Abstract
Endothelial dysfunction, characterized by impaired activation of endothelial nitric oxide (NO) synthase (eNOS) and ensued decrease of NO production, is a common mechanism of various cardiovascular pathologies, including hypertension and atherosclerosis. Laminar blood flow-mediated specific signaling cascades modulate vascular endothelial cells (ECs) structure and functions. We have previously shown that flow-stimulated Gab1 (Grb2-associated binder-1) tyrosine phosphorylation mediates eNOS activation in ECs, which in part confers laminar flow atheroprotective action. However, the molecular mechanisms whereby flow regulates Gab1 tyrosine phosphorylation and its downstream signaling events remain unclear. Here we show that platelet endothelial cell adhesion molecule-1 (PECAM1), a key molecule in an endothelial mechanosensing complex, specifically mediates Gab1 tyrosine phosphorylation and its downstream Akt and eNOS activation in ECs upon flow rather than hepatocyte growth factor (HGF) stimulation. Small interfering RNA (siRNA) targeting PECAM1 abolished flow- but not HGF-induced Gab1 tyrosine phosphorylation and Akt, eNOS activation as well as Gab1 membrane translocation. Protein-tyrosine phosphatase SHP2, which has been shown to interact with Gab1, was involved in flow signaling and HGF signaling, as SHP2 siRNA diminished the flow- and HGF-induced Gab1 tyrosine phosphorylation, membrane localization and downstream signaling. Pharmacological inhibition of PI3K decreased flow-, but not HGF-mediated Gab1 phosphorylation and membrane localization as well as eNOS activation. Finally, we observed that flow-mediated Gab1 and eNOS phosphorylation in vivo induced by voluntary wheel running was reduced in PECAM1 knockout mice. These results demonstrate a specific role of PECAM1 in flow-mediated Gab1 tyrosine phosphorylation and eNOS signaling in ECs.
Keywords: Gab1, PECAM1, laminar flow
1. Introduction
The vascular endothelium, lining inner surface of the vessel wall, is constantly exposed to fluid shear stress generated by blood flow and is the key mediator that converts the mechanical shear stress to the biological signaling [1–3]. Substantial evidence shows that laminar flow modulates endothelial cell (EC) structure and function, and prevents atherosclerosis by inhibiting endothelial dysfunction, vascular smooth muscle cell proliferation, vascular inflammation and platelet aggregation [4]. The activation of endothelial nitric oxide synthase (eNOS) plays a major role in these beneficial effects of laminar flow. We and others have previously reported that laminar flow enhances nitric oxide (NO) production by activating phosphatidylinositol 3-kinase (PI3K) and its downstream serine/threonine protein kinase Akt, which phosphorylates eNOS (Ser1177) in ECs [5, 6]. Moreover, we and others have further shown that Grb2-associated binder1 (Gab1), which is a key member of the family of scaffolding adaptor proteins, plays a crucial role in laminar flow-mediated Akt and eNOS activation as well as ischemia-induced angiogenesis [7–9]. Gab1 possesses an amino-terminus pleckstrin homology (PH) domain, several proline-rich domains, and multiple binding sites for SH2 domain-containing proteins [10]. Phosphorylation of tyrosine 627 (Y627) residue of Gab1 is required for laminar flowmediated eNOS activation [10, 11]. The enzymatic activity of SHP2, a widely expressed cytoplasmic tyrosine phosphatase which binds to tyrosine phosphorylated Gab1, is also critical for flow-induced eNOS activation [11]. However, it remains unclear how the extracellular mechanical signaling of laminar flow is transduced to Gab1 tyrosine phosphorylation and hence the activation of eNOS.
In the present study, we hypothesized that platelet endothelial cell adhesion molecule-1 (PECAM1), which has been shown to be involved in EC mechanosensing [12–15], is a signal transducer of flow-mediated Gab1 tyrosine phosphorylation and its downstream Akt and eNOS activation. In view of appreciated role of Gab1 in the signal transduction of cytokine and growth factor receptors, such as hepatocyte growth factor (HGF) [16–18], the role of PECAM1 in HGF-induced signaling was also examined as a comparison.
Our findings reveal that PECAM1 is required for specific transmission of a flow-induced signal from a mechanosensing complex located on the EC surface. PECAM1 depletion or deletion specifically blocked flow- but not HGF-induced Gab1 Y627 phosphorylation in vitro and in vivo. These findings provide new insights into the atheroprotective mechanisms of laminar flow and exercise, and suggest that the PECAM1/Gab1/eNOS pathway could be a potential target for therapeutic protection against endothelial dysfunction-associated diseases including atherosclerosis, hypertension and stroke.
2. Materials and methods
2.1. Reagents and Antibodies
Wortmannin (PI3K inhibitor), LY294002 (Akt inhibitor), and PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo [3,4-d] pyrimidine, Src inhibitor) were purchased from Calbiochem (San Diego, CA). Anti-Gab1 (#06579), anti-phospho-Src (Y416, #05857), and anti-GAPDH (#AB2302) were from EMD Millipore (Billerica, Massachusetts). Anti-Src (#sc-8995), anti-Gab1 (#sc-6292, #sc-9049), anti-PECAM1 (#sc-8306, for detecting human PECAM1), anti-SHP2 (#sc-7384), and anti-β-actin (#sc-47778) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-Gab1 (Tyr627, #3231), anti-phospho-eNOS (Ser1177, #9571), anti-phospho-Akt (Ser473, #4060), anti-phospho-ERK1/2 (#4370), anti-Akt (#4691), anti-ERK1/2 (#9107) were from Cell Signaling Technology (Danvers, MA). Additional anti-phospho-eNOS (Ser1177, #612393) and anti-eNOS (#610297) were purchased from BD Biosciences (San Diego, CA). Recombinant human HGF (#294-HG-005) and VEGF-A165 (#293-VE-010) was from R&D Systems (Minneapolis, MN). Anti-PECAM1 (#ab28364) was also purchased from Abcam (Cambridge, MA) to detect mouse PECAM1 by Western blotting and en face immunofluorescent staining.
2.2. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from fresh human umbilical cord veins from women with normal pregnancies, with patients’ informed consent. HUVECs were collected in accordance with the University of Rochester human subjects review board procedures that prescribe to the Declaration of Helsinki. HUVECs were seeded in 0.2% gelatin-precoated culture dishes maintained in Medium 200 (Cascade Biologics, Portland, OR) with low-serum growth supplement (LSGS; Invitrogen, Carlsbad, CA), 5% fetal bovine serum (FBS), 100 µg/mL streptomycin and 100 IU/mL penicillin at 37°C in a humidified atmosphere of 95% air and 5% CO2 as previously described [19]. HUVECs were used at passages 3–5 for all the experiments. Confluent cells cultured in 60 mm dishes were exposed to HGF (10 ng/mL) as indicated or laminar flow (fluid shear stress = 12 dyn/cm2) in a cone and plate viscometer in serum free media for 15 min [6]. For the inhibitor studies, cells were pretreated with various inhibitors as indicated for 30 min in serum-depleted medium before stimulation with HGF or flow.
2.3. Small Interference RNA (siRNA) and Transfection
HUVECs were treated with siGenome™ human PECAM1 and SHP2 siRNA duplex obtained from Dharmacon (Pittsburg, PA). The sequence for siRNA oligos were: PECAM1 siRNA, sense 5’-GAAUUCUCGAGACCAGAAUUU and antisense 5’-AUUCUGGUCUCGAGAAUUCUU; SHP2 siRNA, sense 5’-GAACAUCACGGGCAAUUAAUU and antisense 5’-PUUAAUUGCCC-GUGAUGUUCUU. The scrambled siRNA control is a non-targeting siRNA from Dharmacon. For transfection of siRNA, HUVECs were cultured in 60 mm dishes for 24 h at about 80–90% confluence, and then transfection of siRNA was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol as described previously [20]. Flow or HGF stimulation was performed 48 h after siRNA transfection.
2.4. Adenovirus Constructs and Infection
Adenovirus constructs encoding the GFP-tagged human Gab1 were generated using the pAd/CMV/V5-DEST™ Gateway® vector kit from Life Technologies according to the manufacturer’s protocol. The infection of HUVECs with recombinant adenovirus was performed as described previously [21]. Briefly, ECs cultured in 60-mm dishes were infected with recombinant adenoviruses at the multiplicity of infection (MOI, 30) for 24 h in a growth medium, and then exposed to laminar flow or treated with HGF (10 ng/ml). HUVECs were fixed with 3.7% formaldehyde in PBS and the images were captured using a fluorescence microscope (Olympus BX51) with a 60× objective lens (N.A. 1.4, oil).
2.5. Western Blot Analysis
Cells were harvested in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-Glycerolphosphate, 50 mM NaF, 1 mM Na3VO4 and protease inhibitor cocktail (Sigma-Aldrich, MO). After centrifugation at 4 °C for 20 min, protein extract supernatant was collected. The protein concentrations in the lysates were determined using the Bradford protein assay kit (Biorad) using a Beckman spectrophotometer (Fullerton, CA). For Western blots, total cell lysates were separated by SDS-PAGE, transferred to nitrocellulose membranes and were subsequently blocked in LI-COR blocking buffer (LI-COR Biosciences, Lincoln, NE) at room temperature for 1 h. Then the blots were incubated overnight at 4 °C with indicated primary antibodies after being washed 3 times with 1 X Tris buffered saline with 0.1% Tween-20 (TBST), membranes were incubated with IRDye® 680RD Goat anti-Mouse IgG (H+L) or IRDye® 800CW Goat anti-Rabbit IgG (H + L) (1:10,000 dilution; LI-COR). Images were visualized by using an Odyssey Infrared Imaging System and densitometric analysis of blots was performed using NIH Image J software (http://imagej.nih.gov/ij/).
2.6. En face immunofluorescence staining
Three-month-old PECAM1+/+ and PECAM1−/− mice were anesthetized with ketamine/xylazine cocktail (0.13/0.0088 mg/g body weight). Then, the arterial tree was perfused with saline containing 40 USPU/ml heparin from left ventricle for 5 min, followed by perfusion of pre-chilled 4% paraformaldehyde in PBS (pH 7.4) for 10 min. Subsequently, after adipose tissues were removed, the whole aorta was dissected from thoracic aorta to the heart, cut open longitudinally, permeabilized with 0.1% Triton X-100 in PBS for 10 min and blocked with 10% normal goat serum (Invitrogen) in Tris-buffered saline (TBS) containing 2.5% Tween-20 for 1 h at room temperature. Next, aortas were incubated with rabbit anti-PECAM1 in the antibody dilution buffer (3%BSA+TBS-2.5% Tween-20) overnight at 4°C. After rinsing with washing solution (TBS containing 2.5% Tween-20) 5 min for 3 times, aortic segments were incubated with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:1000 dilution) for 1 h at room temperature. Nuclei were counterstained with propidium iodide (PI) (Invitrogen). Finally, after another 3 rinses in the washing solution, aortic specimens were gently placed on a glass slide with the luminal side up, mounted in the ProLong Gold-antifade Mounting Media (Invitrogen) and cured overnight. Aortas were examined by a laser-scanning confocal microscope (FV-1000 mounted on IX81, Olympus) with UPlanFL N 60× Oil lens.
2.7. Mice and genotyping
PECAM1−/− mice in C57BL/6J background were obtained from the Jackson Laboratory (Bar harbor, ME) [22] and were crossed with C57BL/6J mice to obtain PECAM1+/− mice. After that PECAM1+/− males and females were intercrossed to generate PECAM1−/− mice. Mice were genotyped by PCR using primers mPECAM1-F (5’-CAGCCACTGTGTGAGACACAAAGGCAAG-3’), mPECAM1-R (5’-ACCACACACCCAGCAACCCTTTCAGAC-3’) and LTR52 (5’-AAGTGGCGTTACTTAAGCTAGCTTGCCAAC-3’). This primer pair amplifies a 350-bp fragment from the wild-type PECAM1 gene and 230-bp from the deleted allele. The PCR amplification profile was set as follows: 94°C, 4 min; 94°C, 1 min; 58°C, 1 min; 72°C, 1.5 min (35 cycles); 72°C, 7 min; 10°C forever. Tail genomic DNA was subject to regular RT-PCR using 2X GoTaq Master Mix (Promega, Madison, WI, USA). Reaction products are separated in 1.0% agarose gel and visualized with Image Lab 5.1 software (Bio-rad, Hercules, CA, USA).
2.8. Volunteer wheel running and HGF treatment
Three-month-old PECAM1+/+ and PECAM1−/− male mice were divided into sedentary and exercised groups. Voluntary physical activity of active animals (exercised) were subjected to voluntary exercise training by wheel running for 30 min in cages equipped with running wheels (Columbus Instruments, Columbus, OH). Sedentary groups (sedentary) were housed during this time in cages without wheels. After running, mice were anesthetized with an intraperitoneal injection of a mixture of Ketamine (80 mg/kg) and Xylazine (5 mg/kg), then the aorta were collected in ice-cold PBS and surrounding connective tissue was quickly removed under dissection microscope. Aortic lysates were prepared by using Precellys® Minilys Bead Homogenizer (Bertin Technologies, France). Another group of mice were injected (by i.p.) with vehicle (PBS) or recombinant human HGF (0.3 µg/mice). Then aortic lysates were prepared 30 min after the injection for Western blot analysis, n=3–5 per each group. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, Revised 2011) and was approved by Institutional Animal Care and Use Committee at the University of Rochester.
Statistics
Data were expressed as means±SEM. Experiments were repeated for three to five times for quantification. Statistical analysis was performed using GraphPad Prism software 5.0 [23] (GraphPad, La Jolla, CA). Student’s t test and one-way analysis of variance (ANOVA) with post hoc Bonferroni tests were used for comparisons between two groups and multiple comparisons, respectively. A p value less that 0.05 was considered statistically significant.
3. Results
3.1. Depletion of PECAM1 specifically blocks laminar flow- but not HGF-induced Gab1 Tyr627 (Y627) phosphorylation
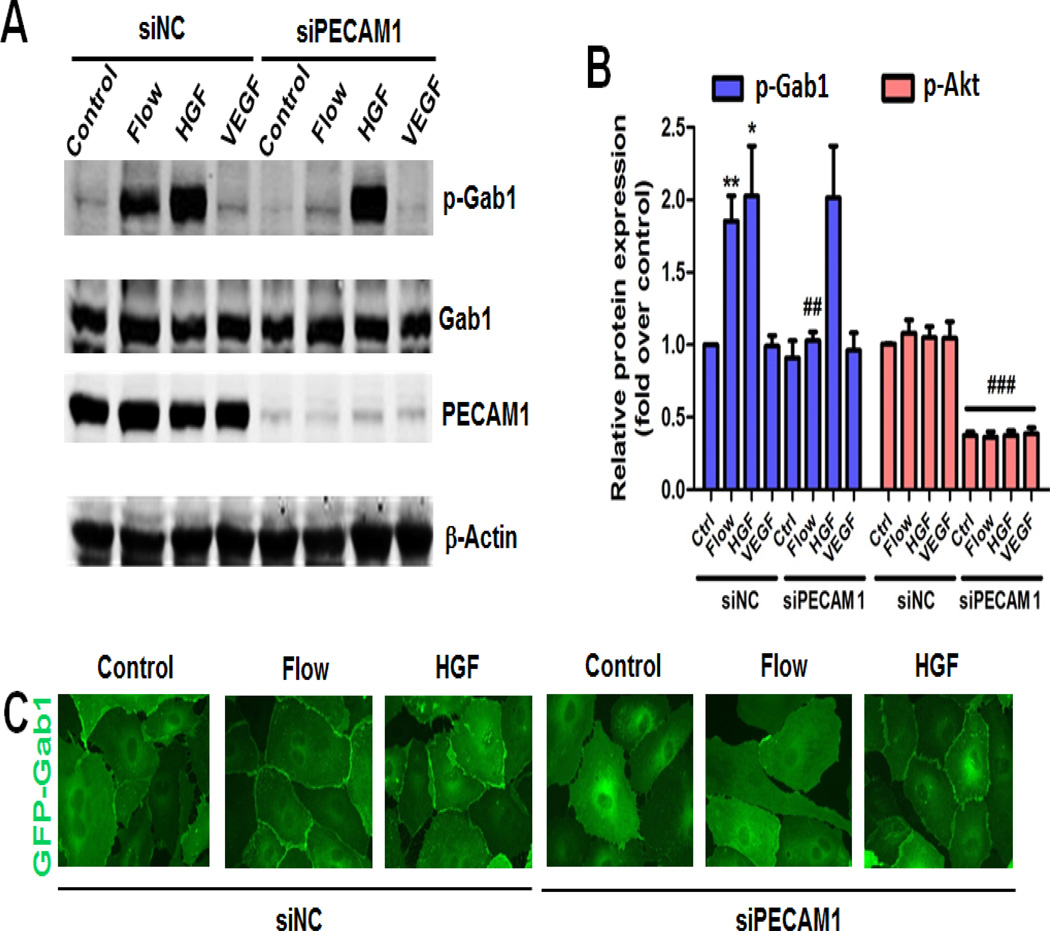
We first examined whether a mechanosensory molecule PECAM1 was involved in Gab1 tyrosine 627 (Y627) phosphorylation. HUVECs were transfected with siRNA targeting PECAM1 (siPECAM1) or non-target siRNA control (siNC) for 48 h. After transfection, cells were subjected to laminar flow for 15 min. Hereafter, the term “flow” denotes “steady laminar flow” (fluid shear stress = 12 dynes/cm2). Since tyrosine phosphorylation of Gab1 in response to growth factors plays a critical role in the signal transduction, we also treated HUVECs with vascular endothelial growth factor (VEGF, 25 ng/mL) or HGF (10 ng/mL) for 15 min for comparison. The cell lysates were collected and Gab1 phosphorylation on Y627 was analyzed by immunoblotting with Y627 phospho-specific Gab1 antibody. As shown in Figure 1A and 1B, flow and HGF, rather than VEGF, potently stimulate Gab1 phosphorylation on Y627. Knockdown of PECAM1 by siRNA dramatically decreased flow-induced Gab1 Y627 phosphorylation. However, HGF-induced Gab1 Y627 phosphorylation was unaffected by PECAM1 knockdown. These results demonstrate that PECAM1 conveys specifically the flow signaling to Gab1, while HGF-induced Gab1 tyrosine phosphorylation is PECAM1-independent.
Figure 1. PECAM1 is critical for flow- but not HGF-induced Gab1 activation.
A, The specific role of PECAM1 in flow-induced Gab1 tyrosine (Y627) phosphorylation. HUVECs were transfected with non-target control siRNA (siNC, 50 nM) or human PECAM1 siRNA (siPECAM1, 50 nM) for 48 h, and then exposed to laminar flow (12 dynes/cm2), HGF (10 ng/mL), or VEGF (25 ng/mL) for 15 min. Cell lysates were prepared and probed with the indicated antibodies by Western blot analysis. B, Densitometry quantification of panel A *P<0.05, **P<0.01 versus control (ctrl); ##P<0.01 versus siNC+flow; ### p<0.01 versus respective control. C, The specific role of PECAM1 in flow-induced Gab1 membrane translocation. HUVECs were transfected with siNC or siPECAM1 as described in A, then infected with recombinant adenovirus constructs encoding the GFP-tagged human Gab1 at the multiplicity of infection (MOI, 30) for 24 h, and then exposed to laminar flow (12 dynes/cm2) or HGF (10 ng/mL) for 15 min. Cells were fixed with 3.7% formaldehyde in PBS and GFP fluorescence was captured using a 60x oil immersion objective lens.
3.2. PECAM1 siRNA blocks flow-induced Gab1 localization at cell-cell contacts
Membrane targeting of Gab1 plays a critical role in mediating tyrosine kinase receptors-triggered signaling [24, 25]. To show Gab1 subcellular localization in the cell-cell contacts by both flow and HGF, we infected HUVECs with adenoviral GFP-tagged Gab1 (Ad-GFP-Gab1) followed by exposure to flow or treatment with HGF for 15 min. Translocation of GFP-Gab1 to cell junctions was observed upon stimulation with flow or HGF. Knockdown of PECAM1 significantly reduced flow-induced GFP-Gab1 translocation to cell-cell contacts, but it did not affect HGF-induced GFP-Gab1 membrane localization in ECs (Figure 1C). Together, our data indicate that PECAM1 is critically and specifically involved in flow-mediated Gab1 localization at cell-cell contacts.
3.3. PECAM1 is critical for flow- but not HGF-induced Akt and eNOS phosphorylation
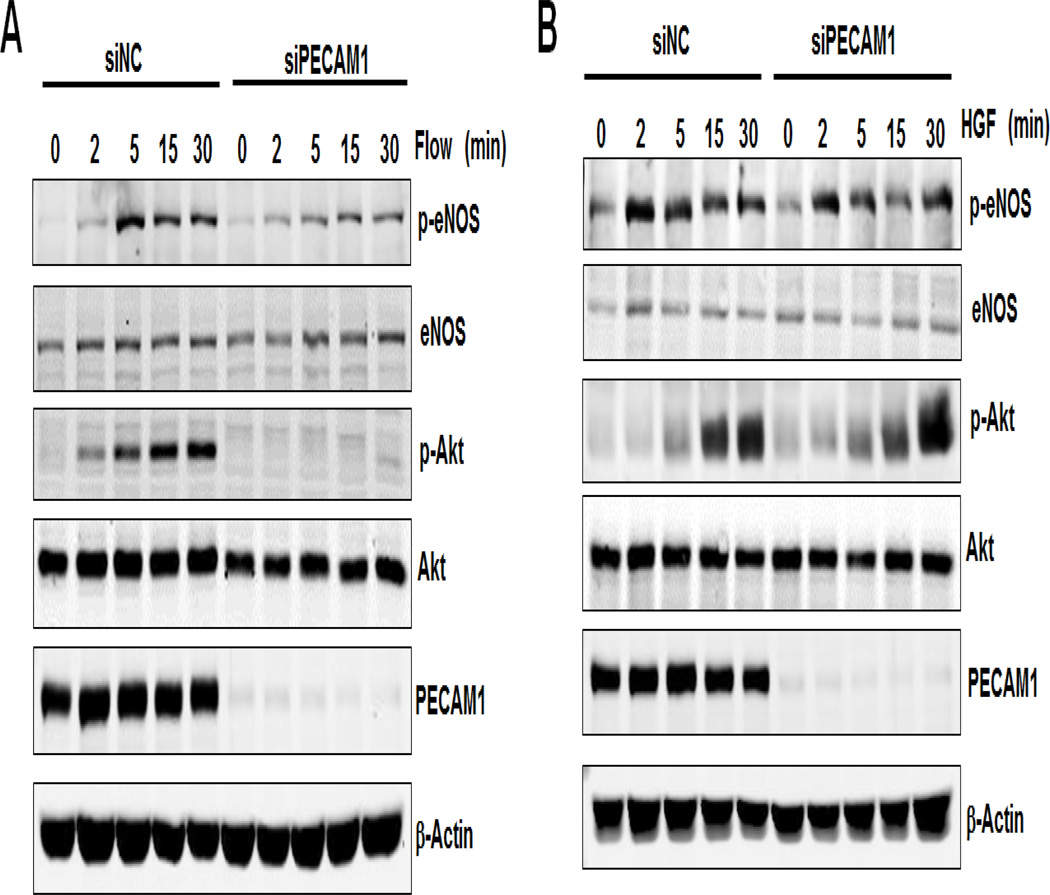
Our previous studies have shown that Gab1 tyrosine phosphorylation functions upstream of Akt and eNOS phosphorylation, which is essential for flow-mediated signaling [19]. Here we examined the role of PECAM1 in flow-induced Gab1 downstream signaling events including phosphorylation of Akt (Ser473) and eNOS (Ser1177). As shown in Figure 2, laminar flow induced phosphorylation of eNOS and Akt, in a time-dependent manner, which was inhibited by PECAM1 siRNA treatment. However, HGF-induced phosphorylation of eNOS and Akt was unaffected by PECAM1 siRNA, suggesting that HGF-induced eNOS and Akt signaling was PECAM1 independent. These data reveal that PECAM1 specifically functions as an upstream mediator of flow-induced Gab1 signaling cascade.
Figure 2. PECAM1 is critical for flow- but not HGF-induced Akt, eNOS and ERK phosphorylation.
HUVECs were transfected with non-target control siRNA (siNC, 50 nM) or human PECAM1 siRNA (siPECAM1, 50 nM) for 48 h, and then exposed to laminar flow (12 dynes/cm2, panel A), or HGF (10 ng/mL, panel B) for 15 min. Cell lysates were prepared and probed with the indicated antibodies by Western blot analysis.
3.4. SHP2 siRNA blocks flow- and HGF-induced Gab1 phosphorylation, membrane localization and phosphorylation status of Akt and eNOS
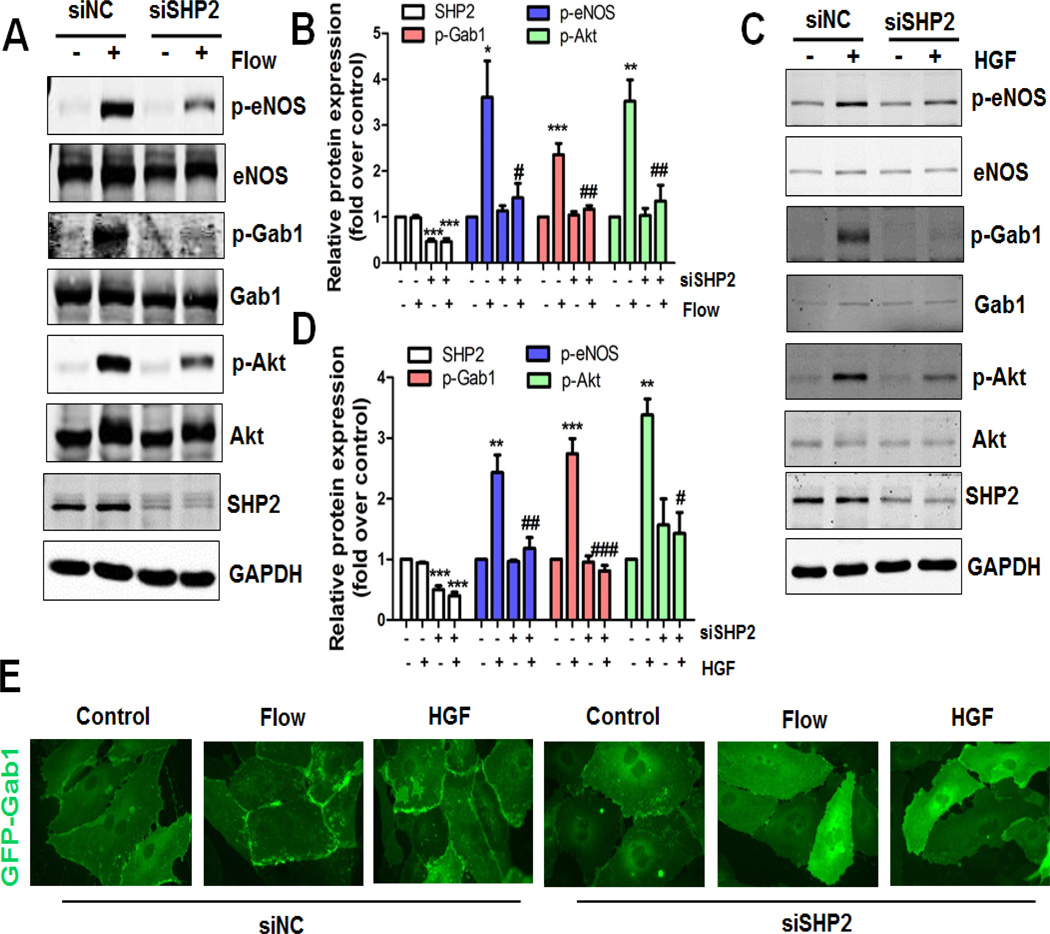
To further explore the molecular mechanisms underlying flow- and HGF-induced Gab1 tyrosine phosphorylation and signaling, we studied the role of SHP2, a critical tyrosine phosphatase that has been reported to interact with PECAM1 mechanosensing complex [14, 26–28]. HUVECs were subjected to flow or treated with HGF for 15 min after transfection with siRNA targeting SHP2 for 48 h. As shown in Figure 3A–3D, SHP2 siRNA almost abrogated both flow- and HGF-induced Gab1 Y627 phosphorylation. Moreover, the signaling events activated by Gab1 phosphorylation including phosphorylation of Akt and eNOS were partially but significantly reduced by SHP2 siRNA treatment. Consistent with the crucial role of SHP2 in Gab1 tyrosine phosphorylation and signaling, both flow- and HGF-induced Gab1 recruitment to the cell-cell border was attenuated by SHP2 siRNA treatment (Figure 3E). Taken together, our findings suggest that SHP2 plays an important role in flow- and HGF-induced Gab1 tyrosine phosphorylation, membrane localization and signaling in ECs.
Figure 3. SHP2 is critical for both flow- and HGF-induced Gab1 activation.
A–D, Critical role of SHP2 in both flow- and HGF-induced Gab1 phosphorylation. HUVECs were transfected with non-target control siRNA (siNC, 50 nM) or human SHP2 siRNA (siSHP2, 50 nM) for 48 h, and then exposed to laminar flow (12 dynes/cm2, panel A), or HGF (10 ng/mL, panel C) for 15 min. Cell lysates were prepared and probed with the indicated antibodies by Western blot analysis. Quantifications of panel A and C were showed in the middle (panel B and D, respectively), *P<0.05, **P<0.01, ***P<0.001 versus control siRNA; #P<0.05, ##P<0.01, ###P<0.001 versus control siRNA + flow or HGF. E, Critical role of SHP2 in both flow- and HGF-induced Gab1 membrane localization. HUVECs were transfected with siNC or siSHP2 for 48 h, then infected with recombinant adenovirus constructs encoding the GFP-tagged human Gab1 at the multiplicity of infection (MOI, 30) for 24 h, and then exposed to laminar flow (12 dynes/cm2) or HGF (10 ng/mL) for 15 min. HUVECs were fixed with 3.7% formaldehyde in PBS and the images were captured using an oil immersion 60× objective lens.
3.5. Inhibition of Src kinase and PI3K blocks flow- but not HGF-induced phosphorylation of Akt and eNOS
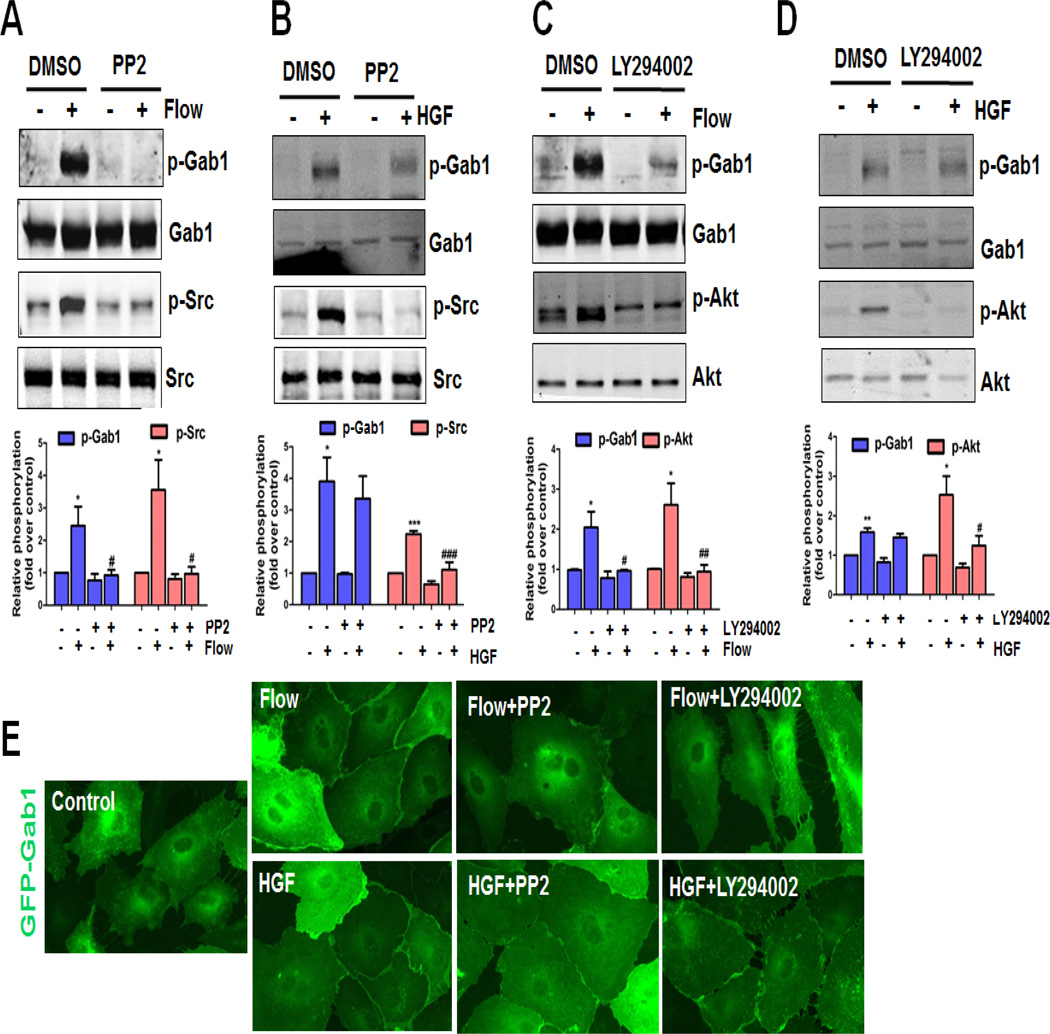
It has been reported that Src kinase is involved in flow [6, 19, 29] and HGF [18, 30, 31] signaling in ECs. In particular, we have previously shown that flow activates the PI3K/Akt/eNOS pathway via Src kinase-dependent transactivation of VEGFR2 [6]. It has also been demonstrated that Gab1 Y627 is phosphorylated by Src in vitro [30]. Hereby, we investigated the role of Src kinase in flow- and HGF-induced Gab1 tyrosine phosphorylation. HUVECs were incubated with 1 µM PP2, a specific Src kinase inhibitor, for 30 min prior to flow or HGF treatment, and Gab1 tyrosine phosphorylation was studied. We found that Gab1 phosphorylation by flow, but not by HGF, was inhibited by PP2 (1 µM), suggesting that flow-mediated Gab1 tyrosine phosphorylation is Src kinase-dependent (Figure 4A–4B).
Figure 4. Src and PI3K are involved in flow- but not HGF-induced Gab1 activation.
Critical role of Src and PI3K in flow-induced Gab1 tyrosine phosphorylation (A and C), but not HGF-induced Gab1 tyrosine phosphorylation (B and D). HUVECs were treated with vehicle (0.1% DMSO) or specific inhibitor of Src activity (PP2, 1 µM), or PI3K (LY294002, 10 µM), then exposed to laminar flow (12 dynes/cm2), or HGF (10 ng/mL) for 15 min. Cell lysates were prepared and probed with the indicated antibodies by Western blot analysis. Quantification of each panel was provided under each panel, *P<0.05, **P<0.01 versus vehicle control (DMSO); #P<0.05, ##P<0.01, ###P<0.001 versus DMSO + flow or HGF. E, Src and PI3K are involved in flow- but not HGF-induced Gab1 membrane localization. HUVECs were infected with recombinant adenovirus constructs encoding the GFP-tagged human Gab1 at the multiplicity of infection (M.O.I., 30) for 24 h, then treated with vehicle (0.1% DMSO) or specific inhibitor of Src activity (PP2, 1 µM), or PI3K (LY294002, 10 µM), before exposure to laminar flow (12 dynes/cm2), or HGF (10 ng/mL) for 15 min. HUVECs were fixed with 3.7% formaldehyde in PBS and the images were captured using an oil immersion 60× objective lens.
Gab1 possesses a functional PH domain which binds to specific phosphatidylinositol lipids within biological membranes and this PH domain is required for the localization of Gab1 at sites of cell-cell contact [24]. Therefore, we examined the role of PI3K in flow-mediated Gab1 tyrosine phosphorylation and membrane localization. Treatment with PI3K specific inhibitor LY294002 (10 µM) inhibited flow-mediated Gab1 tyrosine phosphorylation, without affecting HGF-mediated Gab1 tyrosine phosphorylation (Figure 4C–4D). In agreement with Gab1 phosphorylation, flow- but not HGF-induced Gab1 recruitment to the cell-cell border was attenuated by PP2 and LY294002 (Figure 4E). Taken together, these results indicate that Gab1 tyrosine phosphorylation by flow, but not HGF, is Src- and PI3K-dependent.
3.6. PECAM1 is critical for Gab1 tyrosine phosphorylation in vivo
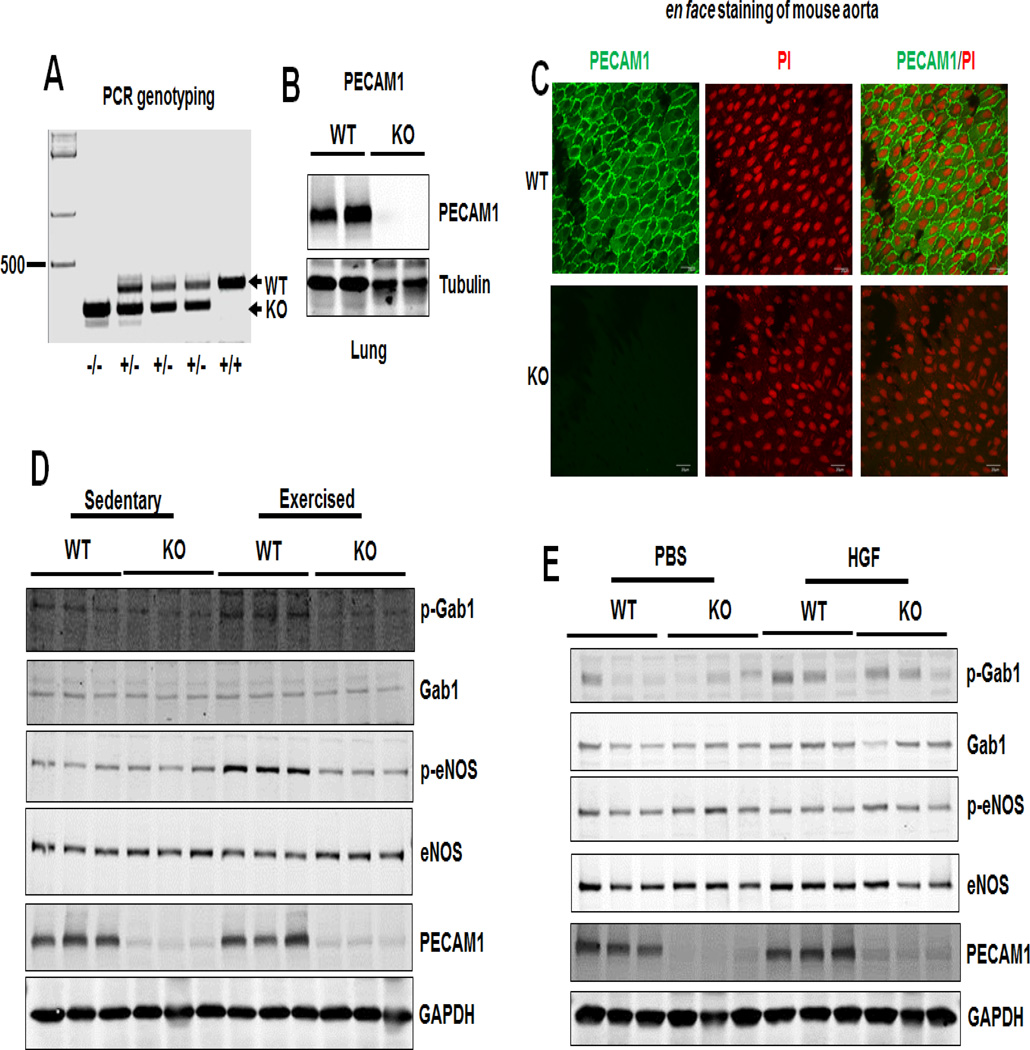
It has been reported that exercise increases blood flow and vessel wall shear stress [32] and acute exercise activates flow-dependent signal transduction to eNOS activation in mouse aorta [33]. To determine the role of PECAM1 in flow and HGF-mediated Gab1 tyrosine phosphorylation in vivo, we subjected PECAM1+/+ (wild type, WT) and PECAM1−/− (knockout, KO) mice to volunteer wheel running or HGF treatment. PECAM1 was deleted in PECAM1−/− mice, confirmed by genotyping (Figure 5A), Western blotting (Figure 5B) and en face immunofluorescent staining of mouse aorta with a specific antibodies to PECAM1 (Figure 5C). Next, we examined whether PECAM1 deficiency in mice would impact exercise-induced phosphorylation of Gab1 (Y627) and eNOS (S1177) in mouse aorta. As shown in Figure 5D, exercise increased Gab1 (Y627) and eNOS (S1177) phosphorylation, without affecting the abundance of total Gab1 and eNOS protein levels. However, in PECAM1−/− mice, exercise-induced Gab1 and eNOS phosphorylation were diminished. Immunoblots of aortic lysate from HGF-treated mouse revealed that Gab1 tyrosine phosphorylation, but not eNOS phosphorylation, was increased by HGF treatment, and increased Gab1 phosphorylation by HGF was unaffected by PECAM1 deletion (Figure 5E). These data indicate that PECAM1 is critical for flow-induced Gab1 tyrosine phosphorylation and eNOS phosphorylation in vivo.
Figure 5. Gab1 is critical for flow-induced Gab1 and eNOS phosphorylation in vivo.
A, Genotyping of PECAM1+/+, PECAM1+/−, PECAM1−/− mice. B, Deletion of PECAM1 in mouse lung. C, En face staining showing PECAM1 deletion in aortic endothelium. PI: propidium iodide. D–E, The phosphorylation levels of Gab1 (Y627) and eNOS (S1177) were diminished in PECAM1−/− mice undergoing exercise but not HGF treatment. PECAM1+/+ and PECAM1−/− male mice were divided into sedentary and exercised groups. Voluntary physical activity of active animals (exercised) were subjected to voluntary exercise training by wheel running for 30 min in cages equipped with running wheels. Sedentary groups (sedentary) were housed during this time in cages without wheels. Another group of mice were injected (by i.p.) with vehicle (PBS) or recombinant human HGF (0.3 µg/mouse) for 30 min. After that, aortic lysates were prepared for Western blot using specific antibodies as indicated.
4. Discussion
Atherosclerotic cardiovascular disease is the leading cause of death and disability worldwide [34–36]. Atherosclerosis develops at sites of disturbed flow, but less in regions with steady laminar flow [2]. Previous studies have shown that laminar flow confers atheroprotection by activating anti-atherogenic, anti-thrombotic and anti-inflammatory signaling pathways, whereas disturbed flow promotes endothelial dysfunction thereby contributing to the development of atherosclerosis-related cardiovascular diseases [37]. Specifically, the endothelium in the atheroprotective regions, in comparison to that in atheroprone regions, shows increased expression of Kruppel-like factor 2 (KLF2), increased production of NO derived from activated eNOS, decreased leukocyte adhesion and vascular permeability, as well as other atheroprotective phenotypes [38]. Our present study provides new insight into laminar flow atheroprotective signaling, in which we reveal that PECMA1 plays a crucial role for flow-induced Gab1 tyrosine phosphorylation and eNOS phosphorylation in vitro and in vivo.
Immediate Ser1177 phosphorylation of eNOS in response to various stimuli, such as Ca2+ elevating agonists, growth factors (such as VEGF), plant-derived polyphenols and laminar flow, increase NO production in ECs [39, 40]. Our previous studies showed that Gab1 functions upstream of Akt and eNOS phosphorylation in response to laminar flow [19]. More recently, we have reported that laminar flow stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS [41, 42]. Besides laminar flow, many growth factors and cytokines (such as HGF) can also induce Gab1 tyrosine phosphorylation, which results in the activation of Ras/MAPK and PI3K/Akt pathways. Kusano et al [28] have previously shown that Gab1 and PECAM1 were two major SHP-2-binding proteins in cultured bovine aortic endothelial cells. Furthermore, Fleming et al. [43] showed that PECAM1, a critical mechanosensing molecule in flow-mediated signaling, contributes to the flow-induced activation of eNOS. However, it remains elusive whether flow-mediated Gab1/eNOS activation is regulated by PECAM1. Our present study indicates that PECAM1 is a key mediator of Gab1 Y627 phosphorylation by laminar flow but not by HGF stimulation. Our in vivo data are consistent with this notion as PECAM1 deficiency abolished exercise-induced Gab1 and eNOS phosphorylation, without affecting HGF-induced Gab1 phosphorylation.
A number of studies have implicated Gab1 in growth factor- and cytokine receptor-mediated signaling [10, 44]. Gab1 deficient mice die between E12.5 and 18.5 due to the defects in heart, placenta, skin, and muscle development [10]. Tyrosine phosphorylation of Gab1 and its recruitment to the HGF receptor, c-Met, have been well studied [45]. Gab1 is a major tyrosine-phosphorylated protein following stimulation of the c-Met receptor by HGF in epithelial cells [46, 47]. Gab1 associates with PI3K and SHP2 after HGF stimulation in epithelial cells [48, 49]. HGF is a pro-angiogenic mitogen for ECs [31], promoting their proliferation and migration [18]. In our present study, PECAM1 depletion blocks flow-induced Gab1 Y627 phosphorylation, but HGF-induced Gab1 Y627 phosphorylation remains intact. These results suggest a specific role of Gab1 Y627 phosphorylation in flow-induced PECAM1 mechanosignaling. The identification of Gab1 tyrosine phosphorylation in regulating multiple endothelial functions in response to distinct stimuli demonstrates the essential role of Gab1, as an integrator of multiple signaling pathways, in maintaining functional integrity of normal and healthy endothelium.
Tzima et al [26] showed that PECAM1 (which senses the mechanical force of shear stress [14]), VE-Cadherin (which functions as an adaptor [26]), and VEGFR2 (which activates PI3K [6]) comprise a mechanosensory complex which mediates EC responses to fluid shear stress. Furthermore, three independent research groups reported that in regions of disturbed flow (the lesser curvature of the aortic arch), PECAM1 promotes the development of atherosclerosis in mice lacking LDL receptor [50] and ApoE [22, 51]. In line with these observations, the Tzima’s group further showed that PECAM1 is essential for disturbed flow-induced vascular remodeling by using the partial ligation model [52]. However, PECAM1 in steady laminar flow area (thoracic and abdominal aorta) had atheroprotective effects [50, 53]. These studies suggest that PECAM1 regulates plaque development in a cell-type-and site (flow pattern)-specific manner [53, 54]. Our previous data show that flow-activated VEGFR2 recruits Gab1 and that phosphorylation of Gab1 leads to recruitment of PI3K, which then transmits a flow signal to Akt and eNOS [6, 19]. Based on these findings, we propose that PECAM1 is required for the formation of laminar flow mechanosensing complex, which recruits Gab1 and activates Akt and eNOS and subsequently NO production in ECs.
SHP2, as a tyrosine phosphatase, has two SH2 domains, which binds to multiple receptor protein tyrosine kinases including PDGF receptor and FGF receptor [55]. The interaction between tyrosine phosphorylated Gab1 and SHP2 plays a crucial role in the activation of eNOS. PECAM1 also interacts with SHP2 upon flow-induced tyrosine phosphorylation on the cytoplasmic domain of PECAM1 [14, 27, 56]. Binding of SHP2 via the SH2 domain enhances its catalytic activity. A possible mechanism for SHP2 is to dock on a “positive” phospho-tyrosine and dephosphorylates a “negative” phospho-tyrosine either on the same protein or another interacting protein. Fleming group demonstrated that SHP2 via interacting Gab1 is crucial for the activation of eNOS by fluid shear stress [11]. Our data show that SHP2 is required for Gab1 membrane relocation, and it is possible that PECAM1, SHP2, and Gab1 form a complex upon laminar flow, which allows the access of SHP2 to its potential substrates, such as c-Src, which is activated by tyrosine dephosphorylation at c-Src Y527. Upon stimulation by laminar flow, PECAM1, as the direct mechanical force sensor and transmitter (through forming the junctional mechanosensing complex), recruits Src to phosphorylate Gab1 on Y627, thereby forming the Gab1/SHP2/PI3K complex to regulate the phosphorylation of ERK, Akt and eNOS. PI3K affects Gab1 membrane localization and tyrosine phosphorylation via a positive feedback pathway. However, upon stimulation with HGF, binding HGF receptor c-Met via Met binding domain in Gab1 induces the signaling by the Gab1/SHP2/PI3K complex. In this case, PECAM1 does not play any significant role, and there is no positive feedback regulation of Gab1 membrane localization and tyrosine phosphorylation by PI3K. Our observations that SHP2 siRNA almost abolished flow- and HGF-induced Gab1 phosphorylation to basal level but only partially reduce Akt/eNOS phosphorylation, suggest that additional SHP2-Gab1-independent pathway(s) might be involved in regulation of Akt/eNOS phosphorylation. This is consistent with our previous observations that inhibition of Gab1 function by Gab1 siRNA knockdown or overexpression of PI3K p85 subunit binding defective Gab1 mutant in endothelial cells partially but significantly reduced laminar flow-induced Akt and eNOS phosphorylation [19]. Collectively, our results reveal that the PECAM1/SHP2/Gab1 signaling pathway plays a critical role in mediating flow-induced Akt/eNOS phosphorylation in endothelial cells.
In summary, our results demonstrate that under conditions of laminar flow, PECAM1 acts upstream of Gab1, promoting Gab1/Akt/eNOS phosphorylation and activation, thereby confers EC responsiveness to flow. The mechano-effector function of Gab1 may open up new therapeutic avenues for the treatment of cardiovascular diseases.
Highlights.
PECAM1 transduces flow induced mechano-signal via Gab1/Akt/eNOS pathway
PECAM1 is critical for exercise induced Gab1/eNOS activation
PECAM1 is not involved in HGF-induced Gab1 activation
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (R01HL109502 and R01HL114570 to Z.G.J), American Heart Association Grant-In-Aid Award (0755916T to Z.G.J.), and the American Diabetes Association (7-12-BS-085 to Z.G.J.). We thank Yingqian Xu and Chelsea Wong for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none declared
References
- 1.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies PF, Civelek M, Fang Y, et al. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99:315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak BR, Back M, Bochaton-Piallat ML, et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications dagger. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117:1082–1089. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 5.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin ZG, Ueba H, Tanimoto T, et al. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Wang W, Ha CH, et al. Endothelial Grb2-associated binder 1 is crucial for postnatal angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1016–1023. doi: 10.1161/ATVBAHA.111.224493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Xiong Y, Huo Y, et al. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci U S A. 2011;108:2957–2962. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shioyama W, Nakaoka Y, Higuchi K, et al. Docking protein Gab1 is an essential component of postnatal angiogenesis after ischemia via HGF/c-met signaling. Circ Res. 2011;108:664–675. doi: 10.1161/CIRCRESAHA.110.232223. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Xu S, Yin M, et al. Essential roles of Gab1 tyrosine phosphorylation in growth factor-mediated signaling and angiogenesis. Int J Cardiol. 2015;181:180–184. doi: 10.1016/j.ijcard.2014.10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixit M, Loot AE, Mohamed A, et al. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res. 2005;97:1236–1244. doi: 10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara K, Masuda M, Osawa M, et al. Is PECAM-1 a mechanoresponsive molecule? Cell Struct Funct. 2001;26:11–17. doi: 10.1247/csf.26.11. [DOI] [PubMed] [Google Scholar]
- 13.Collins C, Osborne LD, Guilluy C, et al. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat Commun. 2014;5:3984. doi: 10.1038/ncomms4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osawa M, Masuda M, Kusano K, et al. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182:753–763. doi: 10.1083/jcb.200801062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aasrum M, Odegard J, Sandnes D, et al. The involvement of the docking protein Gab1 in mitogenic signalling induced by EGF and HGF in rat hepatocytes. Biochim Biophys Acta. 2013;1833:3286–3294. doi: 10.1016/j.bbamcr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Barrow-McGee R, Kermorgant S. Met endosomal signalling: in the right place, at the right time. Int J Biochem Cell Biol. 2014;49:69–74. doi: 10.1016/j.biocel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Manganini M, Maier JA. Transforming growth factor beta2 inhibition of hepatocyte growth factor-induced endothelial proliferation and migration. Oncogene. 2000;19:124–133. doi: 10.1038/sj.onc.1203225. [DOI] [PubMed] [Google Scholar]
- 19.Jin ZG, Wong C, Wu J, et al. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong C, Jin ZG. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J Biol Chem. 2005;280:33262–33269. doi: 10.1074/jbc.M503198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lungu AO, Jin ZG, Yamawaki H, et al. Cyclosporin A inhibits flow-mediated activation of endothelial nitric-oxide synthase by altering cholesterol content in caveolae. J Biol Chem. 2004;279:48794–48800. doi: 10.1074/jbc.M313897200. [DOI] [PubMed] [Google Scholar]
- 22.Stevens HY, Melchior B, Bell KS, et al. PECAM-1 is a critical mediator of atherosclerosis. Dis Model Mech. 2008;1:175–181. doi: 10.1242/dmm.000547. discussion 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu S, Liu Z, Huang Y, et al. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-kappaB activation. Transl Res. 2012;160:114–124. doi: 10.1016/j.trsl.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Maroun CR, Naujokas MA, Park M. Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program. Mol Biol Cell. 2003;14:1691–1708. doi: 10.1091/mbc.E02-06-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frigault MM, Naujokas MA, Park M. Gab2 requires membrane targeting and the Met binding motif to promote lamellipodia, cell scatter, and epithelial morphogenesis downstream from the Met receptor. J Cell Physiol. 2008;214:694–705. doi: 10.1002/jcp.21264. [DOI] [PubMed] [Google Scholar]
- 26.Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 27.Masuda M, Osawa M, Shigematsu H, et al. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997;408:331–336. doi: 10.1016/s0014-5793(97)00457-2. [DOI] [PubMed] [Google Scholar]
- 28.Kusano K, Thomas TN, Fujiwara K. Phosphorylation and localization of protein-zero related (PZR) in cultured endothelial cells. Endothelium. 2008;15:127–136. doi: 10.1080/10623320802125250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spindel ON, Burke RM, Yan C, et al. Thioredoxin-interacting protein is a biomechanical regulator of Src activity: key role in endothelial cell stress fiber formation. Circ Res. 2014;114:1125–1132. doi: 10.1161/CIRCRESAHA.114.301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan PC, Sudhakar JN, Lai CC, et al. Differential phosphorylation of the docking protein Gab1 by c-Src and the hepatocyte growth factor receptor regulates different aspects of cell functions. Oncogene. 2010;29:698–710. doi: 10.1038/onc.2009.363. [DOI] [PubMed] [Google Scholar]
- 31.Maejima Y, Ueba H, Kuroki M, et al. Src family kinases and nitric oxide production are required for hepatocyte growth factor-stimulated endothelial cell growth. Atherosclerosis. 2003;167:89–95. doi: 10.1016/s0021-9150(02)00384-2. [DOI] [PubMed] [Google Scholar]
- 32.Kim B, Lee H, Kawata K, et al. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PLoS One. 2014;9:el11409. doi: 10.1371/journal.pone.0111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cacicedo JM, Gauthier MS, Lebrasseur NK, et al. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–H1265. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2015 Update: A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, Bai P, Little PJ, et al. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev. 2014;34:644–675. doi: 10.1002/med.21300. [DOI] [PubMed] [Google Scholar]
- 36.Xu S, Liu Z, Liu P. HDL cholesterol in cardiovascular diseases: the good, the bad, and the ugly? Int J Cardiol. 2013;168:3157–3159. doi: 10.1016/j.ijcard.2013.07.210. [DOI] [PubMed] [Google Scholar]
- 37.Firasat S, Hecker M, Binder L, et al. Advances in endothelial shear stress proteomics. Expert Rev Proteomics. 2014:1–9. doi: 10.1586/14789450.2014.933673. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Li YS, Chien S. Shear Stress-Initiated Signaling and Its Regulation of Endothelial Function. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt CA, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Ha CH, Jhun BS, et al. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDACS and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon IS, Wang W, Xu S, et al. Histone Deacetylase 5 Interacts with Kruppel-Like Factor 2 and Inhibits its Transcriptional Activity in Endothelium. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming I, Fisslthaler B, Dixit M, et al. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 44.Holgado-Madruga M, Emlet DR, Moscatello DK, et al. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 45.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen L, Holgado-Madruga M, Maroun C, et al. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 47.Tulasne D, Paumelle R, Weidner KM, et al. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol Biol Cell. 1999;10:551–565. doi: 10.1091/mbc.10.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaeper U, Gehring NH, Fuchs KP, et al. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachs M, Brohmann H, Zechner D, et al. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goel R, Schrank BR, Arora S, et al. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1996–2002. doi: 10.1161/ATVBAHA.108.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harry BL, Sanders JM, Feaver RE, et al. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2003–2008. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison M, Smith E, Ross E, et al. The role of platelet-endothelial cell adhesion molecule-1 in atheroma formation varies depending on the site-specific hemodynamic environment. Arterioscler Thromb Vasc Biol. 2013;33:694–701. doi: 10.1161/ATVBAHA.112.300379. [DOI] [PubMed] [Google Scholar]
- 54.Cybulsky MI. Morphing the topography of atherosclerosis: an unexpected role for PECAM-1. Arterioscler Thromb Vasc Biol. 2008;28:1887–1889. doi: 10.1161/ATVBAHA.108.174029. [DOI] [PubMed] [Google Scholar]
- 55.Mannell H, Krotz F. SHP-2 regulates growth factor dependent vascular signalling and function. Mini Rev Med Chem. 2014;14:471–483. doi: 10.2174/1389557514999140506094738. [DOI] [PubMed] [Google Scholar]
- 56.Tai LK, Zheng Q, Pan S, et al. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem. 2005;280:29620–29624. doi: 10.1074/jbc.M501243200. [DOI] [PubMed] [Google Scholar]