Abstract
Objective
To conduct a longitudinal study on age-related nuclear cataracts using the technique of Dynamic Light Scattering (DLS) to determine if cataract progression is associated with the loss of the unbound form of the lens molecular chaperone protein, α-crystallin.
Design
Natural History, cohort study
Subjects
Patients 30 years or older of either sex presenting at the Wilmer Eye Institute Cornea-Cataract Department.
Materials and Methods
We conducted a longitudinal study of age related nuclear cataracts at the Wilmer Eye Institute of Johns Hopkins Hospital in Baltimore, MD. All patients underwent a comprehensive dilated eye exam every 6 months, including slit-lamp grading of their lenses using the Age Related Eye Disease Study (AREDS) clinical lens grading system and obtaining an estimate of the unbound α-crystallin level in the nucleus, the α-Crystallin Index (ACI), using the NASA-NEI DLS device. We used a random effects statistical model to examine the relationship of lens opacity changes over time with ACI changes.
Main Outcome Measures
α-Crystallin Index (ACI) and AREDS Nuclear cataract grade
Results
45 patients (66 eyes) aged 34–79 years with AREDS nuclear lens grades of 0–3.0 were followed every 6 months for a mean of 19 months (range, 6–36 months). We found that lenses with the lowest baseline levels of ACI had the most rapid progression of cataracts, whereas lenses with higher ACI at baseline had no or slower cataract progression. Lenses which lost α-crystallin at the highest rates during the study also had faster progression of nuclear cataracts than did lenses with a slower rate of ACI loss. Kaplan Meier survival curves showed that lenses with the lowest initial ACI had the highest risk of undergoing cataract surgery.
Conclusion
Our longitudinal study corraborates our previous cross-sectional study finding that higher levels of unbound α-crystallin as assessed by ACI are associated with lower risk of cataract formation and that loss of ACI over time is associated with cataract formation and progression. This study suggests that assessment of ACI with the DLS device could be used as a surrogate for lens opacity risk in clinical studies, including for future anti-cataract drug trials, and for assessing adverse nuclear cataract events in studies where cataract development might be a side effect of a drug or device.
Age -related cataract remains the main cause of blindness in the world, despite advances in its surgical treatment. With the rapid aging of the US population, there will be an increase in the economic burden from cataract in this country. Cataract surgery is performed in at least 2.5 million eyes each year in the U.S. and is now the most commonly reimbursed surgical procedure by Medicare1–4.
Nuclear cataract, which is the opacification of the nuclear region of the lens, is the most common type of age related cataract in the U.S5–6. Nuclear cataracts result from misfolding and aggregation of lens proteins, causing the formation of high molecular weight protein aggregates that block, scatter and distort light as it passes through the lens. These opacities cause progressive loss of vision that ultimately requires cataract surgery. A major cause of such protein damage in the lens is chronic oxidative stress7–17.
Recently, it has been found that one of the main lens proteins, α-crystallin, has protective molecular chaperone properties and can prevent the aggregation of lens proteins damaged by oxidative stress or other insults18–23. The α-crystallin molecule has the ability to bind to partially unfolded proteins including β- and γ-crystallins as well as to other proteins in the lens, stabilizing them and preventing uncontrolled aggregation that would produce large, light-scattering elements. For this reason, α-crystallin has been identified as an endogenous anti-cataract lens protein and has become an important focus of study. Many laboratories have been studying its properties and characteristics, using animal lenses as well as eye bank /cadaveric and surgically extracted human lenses. However, until the development of Dynamic Light Scattering (DLS) technology, it has not been possible to study α-crystallin in the intact living human eye.
With the development of the Dynamic Light Scattering (DLS; also called Quasi-elastic Light Scattering or QELS) technique, α-crystallin in the lenses of animals and patients can be detected and measured in vivo, non-invasively and safely8,24–36. Benedek8 developed the first QELS device in the 70’s, followed by others, including Weiss, Bursell, Thurston and co-workers. They studied animal and human normal and diabetic lenses24–30.
The discovery that α-crystallin were endogenous molecular chaperones18–23, led us to monitor and estimate the loss of α-crystallin in the lens in vivo using the new compact fiberoptic-based DLS technology developed for fluid physics experiments in space31–36. We used the DLS clinical device developed by a NASA-NEI team to study the early onset of cataractogenesis in model protein solutions, in live animals, and in clinical experiments31–34. We demonstrated its potential in helping patients to predict the fate of their lenses when exposed to cataract risks. In our earlier papers we first confirmed in the laboratory that the DLS can detect early changes in lens crystallin during cold cataract formation in calf lenses, detecting lens protein changes much earlier than Scheimpflug slit-lamp lens imaging31–35. Next, in a clinical cross-sectional study36 on 380 eyes from 235 subjects aged 7–86 years with lens nuclear opacities ranging from clear to opaque (AREDS lens grades 0–3.8), we found that there was a corresponding loss of α-crystallin, as estimated by the α-crystallin Index (ACI) obtained from the DLS device, which was associated with increasing lens nuclear opacity (P<0.0001). High values of ACI, indicating high levels of unbound α-crystallin, are associated with lower risk for cataract. In the current report, we conducted a longitudinal study to determine whether α-crystallin levels decreased over time, whether lens opacification was associated with the decline in α-crystallin, and whether α-crystallin decrease will lead to cataract surgery.
Materials and Methods
We conducted a natural history/cohort study of patients 30 years of age and older, presenting at the Stark-Mosher Center for Cataract and Corneal Disease of the Wilmer Eye Institute of Johns Hopkins Hospital in Baltimore, MD. Excluded were patients who had tear film disorders, corneal opacities or disorders, uveitis or glaucoma or those who had difficulty fixating, any adverse reaction to dilating drops or inability to return for follow up visits. The study was approved by the Johns Hopkins Medical Institutions (JHMI) Intramural Research Board (IRB) and was HIPAA compliant. All tenets of the Declaration of Helsinki were followed, and all patients gave written informed consent.
All patients underwent a comprehensive dilated eye examination at baseline and then every 6 months, including slit lamp grading of their lenses using the Age Related Eye Disease Study (AREDS) clinical lens grading system (using a Haag Streit BM 900 slit lamp: Haag Streit, Koeniz, Switzerland), and measurement of their α-crystallin Index (ACI) using the NASA-NEI Dynamic Light Scattering (DLS) device as previously described.36 The ACI is an estimate of unbound α-crystallin present in the lens nucleus as reported earlier.36 Unbound α-crystallin is the native molecule (mw about 800,000) not bound to other partially unfolded proteins. It is computed as the sum of intensities from the first 6 particle intervals of the DLS output (the first peak, representing unbound α-crystallin) divided by the sum of the intensities of all 18 particle size intervals, expressed as a percentage (explained in detail in our previous paper, Datiles et al, Archives of Ophthalmology 2008. 126:1687-93.).
For statistical analysis we used SAS software (version 9.2, Cary, NS, USA) to compare the ACI change over time between subgroups. We first compared the baseline characteristics across ACI categories. P values were obtained from ANOVA test for continuous variables and from Chi-square test for categorical variables. A growth curve model (also known as random effects or mixed model) was then used to compare the nuclear opacity/cataract progression rate between subgroups. To evaluate the relationship between ACI categories and incident cataract surgery, Kaplan-Meier survival analyses was performed. Significance level was p<0.05.
Results
We studied 45 subjects (66 eyes) aged 34–79 years, 50% female, with AREDS nuclear lens grades of 0–3.0 at baseline (full scale 0–4). Subjects underwent complete dilated eye examinations including AREDS clinical lens nuclear grading and DLS measurements to obtain ACI every 6 months for a mean of 19 months (range, 6–36 months). Table 1 shows the demographic data of this patient population.
TABLE 1. Characteristics of the Study Population.
This table shows the characteristics of the subjects. Subjects were divided into 3 groups (tertiles) based on baseline ACI. Age shows the mean age (in years) and standard deviation (in parenthesis) for each tertile group. Gender shows how many in each tertile were male; numbers in parenthesis shows percent male. Race shows how many in each tertile were white; number in parenthesis shows percent white. Baseline AREDS Nuclear Grade shows the mean nuclear lens grade for each tertile; numbers in parenthesis show the standard deviation. Follow up time show the mean follow up time for each tertile group in months; numbers in parenthesis show standard deviation.
| Overall | ACI>=10.8 (23 eyes, 17 patients) |
ACI 6.1–10.8 (26 eyes,15 patients) |
ACI <6.1 (17 eyes,13 patients) |
P-value | |
|---|---|---|---|---|---|
| Age in years, mean (sd) | 61.4 (8.5) | 57.2 (8.4) | 63.2 (5.0) | 64.6 (10.9) | 0.0083* |
| Gender, Male, N (%) | 33 (50.0) | 14 (60.9) | 12 (46.2) | 7 (41.2) | 0.41 |
| Race, White, N (%) | 63 (95.5) | 22 (95.7) | 24 (92.3) | 17 (100.0) | 0.50 |
| Baseline AREDS Lens Nuclear Grade, mean (sd) | 1.3 (0.7) | 0.97 (0.67) | 1.41 (0.76) | 1.57 (0.49) | 0.014* |
| Follow-up time (months), mean (sd) | 19.4 (8.1) | 19.9 (9.3) | 20.2 (8.0) | 17.0 (6.5) | 0.44 |
P-value<0.05 (0.05 is the significance level)
We divided the population into tertiles based on baseline ACI level: Group 1 included lenses with ACI greater than 10.8 at baseline; Group 2 included lenses with ACI between 10.8 and 6.1; and Group 3 included lenses with ACI less than 6.1. For each ACI group, we computed the slope of the cataract grade over time.
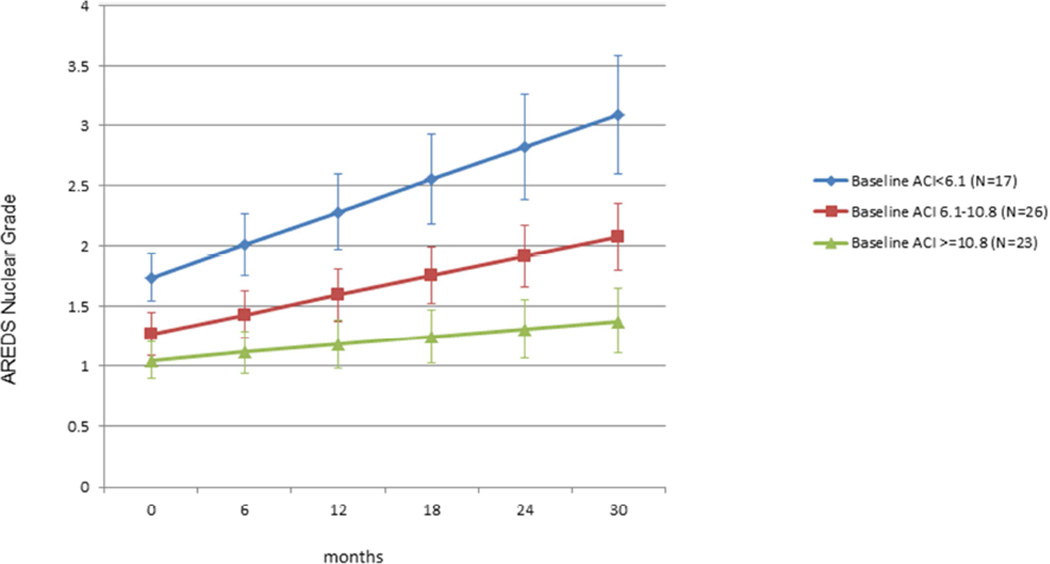
Figure 1 shows the nuclear cataract progression rate for each of the 3 baseline ACI groups. The rate of lens opacity progression increased with decreasing baseline ACI. The rate of increase in nuclear grade per year was 0.1, 0.2 and 0.4 respectively for Groups 1, 2 and 3 (P value of trend <0.0001). Based on model estimates, for each 10 unit-decrease in ACI, there is an associated one AREDS grade increase in nuclear opacity in 4 years.
Figure 1. Nuclear cataract progression based on baseline ACI.
Shows the rate of progression of nuclear cataract based on initial α-Crystallin Index (ACI) level. Eyes were divided into 3 groups (tertiles) based on baseline ACI. Those with the lowest ACI at baseline had the fastest cataract progression; those with highest ACI at baseline had the slowest progression. (P value of trend <0.0001)
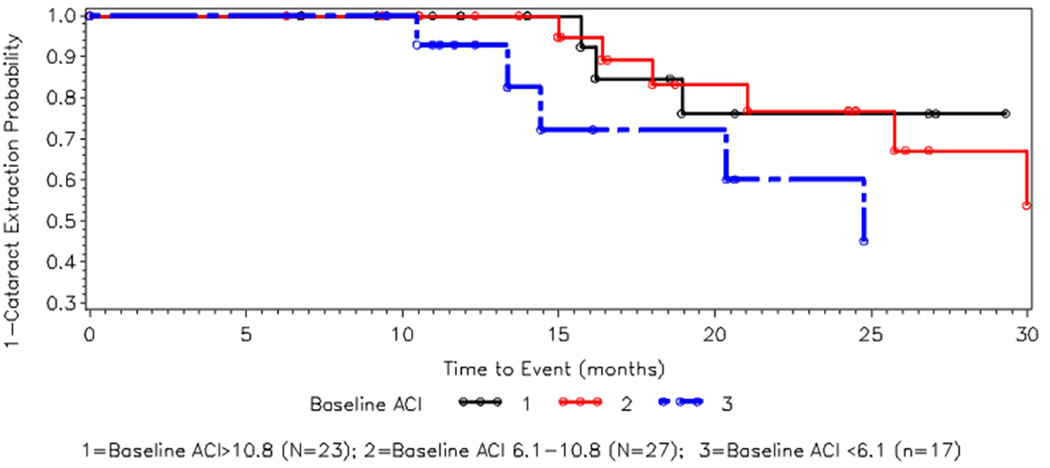
Figure 2 shows the Kaplan-Meier survival curves of the cumulative probability of not having cataract surgery, stratified by baseline ACI status. Those with the lowest baseline ACI (Group 3 as above) had the highest risk for cataract surgery. Group 3 was statistically significant from both Groups 1 and 2 (p=0.03 and p=0.05 respectively). There was no significant difference between Groups 1 and 2.
Figure 2. Kaplan – Meier curve for cataract surgery by baseline ACI.
Kaplan-Meier curve for cumulative risk for cataract surgery. Eyes were divided into 3 groups (tertiles) based on baseline ACI. The x axis shows time since baseline in months. The Y axis shows 1-cumulative probability of cataract surgery (i.e., the chance of not having cataract surgery). Those with lowest ACI (blue broken line) at baseline had highest risk for cataract surgery; those with higher ACI had lower risks for cataract surgery. The open circles indicate censored observation.
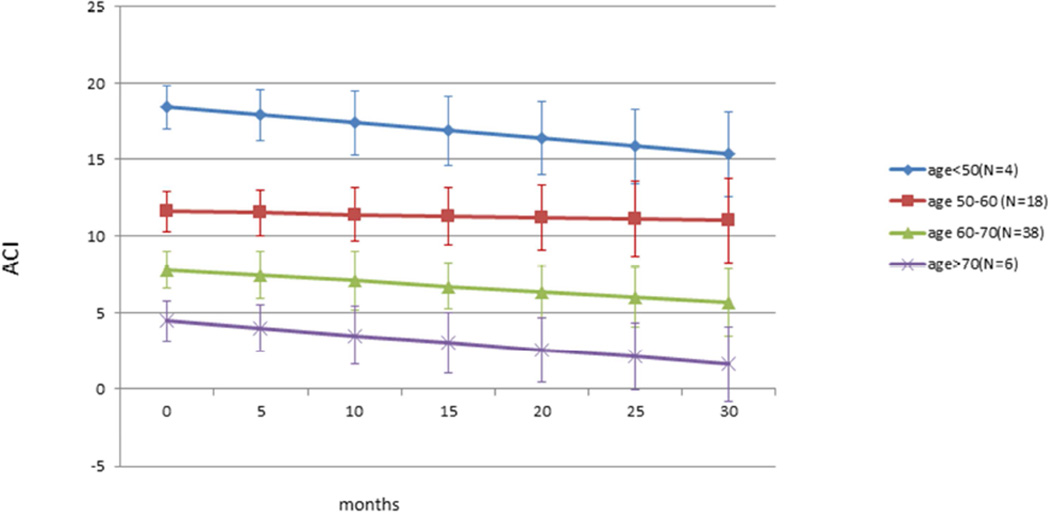
Figure 3 shows the changes in ACI related to aging during follow up studies after dividing the patients by age groups (<50, 50–60, 60–70 and >70). Rates of decline in ACI are similar in all age groups, similar to the age related decrease in ACI reported in our previous cross sectional study.36 Slopes are −0.028, −0.991, −0.068 and −0.102 for <50, 50–60, 60–70 and >70 yr. old groups. Baseline age is highly associated with ACI level (p<0.001).
Figure 3. Age related decrease of ACI (by age groups).
Decrease of ACI with aging. Eyes were divided by age in decades. There was consistent age related decrease in ACI among all groups over time. Rates of decline in ACI are similar in all age groups, similar to the age related decrease in ACI reported in our previous cross sectional study.36
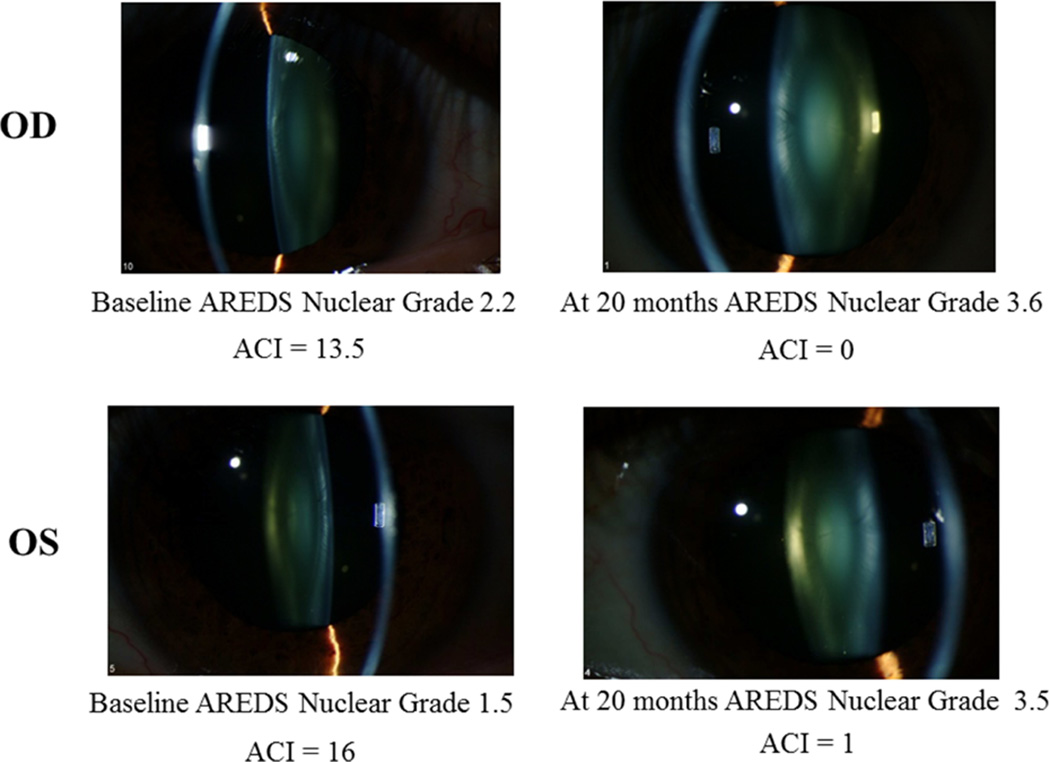
Figure 4 shows slit lamp photographs from a 43 yr. old subject, followed for 20 months. The right eye initially had an early nuclear cataract, AREDS nuclear grade 2.2 and ACI of 13.5, and by 20 months had developed a clinically significant nuclear cataract, AREDS grade 3.6 and with an ACI of zero. For the left eye, initial AREDS nuclear grade was 1.5 and ACI was 16, and at 20 months the eye developed a nuclear cataract with AREDs nuclear grade of 3.5 and ACI of 1.
Figure 4.
Nuclear cataract progression in a patient with corresponding decrease in ACI. The upper two figures are for the right eye of a 43 year old patient, at baseline and 20 months later, and the lower two figures show the left eye of the same patient, at baseline and 20 months later. As the nuclear cataract progressed, the ACI decreased.
Discussion
This longitudinal study shows that a decrease in α-crystallin over time will lead to lens opacification (Figure 1), that α-crystallin levels decrease over time with aging (Figure 3), and that α-crystallin decrease over time will lead to cataract surgery (Figure 2).
Recent research has revealed the fundamental role of α-crystallin in the lens, acting as an endogenous anti-cataract agent in the most common type of age related cataract, nuclear cataract18–23. α-crystallin act as molecular chaperones that stabilize damaged lens proteins and thus prevent them from forming large, high molecular weight aggregates that scatter light, causing nuclear lens opacities. Our findings provide additional evidence of this association. The higher the amount of unbound α-crystallin in the lens, the lower the risk of lens opacity progression or need for surgery. The probability of lens opacity progression and cataract formation is especially high as ACI approaches zero.
These longitudinal study findings are consistent with those from our previous cross sectional study where we found that the lower α-crystallin levels were associated with increased nuclear lens opacity scores36.
In this longitudinal study and our previous cross-sectional study, we found statistically significant decrease of α-crystallin with aging. The hypothesis that as α-crystallin is increasingly used up through its action as a molecular chaperone, the decrease of free α-crystallin accelerates and the risk of apparent lens opacity increases, is consistent with, and confirmed by, observations in these two studies.
In this pilot study, we demonstrate the usefulness of DLS in studying the role of α-crystallin in nuclear cataract formation by following patients as a function of time. Perhaps this technique can be used to assess potential risk factors for nuclear lens opacity such as oxidative stress7–17, toxins7, 37, drugs7, 37, radiation7, 10, 37, trauma37, genetics38–41 or surgical intervention such as vitrectomy42–43. It might also provide a useful outcome variable for evaluation of drugs44 or interventions (with mini-chaperone α-crystallin peptides)45 designed to slow the progression of lens nuclear opacity or document dysfunctional lens syndrome.46
In summary, until recently, clinical detection of lens opacity has relied on slit lamp microscopy or photography. However, by the time these procedures can detect these lens changes, the damage to the lens may already be irreversible. The ability of the DLS device to measure unbound α-crystallin in the lens in vivo while the lens is still clear potentially opens new avenues for lens research including for presbyopia, which has been linked to α-crystallin age related changes47. It allows us to assess the effect of deleterious agents causing lens protein damage before such effects can be detected by slit lamp examination or photography. Change in α-crystallin index could be used as an outcome variable to assess interventions that could slow or accelerate the progression of the disease process, before lens opacities develop.
Acknowledgements
We would like to thank Prof. Su-Long Nyeo, Professor of Physics of the University of Taiwan for assisting in monitoring the calculations of the ACI from the DLS data.
Financial Support:
Partially supported by the NASA-NEI Inter-Agency Agreement, by the Kwok Foundation Fund and the Stark-Mosher Center for Cataract and Corneal Disease Research Fund.
The sponsors/funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation:
Partially presented at the ARVO annual scientific meeting, Florida, May 2012.
Conflict of Interest/Financial Disclosure:
Dr. Rafat Ansari owns the DLS device patent.
None of the other authors have any conflict of interest or financial disclosures.
References
- 1.Brian G, Taylor H. Cataract blindness- challenges for the 21st Century. Bull. of World Health Org. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- 2.Gollogly HE, Hodge DO, St. Sauver J, Erie JC. Increasing incidence of cataract surgery: Population-based study. J Cataract Refract Surg. 2013;39:1383–1389. doi: 10.1016/j.jcrs.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GC, Brown MM, Menezes A, et al. Cataract Surgery cost utility revisited in 2012: A new economic paradigm. Ophthalmology. 2013;120:2367–2376. doi: 10.1016/j.ophtha.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Mariotti AP, Pascolini D. Global estimates of Visual Impairment. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 5.Klein BEK, Klein R, Linton KLP. Prevalence of age related opacities in a population. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 6.West SK. Epidemiologic Aspects of Age Related Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia: Lippincott-Williams & Wilkins Co., Inc.; 2005. pp. 1–15. Ch. 73a. [Google Scholar]
- 7.Zigler JS, Datiles MB. Pathogenesis of Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia: Lipincott-Williams and Wilkins Co. Inc.; 2010. pp. 1–13. Ch. 72B. [Google Scholar]
- 8.Benedek GB. Theory of transparency of the eye. Applied Optics. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 9.Jedziniak JA, Nicoli DF, Baram H, et al. Quantitative verification of the existence of high-molecular-weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Visual Sci. 1978;17:51–57. [PubMed] [Google Scholar]
- 10.Zigman S, Datiles MB, Torczynski E. Sunlight and Human cataracts. Invest Ophthalmol Vis Sci. 1979;18:462–467. [PubMed] [Google Scholar]
- 11.Zigler JS, Jr, Goosey JD. Photosensitized oxidation in the ocular lens: Evidence for photosensitizers endogenous to the human lens. Photochem Photobiol. 1981;33:869–874. doi: 10.1111/j.1751-1097.1981.tb05505.x. [DOI] [PubMed] [Google Scholar]
- 12.Garland D. Role of site-specific, metal catalyzed oxidation in lens aging and cataract: a hypothesis. Exp Eye Res. 1990;50:677–682. doi: 10.1016/0014-4835(90)90113-9. [DOI] [PubMed] [Google Scholar]
- 13.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9:1173–1182. [PubMed] [Google Scholar]
- 14.Lou MF. Thiol regulation in the lens. J Ocul Pharmacol Ther. 2000;16:137–148. doi: 10.1089/jop.2000.16.137. [DOI] [PubMed] [Google Scholar]
- 15.Garner B, Davies MJ, Truscott RJW. Formation of hydroxyl radicals in the human lens is related to the severity of nuclear cataract. Exp Eye Res. 2000;70:81–88. doi: 10.1006/exer.1999.0754. [DOI] [PubMed] [Google Scholar]
- 16.Truscott RJW. Age-related nuclear cataract -oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Beebe DC, Holekamp NM, Shui Y-B. Oxidative damage and the prevention of cataract. Ophthalmic Res. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz J. a-crystallin can act as a molecular chaperone. Proc Natl Acad Sci. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graw J. Crystallins: genes, proteins and diseases. Exp. Eye Res. 1997;378:331–348. [PubMed] [Google Scholar]
- 20.Horwitz J. The function of a-crystallin in vision. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 21.Andley UP. Effects of α-crystallins on lens cell formation and cataract pathology. Curr Mol Med. 2000;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- 22.Christopher KL, Pedler MG, Shieh, et al. α-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim et Biophys Acta - Molecular Cell Research. 2014;1843:309–315. doi: 10.1016/j.bbamcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013;54(14):ORSF54–ORSF59. doi: 10.1167/iovs.13-12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka T, Benedek GB. Observation of protein diffusivity in intact human and bovine lenses with application to cataract. Invest Ophthalmol Vis Sci. 1975;14:449–456. [PubMed] [Google Scholar]
- 25.Weiss JN, Rand LI, Gleason RE, Soeldner JS. Laser light scattering spectroscopy of in vivo human lenses. Invest Ophthalmol Vis Sci. 1984;25:594–598. [PubMed] [Google Scholar]
- 26.Nishio I, Weiss JN, Tanaka T, et al. In vivo observation of lens protein diffusivity in normal and X-irradiated rabbit lenses. Exp Eye Res. 1984;39:61–68. doi: 10.1016/0014-4835(84)90115-5. [DOI] [PubMed] [Google Scholar]
- 27.Benedek GB, Chylack LT, Libondi T, et al. Quantitative detection of the molecular changes associated with early cataractogenesis in living human lenses using dynamic light scattering. Curr Eye Res. 1987;6:1421–1432. doi: 10.3109/02713688709044506. [DOI] [PubMed] [Google Scholar]
- 28.Datiles M, Podgor M, Edwards P. Reproducibility of the Early Cataract Detector (Kowa QELS ECD) 2000. Ophthalmic Surgery. 1988;19:664–666. [PubMed] [Google Scholar]
- 29.Bursell SE, Magnante PC, Chylack LT., Jr . In vivo uses of quasi-elastic light scattering spectroscopy as a molecular probe in the anterior segment of the eye. In: Masters BR, editor. Noninvasive Diagnostic Techniques in Ophthalmology. Vol. 18. New York: Springer; 1990. pp. 342–365. [Google Scholar]
- 30.Thurston GM, Hayden DL, Burrows P, et al. Quasielastic Light Scattering study of the living lens as a function of age. Curr Eye Res. 2000;20:502–510. doi: 10.1076/ceyr.16.3.197.15410. [DOI] [PubMed] [Google Scholar]
- 31.Ansari RR. Ocular Static and Dynamic Light Scattering: a non-invasive diagnostic tool for eye research and clinical practice. J Biomed Opt. 2004;9:22–27. doi: 10.1117/1.1626663. [DOI] [PubMed] [Google Scholar]
- 32.Ansari RR, Datiles M, King JF, Leftwood D. Measuring lens opacity: combining quasielastic light scattering with scheimpflug imaging system. Proceedings of SPIE- Lasers & Ophthalmol. 1998;3246:35–40. [Google Scholar]
- 33.Ansari RR, Datiles MB, King JF. A new clinical instrument for the early detection of cataracts using dynamic light scattering and corneal topography. Proc SPIE Int Soc Opt Eng. 2000;3908:38–49. [Google Scholar]
- 34.Datiles MB, Ansari RR, Reed GF. A clinical study of the human lens with a dynamic light scattering device. Exp Eye Res. 2002;74:93–102. doi: 10.1006/exer.2001.1106. [DOI] [PubMed] [Google Scholar]
- 35.Datiles MB, Ansari RR. Clinical Evaluation of Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia: Lipincott-Williams and Wilkins Co., Inc.; 2004. pp. 1–20. Ch. 72A. [Google Scholar]
- 36.Datiles MB, Ansari RR, Suh KI, et al. Clinical detection of Pre-cataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008;126:1687–1693. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Congdon NG, Chong MA, Botelho P, et al. Clinical Types of Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia, PA: Lippincott Williams and Wilkins Co., Inc.; 2006. pp. 1–25. Ch. 73. [Google Scholar]
- 38.Shiels A, Hejtmancik JF. Genetics of Human cataract. Clinical Genetics. 2013;84:120–127. doi: 10.1111/cge.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hejtmancik JF, Datiles MB. Congenital and Hereditary Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. 2008. pp. 1–19. Ch. 74. [Google Scholar]
- 40.Hammond CJ, Snieder H, Spector TD, et al. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. New Eng J Med. 2000;342:1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 41.Congdon N, Broman KW, Lai H, et al. Nuclear cataract shows significant familial aggregation in an older population after adjustment for possible shared environmental factors. Invest Ophthalmol Vis Sci. 2004;45:2182–2186. doi: 10.1167/iovs.03-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bustros S, Thompson JT, Michels RG, et al. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105:160–164. doi: 10.1016/0002-9394(88)90180-8. [DOI] [PubMed] [Google Scholar]
- 43.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 44.Kador PF, Zigler JS, Clark JI, Datiles MB. Medical Treatment of Cataracts. In: Tasman W, Jaeger E, editors. Duane’s Clinical Ophthalmology. Vol. 1. Philadelphia: Lipincott – Williams and Wilkins Co., Inc.; 2009. pp. 1–15. Ch. 74. [Google Scholar]
- 45.Nahomi RB, Wang B, Raghavan CT, et al. Chaperone peptides of a-crystallin inhibit epithelial cell apoptosis, protein insolubilization and opacification in experimental cataracts. J Biol Chem. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waring GO., IV Diagnosis and Treatment of Dysfunctional Lens Syndrome. [Accessed March 4, 2015];Cataract & Refractive Surgery Today March. 2013 http://crstoday.com/2013/03/diagnosis-and-treatment-of-dysfunctional-lens-syndrome/ [Google Scholar]
- 47.Truscott RJ. Presbyopia. Emerging from a blur towards an understanding of the molecular basis of the most common eye condition. Exp. Eye Res. 2009;88:241–247. doi: 10.1016/j.exer.2008.07.003. [DOI] [PubMed] [Google Scholar]