Abstract
Past studies have demonstrated that inducing several seizures or continuous seizures in neonatal or adult rats results in impairments in learning and memory. The impact of a single acute seizure on learning and memory has not been investigated in mice. In this study, we exposed an adult 129SvEvTac mouse to the inhalant flurothyl until a behavioral seizure was induced. Our study consisted of 4 experiments where we examined the effect of one seizure before or after delay fear conditioning. We also included a separate cohort of animals that was tested in the open field after a seizure to rule out changes in locomotor activity influencing the results of memory tests. Mice that had experienced a single seizure 1 hour, but not 6 hours, prior to training showed a significant impairment in associative conditioning to the conditioned stimulus when compared to controls 24 hours later. There were no differences in freezing one day later for animals that experienced a single seizure 1 hour after associative learning. We also found that an acute seizure reduced activity levels in an open field test 2 hours but not 24 hours later. These findings suggest that an acute seizure occurring immediately before learning can have an effect on the recall of events occurring shortly after that seizure. In contrast, an acute seizure occurring shortly after learning appears to have little or no effect on long-term memory. These findings have implications for understanding the acute effects of seizures on the acquisition of new knowledge.
Keywords: Acute Seizure, Epilepsy, Learning, Memory, Fear Conditioning, Associative
1. Introduction
According to a recent paper using a meta-analytic approach more than 65 million people worldwide suffer from epileptic disorders [1]. This is a much higher estimate than the 50 million reported by the World Health Organization [2]. In the United States alone epilepsy affects 2.3 million adults [3]. The rate of epilepsy in developed countries is approximately 50 per 100,000 individuals per year with the highest rates in the neonatal periods and in the elderly [4, 5]. The conceptual definition of epilepsy was established in 2005, describing epilepsy as a disorder of the brain where there are at least two unprovoked seizures that occur more than 24 h apart [6]. However, there was immediate resistance to this definition since there are several instances where one seizure may be sufficient to classify the patient with epilepsy, and there are other conditions where more than 2 seizures may be required to properly diagnosis the patient with epilepsy [7]. One study provided different hypothetical situations where it would be reasonable to provide a diagnosis of epilepsy after the first seizure [8]. In 2014, a report from the International League Against Epilepsy altered the practical definition of epilepsy [9]. In their revised definition epilepsy may be considered present after one unprovoked seizure in individuals with other associated factors. This revision has taken into account that a single seizure can have long-lasting and significant effects. Indeed, a single unprovoked seizure increases the risk for another seizure by 40–52% [10].
In addition to increased susceptibility to subsequent seizures, damage and reactive plasticity can also have effects on a number of other processes, which lead to comorbidities. Individuals suffering from epileptic seizure disorders are more likely to also suffer from psychiatric disorders such as anxiety, depression, bipolar, and attention deficit hyperactivity disorder (ADHD) [11]; and neurological conditions including sleep and movement disorders [12]. Pain disorders and asthma also show high comorbidity with epilepsy [12]. Another common comorbidity of epilepsy is cognitive impairment, such as memory deficiency. This can even persist once seizures are under control with antiepileptic medication [13]. Antiepileptic medication can directly impact and/or contribute to further memory impairments [14, 15]. Impairments are particularly more prevalent when patients are treated with more than one antiepileptic medication compared to those with monotherapy [16–19]. Persistent cognitive impairment could have significant effects on the daily functioning of affected individuals who may be unable to reliably form new memories or clearly recall old memories. Learning and memory deficits could therefore represent a substantial effect that results from recurrent seizures.
Research utilizing clinical data and animal models has shown that prolonged or recurring seizures can impair different types of learning and memory including spatial, episodic, and emotional [20–23]. While notably, there has been much research using animal models of epilepsy to investigate the chronic effects of regular seizures on cognition [24–28], little research has investigated the effects a single or acute seizure on this same subject. Therefore, while we may have some idea of the degree of long-term cognitive impairments that result from an epileptic condition, it is still important to separate out the effects each seizure may have on the processes of learning and memory. Multiple seizures result in extensive cell loss and other damage that can affect learning and memory, and the application of antiepileptic drugs (AEDs) to combat seizures has been implicated in contributing to such cognitive impairment [29, 30]. Our studies will control for the possible impairments on learning and memory in isolation from the effects of AEDs and damage from multiple seizures.
In the current study we investigated the effect of a single acute flurothyl-induced seizure in mice at various time points before or after associative fear conditioning. We wanted to first determine whether impairments of contextual fear memory previously reported in rats could be extended to associative fear memory in mice [31]. We also wanted to expand on any impairments found. To this end, we looked at a 6-hour pre-training seizure to examine whether a single seizure can affect learning and memory at a more distant time point.
2. Materials and methods
2.1. Animals
We used adult (~60–100 postnatal days old) male and female 129SvEvTac mice that were generated and housed at Baylor University. The home colony room was maintained at an ambient temperature of 22 °C, with a 14-h light and 10-h dark (20:00 to 6:00h) diurnal cycle. The mice were group housed in standard cages and were allowed ad libitum access to food and water. The animal protocol was approved by Baylor University Animal Care and Use Committee and all procedures performed were in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Flurothyl Induction
All seizures were induced under a fume hood inside a clear acrylic (29 cm × 16 cm × 15 cm) inhalation chamber. Flurothyl (bis-2,2,2-trifluroethyl ether), obtained from Sigma-Aldrich (St Louis, MO, USA), was pumped into the chamber using a Harvard Apparatus model 11 Plus syringe pump at a rate of 50 μL per minute.
2.3. Fear Conditioning
Fear conditioning and testing were performed using two Coulbourn Habitest® fear conditioning chambers (26 cm × 22 cm × 18 cm) placed inside of sound dampening, isolation cubicles. The chambers were configured with two acrylic and two metal sides. The chambers were equipped with metal grid floors which delivered mild footshocks. These footshocks were delivered by Coulbourn Precision Animal Shockers which were manually calibrated using an ENV 420 Amp-meter (Med Associates inc.) prior to testing on each day shocks were to be delivered. A white noise tone was delivered by an external PYLE® PRO PCA2 stereo amplifier that played though speakers mounted on the rear of the isolation cubicles. Shocks were delivered and freezing behavior was recorded and measured for each testing day using FreezeFrame 3 software (Coulbourn, Ohio, USA).
We used a delay fear conditioning protocol in which a mouse was placed into the chamber and exposed to the conditioning stimuli as previously described [32]. There was a 2-minute baseline period, which was followed by the presentation of the conditioned stimulus (CS) for 20 seconds (80-dB white noise tone). The white noise was immediately followed by a mild footshock (2 second 0.7-mA) that served as the unconditioned stimulus (US). This was followed by an inter-trial interval of 1 minute, followed by another tone and shock pairing. There was a 20-second interval period following the second pairing. Mice received a total of 2 CS-US pairings. The FreezeFrame monitor system was used to control the timing of the CS and US presentation. After each testing session the chambers were cleaned with 30% isopropyl alcohol.
Mice were tested for cued fear conditioning after a seizure. We used the same protocol to measure both short- and long-term memory. The fear learning chambers were altered prior to testing in order to present a novel context. The texture, color, and shape of the chambers were altered using acrylic inserts and a novel odor (vanilla extract; Adam’s Extracts, USA) was placed under the floor. We monitored freezing behavior for two 3-minute periods inside the chamber. The first period consisted of the new context for 3 minutes. During the second period, we presented the auditory conditioned stimulus (CS) for 3 minutes and measured freezing.
2.4. Open Field
Two Fusion Nodes equipped with multiple Fusion Sensors measured activity level in the open field. The testing arena was a clear acrylic (40 cm × 40 cm × 40 cm) box. The automated measurements by the sensors were recorded using the Fusion software (Omnitech electronics, OH, USA) for each 10 minute trial.
2.5. Experiment 1: One Hour Pre-training Seizure
In our first experiment we wanted to examine the effect of a single acute flurothyl-induced seizure one hour prior to fear conditioning on subsequent fear learning and memory (See Fig. 1A). The mice were transported to a holding room where they were allowed to acclimate for 30 minutes prior to seizure induction. A single mouse was placed into a clean transfer cage and transported from a holding room to the main lab. The animal was then placed into an inhalation chamber under a chemical vent hood where it was exposed to flurothyl until a behavioral seizure (wild running with tonic-clonic seizure) was observed. A control animal was also placed into a second inhalation chamber for the same amount of time in parallel to the experimental animal. After a behavioral seizure was induced, both experimental and control animals were removed from the inhalation chambers and returned to the transfer cages to recover. The mice were transported back to the holding room after a brief recovery period to await the next phase of the experiment.
Fig. 1. Timelines for 1 h pre-, 6 h pre-training seizure experiments, open field experiment, and 1 h post-training seizure experiment.
(A) 1 h pre-training group, (B) 6 h pre-training group, (C) 2 h post-seizure group, (D) 1 h post-training group.
One hour after a seizure was induced mice were transported to a testing room where they underwent delay fear conditioning. The mouse was placed into the chamber for fear conditioning using the protocol that was described above. After the protocol ended, the animal was removed from the chamber and returned to the holding room.
For our studies we define short-term memory as a memory that has been encoded but not consolidated into permanent long-term memory. In our associative learning tests we measured short-term memory 1 hour following fear conditioning. We also tested the animal in the same conditions 24 hours after the seizure was induced to examine LTM. The protocol and measurement parameters were identical to those used in the STM test. After the completion of testing for all animals, the mice were returned to their home cages and transported to their home colony.
2.6. Experiment 2: Six Hour Pre-training Seizure
In our second experiment we examined whether animals that experienced an acute seizure 6 hour prior to delay fear conditioning would show a learning and memory deficit compared to control animals. A single pre-training acute flurothyl seizure was induced inside an inhalation chamber as described in experiment 1. Control animals were again placed into a second inhalation chamber concurrent with animals assigned to the experimental condition (see Fig. 1B). Animals were trained using delay fear conditioning 6 hours after they received a seizure. Then, in order to examine LTM, we tested the mice 24 hours post-seizure. We tested the mice in the same new context and conditions as already described.
2.7. Experiment 3: Open Field
A separate set of mice were examined in a 10-minute open field test to evaluate whether the results of the STM test in experiment 1 might have been influenced by a general decrease in locomotor activity level following a flurothyl seizure or 24 hours after a seizure (see Fig. 1C). A single acute seizure was induced using flurothyl and control mice were used as previously described. Two hours after experiencing a seizure, a mouse was placed into the open field for a 10-minute period. We recorded several measures of activity. The mouse was removed and returned to its home cage after the 10 minute test. The open field arena was cleaned with 30% isopropanol and prepared for the next mouse. All mice were returned to their home colony after testing was completed. The mice were tested again in the open field at a 24 hour post-seizure time point to assess their activity levels in another 10-minute trial.
2.8. Experiment 4: One Hour Post-training Seizure
The previous experiments were conducted to investigate whether an acute seizure impairs the acquisition of a new memory. For our final experiment, we wanted to determine whether an acute seizure impairs the retention of a newly acquired memory (See Fig. 1D).
The mice were first trained using delay fear conditioning. One hour after the training phase of delay fear conditioning, we induced a flurothyl seizure in the mice. One hour following the seizure we tested the STM of mice who had experienced a post-training seizure. On the next day (24 hours following the seizure) the mice were transported to the testing room where they were again placed into the chamber and LTM was examined.
2.9. Data Analysis
All data were analyzed using Prism 6 (GraphPad Software, Inc., La Jolla, CA). For all comparisons, the level of significance was set at p < 0.05. Males and females were combined per group since no statistically significant differences were found between them. Animals were monitored throughout the experiments for changes in weight and no significant differences were found.
3. Results
3.1. Flurothyl-induced Brief Seizures
In the mice that experienced flurothyl-induced acute seizures, the latency to the first seizure across all experiments was 144.1 ± 4.3 seconds (mean ± standard error of the mean). The duration of the behavioral seizure was approximately 60–70 seconds or less from the time the animals were removed from the inhalation chamber. The animals were allowed to rest for at least one hour after seizure induction. None of the mice had spontaneous seizures during any of the experiments.
3.2. One Hour Pre-training Seizure
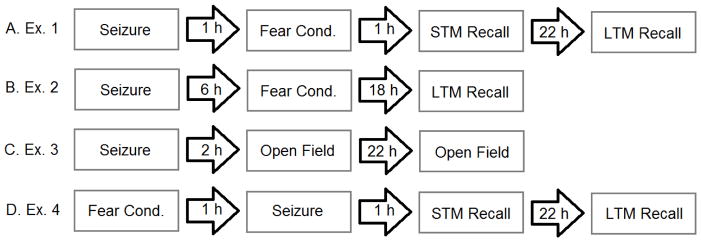
The results of memory tests revealed that long-term memory for a conditioned stimulus was impaired a day later following a 1-hour pre-training seizure. Mice that were given a seizure and then examined in delay fear conditioning showed an increase in freezing during all aspects of the training phase. There was a main effect of group during the training phase of delay fear conditioning [F(1, 13)= 46.19, p < 0.0001] (Fig. 2A). The STM test was performed one hour after delay fear conditioning. The seizure group had a significant increase in freezing in the new context [t(1, 12) = 2.82, p < 0.05] but did not show a significant difference compared to control mice when the CS was presented [t(1, 12) = 1.79, p = 0.09] (Fig. 2B: left two graphs). Twenty four hours after we induced seizures we tested the mice for alterations in LTM. We found that mice that had experienced a single seizure 1 hour prior to training showed significant impairment of associative conditioning 24 hours later compared to controls when presented with the conditioned stimulus [t(1, 12) = 3.48, p < 0.05]. However, the seizure mice were no different in their freezing behavior in the new context compared to controls [t(1, 12) = 1.85, p = 0.08] (Fig. 2B: right two graphs).
Fig. 2. Freezing during fear conditioning training and during associative memory tests with a single seizure 1 hour prior to training.
(A) Freezing during fear conditioning in the 1 h pre-training group as compared to controls that did not experience seizures. (B) Freezing levels of mice at the 1 h time point after seizures [Short Term Memory (STM)] and 24 hrs [Long Term Memory (LTM)] after a seizure. The bars are the mean value and the error bars denote SEM. * = p < .05
3.3. Six Hour Pre-training Seizure
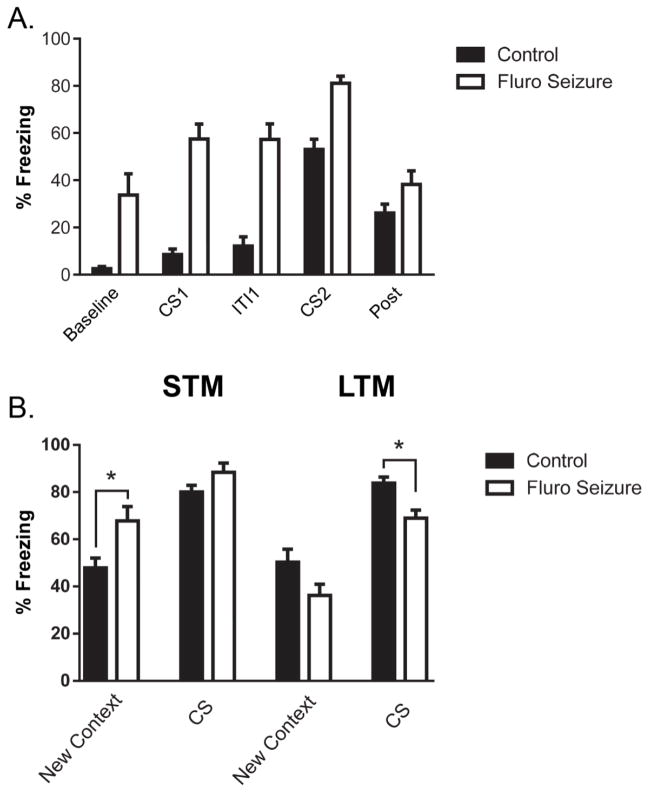
A 6-hour pre-training seizure did not affect associative memory one day later. Mice that had experienced a seizure 6 hours prior to training did not show any significant difference in freezing behavior compared to controls during delay fear conditioning training [F(1, 9) = 3.1, p = 0.11] (Fig. 3A). There was also no difference 24 hours after a seizure when presented with the conditioned stimulus [t(1, 9) = 0.81, p = 0.43] or in the new context [t(1, 9) = 0.76, p = 0.46] (Fig. 3B). However, both groups did demonstrate an increase in freezing when presented with the CS compared to the new context condition. A paired t-test revealed an increase in freezing for the control mice across the new context compared to CS condition [t(1, 5) = 3.9, p < 0.05]. Similar results were found in the seizure group [t(1, 4) = 4.3, p < 0.05].
Fig. 3. Freezing during fear conditioning training and during associative memory tests with a single seizure 6 hours prior to training.
(A) Freezing during fear conditioning 6 hrs after a single seizure. (B) Freezing levels of mice 24 hrs after a single seizure in the new context and conditioned stimulus (CS) condition. The bars are the mean value and the error bars denote SEM.
3.4. Open Field
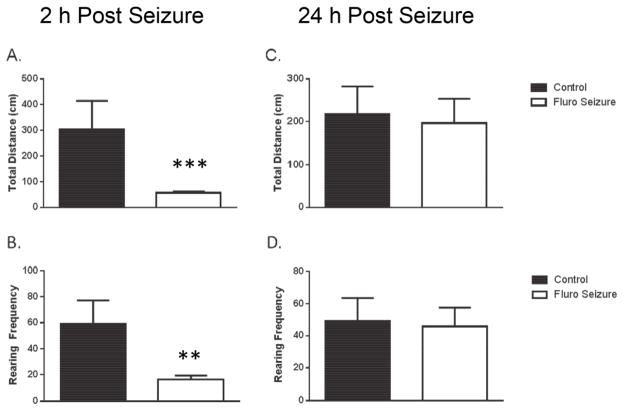
For experiment three we wanted to determine if a single seizure affected the activity levels of our mice during the short- and long-term memory tests. We found that an acute flurothyl seizure resulted in a significant decrease in activity 2 hours after the seizure was induced but the effect was not long-lasting. For the 2 hour analysis we used the Mann-Whitney U test because the variance of the data violated the assumption of homogeneity. The F test for the analysis for locomotor activity was [F(7, 6) = 423, p < 0.001] and for horizontal movement frequency was [F(7,6) = 42.1. We found a significant reduction in locomotor activity at the 2-hour time point [U = 1, p < 0.001] (Fig. 4A) and a significant reduction in horizontal movement frequency [U = 4.5, p < 0.01] (Fig. 4B). The effect on activity was temporary. When tested at 24 hours after seizure induction there was no difference in total activity or in movement frequency in mice that experienced a single flurothyl seizure compared to control mice in the open field test 24 hours after a seizure [t(1, 13) = 0.24, p = 0.81] (Fig. 4C); [t(1, 13) = 0.18, p = 0.86] (Fig. 4D).
Fig. 4. A flurothyl seizure transiently reduces activity levels.
In the open field test we report (A) locomotor activity at the 2 h time point and (B) movement frequency. (C) Total activity and (D) movement frequency in control and seizure mice. The bars are the mean value and the error bars denote SEM. ** = p < .01; *** = p < .001
3.5. One Hour Post-training Seizure
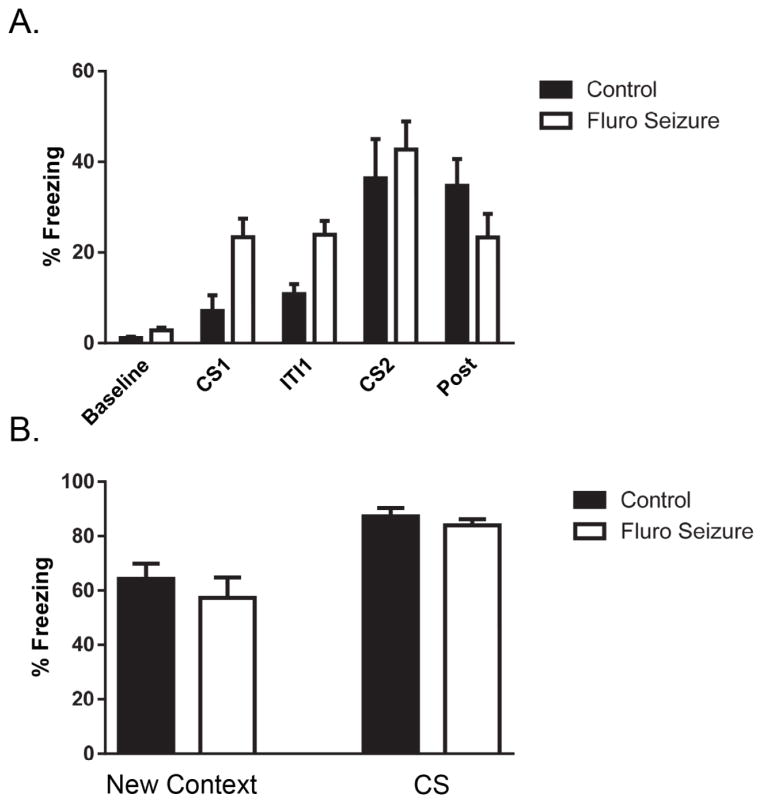
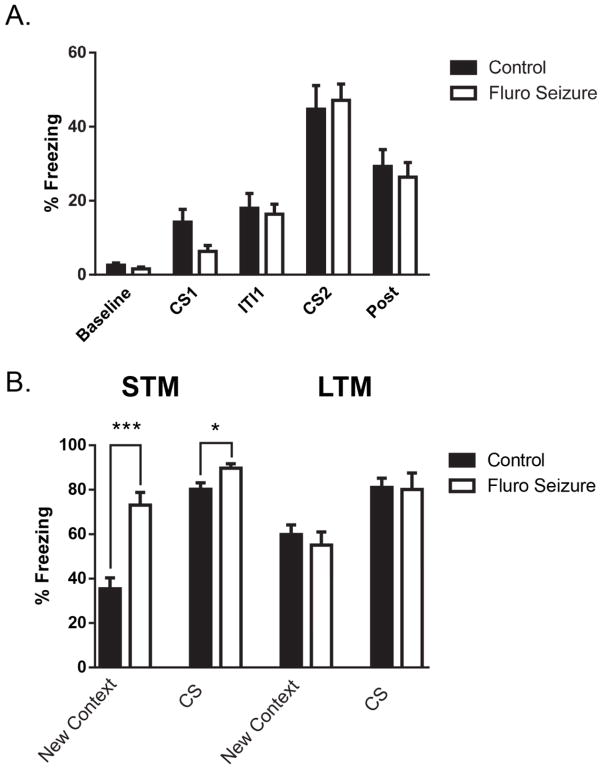
We found that a single post-training seizure did not affect associative memory when recalled a day later. There was no difference in freezing during training for mice that had experienced a seizure 1 hour after training [F(1, 30) = 0.39, p = 0.53] (Fig. 5A). We found that the seizure group had an increase in freezing in the New Context testing phase during the STM test [t(1,19) = 4.9, p < 0.0001] (Fig. 5B left graphs). A similar significant increase in freezing was found in the 3-minute period when the CS was presented [t(1,19) = 2.34, p < 0.05] 1 hour after a seizure (Figure 5B left graphs). The effect was not long-lasting. At the 24-hour time point after the seizure was induced there was no difference between the groups in the new context [t(1,19) = 0.66, p = 0.51] or in the CS condition [t(1,19) = 0.12, p = 0.90] (Fig. 5B right graphs).
Fig. 5. Freezing during fear conditioning training and during associative memory tests with a single seizure 1 hr after training.
(A) There was no difference in freezing during training prior to a 1 h post-training seizure. (B) Mice that experienced a single seizure 1 h after training had an increase in freezing in the New Context and during the CS presentation 1 h after the seizure. At 22 h after a seizure mice showed no difference in freezing in the new context or when presented with the CS. The bars are the mean value and the error bars denote SEM. * = p < .05 ; *** = p < .001
4. Discussion
In 2010, Dr. McAuley and colleagues conducted a study with the Comprehensive Epilepsy Program at Ohio State to examine what the highest concerns were for individuals with epilepsy. The second most important concern for those with epilepsy was their memory and it ranked as their third most frequent concern [33]. This is in contrast to the reports from practitioners where they ranked memory concerns as number 12. This contrast demonstrates the discrepancy in the concerns of patients with epilepsy compared to practitioners. There is accumulating evidence that cognitive impairments are an important consideration for those afflicted with epilepsy. In our study we induced seizures 1 hour prior to associative fear conditioning and observed associative learning deficits when we examined their memory 24 hours later. We did not observe deficits in associative memory when the seizure was induced after associative conditioning. The results from our studies provide evidence that the ability to form memories may be more sensitive to seizure disruption compared to memories acquired before seizure induction.
Our results support a similar study that examined the influence of acute seizures on spatial and associative learning [31]. They induced seizures by an intraperitoneal injection of PTZ in adult rats. In one set of experiments they induced a seizure 30 minutes prior to trials in the Morris Water Maze. In another experiment they induced a PTZ seizure 30 minutes prior to contextual fear conditioning. In both tests they found deficits in the acquisition of learning. They also examined the effect of a seizure induced immediately after the animals were tested in the MWM and fear conditioning test and found no significant memory impairment 24 hours later.
One difference between our study and the Mao et al. (2009) study is that they found that a seizure induced before fear conditioning results in a learning deficit when the animal is tested 1 hour after fear conditioning training. We did not observe this difference in our study. One reason for this difference may be that the PTZ seizure induction method produces more severe seizures. They report that the PTZ seizure duration is 60–70 seconds. The flurothyl seizure induction method we used rapidly results in a seizure within minutes of exposure [34]. The behavioral seizures in our study lasted for 20–30 seconds after the animals were removed from the inhalation chamber. Another concern with the use of flurothyl seizures is that there is a significant reduction in locomotor activity in the mice. We observed an increased level of freezing when we examined the freezing behavior of the seizure group 2 hours after seizures. Since freezing is the measurement of learning in the fear conditioning test, it appeared as though the animals had an enhancement of learning. However, we repeatedly observed increased freezing whenever we examined the freezing behavior of the animals within 2 hours after seizures. Therefore, we examined locomotor behavior of another cohort of mice in an open field test. We administered a flurothyl seizure to the mice and observed their locomotor activity 2 hours and 24 hours later in an open field test. We found a significant reduction in activity in the 2-hour test. This reduction may explain why the animals had more freezing after seizure induction. It is important to note that the decrease in activity was not present at the 24-hour time period. Therefore, any change of freezing at the 24-hour time point does not appear to be due to a change in activity levels.
The influence of PTZ on activity levels may help to explain another difference between the Mao et al, 2009 paper and our study. They report short-term memory deficits in both retrieval and consolidation of a fear memory, which is shown through a reduction in freezing behavior in fear conditioning. It is not clear whether PTZ induces acute hyperactivity in rodents. It is possible that the reduction in freezing after seizures could be due to a change in locomotor activity, which would then influence freezing behavior in the animals at this time point. Future studies examining learning and memory after seizures should include measures of locomotion to rule out the influence on activity levels.
Our observations on the influence of seizures on memory are in line with our understanding of the consolidation of memories. In order for a short-term memory to become a long-term memory there is a necessary period of consolidation. This process is believed to require a few hours to complete and requires protein synthesis. These assumptions are supported by experiments using anisomycin, which blocks protein synthesis. When given prior to fear conditioning it results in memory deficits [35]. Similar results are found if the drug is given 1–2 hours after fear conditioning, but little impairment occurs if the drug is given 4–6 hours after training. We included a group that received a seizure 6 hours prior to fear conditioning and found no impairment in memory. It may be that the mechanism of memory impairment observed here is similar to anisomycin, preventing/disrupting protein synthesis. Additional studies will address how seizures impact the role of protein synthesis in memory consolidation.
Another future direction will be to examine why a seizure prior to acquisition has an impact on learning while a seizure during the consolidation phase has no impact on learning. One possibility is that flurothyl seizures may have selectively impacted the lateral amygdala. In a previous study, investigators infused the GABAA agonist muscimol into the lateral amygdala to temporarily inhibit this region [36]. They found this inactivation blocked the acquisition of fear conditioning. This effect was not supported when muscimol is administered after fear conditioning. Therefore, it is hypothesized that this area plays a strong role in the acquisition of memories. Another possible mechanism could include NMDA receptors. It has previously been shown that the NMDA antagonist MK-801 disrupts contextual fear conditioning when administered prior to learning, but does not disrupt contextual fear conditioning when administered after the training session [37]. Future studies could examine whether seizures induce changes in specific areas of the amygdala and determine the role of NMDA receptors within the different regions of the amygdala and hippocampus.
Our research contributes to a large body of evidence that seizures have long-lasting impacts on learning and behavior. Several of the studies have focused on the long-term effects of seizures on spatial learning and memory and often use the Morris water maze task. In this task that is strongly dependent on the hippocampus, the rodent navigates a circular pool to find a hidden platform [38, 39]. Several studies have found that seizures during adulthood [25] and during early development [40–44] result in spatial learning deficits. Studies using fear conditioning have also found that seizures result in learning and memory deficits. Rats that received kainate or electrical stimulation of the amygdala have deficits in contextual memory [45]. A different study found impaired extinction in mice with pilocarpine-induced seizures [46].
One issue with previous studies examining learning and memory in young and adult subjects is that there are numerous structural and biochemical changes that can occur between the induction of seizures and the examination of learning and memory deficits. There is an increase in cell death, mossy fiber sprouting in the hippocampus, and gliosis [47–49]. However, the impact of a single flurothyl seizures on cell death in adult rodents has not been examined. Many of the studies have examined the long-term effect of many flurothyl seizures and have found little cell death [34, 50, 51]. Flurothly seizures result in changes in spine loss when induced during early development [52] and seem to have less impact on dendrite morphology when induced later in development [42]. Flurothyl seizures have been shown to lead to mossy fiber sprouting similar to other chemoconvulsants while cell loss is absent unless seizures are repeatedly induced [53]. Future studies could examine whether flurothyl seizures result in immediate cell death during adulthood. Such immediate cell death could help to explain our results but future studies could also include measures of spine loss.
It is difficult to tease out the impacts of seizures when there are concurrent alterations in the brain that strongly affect learning and memory. For our study we examined a relatively short time to measure alterations in long-term memory. One paper found that a single flurothyl seizure in rats results in spatial learning deficits several days after the seizure [54]. We plan to add later time points to determine the long-lasting impacts of a single seizure. As converging lines of evidence occur over time we plan to fill the gaps to determine the acute and long-term effects of seizures on cognitive function.
5. Conclusions
In addition to the long-term risk of future seizures, a single short seizure can impair the ability to learn a new task. We observed that a seizure before an associative learning task resulted in long-term learning deficits. However, learning occurring more remotely after a seizure was not impaired by the seizure, and learning that occurs shortly after a brief seizure was not impaired. This information should be taken into account when considering how epileptic conditions might affect the quality of life and ability to live independently for an individual diagnosed with epilepsy. Furthermore, by examining the biochemical changes that occur after a single seizure new therapeutics could be generated to address the effect of seizures on learning, and perhaps influence epileptogenesis.
Highlights.
A single flurothyl seizure impairs the acquisition of learning.
A single flurothyl seizure does not impair the retention of learning.
A flurothyl seizure results in a transient suppression of locomotor activity.
Acknowledgments
This research was funded by the Epilepsy Foundation and NIH NS056664 to JNL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Epilepsy Fact Sheet. 2015. [Google Scholar]
- 3.Kobau R, Luo Y-H, Zack MM, Helmers S, Thurman DJ. Epilepsy in adults and access to care--United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:909–13. [PubMed] [Google Scholar]
- 4.Banerjee PN, Filippi D, Allen Hauser W. The descriptive epidemiology of epilepsy-a review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe - a systematic review. Eur J Neurol. 2005;12:245–53. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 7.Beghi E, Berg A, Carpio A, Forsgren L, Hesdorffer DC, Hauser WA, et al. Comment on epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:1698–9. doi: 10.1111/j.1528-1167.2005.00273_1.x. author reply 1701–2. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva V, Sanchez-Alvarez JC, Pena P, Puig JS, Caballero-Martinez F, Gil-Nagel A. Treatment initiation in epilepsy: an expert consensus in Spain. Epilepsy Behav. 2010;19:332–42. doi: 10.1016/j.yebeh.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 10.Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965–72. doi: 10.1212/wnl.41.7.965. [DOI] [PubMed] [Google Scholar]
- 11.Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53:1095–103. doi: 10.1111/j.1528-1167.2012.03500.x. [DOI] [PubMed] [Google Scholar]
- 12.Ottman R, Lipton RB, Ettinger AB, Cramer JA, Reed ML, Morrison A, et al. Comorbidities of epilepsy: results from the Epilepsy Comorbidities and Health (EPIC) survey. Epilepsia. 2011;52:308–15. doi: 10.1111/j.1528-1167.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- 13.Titiz AS, Mahoney JM, Testorf ME, Holmes GL, Scott RC. Cognitive impairment in temporal lobe epilepsy: role of online and offline processing of single cell information. Hippocampus. 2014;24:1129–45. doi: 10.1002/hipo.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5:435–9. doi: 10.1016/j.yebeh.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. doi: 10.1016/j.yebeh.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Thompson PJ. Antiepileptic drugs and memory. Epilepsia. 1992;33(Suppl 6):S37–40. [PubMed] [Google Scholar]
- 17.Thompson PJ, Trimble MR. Anticonvulsant serum levels: relationship to impairments of cognitive functioning. J Neurol Neurosurg Psychiatry. 1983;46:227–33. doi: 10.1136/jnnp.46.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trimble MR. Anticonvulsant drugs and cognitive function: a review of the literature. Epilepsia. 1987;28(Suppl 3):S37–45. doi: 10.1111/j.1528-1157.1987.tb05776.x. [DOI] [PubMed] [Google Scholar]
- 19.Trimble MR, Thompson PJ. Anticonvulsant drugs, cognitive function, and behavior. Epilepsia. 1983;24(Suppl 1):S55–63. doi: 10.1111/j.1528-1157.1983.tb04644.x. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen B, Dam M. Memory disturbances in epileptic patients. Acta Neurol Scand Suppl. 1986;109:11–4. doi: 10.1111/j.1600-0404.1986.tb04859.x. [DOI] [PubMed] [Google Scholar]
- 21.Dodrill CB. Progressive cognitive decline in adolescents and adults with epilepsy. Prog Brain Res. 2002;135:399–407. doi: 10.1016/S0079-6123(02)35037-4. [DOI] [PubMed] [Google Scholar]
- 22.Breier JI, Plenger PM, Castillo R, Fuchs K, Wheless JW, Thomas AB, et al. Effects of temporal lobe epilepsy on spatial and figural aspects of memory for a complex geometric figure. J Int Neuropsychol Soc. 1996;2:535–40. doi: 10.1017/s1355617700001703. [DOI] [PubMed] [Google Scholar]
- 23.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–55. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannesson DK, Howland J, Pollock M, Mohapel P, Wallace AE, Corcoran ME. Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J Neurosci. 2001;21:4443–50. doi: 10.1523/JNEUROSCI.21-12-04443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert TH, Hannesson DK, Corcoran ME. Hippocampal kindled seizures impair spatial cognition in the Morris water maze. Epilepsy Res. 2000;38:115–25. doi: 10.1016/s0920-1211(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 26.Mortazavi F, Ericson M, Story D, Hulce VD, Dunbar GL. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005;7:629–38. doi: 10.1016/j.yebeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Genkova-Papazova MG, Lazarova-Bakarova MB. Pentylenetetrazole kindling impairs long-term memory in rats. Eur Neuropsychopharmacol. 1995;5:53–6. doi: 10.1016/0924-977x(94)00134-w. [DOI] [PubMed] [Google Scholar]
- 28.Zhou JL, Shatskikh TN, Liu X, Holmes GL. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci. 2007;25:3667–77. doi: 10.1111/j.1460-9568.2007.05598.x. [DOI] [PubMed] [Google Scholar]
- 29.Becker A, Grecksch G, Brosz M. Antiepileptic drugs--their effects on kindled seizures and kindling-induced learning impairments. Pharmacol Biochem Behav. 1995;52:453–9. doi: 10.1016/0091-3057(95)00137-l. [DOI] [PubMed] [Google Scholar]
- 30.Liu RS, Lemieux L, Bell GS, Hammers A, Sisodiya SM, Bartlett PA, et al. Progressive neocortical damage in epilepsy. Ann Neurol. 2003;53:312–24. doi: 10.1002/ana.10463. [DOI] [PubMed] [Google Scholar]
- 31.Mao RR, Tian M, Yang YX, Zhou QX, Xu L, Cao J. Effects of pentylenetetrazol-induced brief convulsive seizures on spatial memory and fear memory. Epilepsy Behav. 2009;15:441–4. doi: 10.1016/j.yebeh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Lugo JN, Brewster AL, Spencer CM, Anderson AE. Kv4. 2 knockout mice have hippocampal-dependent learning and memory deficits. Learn Mem. 2012;19:182–9. doi: 10.1101/lm.023614.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAuley JW, Elliott JO, Patankar S, Hart S, Long L, Moore JL, et al. Comparing patients’ and practitioners’ views on epilepsy concerns: a call to address memory concerns. Epilepsy Behav. 2010;19:580–3. doi: 10.1016/j.yebeh.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 35.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–66. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould TJ, McCarthy MM, Keith RA. MK-801 disrupts acquisition of contextual fear conditioning but enhances memory consolidation of cued fear conditioning. Behav Pharmacol. 2002;13:287–94. doi: 10.1097/00008877-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 39.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 40.Cornejo BJ, Mesches MH, Benke TA. A single early-life seizure impairs short-term memory but does not alter spatial learning, recognition memory, or anxiety. Epilepsy Behav. 2008;13:585–92. doi: 10.1016/j.yebeh.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Spatial learning deficits without hippocampal neuronal loss in a model of early-onset epilepsy. Neuroscience. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura M, Gu X, Swann JW. Seizures in early life suppress hippocampal dendrite growth while impairing spatial learning. Neurobiol Dis. 2011;44:205–14. doi: 10.1016/j.nbd.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–48. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu CL, Huang LT, Liou CW, Wang TJ, Tung YR, Hsu HY, et al. Lithium-pilocarpine-induced status epilepticus in immature rats result in long-term deficits in spatial learning and hippocampal cell loss. Neurosci Lett. 2001;312:113–7. doi: 10.1016/s0304-3940(01)02202-9. [DOI] [PubMed] [Google Scholar]
- 45.Kemppainen EJ, Nissinen J, Pitkanen A. Fear conditioning is impaired in systemic kainic acid and amygdala-stimulation models of epilepsy. Epilepsia. 2006;47:820–9. doi: 10.1111/j.1528-1167.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 46.Lesting J, Geiger M, Narayanan RT, Pape HC, Seidenbecher T. Impaired extinction of fear and maintained amygdala-hippocampal theta synchrony in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:337–46. doi: 10.1111/j.1528-1167.2010.02758.x. [DOI] [PubMed] [Google Scholar]
- 47.Henshall DC, Meldrum BS. Cell death and survival mechanisms after single and repeated brief seizures. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 48.de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–95. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 49.Wolf HK, Aliashkevich AF, Blumcke I, Wiestler OD, Zentner J. Neuronal loss and gliosis of the amygdaloid nucleus in temporal lobe epilepsy. A quantitative analysis of 70 surgical specimens. Acta Neuropathol. 1997;93:606–10. doi: 10.1007/s004010050658. [DOI] [PubMed] [Google Scholar]
- 50.Holmes GL. Effects of seizures on brain development: lessons from the laboratory. Pediatr Neurol. 2005;33:1–11. doi: 10.1016/j.pediatrneurol.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang LT, et al. Consequences of recurrent seizures during early brain development. Neuroscience. 1999;92:1443–54. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 52.Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–68. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Q, Hu Y, Holmes GL. Effect of topiramate on cognitive function and activity level following neonatal seizures. Epilepsy Behav. 2005;6:529–36. doi: 10.1016/j.yebeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Mares J, Pometlova M, Deykun K, Krysl D, Rokyta R. An isolated epileptic seizure elicits learning impairment which could be prevented by melatonin. Epilepsy & Behavior. 2012;23:199–204. doi: 10.1016/j.yebeh.2011.11.018. [DOI] [PubMed] [Google Scholar]