Abstract
The pulvinar is the largest thalamic nucleus in primates, and one of the most mysterious. Endeavors to understand its role in vision have focused on its abundant connections with the visual cortex. While its connectivity mapping in the cortex displays a broad topographic organization, its projections are also marked by considerable convergence and divergence. As a result, the pulvinar is often regarded as a central forebrain hub. Moreover, new evidence suggests that its comparatively modest input from structures such as the retina and superior colliculus may critically shape the functional organization of visual cortex, particularly during early development. Here we review recent studies that cast fresh light on how the many convergent pathways through the pulvinar contribute to visual cognition.
Keywords: thalamus, visual cortex, primate, human, superior colliculus, vision
The enigmatic pulvinar
The pulvinar is often considered a convergence point for sharing information widely in the cerebral cortex. The prominent interchange of connections between the cortex and pulvinar has been explored in detail using anatomical tracing methods in the nonhuman primate, with the intricate results summarized in a number of reviews [1–4] (see Figure 1). The notion that the pulvinar is a “connectional hub” has certain connotations, for example it integrates convergent information and then transmits processed signals. Whether such connotations are apt for understanding pulvinar function is not yet known. Another word that is frequently used to describe the role of the pulvinar is “relay”. The relay concept borrows from the known function of first-order thalamic nuclei to communicate peripheral sensory information into the cerebral cortex, but in the case of the pulvinar it often refers to the exchange of information between cortical areas (see Box 1). That the pulvinar acts as a cortical relay in some capacity is beyond doubt, particularly since both its primary driving input and primary output target is the cortex. However, researchers still struggle in answering the question, “What does the pulvinar do?” Despite a growing understanding of the anatomy, combined with a large number of physiological and behavioral observations, the pulvinar has yet to lose its customary descriptors “enigmatic”, “mysterious”, and, perhaps most damningly, “dangerous”, at least as far as scientific careers are concerned.
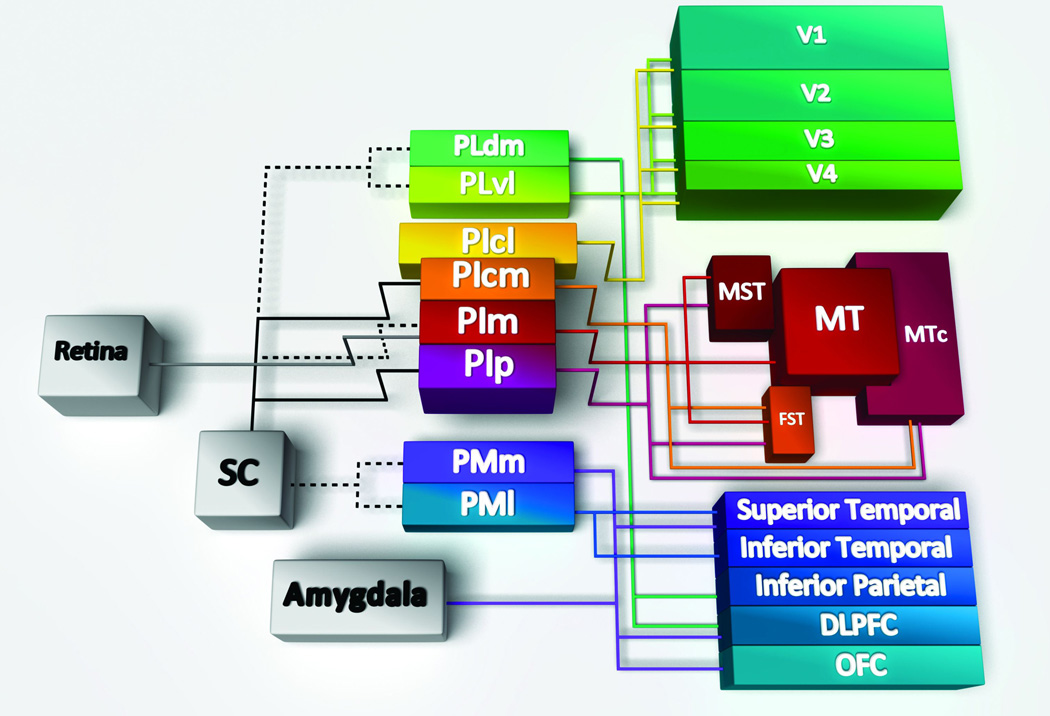
Figure 1. Connectivity of the pulvinar subregions.
The pulvinar has significant reciprocal connectivity with the cortex, here summarized in cartoon form with lines depicting bidirectional connections except the connections from the retina and superior colliculus (SC), which are unidirectional. Hatched lines indicate reported connections that are controversial or have not been verified. Specific subdivisions within the inferior pulvinar (PI) and lateral pulvinar (PL) send and receive projections from both dorsal and ventral streams of the visual cortex. The medial subdivision of the inferior pulvinar (PIm) is recipient of input from the retina, and a disputed input from the superior colliuculus (SC; hatched line). The PIm in turn relays to the middle temporal (MT) area, the medial superior temporal area (MST) and the fundus of the superior temporal area (FST); all components of the dorsal stream. The central medial (cm) and posterior (p) subdivisions of the PI also connect with dorsal stream areas MST, FST and the crescent of the middle temporal area (MTc). The central lateral subdivision of the PI (PIcl) and the ventrolateral (vl) subdivision of PL are heavily connected with the ventral stream associated areas V1, V2, V3 and V4. Other subdvisions have indirect connectivity with the visual cortex. The dorsal medial (dm) subdivision of PL projects to the inferior parietal cortex and the dosolateral prefrontal cortex (DLPFC). The medial pulvinar (PM), which possesses a lateral (l) and medial (m) subdivision projects to the temporal and parietal cortex, while the PMm also projects to the DLPFC, orbitofrontal cortex (OFC) and amygdala. The PM has been suggested to be recipient of input from the SC (hatched line), as has the PL
Box 1: Cortical domination of the pulvinar.
The repeated demonstration of tightly intermixed populations of neurons with heterogeneous physiological properties appears to be a characteristic of the pulvinar, and one that may reflect its relative paucity of local interconnectivity. Whether the computations of the pulvinar draw critically on the local convergence of diverse inputs, such as those stemming from the cortex, superior colliculus, and retina, or whether the pathways that run through it in parallel have minimal interaction, it is clear that the main input to the pulvinar in all of its basic subdivisions is from the cerebral cortex. As with other thalamic nuclei, the direct synaptic inputs and outputs of the pulvinar can be broadly separated into those whose position and strength confers a direct activation (“driving inputs”) and those whose contribution is weaker and less well understood (“modulatory inputs”) [78]. The cortical pulvinar afferent origins of these two types of inputs differ, with the former arising from pyramidal cells in layer 5 and the latter from those in layer 6. However, the driving inputs arising from cortical layer 5 appear similar to the ascending inputs from the retina and superior colliculus, including their large, proximal synapses and presynaptic expression of the VGLUT2 vesicular glutamate transporter [79]. PIm afferents can also be classified as driver or modulator based on their expression of the calcium-binding proteins parvalbumin and calbindin, respectively [4]
The anatomical subdivisions of the pulvinar distinguish themselves not only in the types of cortical input they receive but also in their ascending inputs. As such, it is interesting to speculate on an evolutionary scenario in which secondary thalamic nuclei, as with so many other cortical projection targets in the brainstem and spinal cord, followed a trajectory of increasing cortical innervation. In that sense, cortical control over the thalamus expanded in a manner analogous to cortical control over the motor system. In large primates, the direct control over primary motor neurons allows the cortex exquisite control over fine manual movements, conferring an unusually high level of dexterity [80, 81]. In the case of the pulvinar, the increased cortical input may confer sharing of visual information broadly over the cortex, tight visuomotor coordination, attentional control, or some combination of these elements. While anatomical projections, neural correlates, and lesion studies provide some hints, the essence of this functional organization remains an enduring puzzle.
In this review, we highlight new discoveries vis-à-vis the pulvinar that provide insight into aspects of its functional anatomy, and particularly how different types of signals are integrated within its different subdivisions. To this end, we focus upon recent anatomical, physiological, and behavioral results that, together with fresh theoretical concepts, sketch out a somewhat new perspective on aspects of pulvinar function. We begin in the first section by reviewing recent findings that point to a rather surprising role for the pulvinar in directly relaying retinal information to the cortex during an early developmental bottleneck. In the second section, we outline a more traditional view of pulvinar organization in the adult, reviewing new findings that speak to the integration of cortical and subcortical visual signals in its subdivisions. In the final section, we consider conclusions from human experiments that suggest that the visual pulvinar circuitry contributes to high-level aspects of human cognition and that its disruption can underlie a range of cognitive deficits.
An early visual pathway involving the pulvinar
What is the purpose of the pulvinar in visual cognition and why did it emerge? Questions such as this are often framed in terms of the functional capabilities of the adult. While this is a valid approach for addressing the complex relationship between structure and function, it can also be a teleological trap, as it neglects an aspect that is of highest importance in evolution: development. It may be through the lens of development that the pulvinar will ultimately make the most sense. We thus begin by reviewing the literature that has given rise to an emerging theory of pulvinar function, which holds that one of its most important roles in the primate is in early development. The early pulvinar relay of retinal information to the cortex is thought to be critical for at least two reasons: it supports visual behavior in the newborn, and it facilitates normal maturation of extrastriate cortex.
Vision in newborns
In humans, vision during the first weeks of life is characterized by poor acuity and shape perception, but reasonably good motion perception [5, 6]. Conventional opinion suggests a newborn’s interaction with the visual world initially draws upon innate circuits in the superior colliculus. Then after approximately two months, the major visual pathway through the lateral geniculate nucleus (LGN) to the primary visual cortex (V1) takes over and dominates adult vision [7–9]. This appears to be reflected in the ordered appearance of orientation and spatial frequency selectivity, followed by direction selectivity and finally stereoscopic depth perception [10]. On the other hand, the geniculostriate pathway of the newborn macaque is at least partially functional, with neurons in V1 and V2 exhibiting “adult-like” receptive fields at this stage [11]. Thus it is unclear to what extent distinct pathways are used to support newborn versus adult visual behavior.
Recent studies in the marmoset (a New World simian with a similar visual system assembly to other primates [12]) have offered a new perspective on the development of the visual system. Specifically, these studies have identified a transient pathway present in early life that projects directly from the retina to the pulvinar, without involvement of the superior colliculus [13]. While in adult primates this retinopulvinar pathway is present, it is much sparser [14–17], and intraocular injection of anterograde tracer throughout the lifespan have demonstrated a large number of labeled terminals in the medial part of the inferior pulvinar (PIm) in early life [16]. Moreover, microscopic analysis reveals that the ganglion cells entering PIm terminate on neurons that project to the middle temporal (MT) area (Figure 1) [17] a cortical area associated with the dorsal visual stream (see Glossary). As with the genioculostriate projections, the main pathway from V1 to MT is physically in place at this stage, but likely not yet mature [18]. Together, these findings have led to the opinion that the visual pathway in which PIm relays retinal signals to MT is used to support visually guided behavior.
All components of the retino-pulvinar-MT pathway are abundant at birth (Figure 2A, blue and red arrows, respectively) but normally regress in the first months of life (Figure 2B). However, under certain irregular conditions, such as when the predominant LGN-V1 pathway is damaged very early in life, the retino-pulvinar-MT pathway can persist and remain robust into adulthood (see Box 2). This has been demonstrated experimentally in marmosets, where the magnitude of the pathway was assessed in animals that had undergone a neonatal lesion of V1 [19]. Under such conditions, the components of the retino-pulvinar-MT pathway did not diminish after the first postnatal weeks, but instead remained largely intact for a significant period (Figure 2C). This was true of both the retinal innervation of PIm, as well its projections to MT. In animals receiving adult V1 lesions, PIm was no more prominent than in controls.
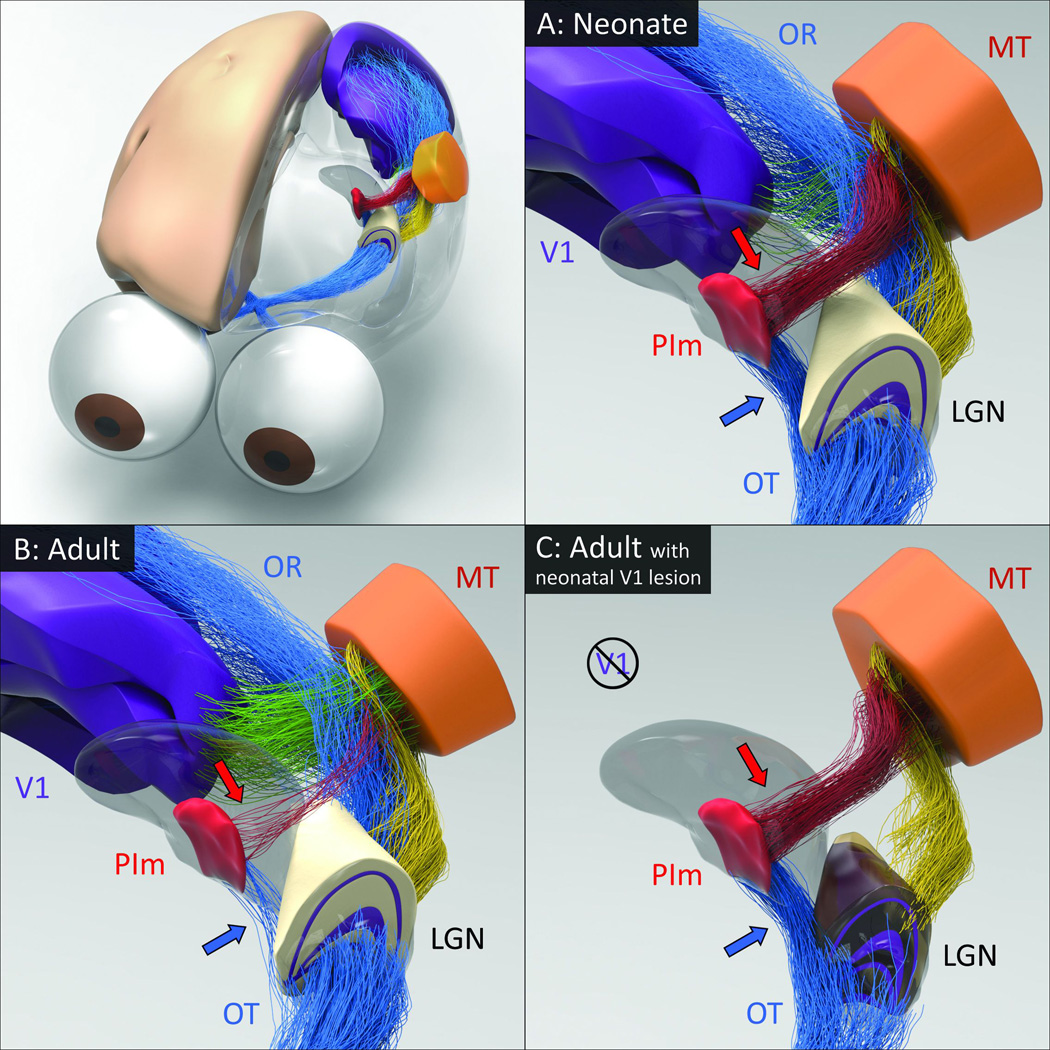
Figure 2. The developmental trajectory of the retino-pulvinar-MT pathway and the effects of early-life damage to V1.
A: In the marmoset neonate, a prominent direct pathway (blue arrow) carries retinal information through the optic tract (OT) to the medial division of the inferior pulvinar (PIm), in addition to the lateral geniculate nucleus (LGN). A thalamocortical pathway from PIm (red arrow) is thought to pass this image information to cortical area MT, thus completing the early visual pathway to the extrastriate cortex. B: During normal development, as the LGN pathway matures and begins to dominate visual input to the cortex through the optic radiations (OR), the early visual pathway through PIm regresses. C: When animals develop in the context of an early life V1 lesion, this regression fails to occur. The LGN undergoes significant degeneration and both the afferent and efferent components of the PIm visual pathway remain intact. It may be for this reason that early life V1 lesions lead to a significant retention of vision. However, following a lesion of V1 in adulthood (not shown), the degeneration of the LGN is not accompanied by a strenghtening of the PIm-MT pathway, which has already regressed. Thus subjects with adult V1 lesions experience blindness.
Box 2: Developmental modification of the human pulvinar.
In congenitally anophthalmic people, where the eyes fail to develop, the connections into and within the visual cortex are taken over by other sensory systems, particularly the auditory system. However, the question remaining is how auditory information reaches the visual cortex. A recent study [82] showed that human motion area MT+ contains a tonotopic map in these anophthalmic participants, suggesting that this activity is early in auditory hierarchy. A later study from the same group [83] indicated that there was no auditory activation in the lateral geniculate nucleus, suggesting that the input did not project from that thalamic nucleus. There was, however, significantly higher activation in the superior colliculus of anophthalmic (and early blind) participants to auditory stimulation compared to sighted controls. Given the pattern of auditory activation in the superior colliculus and the tonotopic organization of MT+, the inferior pulvinar may also play a role in this auditory processing, enabling the redirection of information to MT+. Such a pathway would be consistent with studies in early and congenitally blind individuals showing direction selectivity in MT+ [84–87]. While none of these studies showed activation of the pulvinar itself, it is the case that even visual stimulation in sighted individuals often does not significantly activate the pulvinar. Future work should target this structure with a variety of stimulus types, ideally at higher field strength to boost the signal in that region.
The persistence of this visual pathway following early-life V1 lesions may explain previous observations in both humans and monkeys, in which vision has been shown to remain intact despite the absence of V1. Normally, damage to V1 in the adult leads to the elimination of conscious vision, with only vague traces of visual behavior sometimes expressed in the form of “blindsight” [20]. However, the residual vision in humans and nonhuman primates with damage to V1 very early in life show a notably higher level of residual vision. For example, patients who had experienced perinatal infarctions to V1, when tested later, were much better in their visual performance than those who had acquired comparable damage during adolescence [21, 22]. Similarly, monkeys having received a lesion to area V1 during the second month of life showed much greater residual vision as adults than those having received adult lesions [23]. In light of the findings described above, the most obvious candidate for this unusual preservation in vision following an early life V1 lesion is the retino-pulvinar-MT pathway that, while normally transient, remains in place when the LGN pathway fails to evolve dominance (see Outstanding Questions Box).
Box 3: Outstanding questions.
Given the pivotal role of early visual input to the pulvinar, along with the capacity for preserved vision neonatal V1 damage, what would be the visual consequences of early pulvinar damage?
What specific role does the pulvinar play in the preservation of vision following injury restricted to the primary visual cortex (V1) especially in early life?
What is the role of the thalamic reticular nucleus (TRN) in regulating pulvinar function through inhibition of its projection neurons?
How do neuromodulatory systems impact the development of the pulvinar, as well as its principal attentional and sensorimotor functions in the adult?
Do the retinal efferents targeting the pulvinar collateralize with those of the superior colliculus or LGN? If so, how does this collateralization regress to reach its adult form?
Critical early input to the dorsal visual stream
In addition to its role in providing visual information to support the survival of the newborn, the early retino-pulvinar-MT pathway is thought to have an additional role in steering the organization and maturation of extrastriate cortex, and in particular the dorsal visual stream. In primates, connections between the pulvinar and extrastriate cortex are present at birth, as are those between the LGN and V1 [24, 25]. However, as indicated above, the existence of the projection alone does not indicate the level of maturation. Throughout the visual cortex, experience plays an important role in establishing, eliminating or refining synapses to produce a stereotypical mature pattern of connections.
Postnatal maturation of the visual cortex is sometimes conceived as a progression that propagates from visual area to visual area in a manner that roughly follows the cortical hierarchy. Although V1 is at the base of the hierarchy, area MT is seen to mature in monkeys at least as early as V1, as assessed by cell markers such as nonphosphorylated neurofilament. [18]. One possible explanation for this early maturation of MT is the initial visual input from the retino-pulvinar-MT pathway. This constant visual signal may initiate experience-dependent changes in area MT that set the dorsal stream on a path toward accelerated maturation compared to the ventral stream. Behaviorally, the early reliance on a dorsal stream pathway may also explain why motion perception develops before form perception in infant macaques [8, 9].
The switch in dominance from the retino-pulvinar-MT pathway to the LGN-V1 pathway is a major developmental milestone. After this time, MT receives most of its visual input from cortical visual areas and its pulvinar inputs decrease in number. Among cortical areas, V1 sends pronounced direct projections to MT. The increase in V1 input is concurrent with the decline of the PIm input, resulting in a change in the dominance of driving input to MT [13]. Based on evidence from rodent studies in other systems, this switch is likely accompanied by increased reliability in the synaptic drive of V1 projection neurons in layers 2/3 [26] along with the development of perisomatic inhibition of projection neurons to extrastriate cortex [27, 28], leading to a more refined visual topography. Thus, after the retino-pulvinar-MT pathway has served its role in early life vision and shaped the dorsal visual pathway, it is soon surpassed by the LGN-V1 pathway, whose detail vision and object specialization are critical for multiple aspects of primate visual cognition [12]. The LGN is the target of vast majority of retinal ganglion cells in the adult, and is thought to be the exclusive target of the 80% of ‘P’ type neurons that support high resolution vision [29, 30].
In the adult, the retinal contribution to the pulvinar is strongly diminished (Figure 2B), with the primary driving input to virtually all of its subdivisions coming from the cortex. Nonetheless, subcortical visual inputs continue to guide some pulvinar functions, with those arising from the superior colliculus playing perhaps the largest role. In the next section, we briefly review how these ascending inputs, along with the most dominant projections from the cortex, combine to shape activity in different portions of the pulvinar.
Integration of sensory and cognitive signals in the pulvinar
One approach to understanding the pulvinar’s anatomical scheme is to investigate, at a given location, the specific contributions of inputs originating in different structures (Box 2). In this section, we outline some of the connections to the main subdivisions of the pulvinar, placing focus on classic and recent experiments that shed light on the contribution of its subcortical visual inputs. In compiling such data, attention must be given to methods because anatomical localization methods have improved over time, and some of the earlier results still require replication or verification (see Outstanding Questions Box).
Inferior Pulvinar
All regions of the pulvinar receive converging information from the cerebral cortex. In addition, many, if not most, parts of the pulvinar receive some input from structures other than the cerebral cortex [15, 31]. The contribution of noncortical input is particularly conspicuous in the pulvinar’s inferior portion, including the retinorecipient PIm region discussed in detail above (Figure 1). The most prominent ascending visual pathway to the inferior pulvinar passes through the superior colliculus. From a comparative standpoint, this pathway likely evolved in parallel with that through the LGN and thus has putative homologs across a wide range of vertebrates [32]. This pathway is prominent in mammals and most studied in rodents [33]. Yet in adult primates even the inferior pulvinar is driven primarily by the visual cortex, as demonstrated in a seminal electrophysiological study [34]. Removal of V1 led to the near abolishment of activity in the pulvinar, with a few residual responses attributed to the retino-collo-pulvinar visual pathway [35], though there is some uncertainty as to the precise recording locations in this early work.
Is it possible then to identify a functional role for the well-established projections from the superficial layers of the superior colliculus to the inferior pulvinar? This pattern of projections has been demonstrated in a range of primates [36, 37] and other mammals such as the gray squirrel [38]. It is interesting to consider how different types of inputs might shape function at the neuronal and behavioral level. For example, pulvinar cells receiving input from the superior colliculus could have a fundamentally different function than those receiving input from the cortex. Assessing the origin of input to a single neuron is very difficult experimentally and normally out of reach for the electrophysiologist. However, in a recent study, neurons were classified based on their input by combining microelectrode recordings with antidromic and orthodromic stimulation. Using action potential timing following electrical pulses directed to the superior colliculus and MT, it was possible to estimate whether a given PIm neuron received projections from the superior colliculus, area MT, or both [39–41], and also whether it projected to area MT. With this information at hand, it was found that local populations of projection neurons were highly heterogeneous with regard to their sources of input and the types of signals they carried.
Of particular interest were so-called “relay neurons”, which received input from the superior colliculus and then projected to area MT. Such neurons had been hypothesized as a means for the superior colliculus to provide feedback to the cortex about the eye movements it had just executed. Such relay neurons were located in two subdivisions of the inferior pulvinar (medial, PIm; and posterior, PIp) and the properties of such neurons closely resembled those of the superior colliculus [40, 41], including brisk visual responses, diminished spiking at the time of a saccade, and little if any direction selectivity. Remarkably, neighboring neurons in the same population could be very different. Cells identified as likely receiving direct input from MT, for example, possessed very high direction selectivity, similar to the cellular characteristics observed in MT itself. These findings demonstrate a high level of convergence of cortical and superior colliculus input into a small subdivision of the inferior pulvinar. However, the resulting diversity in cell responses appears to reflect exclusive targeting of cells, whose response characteristics retain that of the input cells, with minimal lateral mixing of signals within the inferior pulvinar itself. These findings offer a glimpse into the complexity of pulvinar organization. In contrast to the columnar organization of the cerebral cortex, individual neurons within the same region of the same subnucleus appear to contribute to very different aspects of visual cognition.
It bears brief mention that the physiological demonstration of relay neurons is at odds with conclusions from anatomical tracing studies suggesting that no such relay exists through PIm. Specifically, anterograde tracer injected into the superior colliculus was shown to have minimal spatial overlap with the cell bodies of MT-projecting neurons in PIm [37], suggesting that PIm does not receive superior colliculus input, though a subsequent study using polysynaptic retrograde tracing in MT left open the possibility [42]. The existence of a collo-pulvinar-MT relay pathway is an important issue whose details are likely to resolve in time. It is possible that the existing data can be reconciled, for example, if neurons in PIm gain their synaptic input through dendritic terminations in neighboring pulvinar subregions.
Lateral Pulvinar
The lateral pulvinar, like the other classically defined pulvinar subregions, is heterogeneous with respect to its cortical innervation, and to some extent its immunochemical markers [43]. The ventrolateral subdivision of the lateral pulvinar (PLvl; Figure 1) has been shown to receive considerable input from early visual cortical regions (ventral stream associated), to which it also sends reciprocal projections [2, 31, 44]. While its innervation is dominated by the cortex, it has also been reported to receive input from the superior colliculus and pretectum [31], though these initial reports have not been verified with more modern methods. Moreover, the potential functional consequences of these inputs are unknown. However, three recent experimental approaches have suggested the projections of the lateral pulvinar may have a strong role in regulating activity within the cortex, with downstream consequences for visual processing and behavior.
One of these approaches [45] investigated the effects of blocking signals in the lateral pulvinar on the responses to stimuli in the visual cortex. One study inactivated the lateral pulvinar of the Galago, a small prosimian primate whose pulvinar organization and basic visual circuitry is similar to other primates. Surprisingly, inactivation of the lateral pulvinar led to a temporary loss of neuronal responsiveness within the upper layers of V1. Conversely, chemical excitation of this region increased neuron responsiveness in V1. The lateral pulvinar projections to V1 are directed predominantly to cortical layer 1 and are thought to be principally modulatory in their nature. Nonetheless, at face value the results suggest that the nature of this input is much more than modulatory, essentially controlling the LGN-derived visual signals leaving the primary visual cortex. Because this is such a strong departure from the view of the pulvinar as a hub or relay, these results need to be replicated and examined in more detail to understand their implications fully.
A second experimental approach used reversible inactivation of another portion of the lateral pulvinar, in this case with notable behavioral consequences. Early studies demonstrated that inactivation of a more dorsal portion of the lateral pulvinar (PLdm) in the macaque caused a systematic shift in visual attention away from visual space contralateral to the inactivation [46, 47]. More recent work has demonstrated that such inactivation leads to symptoms that appear very similar to perceptual neglect in humans but that may be, at their core, more closely related to the initiation of action or motivation than to perception per se. For example, following lateral inactivation that included PLdm, monkeys were reluctant to reach and grasp with their contralateral limb and displayed signs of dyspraxia [48]. At the same time, an increase in the motivational salience of a target stimulus was able to overcome what had appeared to be perceptual neglect [49]. The mechanistic basis of these deficits, which have features in common with the inactivation of both the parietal cortex [50] and the superior colliculus [51], is not well understood. As both of the parietal cortex and superior colliculus have been reported to provide input to the dorsal portion of the lateral pulvinar [31, 52] (though see caveats above regarding the superior colliculus input), the observed behavior may reflect a disruption of a circuit that draws signals carried in both corticothalamic and collothalamic projections. Since lateral pulvinar inactivation appears to affect perception, attention, intention, and coordination, it is likely that a deeper understanding of this area will involve the development and use of experimental paradigms to measure activity during active movement [53].
A third approach involves the simultaneous measurement of neural activity in the pulvinar and its corresponding areas in the extrastriate visual cortex. A recent study recorded neural activity from the lateral and inferior portions of the ventral pulvinar together with cortical areas V4 and TEO of the macaque [54]. During an attentional task, several measures of neural coordination suggested that the pulvinar exerts a common input synchronizing influence over interconnected cortical areas and may thus regulate information transmission related to visual cognition.
Medial Pulvinar
The medial pulvinar is greatly expanded in humans and may be an important contributor to cognition. In monkeys, tracer studies have demonstrated that the medial pulvinar receives input from the multiple cortical areas, including widespread prefrontal and temporal cortical regions [55] (Figure 1). Some zones within the medial pulvinar are suggested to receive input from intermediate and deep layers of the superior colliculus [31], a projection that parallels the superficial layer projection of the superior colliculus to the inferior pulvinar. The existence of this collicular projection to the medial pulvinar, together with the known projections from the medial pulvinar to the amygdala [56], have led to the speculation, or assertion, that the pulvinar is a subcortical relay for certain types of visual information to the amygdala [55, 56]. As such a connection conforms to the general mammalian pattern of collothalamic input to the amygdala [57], there is reason to believe that it may be a viable pathway in primates [58], though it remains to be demonstrated. As with the other pulvinar subdivisions, however, the bulk of input to the medial pulvinar originates in the cortex.
The response selectivity of medial pulvinar neurons is difficult to summarize, but more than in other pulvinar subdivisions responses tend to be selective for complex stimuli, including social and fear-inducing images [59, 60]. The latter responses are of particular interest for investigators postulating a specialized collo-pulvino-amygdala pathway [61, 62], as they are thought to provide a substrate for a subcortical pathway mediating unconscious emotional responses. Finally, the medial pulvinar has at least one other source of visual input: though almost certainly negligible at a functional level, direct retinal projections have been reported [15, 63].
Human pulvinar characteristics
Comparatively, humans possess the largest pulvinar, though it is of an expected size given the overall brain volume [64]. Its exploration is challenging for some (oft-repeated) reasons, but most importantly the low spatial resolution of functional and anatomical data that can be obtained in vivo. While some aspects of its functional organization can be inferred from the macaque, it is likely to differ in some ways that reflect, for example, human neocortical adaptations. Recent human studies have provided a framework for understanding the coarse structure of the human pulvinar and some of its connections using diffusion tractography [65], spontaneous activity correlation [66], and functional activation during behavioral tasks [67], or a combination of these approaches [68].
By combining detailed fMRI retinotopic mapping, diffusion tractography and resting state functional connectivity, a ventral/ dorsal difference in connectivity pattern of the pulvinar has recently been demonstrated [68]. Specifically, the ventral portions of the pulvinar connected strongly with V1 and extrastriate visual areas while the dorsal portions connected strongly with parietal and frontal regions. Within the dorsal and ventral regions of the pulvinar there are two distinct retinotopic maps, suggesting multiple functional domains within the human pulvinar, potentially corresponding to the primate subdivisions. In the absence of electrophysiological data, this work provides some basic insight as to the organization of the human pulvinar.
Influence on human cognition
As we begin to discover the circuitry of the human pulvinar, the importance of the pulvinar for human cognition is increasingly apparent. This is particularly true regarding its potential contribution to the many unique human adaptations related to tool use, language, and, more generally, hemispheric specialization, whose investigation is only just beginning. The expansion of the nucleus in humans may lead naturally, by virtue of its larger number of cells and connections, to functions that are qualitatively and quantitatively different than those in smaller primates. This increase in size relative to other thalamic structures [64] may result in the pulvinar contributing to a wider range of cognitive abilities than in other primates. In that sense, simple scaling rules may change the balance in a way that results in larger-brained primates having increased corticospinal control over their movements [69].
Most knowledge of the human pulvinar has been obtained from patients with pulvinar lesions and functional MRI studies. In both cases, the most widely studied aspect of cognition has been visual attention. In certain patients with pulvinar lesions, attentional deficits can be severe and resemble hemineglect caused by cortical lesions [70]. Some debate exists, however, about the degree to which attention modulates fMRI pulvinar responses. For example, it has been reported that attention was critical for visual responses [71], while another study found that attention had only a minor contribution [72]. These differences could be due to methodological differences, as the former study of used stimuli optimized for activating early visual cortex, whereas the latter used stimuli optimal for activating areas with larger receptive fields such as motion area hMT+. Multivariate analyses have demonstrated that attention is critical for decoding orientation and position information from pulvinar responses [73] One proposed role for the human pulvinar is the filtering of distracting stimuli, a critical component of focused attention [74]. As deficits in distractor filtering are associated with schizophrenia, it may not be a coincidence that subdivisions of the pulvinar are affected in this disease [75].
In addition to a role in attention, some evidence suggests that the pulvinar may selectively receive and transmit certain kinds of visual information to the amygdala. One such type of information is the emotional expression of faces. Face processing is a critical function for human understanding of emotion and empathy and is critical for interpreting signs of danger. In a patient with extensive bilateral damage to the occipital lobe, who lacks any conscious vision, fMRI showed that there was activation of the right amygdala in response to emotionally expressive faces, but not neutral ones [76]. Given the absence of the LGN-V1 pathway, one potential pathway to support this activation is that, described above, by which retinal information passes through the superior colliculus to the pulvinar. While there has not yet been a definitive study demonstrating that the retino-collo-pulvinar pathway can pass visual information to the amygdala, there is a fair amount of indirect evidence. For example, one patient with complete loss of the pulvinar unilaterally had a selective deficit in which he was unable to recognize fearful faces in the visual field contralateral to the lesion [77]. Whether the direct pulvinar pathway to the amygdala plays an important role in human cognition, either during development or adulthood, is a topic of great interest that has yet to be resolved.
Concluding remarks
Although the past decade has seen a considerable increase in the understanding of the connectivity and anatomical subdivisions of the primate pulvinar, the functional role of this structure in nonhuman primates, and more specifically humans, remains a mystery. The recent finding that the pulvinar may have a critical role in supporting an early visual pathway and guiding maturation of the cortex, has placed new emphasis on the developmental importance of this thalamic nucleus. Much has been learned about the convergence of cortical and subcortical signals within the diverse pulvinar subdivisions, though many questions remain, including the manner in which such signals are used to guide behavior. For humans, improvements in non-invasive brain imaging techniques are gradually improving the capacity to differentiate between pulvinar subdivisions, allowing the structural and functional properties to be assessed and compared with behavior under normal conditions and in disease states. Nonetheless, despite the convergence of experimental methods, each improving over time, the essence of the pulvinar function and its relationship to perception and goal-directed behavior remains elusive.
Box: Trends Box.
The pulvinar plays a critical role in the early development of the primate dorsal visual stream, relaying visual information directly from the retina to the cortex before the more prominent pathway through the lateral geniculate nucleus to V1 is fully mature.
Abnormal retention of this early visual pathway through the pulvinar may explain the preservation of vision when lesions to V1 occur during infancy.
In the adult, subcortical and cortical signals converge in multiple pulvinar subdivisions, though there is minimal evidence that the information from the two sources is actively integrated.
Most research on the human pulvinar has been directed toward attention and emotional processing, though it is hypothesized to have a broader role in human cognition.
Acknowledgments
The authors would like to thank Phil Owen for assistance with figures and Mary Baldwin, Aidan Murphy and Jude Mitchell for their insightful comments on earlier versions. J.A.B is supported by a National Health and Medical Research Senior Research Fellowship (APP1077677) and Project Grants (APP1042893;) H.B. is a Royal Society University Research Fellow. This work was supported, in part,by the Intramural Research Program of the National Institute of Mental Health (D.A.L) and a Royal Society International Exchange Grant to H.B. and J.A.B.
Glossary
- Blindsight
Is the ability of people who are recipient of a lesion of the primary visual cortex (V1) to respond to visual stimuli that they do not consciously see, for example, the ability to preshape hand for object when grasping in the blind field. The concept of blindsight challenges the principle that behavior requires conscious visual perception
- Dorsal Stream
or “where“ pathway; under the two-stream hypothesis is purported to be involved in the guidance of actions (e.g. reaching) and recognizing where objects are in space. This pathway emerges in the primary visual cortex (V1) and continues into parietal lobe. The middle temporal (MT) area is a major component of this stream. Lesions of the dorsal stream result in a number of disorders including: akinetopsia, the inability to perceive motion; hemineglect, is the inability to perceive stimuli on one side of the body or environment, and is usually contralateral to the side of the lesion; apraxia, the inability to produce discretionary or volitional movement
- Superior colliculus
The superior colliculus (SC), or optic tectum in nonmamallian vertebrates is a midbrain structure. This laminated structure, at least in mammals, comprises approximately 7 layers subdivided into 3 zones. The top three layers are incorporated into the superficial layers, the next two the intermediate layers and the final three the deep layers. Functionally, the SC is involved in guiding behavioral responses towards specific points of egocentric space. The superficial layers are sensory related (retinorecipient), while the deep layers are more motor-related and involved in activating eye movements, while the intermediate layers are believed to be multi-sensory. In the nonhuman primates studies of the SC have principally involved in the study of saccadic eye movements
- Ventral stream
or “what“ pathway; under the two-stream hypothesis is associated with object recognition and form representation. The stream emerges from the primary visual cortex (V1) and continues into the inferior temporal lobe. The second (V2) and fourth (V4) visual areas are major components. Extraretinal factors such as working memory and attention also participate in the processing of information in the ventral stream. Lesions of areas in the ventral stream result in conditions, including: achromatopsia, inability to perceive color; and, prosopagnosia, an inability to recognize faces
- Extrastriate cortex
Visual cortical areas in the occipital lobe beyond V1, including areas within both the dorsal and ventral visual streams. The definition also includes V2 and V3, which are still classified as ‘early’ visual areas that respond to many different aspects of vision
- Saccade
A rapid eye movement to change the direction of gaze in which both eyes move simultaneously. One of the mechanisms to allow us to visually explore our environment, saccades often reflect changes of attention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams MM, et al. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. The Journal of comparative neurology. 2000;419:377–393. doi: 10.1002/(sici)1096-9861(20000410)419:3<377::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain research reviews. 2007;55:285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shipp S. The functional logic of cortico-pulvinar connections. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones EG. The Thalamus. Cambridge University Press; 2007. [Google Scholar]
- 5.Atkinson J. Human visual development over the first 6 months of life. A review and a hypothesis. Human neurobiology. 1984;3:61–74. [PubMed] [Google Scholar]
- 6.Atkinson J, Braddick O. From genes to brain development to phenotypic behavior: "dorsal-stream vulnerability" in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Progress in brain research. 2011;189:261–283. doi: 10.1016/B978-0-444-53884-0.00029-4. [DOI] [PubMed] [Google Scholar]
- 7.Bronson G. The postnatal growth of visual capacity. Child development. 1974;45:873–890. [PubMed] [Google Scholar]
- 8.Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Visual neuroscience. 2004;21:851–859. doi: 10.1017/S0952523804216054. [DOI] [PubMed] [Google Scholar]
- 9.Kiorpes L, et al. Development of sensitivity to global form and motion in macaque monkeys (Macaca nemestrina) Vision research. 2012;63:34–42. doi: 10.1016/j.visres.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braddick O, Atkinson J. Development of human visual function. Vision research. 2011;51:1588–1609. doi: 10.1016/j.visres.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, et al. Receptive-field subfields of V2 neurons in macaque monkeys are adult-like near birth. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2639–2649. doi: 10.1523/JNEUROSCI.4377-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell JF, Leopold DA. The marmoset monkey as a model for visual neuroscience. Neuroscience research. 2015;93:20–46. doi: 10.1016/j.neures.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner CE, et al. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17073–17085. doi: 10.1523/JNEUROSCI.3269-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowey A, et al. Retinal ganglion cells labelled from the pulvinar nucleus in macaque monkeys. Neuroscience. 1994;61:691–705. doi: 10.1016/0306-4522(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa S, Tanaka S. Retinal projections to the pulvinar nucleus of the macaque monkey: a re-investigation using autoradiography. Experimental brain research. 1984;57:151–157. doi: 10.1007/BF00231141. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien BJ, et al. The retinal input to calbindin-D28k–defined subdivisions in macaque inferior pulvinar. Neuroscience letters. 2001;312:145–148. doi: 10.1016/s0304-3940(01)02220-0. [DOI] [PubMed] [Google Scholar]
- 17.Warner CE, et al. Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Frontiers in neuroanatomy. 2010;4:8. doi: 10.3389/neuro.05.008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourne JA, Rosa MG. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT) Cerebral cortex. 2006;16:405–414. doi: 10.1093/cercor/bhi119. [DOI] [PubMed] [Google Scholar]
- 19.Warner CE, et al. Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Current biology : CB. 2015;25:424–434. doi: 10.1016/j.cub.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Leopold DA. Primary visual cortex: awareness and blindsight. Annual review of neuroscience. 2012;35:91–109. doi: 10.1146/annurev-neuro-062111-150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tinelli F, et al. Blindsight in children with congenital and acquired cerebral lesions. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:1636–1647. doi: 10.1016/j.cortex.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Kiper DC, et al. Vision after early-onset lesions of the occipital cortex: I. Neuropsychological and psychophysical studies. Neural plasticity. 2002;9:1–25. doi: 10.1155/NP.2002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore T, et al. Greater residual vision in monkeys after striate cortex damage in infancy. Journal of neurophysiology. 1996;76:3928–3933. doi: 10.1152/jn.1996.76.6.3928. [DOI] [PubMed] [Google Scholar]
- 24.Rakic P. Prenatal development of the visual system in rhesus monkey. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- 25.Shatz CJ, Rakic P. The genesis of efferent connections from the visual cortex of the fetal rhesus monkey. The Journal of comparative neurology. 1981;196:287–307. doi: 10.1002/cne.901960208. [DOI] [PubMed] [Google Scholar]
- 26.Stern EA, et al. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 27.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 28.Hensch TK, et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry VH, Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984;12:1125–1137. doi: 10.1016/0306-4522(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 30.Perry VH, et al. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Benevento LA, Standage GP. The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. The Journal of comparative neurology. 1983;217:307–336. doi: 10.1002/cne.902170307. [DOI] [PubMed] [Google Scholar]
- 32.Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. 2005 [Google Scholar]
- 33.Tohmi M, et al. The extrageniculate visual pathway generates distinct response properties in the higher visual areas of mice. Current biology : CB. 2014;24:587–597. doi: 10.1016/j.cub.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 34.Bender DB. Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain research. 1983;279:258–261. doi: 10.1016/0006-8993(83)90188-9. [DOI] [PubMed] [Google Scholar]
- 35.Bender DB. Electrophysiological and behavioral experiments on the primate pulvinar. Progress in brain research. 1988;75:55–65. doi: 10.1016/s0079-6123(08)60465-3. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin MK, Kaas JH. Cortical projections to the superior colliculus in prosimian galagos (Otolemur garnetti) The Journal of comparative neurology. 2012;520:2002–2020. doi: 10.1002/cne.23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stepniewska I, et al. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Visual neuroscience. 2000;17:529–549. doi: 10.1017/s0952523800174048. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin MK, et al. Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): evidence for four subdivisions within the pulvinar complex. The Journal of comparative neurology. 2011;519:1071–1094. doi: 10.1002/cne.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berman RA, Wurtz RH. Exploring the pulvinar path to visual cortex. Progress in brain research. 2008;171:467–473. doi: 10.1016/S0079-6123(08)00668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman RA, Wurtz RH. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:373–384. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyon DC, et al. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65:270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez C, et al. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. The Journal of comparative neurology. 2000;419:61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Rezak M, Benevento LA. A comparison of the organization of the projections of the dorsal lateral geniculate nucleus, the inferior pulvinar and adjacent lateral pulvinar to primary visual cortex (area 17) in the macaque monkey. Brain research. 1979;167:19–40. doi: 10.1016/0006-8993(79)90260-9. [DOI] [PubMed] [Google Scholar]
- 45.Purushothaman G, et al. Gating and control of primary visual cortex by pulvinar. Nature neuroscience. 2012;15:905–912. doi: 10.1038/nn.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen SE, et al. Contributions of the pulvinar to visual spatial attention. Neuropsychologia. 1987;25:97–105. doi: 10.1016/0028-3932(87)90046-7. [DOI] [PubMed] [Google Scholar]
- 47.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends in neurosciences. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- 48.Wilke M, et al. Pulvinar inactivation disrupts selection of movement plans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilke M, et al. Effects of pulvinar inactivation on spatial decision-making between equal and asymmetric reward options. Journal of cognitive neuroscience. 2013;25:1270–1283. doi: 10.1162/jocn_a_00399. [DOI] [PubMed] [Google Scholar]
- 50.Hwang EJ, et al. Inactivation of the parietal reach region causes optic ataxia, impairing reaches but not saccades. Neuron. 2012;76:1021–1029. doi: 10.1016/j.neuron.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. Journal of neurophysiology. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- 52.Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Visual neuroscience. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- 53.Grieve KL, et al. The primate pulvinar nuclei: vision and action. Trends in neurosciences. 2000;23:35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 54.Saalmann YB, et al. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romanski LM, et al. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. The Journal of comparative neurology. 1997;379:313–332. [PubMed] [Google Scholar]
- 56.Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain research. 1976;104:142–147. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- 57.Day-Brown JD, et al. Pulvinar projections to the striatum and amygdala in the tree shrew. Frontiers in neuroanatomy. 2010;4:143. doi: 10.3389/fnana.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nature reviews. Neuroscience. 2010;11:697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- 59.Maior RS, et al. The monkey pulvinar neurons differentially respond to emotional expressions of human faces. Behavioural brain research. 2010;215:129–135. doi: 10.1016/j.bbr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Van Le Q, et al. Pulvinar neurons reveal neurobiological evidence of past selection for rapid detection of snakes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19000–19005. doi: 10.1073/pnas.1312648110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Gelder B, et al. Emotion in the brain: of low roads, high roads and roads less travelled. Nature reviews. Neuroscience. 2011;12:425. doi: 10.1038/nrn2920-c1. author reply 425. [DOI] [PubMed] [Google Scholar]
- 62.Liddell BJ, et al. A direct brainstem-amygdala-cortical 'alarm' system for subliminal signals of fear. NeuroImage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Itaya SK, Van Hoesen GW. Retinal projections to the inferior and medial pulvinar nuclei in the Old-World monkey. Brain research. 1983;269:223–230. doi: 10.1016/0006-8993(83)90131-2. [DOI] [PubMed] [Google Scholar]
- 64.Chalfin BP, et al. Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates, and mammals. The Journal of comparative neurology. 2007;504:265–274. doi: 10.1002/cne.21406. [DOI] [PubMed] [Google Scholar]
- 65.Leh SE, et al. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. International journal of biomedical imaging. 2008;2008:789539. doi: 10.1155/2008/789539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raichle ME, et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barron DS, et al. Human pulvinar functional organization and connectivity. Human brain mapping. 2015;36:2417–2431. doi: 10.1002/hbm.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arcaro MJ, et al. The Anatomical and Functional Organization of the Human Visual Pulvinar. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:9848–9871. doi: 10.1523/JNEUROSCI.1575-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herculano-Houzel S, et al. Corticalization of motor control in humans is a consequence of brain scaling in primate evolution. The Journal of comparative neurology. 2015 doi: 10.1002/cne.23792. [DOI] [PubMed] [Google Scholar]
- 70.Karnath HO, et al. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain : a journal of neurology. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- 71.Kastner S, et al. Functional imaging of the human lateral geniculate nucleus and pulvinar. Journal of neurophysiology. 2004;91:438–448. doi: 10.1152/jn.00553.2003. [DOI] [PubMed] [Google Scholar]
- 72.Smith AT, et al. Dissociating vision and visual attention in the human pulvinar. Journal of neurophysiology. 2009;101:917–925. doi: 10.1152/jn.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischer J, Whitney D. Attention gates visual coding in the human pulvinar. Nature communications. 2012;3:1051. doi: 10.1038/ncomms2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strumpf H, et al. The role of the pulvinar in distractor processing and visual search. Human brain mapping. 2013;34:1115–1132. doi: 10.1002/hbm.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kemether EM, et al. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Archives of general psychiatry. 2003;60:983–991. doi: 10.1001/archpsyc.60.9.983. [DOI] [PubMed] [Google Scholar]
- 76.Pegna AJ, et al. Discriminating emotional faces without primary visual cortices involves the right amygdala. Nature neuroscience. 2005;8:24–25. doi: 10.1038/nn1364. [DOI] [PubMed] [Google Scholar]
- 77.Ward R, et al. Emotion recognition following human pulvinar damage. Neuropsychologia. 2007;45:1973–1978. doi: 10.1016/j.neuropsychologia.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 78.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. Journal of neurophysiology. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 79.Balaram P, et al. VGLUT2 mRNA and protein expression in the visual thalamus and midbrain of prosimian galagos (Otolemur garnetti) Eye and brain. 2011;2011:5–15. doi: 10.2147/EB.S16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain, behavior and evolution. 1983;23:165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- 81.Heffner R, Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain, behavior and evolution. 1975;12:161–200. doi: 10.1159/000124401. [DOI] [PubMed] [Google Scholar]
- 82.Watkins KE, et al. Early auditory processing in area V5/MT+ of the congenitally blind brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18242–18246. doi: 10.1523/JNEUROSCI.2546-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coullon GS, et al. Subcortical functional reorganization due to early blindness. Journal of neurophysiology. 2015 doi: 10.1152/jn.01031.2014. jn 01031 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poirier C, et al. Auditory motion perception activates visual motion areas in early blind subjects. NeuroImage. 2006;31:279–285. doi: 10.1016/j.neuroimage.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 85.Poirier C, et al. Specific activation of the V5 brain area by auditory motion processing: an fMRI study. Brain research. Cognitive brain research. 2005;25:650–658. doi: 10.1016/j.cogbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 86.Strnad L, et al. Multivoxel pattern analysis reveals auditory motion information in MT+ of both congenitally blind and sighted individuals. PloS one. 2013;8:e63198. doi: 10.1371/journal.pone.0063198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang F, et al. Auditory motion processing after early blindness. Journal of vision. 2014;14:4. doi: 10.1167/14.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]