variable than the previous initial CS – response for anterior VP sites (or initial CS+ response). Further, after aversion conditioning CS+ responses were difficult to detect at all among anterior VP sites (1% decrease). Overall, it appears that posterior VP neurons may more faithfully track hedonic impact in firing rate than anterior or mid-VP neurons located outside rostral to the anatomical hotspot. Future studies may be able to further test this localization of function hypothesis.
In terms of a population code, among VP neurons that responded rapidly (<250 ms) to the infusion of sweet tastes into the mouth, the proportions of “excitations” to “inhibitions” was similarly changed when the hedonic value of was shifted by discriminative aversion conditioning. The posterior VP units tracked the change in hedonic value quite accurately. That is, 90-100% of responsive cells responded to the CS+ and CS− before conditioning with excitations. After conditioning the CS− persisted in evoking 75% excitations, whereas the CS+ response became dominated by 75% inhibitions in the same posterior VP units. However, the mid/anterior VP units failed to discriminate as reliably between the CS+ and CS− tastes post-conditioning: both tastes each elicited very few responses (0-1 units per response type). The overall decrease in number of units responding to sweet tastes post-training, compared to pre-training, may indicate an additional property of encoding by the VP.
4.2 What is driving the VP coding of hedonia and reward learning?
Within the posterior VP hotspot, previous studies have shown that ‘liking’ reactions elicited by sweet tastes are enhanced by exogenous opioid and orexin stimulation produced by agonist microinjections (Smith and Berridge 2005; Ho and Berridge 2013). Conversely, excessive ‘disgust’ reactions are elicited by sucrose taste after either temporary inhibition of posterior VP neurons by pharmacological GABAergic microinjections, or neuronal destruction of hotspot neurons via excitotoxin lesions (Ho and Berridge 2014; Shimura et al 2006; Cromwell and Berridge 1993). Further, stimulation of neuronal excitation in VP, by preventing GABAergic inhibition via VP biculline microinjection, has been suggested to block the induction of a learned taste aversion (Inui et al. 2007). Similarly, optogenetic pilot evidence has emerged suggesting that ‘liking’ reactions to sweet tastes may be enhanced by direct excitation via channelrhodopsin-2 stimulation in posterior VP neurons (Castro and Berridge 2013). In other words, taken together, converging evidence is consistent with the hypothesis that excitation of neurons in the posterior VP contributes to the pleasantness of a taste. Conversely, this evidence is consistent with the notion that ‘disgust’ is both caused and coded by inhibitions in taste-elicited firing of posterior VP neurons.
However, as a caveat, it is not yet clear whether a simple rate code is the primary mechanism of VP hedonic mediation. For example, VP biculline microinjections fail to enhance normal unconditioned ‘liking’ reactions to sweet tastes, nor to block ‘disgust’ reactions elicited by bitter tastes, even though bicuculline microinjections do increase ‘wanting’ to eat or consumption of palatable tastes (Smith and Berridge, 2005, Shimura at al. 2006). For palatability enhancements by VP opioid or orexin stimulation especially, it seems plausible that more complex pattern codes might be involved, involving excitations but requiring additional temporal features (Smith et al., 2011).
The NAc-VP circuit has been suggested to involve opposite polarity between neuronal firing in the two structures: NAc inhibitions can produce VP excitations via disinhibition from GABAergic NAc-VP projections. In NAc, several studies report that neurons are generally inhibited by palatable sweet tastes but excited by aversive tastes (Roitman et al 2005, Nicola et al. 2004, Taha and Fields 2006). Furthermore, NAc responses to a sweet taste switch from predominantly inhibitions before conditioned taste aversions to predominantly excitations after taste aversion learning (Roitman et al 2010). NAc opioids, acting in a hedonic hotspot located in the rostrodorsal quadrant of NAc medial shell (Pecina et al. 2006), may further enhance hedonic impact by predominantly inhibiting NAc firing rate (Hakan et al. 1992, Hakan et al. 1994, Hakan and Eyl 1995). By contrast, in VP, we found that palatable tastes excited VP neurons, whereas aversive tastes inhibited VP neurons. This opposite pattern fits the NAc-VP disinhibition hypothesis mentioned above. A NAc-VP circuit conclusion may also be consistent with the report that induction of a learned taste aversion is accompanied by transfer of manganese from NAc to VP (Inui et al. 2011). VP roles in learned motivation is also consistent with previous reports of VP responses to auditory CS+ cues (tones as Pavlovian CSs) that predict palatable versus aversive tastes (Tindell et al. 2004, 2005a, and 2005b, 2006 & 2009). Overall, the inversion of neural coding of ‘liked’ vs. ‘disliked’ tastes between the NAc and VP may create a key circuit in the neural coding of both innate and learned hedonic impact.
4.3 Corticolimbic circuitry
Besides NAc, other limbic structures that project information to the VP include the orbitofrontal cortex and insular cortex, which contain regions that code the hedonic impact of tastes (O'Doherty et al. 2000, Small et al. 2001, Kringelbach et al. 2003). Neural correlates of palatability devaluation have also been found in the lateral hypothalamus and brainstem parabrachial nucleus, which interact with VP neurons (Burton et al. 1975, Rolls et al. 1989, Nakamura and Norgren 1995, Critchley and Rolls 1996, Giza et al. 1997, Schoenbaum et al. 1998, Reilly 1999, Rolls 2000, Schultz 2000, de Araujo et al. 2006, Peciña et al. 2006, Simon et al. 2006). Small increments in taste palatability encoding (e.g. caused by a weak salt-appetite induction) have been reported in gustatory relay nuclei of the brainstem, including the parabrachial nucleus and the nucleus of the solitary tract (Jacobs et al. 1988, McCaughey and Scott 2000).
5. Conclusion
Neuronal firing by VP neurons, especially in the posterior region of VP, tracked the shift in hedonic value that occurred when a sweet taste that was initially liked became aversive through LiCl pairings. Posterior VP neurons typically responded to ‘liked’ tastes with an increase in firing rate, before and after learning, but responded to the learned ‘disgusting’ taste with an inhibition in firing rate below baseline.
Our findings demonstrate that the VP is able to encode a learned reversal in hedonic value of a particular taste. This finding, in conjunction with a previous study that found that the VP can track the change in opposite enhancement in hedonic value of an intensely salty taste from aversive to positively hedonic, via physiological salt depletion (Tindell et al. 2006), provides evidence that neuronal firing in the posterior VP encodes shifts in hedonic value between ‘liking’ and ‘disgust’.
Supplementary Material
Table 1.
VP unit counts and percentages for ‘safe’ CS− and ‘novel’ CS+ on training days and the ‘safe’ CS− and ‘hedonically diminished’ CS+ tastes on the test day, organized by anatomical location (all VP units, anterior VP units, posterior VP units) and response type (excitations vs. inhibitions). Two types of percentages are listed: “Total” indicates percentages of total units for that region (i.e. the number of units that changed rate/total unit count) and “Responsive” indicates percentages of responsive units (i.e. the number of units that changed rate/number of responsive units)
| Units | Total | Responsive | Units | Total | Responsive | Units | Total | Responsive | Units | Total | Responsive | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All VP Units | Total Unit Count | 0 | NA | NA | 4 | NA | NA | 7 | NA | NA | 7 | NA | NA |
| Increased Rate | 1 | 27.5% | 100% | 4 | 31.8% | 87.5% | 10.8% | 66.7% | 2.7% | 20% | |||
| Decreased Rate | 0% | 0% | 4.5% | 12.5% | 5.4% | 33.3% | 10.8% | 80% | |||||
| Anterior VP Units | Total Unit Count | 4 | NA | NA | NA | NA | 1 | NA | NA | 1 | NA | NA | |
| Increased Rate | 28.6% | 100% | 55.6% | 83.3% | 9.1% | 50% | 0% | 0% | |||||
| Decreased Rate | 0% | 0% | 11.1% | 16.7% | 9.1% | 50% | 9.1% | 100% | |||||
| Posterior VP Units | Total Unit Count | 6 | NA | NA | 5 | NA | NA | 6 | NA | NA | 6 | NA | NA |
| Increased Rate | 26.9% | 100% | 25.7% | 90% | 11.5% | 75% | 3.8% | 25% | |||||
| Decreased Rate | 0% | 0% | 2.9% | 10% | 3.8% | 25% | 11.5% | 75% |
Highlights.
Pairing nausea with a palatable taste sufficed to induce a learned taste aversion.
We found that safe hedonic tastes elicited excitatory increases in firing rate of VP neurons.
Aversion learning reversed the VP response into a conditioned decrease in neuronal firing rate.
Acknowledgements
This work was supported by funds from NIDA/NIH (grant numbers DA017752 awarded to J. Wayne Aldridge, DA007281 awarded to Terry Robinson, and DA015188 awarded to Kent Berridge), by NEI/NIH (grant number EY017878 awarded to Peter Hitchcock), and by NIMH/NIH (grant number MH63649 awarded to Kent Berridge).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. Modulation of Taste Affect by Hunger, Caloric Satiety, and Sensory-Specific Satiety in the Rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. Journal of Comparative and Physiological Psychology. 1981;95:363–382. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- Best MR, Batson JD. Enhancing the expression of flavor neophobia: some effects of the ingestion-illness contingency. J Exp Psychol Anim Behav Process. 1977;3:132–43. doi: 10.1037//0097-7403.3.2.132. [DOI] [PubMed] [Google Scholar]
- Calder A, Beaver J, Davis M, van Ditzhuijzen J, Keane J, Lawrence A. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, West EA. When a good taste turns bad: Neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology. 2014;76:360–369. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Feigin MB, Sclafani A, Sunday SR. Species differences in polysaccharide and sugar taste preferences, Neurosci. Biobehav. Rev. 1987;11:231–240. doi: 10.1016/s0149-7634(87)80031-3. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–883. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res. 1991;555:1–9. doi: 10.1016/0006-8993(91)90852-m. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Berridge KC. Taste reactivity as a measure of the neural control of palatability. Progress in Psychobiology and Physiological Psychology. 1985;2 [Google Scholar]
- Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW. A multiwire microelectrode for single unit recording in deep brain structures. J Neurosci Methods. 1990;32:143–148. doi: 10.1016/0165-0270(90)90170-k. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur J Neurosci. 2014;40(10):3556–72. doi: 10.1111/ejn.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW. A multiwire microelectrode for single unit recording in deep brain structures. J Neurosci Methods. 1990;32:143–148. doi: 10.1016/0165-0270(90)90170-k. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlis GH, Frank ME, Pfaffmann C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psychol. 1980;94:932–42. doi: 10.1037/h0077809. [DOI] [PubMed] [Google Scholar]
- Parker LA. Conditioned flavor avoidance and conditioned gaping: Rat models of conditioned nausea. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.09.070. In Press. [DOI] [PubMed] [Google Scholar]
- Parker L, Jensen K. Food Aversions: Taste Reactivity Responses Elicited by Lithium-Paired Food. Pharm Biochem & Behavior. 1992;41:239–240. doi: 10.1016/0091-3057(92)90090-3. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversions: Behavioral and Neural Processes. Oxford Univ Press; NY: 2009. pp. 92–113. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Elsevier Inc.; Amsterdam, the Netherlands: 2007. [Google Scholar]
- Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol. 1983;97:140–53. [PubMed] [Google Scholar]
- Pfaffmann C, Frank M, Norgren R. Neural mechanisms and behavioral aspects of taste. Annu Rev Psychol. 1979;30:283–325. doi: 10.1146/annurev.ps.30.020179.001435. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45(4):587–97. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem. 2010;17(11):539–46. doi: 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Einberg LT, Nissenbaum JW. Influence of saccharin on Polycose, sucrose, and glucose intake and preference in rats. Neurosci. Biobehav. Rev. 1987;11:223–229. doi: 10.1016/s0149-7634(87)80030-1. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW, Vigorito M. Starch preference in rats, Neurosci. Biobehav. Rev. 1987;11:253–262. doi: 10.1016/s0149-7634(87)80033-7. [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. The Journal of Neuroscience. 2005;25(38):8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci USA. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Parsley KP, Schwartz ZM, Cheng AY. On lateral septum-like characteristics of outputs from the accumbal hedonic ‘hotspot’ of Peciña and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J Comp Neurol. 2013;521:50–68. doi: 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. The neuroscience of pleasure. Focus on “Ventral pallidum firing codes hedonic eward: when a bad taste turns good”. J Neurophysiol. 2006;96:2175–2176. doi: 10.1152/jn.00727.2006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueji K. Brain mechanisms of flavor learning. Front Syst Neurosci. 2011;5:76. doi: 10.3389/fnsys.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.