Abstract
Five Sardinian patients presented in their 5th or 6th decade with progressive limb girdle muscle weakness but their muscle biopsies showed vacuolar myopathy. The more or less abundant subsarcolemmal and intermyofibrillar vacuoles showed intense, partially α-amylase resistant, PAS-positive deposits consistent with polyglucosan. The recent description of late-onset polyglucosan myopathy has prompted us to find new genetic defects in the gene (GYG1) encoding glycogenin-1, the crucial primer enzyme of glycogen synthesis in muscle.
We found a single homozygous intronic mutation harbored by five patients, who, except for two siblings, appear to be unrelated but all five live in central or south Sardinian villages.
Keywords: Polyglucosan myopathy, Glycogenin deficiency, Glycogenosis, GYG1
1. Introduction
The three main glycogen synthetic enzymes are glycogenin1 (GYG1), glycogen synthase (GYS1), and glycogen branching enzyme (GBE1).
The best known mutations in the gene (GBE1) encoding glycogen branching enzyme have been associated with variably severe clinical forms of glycogenosis type IV (GSD IV), all of which cause accumulation of a poorly branched and poorly spherical amylopectin-like glycogen (polyglucosan) [1]. A late-onset severe form of GSD IV, dubbed as adult polyglucosan body disease (APBD), is characterized by central leukodystrophy, peripheral neuropathy, neurogenic bladder, and inconsistently dementia [2,3].
Recently recognized pathogenic mutations in the gene (GYS1) have been associated with profound glycogen depletion in muscle (glycogenosis type 0), resulting in exercise intolerance, weakness, and myalgia with sudden cardiac death [4,5]. Lack of glycogen substrate was suggested by abnormal ischemic or non-ischemic forearm exercise, a typical test indicating excessive normal muscle glycogen storage due to defects of glycogen breakdown (GSD V, McArdle disease; GSD VII, Tarui disease).
A most unusual glycogenosis type 0 was identified in a 27-year-old man who had exercise intolerance and slight weakness but also suffered from ventricular fibrillation from which he was saved by application of a defibrillator. His muscle biopsy showed severe lack of glycogen due to biallelic mutations in the gene (GYG1) encoding the crucial glycogen synthetic enzyme glycogenin present in skeletal and cardiac muscle [6].
An even most unusual observation linked homozygous and compound heterozygous mutations in the GYG1 gene in seven patients of various ethnic background presenting with late-onset weakness and polyglucosan accumulation – rather than lack of glycogen – in their muscle biopsies and no cardiopathy [7]. It was further noted that the severe mutations resulted in depletion of glycogenin-1 or impairment of glycogenin-1 interaction with glycogen synthase, but the cause of polyglucosan accumulation remains difficult to understand. More recently, Colombo et al. [8] reported two sisters presenting adult onset limb girdle weakness associated with an intronic GYG1 variant previously encountered in 4/7 cases of the seminal paper [7], and Luo et al. described a woman manifesting proximal limb weakness associated a homozygous missense variants [9].
Here, we report on five Sardinian patients presenting late-onset polyglucosan body myopathy associated with a single GYG1 homozygous intronic mutation.
2. Materials and methods
2.1. Patients
Five adult patients with polyglucosan myopathy from four unrelated Sardinian families were included in this study. After informed consent, all five patients underwent open biopsy of the quadriceps muscle for histochemical studies.
2.2. Muscle pathology and biochemistry
Proximal muscle biopsies (quadriceps) were studied extensively with traditional histochemical battery, including periodic acid-Schiff (PAS) stain before and after digestion with α-amylase (diastase). Detailed electron microscopy analysis was performed in four patients as described by Malfatti et al. [10].
Biochemical analyses were performed in frozen muscle specimens in various laboratories, including values for the following enzymes: acid α-glucosidase, glycogen branching enzyme, and all glycogenolytic and glycolytic enzymes.
2.3. Molecular genetic analysis
Genomic DNA was extracted from blood and frozen skeletal muscle by standard methods and Sanger sequencing was used for the GYG1 gene. Briefly, exon 2 was amplified by PCR using primers hGYG1-Ex2F 5′-CCA AAG GGC TAC AGC TTG AT and hGYG1-Ex2R 5′CTC TAC CCG GTG CTC AAT TC. Amplified exon 2 fragment was sequenced with forward or reverse primer.
3. Results
3.1. Clinical findings
Clinical data for all 5 patients are summarized in Table 1. Onset of their weakness started at age 40 to age 60. Their initial complaints included progressive muscles weakness affecting both girdles, causing waddling gait, difficulty in climbing stairs and lifting arms. Notably, two patients, P1 and P5, also had exercise intolerance or myalgia. All patients showed slow progression, with impaired walking, inability to climbing stairs and rising from the floor with positive Gowers sign.
Table 1.
Clinical and laboratory findings in the five patients.
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | |
|---|---|---|---|---|---|
| Gender | M | M | F | F | F |
| Age at onset | 40 | 53 | 55 | 60 | 45 |
| Initial symptoms | Exercise intolerance, myalgia; progressive weakness in scapular and pelvic girdle |
Difficulty walking and climbing stairs |
Weakness in cervical girdle; slow progression of weakness in pelvic girdle |
Weakness in scapular girdle, slow progression of weakness in pelvic girdle |
Difficulty walking and climbing stairs. Exercise intolerance, progressive weakness in scapular and pelvic girdle |
| Age at examination | 63 | 65 | 77 | 80 | 65 |
| Clinical features |
|
|
|
|
|
| Serum CK | 116 U/L (n.v. 55–170) | 254 (n.v. 55–170) | 78 U/L (n.v. 55–170) | 155 U/L (n.v. 55–170) | 95 (n.v. 55–170) |
| Cardiac examination | ECG: bradychardia:53/min EcoCG: global systolic function at lower limits (ischemic cardiomyopathy) |
ECG: normal EcoCG: dilation of aortic root; slight left atrial dilation |
ECG: normal EcoCG: sclerosis of aortic valve, slight mitral and tricuspid insufficiency |
ECG: normal EcoCG: slight mitral and tricuspid insufficiency, hypertensive cardiomyopathy. |
ECG: normal EcoCG: slight mitral and tricuspid insufficiency. |
| EMG | Myopathic pattern proximal; mixed pattern (myopathic and neurogenic indicating radiculopathy) distal. |
Neurogenic findings indicating radiculopathy. |
Myopathic finding with rare fibrillation potentials on deltoid muscle |
Clear myopathic pattern more proximal |
Clear myopathic pattern more proximal |
| Muscle pathology: light microscopy |
Partially α-amylase resistant PAS-positive inclusions |
Partially α-amylase resistant PAS-positive but not abundant inclusions |
Partially α-resistant PAS-positive inclusions visible in scattered fibers |
Numerous vacuoles in many fibers. α-amylase-resistant PAS-positive inclusions |
Nonspecific myopathic changes without vacuoles in two different biopsy (2009–2015) |
Their cardiac examinations revealed decreased global systolic function due to concomitant ischemic cardiomyopathy in patient 1 and only mild changes compatible with their late ages in the other patients (slight left atrial dilation in patient 2, mild mitral and tricuspid valve insufficiency in patients 3, 4 and 5 revealed by echocardiography).
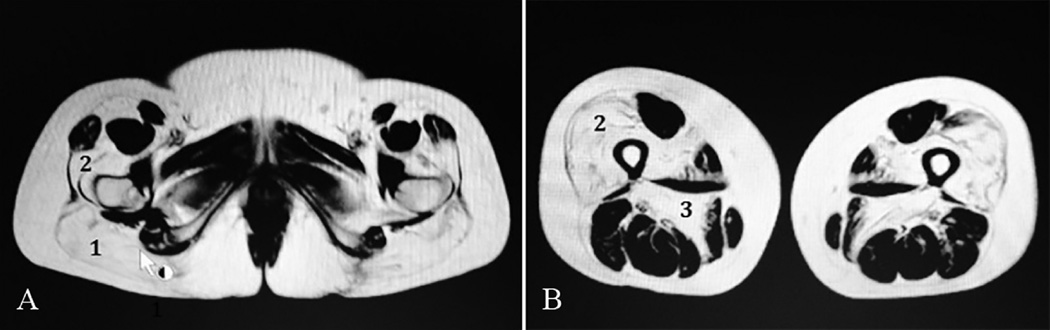
Serum CK values were invariably modestly increased. Electromyography (EMG) showed a mixed myopathic and neurogenic pattern in patient 2, but a clear myopathic pattern in patient 4. Muscle MRI studies were performed and a representative image of lower limbs in patient 5 shows fatty acid replacement of the major gluteus (Fig. 1A) and of both vastus lateralis and adductor magnus muscles (Fig. 1B).
Fig. 1.
Muscle MRI from Patient 5 shows fatty replacement at hip level (A), of notably the major gluteus (1) and vastus lateralis (2), and at thigh level (B), of notably the vastus lateralis (2) and the adductor magnus (3).
3.2. Morphological and biochemical analysis
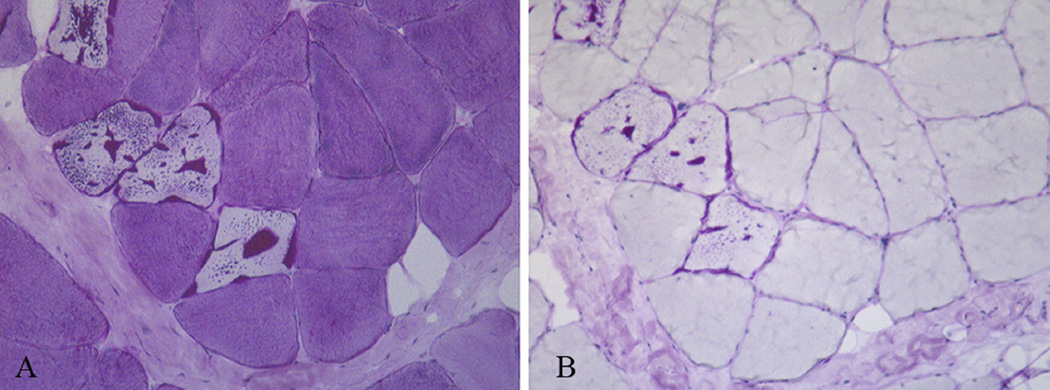
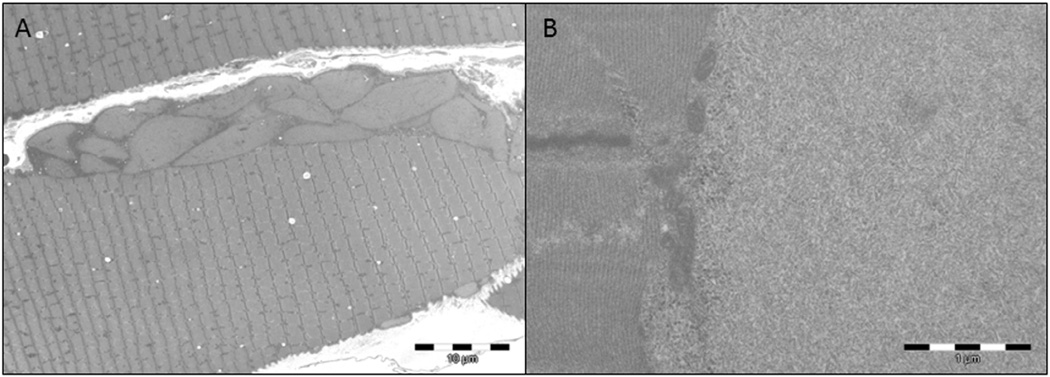
Light and electron microscopic investigations of muscle revealed characteristic alterations. Thirty percent of the fibers harbored vacuoles in the central and subsarcolemmal areas, containing intense PAS-positive inclusions, often surrounded by a halo of less intense PAS-positive material: treatment of muscle fibers with extensive digestion by α-amylase resulted in residual PAS-positive polyglucosan whereas the less intense PAS-positive normal glycogen disappeared (Fig. 2A,B). Electron microscopy revealed the typical polyglucosan structure, consisting of ovoid structure composed of partly filamentous material. A thin rim of normal glycogen particles and normal-looking mitochondria surrounded the polyglucosan bodies (Fig. 3A,B). Muscle biopsy from Patient 5 failed to show inclusions both in light and electron microscopy.
Fig. 2.
Serial sections of vastus lateralis muscle stained strongly with PAS reaction but incompletely digested by alpha-amylase.
Fig. 3.
Electron microscopy shows presence of subsarcolemmal abnormal glycogen material corresponding to polyglucosan bodies (A). At higher magnification, polyglucosan bodies are composed of poorly organized filamentous material (B).
Biochemical analyses in frozen muscle biopsy from patient 1 found normal activities of the following enzymes: acid α-glucosidase, glycogen branching, all glycogen breakdown and glycolytic enzymes (including phosphofructokinase).
3.3. Molecular genetic analysis
The presence of abundant normal glycogen in addition to polyglucosan suggested us to sequence the gene (AGL) encoding glycogen debranching enzyme, which resulted normal.
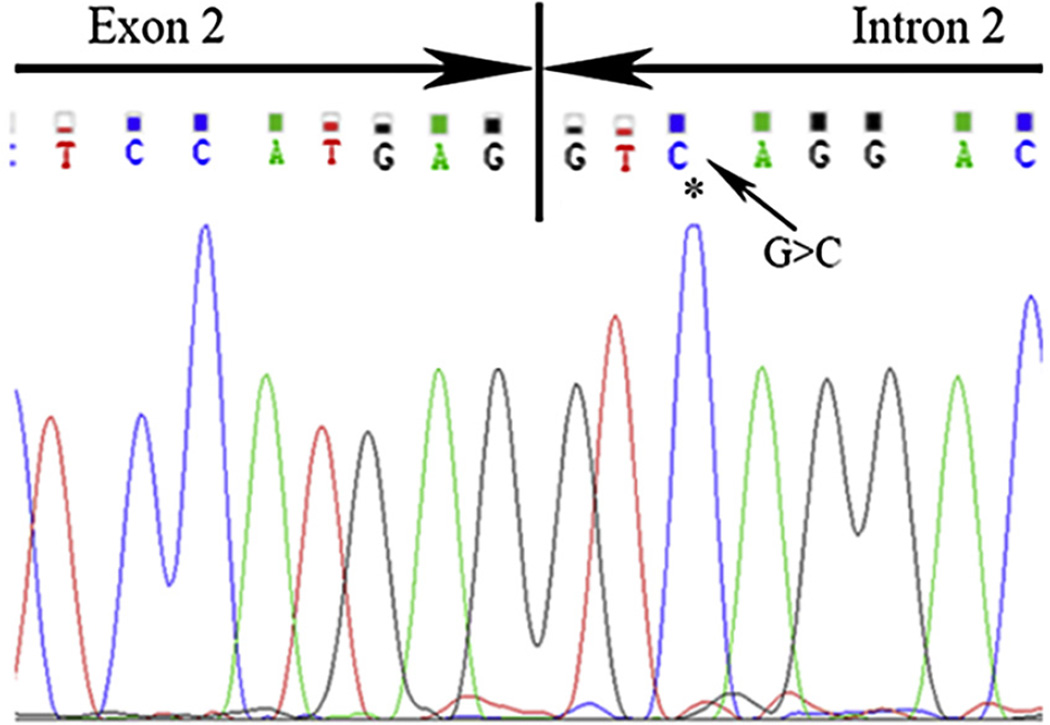
Next we analyzed glycogenin, which has been shown to cause polyglucosan in muscle [7]. A single homozygous pathogenic variant of GYG1 is identified in all five patients (Table 1) and consists of an intronic (c,143 + 3G > C) change (Fig. 4). This intronic change on a splice donor site has altered proper splicing according to the data reported in a previous paper [7].
Fig. 4.
Intronic mutation (c.143 + 3G > C) indicated by arrow causes aberrant splicing of the GYG1 mRNA.
4. Discussion
Our findings in the five patients confirm that a common autosomal recessive intronic GYG1 mutation causes (or is associated with) late-onset polyglucosan myopathy as recently reported in ten unrelated patients with different ethnic backgrounds and harboring pathogenic GYG1 variants [7–9].
The clinical phenotype is homogenous, consisting in late-onset mildly progressive limb-girdle weakness. Patient 3 showed distal involvement, which contrasts with that reported in a previous paper [7], where two patients (P2, P7) showed distal leg involvement, and one (P6) showed uniquely hands and fingers muscle weakness. Of note, a revealing symptom in P1 and P5 was exercise intolerance. P5 differed from the other patients also because she had a mildly myopathic muscle biopsy without inclusions, but MRI axial views of her pelvic and proximal thighs clearly showed fatty replacement of muscles as indicated in recently reported patients [8,9]. Exercise intolerance is a relatively new clinical finding of GYG1-related polyglucosan body myopathy, reported also in a recent paper [9].
By contrast, the firstly described GYG1 patient with glycogenosis type 0 illustrated a different clinical phenotype, characterized by young-onset cardiomyopathy and exercise intolerance, without PAS-positive inclusions but profound glycogen depletion in his muscle biopsy and accumulation of abnormal glycogen in his heart. In further contrast to the patients with GYG1-related polyglucosan body myopathy, the patient with glycogenosis type 0 had more abundant residual glycogenin-1 [6].
Very interestingly, we have identified the same mutation in all the patients. This intronic variant affecting the splice donor site and blocking glycogenin-1 biosynthesis segregated with the disease.
Polyglucosan body myopathy is but one example of several polyglucosan storage diseases, some of which are well known, including fatal infantile GSD IV or adult polyglucosan body disease (APBD) [2,11], some cases of GSD VII [12], teen age-onset myoclonus epilepsy (Lafora disease) [13], and a new form of polyglucosan myopathy with cardiomyopathy due to mutations in RBCK1 gene [14].
Several pathogenic mechanisms have been associated with polyglucosan accumulation. An obvious pathogenic mechanism was associated with the imbalanced ratio of the two glycogen synthetic enzymes, glycogen synthase (GYS) and glycogen branching (GBE), favoring the advantage of GYS over GBE activity. An obvious polyglucosan deposit in multiple tissues is due to loss of GBE activity due to the polymorphic phenotypes associated with GSD IV. However, a subtler GYS/GBE imbalance was identified by excessive activity of GYS-1, which caused less conspicuous polyglucosan deposit in skeletal muscle from patients with phosphofructokinase (PFK) deficiency (GSD VII, Tarui disease) [15]. This was due to accumulation in muscle of glucose-6-phosphate (G6P), an upstream substrate of PFK deficiency and a physiologic activator of GYS-1. This phenomenon was documented later by serendipitously generating transgenic mice with deficient acid maltase and with upregulation of GYS-1, resulting in abundant polyglucosan generation in skeletal muscle [16]. A final discovery of a gain-of-function mutation in equine GYS-1 revealed the polyglucosan myopathy in some breeds of horses [17].
Different pathogenic mechanisms were involved in two very similar disorders. APBD and Lafora disease both cause severe polyglucosan deposition mostly in central and peripheral nervous systems, but also in other tissues, including skeletal muscle. APBD is due to two common GBE1 founder mutations in Ashkenazi Jewish patients [18], but the most common mutations due to Lafora disease affect two distinct genes, one (EPM2A) encoding the laforin protein, a glycogen phosphatase, the other (EPM2B) encoding the malin protein, a ubiquitin 3 ligase [13]. The pathogenesis of polyglucosan accumulation in Lafora disease is not completely clear but there is good evidence that mutation in the glucan phosphatase laforin leads to hyperphosphorylated glycogen, which, in turn, leads to polyglucosan formation. Mutations in malin, a ubiquitin ligase, may impede the normal removal of laforin and excess laforin also causes polyglucosan formation [19].
Interestingly, a new form of polyglucosan storage disease, either limited to muscle or involving heart and muscle, was defined genetically by mutations in another ubiquitin ligase gene, RBCK1, affecting ten patients from eight families [14]. The ten patients were characterized by a juvenile-onset myopathy but eight of them had a rapidly progressive cardiomyopathy, which required cardiac transplantation in four. Notably, some patients with mutations in the same gene had a different phenotype, with failure to thrive, chronic autoinflammation, and recurrent episodes of sepsis [20]. They also accumulated polyglucosan in muscle, heart, and liver, but a careful genotype–phenotype correlation analysis will be needed to clarify the different tissue involvement in these allelic disorders.
Yet another problem remains to be clarified to explain the distinct clinical presentations between two allelic disorders, both due to mutations in the GYG1 gene: (i) a juvenile glycogen-depleted myopathy and a severe cardiopathy; and (ii) a late-onset myopathy with polyglucosan storage in skeletal muscle but without cardiomyopathy. The hypothesis suggested by Malfatti is based on the finding of different residual amounts of glycogenin-1 in the two conditions [7]. Abundant residual presence of glycogenin-1 would explain the complete early loss of muscle glycogen and early accumulation of abnormal glycogen in the heart. However, mixed components of normal glycogen and abundant polyglucosan are present in muscle from patients with almost absent residual glycogenin-1, which is not mandatory as a primer for glycogen synthase, although alternative primers remain to be investigated. The histopathological comparisons of polyglucosan body myopathies with different etiologies could shed some light on the comprehension of mechanisms leading to polyglucosan formation and how they contribute to muscle weakness [21].
Acknowledgements
This research has been funded by grants from the Keith B. Hayes Foundation and from the APBD Research Foundation.
References
- 1.Schochet SS, McCormick WF, Zellweger H. Type IV glycogenosis (amylopectinosis). Light and electron microscopic observations. Arch Pathol. 1970;90:354–363. [PubMed] [Google Scholar]
- 2.Robitaille Y, Carpenter S, Karpati G, DiMauro S. A distinct form of adult polyglucosan body diseases with massive involvement of central and peripheral neuronal processes and astrocytes. Brain. 1980;103:315–336. doi: 10.1093/brain/103.2.315. [DOI] [PubMed] [Google Scholar]
- 3.Mochel F, Schiffmann R, Steenweg M, et al. Adult polyglucosan body disease: natural history and key MRI findings. Ann Neurol. 2012;72:433–441. doi: 10.1002/ana.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukigara S, Liang W-C, Komaki H, et al. Muscle glycogen storage disease 0 presenting recurrent syncope with weakness and myalgia. Neuromuscul Disord. 2012;22:162–165. doi: 10.1016/j.nmd.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JM, Levandovskiy V, MacKay N, et al. Identification of a novel mutation in GSY1 (muscle-specific glycogen synthase) resulting in sudden cardiac death, that is diagnosable from skin fibroblasts. Mol Genet Metab. 2009;98:378–382. doi: 10.1016/j.ymgme.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Moslemi A-R, Lindberg C, Nilsson J, Tajsharghi H, Andersson B, Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N Engl J Med. 2010;362:1203–1210. doi: 10.1056/NEJMoa0900661. [DOI] [PubMed] [Google Scholar]
- 7.Malfatti E, Nilsson J, Hedberg-Oldfors C, et al. A new muscle glycogen storage disease associated with glycogenin-1 deficiency. Ann Neurol. 2014;76:891–898. doi: 10.1002/ana.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo I, Pagliarani S, Testolin S, et al. Longitudinal follow-up and muscle MRI pattern of two siblings with polyglucosan body myopathy due to glycogenin-1 mutation. J Neurol Neurosurg Psychiatry. 2015 doi: 10.1136/jnnp-2015-310553. (in press). [DOI] [PubMed] [Google Scholar]
- 9.Luo S, Zhu W, Yue D, et al. Muscle pathology and whole-body MRI in a polyglucosan myopathy associated with a novel glycogenin-1 mutation. Neuromuscul Disord. 2015;25:780–785. doi: 10.1016/j.nmd.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Malfatti E, Olivé M, Taratuto AL, et al. Skeletal muscle biopsy analysis in reducing body myopathy and other FHL1-related disorders. J Neuropathol Exp Neurol. 2013;72:833–845. doi: 10.1097/NEN.0b013e3182a23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochel F, Knight MA, Tong W-H, et al. Splice mutation in the iron-sulfur cluster scaffold protein ISCU causes myopathy with exercise intolerance. Am J Hum Genet. 2008;82:652–660. doi: 10.1016/j.ajhg.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malfatti E, Birouk N, Romero NB, et al. Juvenile-onset permanent weakness in muscle phophofructokinase deficiency. J Neurol Sci. 2012;316:173–177. doi: 10.1016/j.jns.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Lohi H, Turnbull J, Zhao XC, et al. Genetic diagnosis in Lafora disease. Neurology. 2007;68:996–1001. doi: 10.1212/01.wnl.0000258561.02248.2f. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson J, Schoser BGH, Laforet P, et al. Polyglucosan body myopathy caused by defective ubiquitin ligase RBCK1. Ann Neurol. 2013;74:914–919. doi: 10.1002/ana.23963. [DOI] [PubMed] [Google Scholar]
- 15.Hays AP, Hallett M, Delfs J, et al. Muscle phosphofructokinase deficiency: abnormal polysaccharide in a case of late-onset myopathy. Neurology. 1981;31:1077–1086. doi: 10.1212/wnl.31.9.1077. [DOI] [PubMed] [Google Scholar]
- 16.Raben N, Danon MJ, Lu N, et al. Surprises of genetic engineering: a possible model of polyglucosan body disease. Neurology. 2001;56:1739–1745. doi: 10.1212/wnl.56.12.1739. [DOI] [PubMed] [Google Scholar]
- 17.McCue ME, Valberg SJ, Miller MB, et al. Glycogen synthase (GYS1) mutation causes a novel skeletal muscle glycogenosis. Genomics. 2008;91:458–466. doi: 10.1016/j.ygeno.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akman HO, Kakhlon O, Coku Y, et al. Deep intronic GBE1 mutation in manifesting heterozygous patients wit adult polyglucosan body disease. JAMA Neurol. 2015;72:441–445. doi: 10.1001/jamaneurol.2014.4496. [DOI] [PubMed] [Google Scholar]
- 19.DePaoli-Roach AA, Tagliabracci VS, Segvich DM, Meyer CM, Irimia JM, Roach PJ. Genetic depletion of the malin E3 ubiquitin ligase in mice leads to Lafora bodies and accumulation of insoluble laforin. J Biol Chem. 2010;285:25372–25381. doi: 10.1074/jbc.M110.148668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boisson B, Laplantine E, Prando C, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedberg-Oldfors C, Oldfors A. Polyglucosan storage myopathies. Mol Aspects Med. 2015 doi: 10.1016/j.mam.2015.08.006. [DOI] [PubMed] [Google Scholar]