Abstract
Objective
To identify disparities in utilization of end of life (EoL) resources by gynecologic oncology (GO) patients.
Methods
This retrospective analysis of the medical records of GO patients treated 1/2007–12/2011 and deceased 1/2012 – 8/2014 evaluated patient demographics, disease characteristics, and utilization of EoL resources. Chi-square, Fisher’s exact test, Mann Whitney and Kruskal-Wallis tests were used for statistical analysis.
Results
Of 189 patients analyzed, 113 (60%) were white, 38 (20%) Hispanic, 31 (16%) black, and seven (4%) Asian. Ninety-five (48%) had ovarian cancer, 51 (26%) uterine, 47 (23%) cervical, seven (3%) vulvar/vaginal. In the last 30 days of life (DoL), 18 (10%) had multiple hospital admissions, 10 (5%) admitted to the Intensive Care Unit (ICU), 30 (16%) multiple Emergency Room (ER) visits, 45 (24%) received aggressive medical care and eight (4%) received chemotherapy in the final 14 DoL. Furthermore, 54 (29%) had no Supportive Care referral and 29 (15%) no hospice referral. Only 46 (24%) had a Medical Power of Attorney (PoA) or Living Will (LW) on file.
Non-white race was associated with increased odds of dying without hospice (OR 3.07; 95%CI [1.27, 2.46], p=0.013). However, non-white patients who enrolled in hospice did so earlier than white patients (42 v. 27 days before death, p=0.054). Non-white patients were also significantly less likely to have PoA/LW documentation (24% v. 76%, p=0.009) even if enrolled in hospice (12% v. 31%, p=0.007).
Conclusions
Significant racial disparities in hospice enrollment and PoA/LW documentation were seen in GO patients. This warrants further study to identify barriers to use of EoL resources.
Introduction
The complex fields of palliative care and hospice are becoming increasingly important components of healthcare. Within the United States, cancer remains a leading cause of death.[1] For patients with cancer, there is often an opportunity to prepare for and to plan in advance for death. The World Health Organization defines palliative care as “an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness.”[2] Currently, palliative care and hospice services are underutilized in the end of life care planning process. A 2012 retrospective review of 215,311 Medicare patients with cancer found that only 54% of patients received a hospice referral at any time prior to death.[3] Of those Medicare patients who were referred to hospice prior to death, eight percent had hospice care initiated a mere three days prior to death.[3] This same study found that 65% of patients were hospitalized within the last month of life, 25% were admitted to the ICU in the last month of life, 15% received chemotherapy in the last two weeks of life and 15% underwent a life-prolonging procedure within the last month of life.[3]
There is evidence to support avoiding such aggressive care at the end of life. A study by Wright et al. found that patients receiving aggressive interventions near the end of life were more likely to report a poor quality of life and their caregivers were more likely to suffer greater bereavement.[4] When providers and patients discussed the goals of end of life care, there was a decrease in aggressive interventions such as ventilation, resuscitation and ICU stays and a simultaneous increase in hospice enrollment.[4] Importantly, patients engaging in such conversations did not experience increased depression or anxiety.[4] In addition, more invasive care does not necessarily lead to longer survival. A 2015 study by Lee et al. of over 600 patients with cancer found that patients who utilized palliative care services for longer prior to death had increased overall survival.[5]
Optimizing the quality of end of life care is an important component of caring for oncology patients. Accordingly, several medical organizations, including the American Society of Clinical Oncology Quality Oncology Practice Initiative®, the Physician Consortium for Performance Improvement® and the National Quality Forum have created quality of care guidelines urging against intensive and invasive medical care at the end of life.[6–8] Many researchers use the following markers identified by the National Quality Forum to characterize what constitutes aggressive medical care at the end of life: chemotherapy administration within the last 14 days of life, more than one emergency room visit in the last 30 days of life, more than one hospital admission in the last 30 days of life, more than 14 days spent admitted to the hospital in the last 30 days of life, intensive care unit (ICU) admission in the last 30 days of life, death in the hospital, and hospice admission during the last three days of life.[7, 9–11] These organizations have also recommended completing advance directives and referrals to palliative care and hospice in a timely manner prior to death.[6–8]
Barriers to utilizing hospice and palliative care services do not affect all patients equally. Studies suggest that racial and socioeconomic factors hinder appropriate utilization of palliative care and hospice.[12–15] When compared to white patients, minorities from lower socioeconomic groups experience reduced rates of advance directive formation, increased likelihood of being hospitalized in their final 90 days of life, increased ICU admissions, increased ER visits and decreased likelihood of hospice enrollment at the end of life.[12–14]
Utilization of palliative care services and maintenance of an optimal quality of life have an important role in treating gynecologic oncology patients. Understanding what disparities exist is necessary in order to better meet the needs of these patients and their caregivers. Much of the current literature analyzing end of life resource utilization among gynecologic oncology patients examines outcomes and patterns of care from five or more years ago or focuses on a single disease site.[15–17] Our objective was to identify current disparities in utilization of palliative care and hospice resources among all gynecologic oncology patients.
Methods
After obtaining Institutional Review Board approval, we performed a retrospective analysis of the medical records of gynecologic oncology patients treated at The University of Texas MD Anderson Cancer Center (MDACC) from January 2007 through December 2012 and deceased from January 2012 through August 2014. The end point of August 2014 was chosen because initiatives to raise awareness of end of life quality care goals were launched at the institution after this date and patients receiving care after this time period should be part of a separate analysis. The electronic medical record was reviewed for patient demographics including age at death, self-reported racial identification, relationship status, education level, insurance type, zip code, medical comorbidities, parity, religious affiliation, disease stage and pathology, type of therapy received (surgery, chemotherapy and/or radiation treatment), treatment course including the length of time between diagnosis and death and the length of time between the last cancer recurrence and death, and whether or not the patient underwent consultation for enrollment in a Phase I trial. Median household income was calculated based on publically available United States census information associated with a patient’s zip code. The medical record was also reviewed for end of life quality of care metrics including utilization of palliative care (referred to as Supportive Care at this institution), timing of enrollment in hospice, location of death, number of ER visits in the final 30 days of life, hospital admissions in the final 30 days of life, ICU admissions in the final 30 days of life, receipt of chemotherapy in final 14 days of life and completion of advance directives including a Living Will, Medical Power of Attorney, and Do Not Resuscitate (In-hospital and Out-of-Hospital) order. The aforementioned quality of care metrics used to evaluate care at the end of life were derived from recommendations from the National Quality Forum and the American Society of Clinical Oncology Quality Oncology Practice Initiative ® and from previously published works.[6, 7, 18, 19] Patients were excluded from analysis if they had not received consistent treatment at MDACC but had only been seen for consultation purposes or if they had transferred their care to another institution prior to death. Patients were also excluded if the medical documentation was incomplete rendering the reviewer unable to analyze the medical care received during the last three months of life. Differences between groups for categorical variables were evaluated using Chi-square test, and Fisher’s exact test when necessary as needed. Mann Whitney and Kruskal-Wallis tests were used to assess differences between groups for continuous variables. Univariate logistic regression analysis was performed to assess the association between key independent variables and the dependent variables of interest. Variables found to be significant at the p=0.25 level by univariate analysis were included in the multivariate analysis. P-values of <0.05 were considered statistically significant.
Results
The initial query of the medical records at the MDACC identified 877 patients with a gynecologic malignancy seen at least once from January 2007 through December 2011 and who died from January 2012 through August 2014. Of these 877 patients, 688 patients were excluded from analysis due to being seen only in consultation and not receiving continuing treatment at MDACC, transferring care prior to death, or incomplete documentation of medical care during the final three months of life. Characteristics of the remaining 189 patients are shown in Table 1. Of the 189 charts analyzed, 113 (60%) of the patients were white, 38 (20%) were Hispanic, 31 (16%) were black, and seven (4%) were Asian. Eighty-six (45%) of the patients had ovarian cancer, 51 (27%) had uterine cancer, 38 (20%) had cervical cancer and five (3%) had vulvar or vaginal cancer and nine (5%) patients had more than one type of cancer. Median household income, calculated from Census Bureau data by zip code, was $54,600. Over half of the study population had private insurance (56%) and just under one-third of patients were Medicare beneficiaries (31%). The remaining patients were Medicaid recipients (5%), self-pay (4%), or were uninsured (4%). Most patients, 120 (63%), had received at least a high school education (63%) and 87 (46%) had undergone consultation for consideration in a Phase I trial.
Table 1.
Patient Characteristics
| Characteristic | Patients N =189 (%) |
|---|---|
|
| |
| Age: | |
| Median (Range) | 56 (21 – 85) |
|
| |
| Race/Ethnicity: | |
| Caucasian | 113 (60) |
| Hispanic | 38 (20) |
| African American | 31 (16) |
| Asian | 7 (4) |
|
| |
| Cancer Type: | |
| Ovary | 86 (45) |
| Uterus | 51 (27) |
| Cervix | 38 (20) |
| Vagina/Vulva | 5 (3) |
| Cervix and Uterus | 2 (1) |
| Ovary and Uterus | 7 (4) |
|
| |
| Household Income: | |
| Median (Range) | $54,630 ($18K–157K) |
|
| |
| Insurance: | |
| Private | 107 (56) |
| Medicare | 58 (31) |
| Medicaid | 10 (5) |
| Self-pay | 7 (4) |
| Uninsured | 7(4) |
|
| |
| Education: | |
| Grade School | 10 (5) |
| Some High School | 19 (11) |
| High School | 42 (22) |
| Some College | 32 (17) |
| Bachelors Degree | 29 (15) |
| Masters Degree | 4 (2) |
| Vocational School | 12 (6) |
| Advanced Degree | 13 (7) |
| Unknown | 28 (15) |
|
| |
| Phase I Trial Involvement | 87 (46) |
| Evaluated for Phase I Trial | |
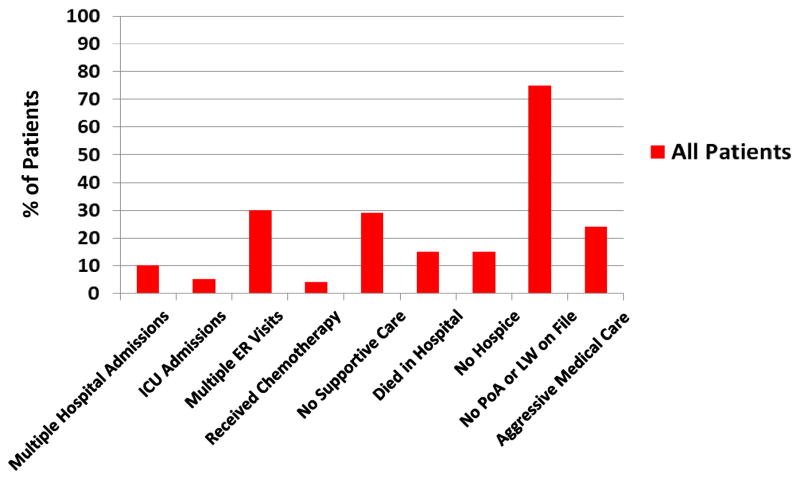
As shown in Figure 1, among all gynecologic oncology patients during the final 30 days of life, 10% had multiple hospital admissions, five percent were admitted to the ICU and 16% had multiple ER visits. Four percent received chemotherapy in the final 14 days of life, 29% had no Supportive Care referral and 15% died without a referral to hospice. Fifteen percent died in the hospital while 84% died in hospice. Of those who enrolled in hospice, however, 15 (8%) enrolled in hospice in the final three days of life or less. Of note, 11% (n=3) of the patients who died while admitted to the hospital had attempted to enroll in hospice during that admission but passed away prior to doing so. Median hospice enrollment was 21.5 days prior to death. Overall, 45 (24%) patients received aggressive medical care during the final 30 days of life. Three-quarters of patients, 144 (76%), did not have a Medical Power of Attorney or Living Will on file.
Figure 1.
Utilization of End of Life Care (All Patients)
Median household income was not associated with significant differences in Supportive Care or hospice usage nor in documentation of Medical Power of Attorney or Living Will. Whether or not a patient underwent Supportive Care consultation, received chemotherapy in the final 14 days of life, or had multiple ER visits, ICU and hospital admissions or aggressive medical care in the final 30 days of life did not vary by race, median household income, educational level, marriage status, insurance type or type of therapy (surgery, chemotherapy and/or radiation treatment). As would be expected, hospice enrollees had less aggressive care including chemotherapy (2% v. 16%, p=0.003), ER visits (13% v 29%, p=0.03), hospital admissions (8% v. 19%, p=0.04) and ICU admission (3% v. 19%, p=0.002) during the final 30 days of life.
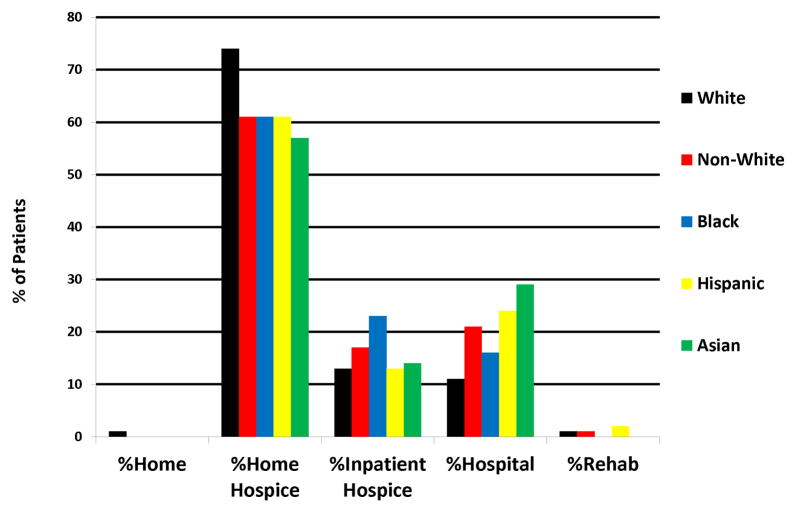
Figure 2 shows the location of death of all patients by race. Most patients, regardless of race, preferred to pass away at home with 74% of white patients (n=113), 61% of black patients (n=31), 61% of Hispanic patients (n=38) and 57% of Asian patients (n=4) doing so. Black patients had the highest percentage of inpatient hospice deaths (23%) and Asian patients the highest percentage of hospital deaths (29%).
Figure 2.
Location of Death by Race
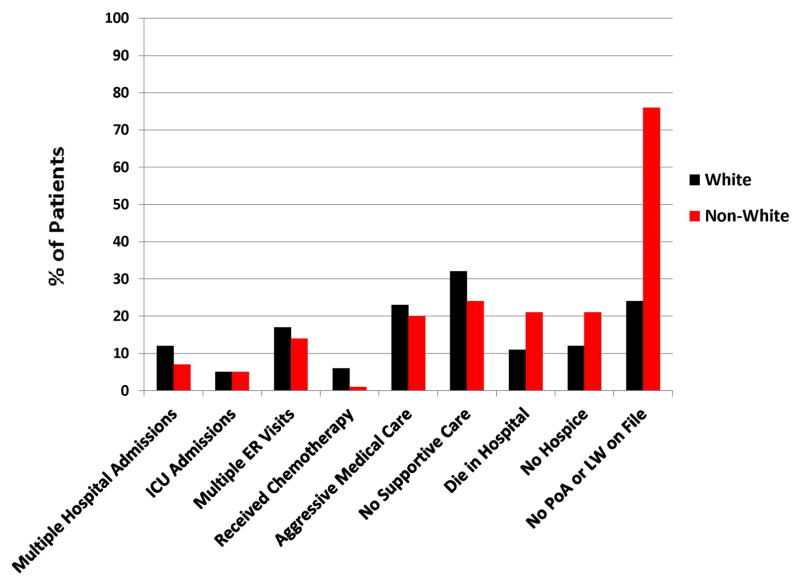
Figure 3 shows the end of life care quality indicators by race. There were no significant associations found between the following outcomes and patient race through univariate analysis: multiple hospital admissions (12% v. 7%, p=0.27), ICU admissions (5% v. 5%, p=0.99), ER visits (17% v. 14%, p=0.27), receipt of chemotherapy (6% v. 1%, p=0.10), aggressive medical care (23% v. 20%, p=0.5) and lack of Supportive Care usage (32% v. 24%, p=0.22). However, after controlling for race, marital status, education and whether or not the patient underwent consultation for enrollment in a Phase I trial in the multivariate analysis, non-white race was associated with increased odds of dying without hospice (21% v. 11%) (OR 3.07; 95%CI [1.27, 2.46], p=0.01). Interestingly, non-white patients who enrolled in hospice did so earlier than white patients (42 v. 27 days before death, p=0.054). In multivariate analysis, non-white patients were also significantly less likely to have documentation of Medical Power of Attorney or Living Will documentation (24% v. 76%, p=0.009) even if enrolled in hospice (12% v. 31%, p=0.007). Tables 2 and 3 summarize the univariate and multivariate logistic regression analyses described above.
Figure 3.
Utilization of End of Life Care by Race
Table 2.
Univariate and Multivariate Logistic Regression for Dying without Hospice
| Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR | 95%CI | P | OR | 95%CI | P |
|
| ||||||
| Race | ||||||
| White | - | - | - | - | - | - |
| Non-white | 2.04 | 0.94, 4.43 | .07 | 3.07 | 1.27, 7.46 | 0.01 |
|
| ||||||
| Marital status | ||||||
| Married | - | - | - | |||
| Not married | 1.67 | 0.77, 3.64 | .19 | |||
|
| ||||||
| Education | ||||||
| ≤ High school graduate | - | - | - | - | - | - |
| > High school education | 2.24 | 0.80, 6.33 | .13 | 2.93 | 0.99, 8.62 | 0.05 |
|
| ||||||
| Consultation for Phase 1 trial | ||||||
| Yes | - | - | - | |||
| No | 1.69 | 0.27, 1.32 | .20 | |||
|
| ||||||
| Age (years) | 1.01 | 0.98, 1.05 | 0.52 | |||
|
| ||||||
| Insurance | 0.99 | |||||
| Medicare/Medicaid | - | - | - | |||
| Private insurance | 0.88 | 0.39, 1.98 | 0.76 | |||
| Self-pay | 0.78 | 0.09, 7.07 | 0.82 | |||
| Uninsured | 0.78 | 0.09, 7.07 | 0.82 | |||
|
| ||||||
| Have children | ||||||
| Yes | - | - | - | |||
| No | 0.38 | 0.16, 2.00 | 0.38 | |||
|
| ||||||
| English-speaking | ||||||
| Yes | - | - | - | |||
| No | 1.43 | 0.38, 5.46 | 0.60 | |||
|
| ||||||
| Disease site | 0.39 | |||||
| Cervix | - | - | - | |||
| Uterus | 0.86 | 0.30, 2.47 | 0.78 | |||
| Ovary | 0.59 | 0.22, 1.59 | 0.30 | |||
| Vulva/vagina | 2.67 | 0.38, 18.74 | 0.32 | |||
Final multivariate regression after backwards stepwise elimination of non-significant covariates
Table 3.
Univariate and Multivariate Logistic Regression for Having an Advance Directive on File
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR | 95%CI | P | OR | 95%CI | P |
|
| ||||||
| Race | ||||||
| White | - | - | - | - | - | - |
| Non-white | 0.37 | 0.18, 0.79 | .01 | 0.40 | 0.18, 0.85 | 0.02 |
|
| ||||||
| Marital status | ||||||
| Married | - | - | - | |||
| Not married | 1.56 | 0.79, 3.07 | .20 | |||
|
| ||||||
| Education | ||||||
| ≤ High school graduate | - | - | - | |||
| > High school education | 1.49 | 0.66, 3.35 | .34 | |||
|
| ||||||
| Consultation for Phase 1 trial | ||||||
| Yes | - | - | - | |||
| No | 1.01 | 0.52, 1.96 | .99 | |||
|
| ||||||
| Age (years) | 1.04 | 1.01, 1.07 | 0.02 | 1.04 | 1.01, 1.07 | 0.02 |
|
| ||||||
| Insurance | 0.71 | |||||
| Medicare/Medicaid | - | - | - | |||
| Private insurance | 0.72 | 0.36, 1.43 | 0.39 | |||
| Self-pay | 0.39 | 0.04, 3.47 | 0.00 | |||
| Uninsured | 0.00 | 0.00, - | 1.00 | |||
|
| ||||||
| Have children | ||||||
| Yes | - | - | - | |||
| No | 1.27 | 0.52, 3.12 | 0.60 | |||
|
| ||||||
| English-speaking | ||||||
| Yes | - | - | - | |||
| No | 0.83 | 0.22, 3.12 | 0.78 | |||
|
| ||||||
| Disease site | 0.47 | |||||
| Cervix | - | - | - | |||
| Uterus | 2.14 | 0.74, 6.21 | 0.16 | |||
| Ovary | 2.11 | 0.79, 5.65 | 0.14 | |||
| Vulva/vagina | 1.42 | 0.13, 14.96 | 0.77 | |||
Discussion
In this analysis, we found significant racial disparities in hospice enrollment and documentation of Medical Power of Attorney and Living Will among gynecologic oncology patients. With respect to hospice enrollment, after adjusting for race, marital status, education and whether or not the patient underwent consultation for enrollment in a Phase I trial, we found that non-white race was associated with three-fold higher odds of never enrolling in hospice prior to death. An in-depth explanation for this observation is beyond the scope of this paper; however, other studies have suggested that disparities in hospice enrollment are a result of distrust of the health care system by minorities and a lack of education regarding the role of palliative care and hospice.[16, 20] Rosenfeld et al. found that one-third of terminally ill minority patients believed that they would have received superior care if they had been of another race and associated this distrust with a reluctance to enroll in hospice.[20] In this same study, the authors also found that the majority of patients reported wishing to die at home (75%) and to be pain-free and comfortable (75%).[20] However, despite wishing to die pain-free and comfortable, over half (53%) of these patients reported that they would want continued medical treatment up until the end of life even if it meant diminishing comfort and increasing pain.[20] These are contradicting goals of care and may result from a lack of communication among clinicians, patients and caregivers, as well as a possible lack of education about end of life care. Among the patients who participated in the survey, none of them reported having had a discussion regarding hospice with their providers.[20]
Educating patients and medical providers has the potential to improve palliative care and hospice utilization.[21] This was demonstrated by an educational and training initiative of the Veterans Association which increased veterans’ utilization of palliative care services from 29% to 42% and hospice enrollment from eight percent to 22% over a three year-period.[21] While we identified that minority gynecologic oncology patients are especially at risk of not receiving hospice care at our institution, it is important to note that 29% of all patients included in our analysis never received a Supportive Care referral prior to death and 15% never received a hospice referral prior to death. These are potential areas to improve education for all patients and medical providers with additional effort spent on reaching minorities.
We found an even larger racial disparity in the documentation of Medical Power of Attorney and Living Will. Seventy-six percent of non-white patients did not have a Medical Power of Attorney or a Living Will on file, compared to just 24% of white patients. Furthermore, this disparity was not corrected even after enrollment in hospice, though it did lessen to 31% of non-white patients not having the proper documentation compared to 12% of white patients. This persistent disparity suggests that hospice utilization and completion of advance directives require separate educational efforts in order to eliminate disparities.
One of the limitations of this analysis is that it is a retrospective review of medical records at a single, highly specialized institution and of small sample size compared to studies conducted through national Medicare databases. Therefore, the findings reported here may not be widely generalizable. This analysis depended on the degree of accuracy of documentation in the medical record. Only those patients with information available regarding events during the final months of life were available for analysis and the possibility of bias must be acknowledged. Strengths of this analysis are that, because we did not rely on national databases which have a delay in updating data, we were able to analyze outcomes from the past three years and were able to capture additional data points not included in larger databases. One of these additional data points was documentation of Medical Power of Attorney and Living Will which were not previously identified as an area of disparity for gynecologic oncology patients.
Reducing the disparities among patients who utilize hospice services and complete advance directives is a significant challenge when delivering care to women with gynecologic malignancies. We have identified an opportunity for improvement in communication and education regarding end of life care goals among clinicians, patients and caregivers. Further research is needed, however, in order to better characterize the specific barriers to utilization of palliative care and hospice services among this patient population which led to such disparities.
Highlights.
We investigated racial and socioeconomic disparities in utilization of end of life care among gynecologic oncology patients.
We found racial minorities were less likely to enroll in hospice.
Racial minorities were also less likely to complete Medical Power of Attorney and Living Will documentation.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SLBSX, Jiaquan MD, Kochanek Kenneth DMA. U.S.D.o.H.a.H.S.C.f.D.C.a.P.N.C.f.H.S.N.V.S. System, editor. National Vital Statistics Reports Deaths: Final Data for 2010. U.S. Department of Health and Human Services; 2010. http://www.cdc.gov/nchs. [Google Scholar]
- 2.Organization, W.H. WHO Definition of Palliative Care. 2014 [cited 2014 July 29]; Available from: http://www.who.int/cancer/palliative/definition/en/
- 3.Morden NE, Jacobson CCJO, Berke EM, Bynum JP, Murray KM, Goodman DC. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Affairs. 2012;31(4):786–796. doi: 10.1377/hlthaff.2011.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AAMZ, Baohui, Ray Alaka, MD, Mack Jennifer W, MD, MPH, Trice Elizabeth, MD, PhD, Balboni Tracy, MD, MPH, MItchell Susan L, MD, Jackson Vicki A, MD, MPH, Block Susan D, MD, Maciejewski Paul K, PhD, Prigerson Holly G., PhD Associations Between End-of-Life Discussions, Patient Mental Health, Medical Care Near Death, and Caregiver Bereavement Adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YJ, YJ, Lee JW, Yoon J, Nah JR, Choi WS, Kim CM. Association between the duration of palliative care service and survival in terminal cancer patients. Support Care Cancer. 2015;23(4):1057–62. doi: 10.1007/s00520-014-2444-4. [DOI] [PubMed] [Google Scholar]
- 6.Oncology, A.S.o.C. Quality Oncology Practice Initiative. [cited 2015#x0005D; Available from: http://qopi.asco.org/program.html.
- 7.Forum, N.Q. National Voluntary Consensus Standards for Quality of Cancer Care. 2009 May; Available from: http://www.qualityforum.org/Publications/2009/05/National_Voluntary_Consensus_Standards_for_Quality_of_Cancer_Care.aspx.
- 8.Association, A.M. Physician Consortium for Performance Improvement. [cited 2015] Available from: https://www.ama-assn.org/ama/pub/physician-resources/physician-consortium-performance-improvement/about-pcpi.page?
- 9.Mack JW, CA, Keating NL, Taback N, Huskamp HA, Malin JL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. Journal of Clinical Oncology. 2012;30(35):4387–95. doi: 10.1200/JCO.2012.43.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Acevedo M, HL, Broadwater G, Kamal AH, Abernethy AP, Berchuck A, et al. Timing of end-of-life care discussion with performance on end-of-life quality indicators in ovarian cancer. Gynecologic Oncology. 2013 doi: 10.1016/j.ygyno.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 11.von Gruenigen V, DB, Gibbons H, Hutchins J, Green A. Indicators of survival duration in ovarian cancer and implications for aggressiveness of care. Cancer. 2008;112(10):2221–7. doi: 10.1002/cncr.23391. [DOI] [PubMed] [Google Scholar]
- 12.Frahm K, Brown LM, Hyer K. Racial disparities in end-of-life planning and services for deceased nursing home residents. J Am Med Dir Assoc. 2012;13(9):7–11. doi: 10.1016/j.jamda.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Frahm K, Brown LM, Hyer K. Racial Disparities in Receipt of Hospice Services Among Nursing Home Residents. Am J Hosp Palliat Care. 2013 doi: 10.1177/1049909113511144. [DOI] [PubMed] [Google Scholar]
- 14.Naya P, Qiu F, Watanabe-Galloway S, Boilesen E, Wang H, Lander L, Islam M. Disparities in End of Life Care for Elderly Lung Cancer Patients. J Community Health. 2014 doi: 10.1007/s10900-014-9850-x. [DOI] [PubMed] [Google Scholar]
- 15.Fairfield KM, KM, Wierman HR, Han PK, Hallen S, Miesfeldt S, Trimble EL, Warren JL, Earle CC. Disparities in hospice care among older women dying with ovarian cancer. Gynecologic Oncology. 2012;126(3):509–510. doi: 10.1016/j.ygyno.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 16.Wright AAH, Laura A, Earle Craig C, Keating Nancy L. End-of-Life Care for Older Patients With Ovarian Cancer Is Intensive Despite High Rates of Hospice Use. JCO. 2014;55:5383. doi: 10.1200/JCO.2014.55.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown AJS, Charlotte C, Prescott Lauren S, Taylor Jolyn S, Ramondetta Lois M, Bodurka Diane C. Missed opportunities: Patterns of medical care and hospice utilization among ovarian cancer patients. Gynecologic Oncology. 2014;135:244–248. doi: 10.1016/j.ygyno.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earle CC, Neville Bridget A, Landrum Mary Beth, Ayanian John Z, Block Susan D, Weeks Jane C. Trends in the Aggressiveness of Cancer Care Near the End of Life. Journal of Clinical Oncology. 2004;22(2):315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 19.T.P.C.f.P.I. National Committee for Quality Assurance, editor. Palliative and End of Life Care Physician Performance Measurement Set. American Medical Association and National Committee for Quality Assurance; 2008. http://www.ama-assn.org/ [Google Scholar]
- 20.Rosenfeld P, DJ, Hanen S, et al. Are there racial differences in attitudes toward hospice care? A study of hospice-eligible patients at the Visiting Nurse Services of New York. Am JHosp & Palliat Med. 2007;24(5):408–416. doi: 10.1177/1049909107302303. [DOI] [PubMed] [Google Scholar]
- 21.Edes TSS, Casarett D. Increasing access and quality in Department of Veterans Affairs care at the end of life: a lesson in change. J Am Geriatr Soc. 2007;55(10):1645–9. doi: 10.1111/j.1532-5415.2007.01321.x. [DOI] [PubMed] [Google Scholar]