Abstract
Background
Kidney disease disproportionately affects minority populations including African Americans and Hispanics; therefore, understanding the relationship of kidney function to cardiovascular (CV) outcomes within different racial/ethnic groups is of considerable interest. We investigated the relationship between kidney function and CV events and assessed effect modification by race/ethnicity in the Women’s Health Initiative.
Study Design
Prospective cohort study
Setting & Participants
Baseline serum creatinine concentrations (assay traceable to isotope-dilution mass spectrometry standard) of 19,411 postmenopausal women aged 50–79 years who self-identified as either non-Hispanic white (n=8921), African American (n=7436), or Hispanic (n=3054) were used to calculate estimated glomerular filtration rates (eGFRs).
Predictors
Categories of eGFR (exposure); race/ethnicity (effect modifier).
Outcomes
The primary outcome was the composite of three physician-adjudicated CV events: myocardial infarction (MI), stroke, or CV-related death.
Measurements
We evaluated the multivariable-adjusted associations between categories of eGFR and CV events using proportional hazards regression and formally tested for effect modification by race/ethnicity.
Results
Over a mean follow-up of 7.6 years, 1424 CV events (653 MI, 627 strokes, 297 CV-related deaths) were observed. The association between eGFR and CV events was curvilinear; however, the association of eGFR with CV outcomes differed by race (P=0.006). In stratified analyses, we observed that the U-shaped association was present in non-Hispanic whites, whereas African American participants had a rather curvilinear relationship with lower eGFR being associated with higher CV risk and higher eGFR with reduced CV risk. Analyses among Hispanic women were inconclusive owing to few Hispanic women having very low or high eGFR and very few events occurring in these categories.
Limitations
Lack of urinary albumin measurements; residual confounding by unmeasured or imprecisely measured characteristics.
Conclusions
In postmenopausal women, the patterns of association between eGFR and CV risk differed between non-Hispanic whites and African American women.
INDEX WORDS: cardiovascular disease (CVD), CV risk, CV events, myocardial infarction (MI), stroke, CV death, renal function, estimated glomerular filtration rate (eGFR), serum creatinine, race/ethnicity, Hispanic, African American, kidney disease, Women’s Health Initiative (WHI)
Chronic kidney disease (CKD), defined by a glomerular filtration rate (GFR) <60 ml/min/1.73m2 or an albumin-creatinine ratio (ACR) ≥ 30mg/g, affects over 25 million Americans and its prevalence is increasing.(1, 2) Both markers of kidney function are directly and independently associated with all-cause and cardiovascular (CV) mortality.(3, 4) Furthermore, evidence is accumulating that minority populations are disproportionately affected by kidney disease, with African Americans having a higher risk of CKD that is more likely to progress to end-stage renal disease (ESRD) compared to whites.(5) Faster kidney function decline and increased risk of ESRD also exist in Hispanics compared to non-Hispanics.(6, 7)
Recent evidence suggests that the association between CKD and CV outcomes may differ by race.(4, 8) While previous studies have reported gender differences in the associations of estimated glomerular filtration rate (eGFR) and albuminuria with CV and all-cause mortality, relatively little is known about the association between decreased kidney function and CV risk and its potential modification by race or ethnicity among postmenopausal women in the United States.(9–13) Because most of the existing studies have predominantly examined white women, more information is needed on women of other racial/ethnic groups.
The multiethnic Women’s Health Initiative (WHI) prospective cohort study of postmenopausal women, for whom creatinine concentrations were measured in a large subset at baseline, provides an opportunity to assess the associations of GFR with myocardial infarction (MI), stroke, and CV death. We hypothesized that these associations would differ among racial and ethnic groups.
Methods
Study Design and Population
The WHI is a large prospective, multicenter cohort study that focuses on strategies for preventing heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women aged 50–79 years recruited from September 1993 through December 1998. Subjects were post-menopausal if they had had some combination of the following: hysterectomy, double oophorectomy, conclusion of menstrual bleeding, vasomotor symptoms, or prescribed hormone therapy. The WHI study consists of an observational study cohort and three clinical trial components (a hormone therapy component, a dietary modification component, and a calcium/vitamin D component). Details of the eligibility criteria, data collection, and ascertainment of health outcomes have been reported previously (see Item S1, available as online supplementary material, for WHI clinical trial and observational study design). (14, 15)
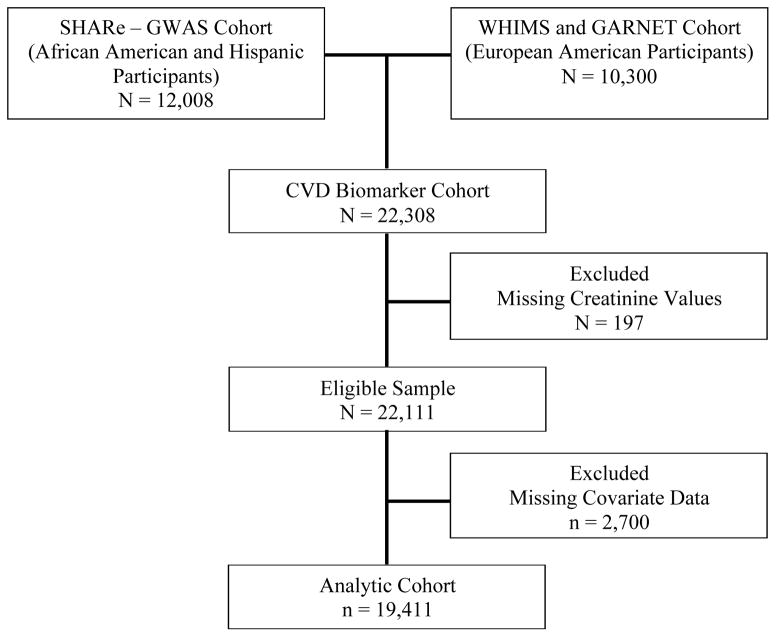
Our analysis was restricted to a subset of WHI participants who participated in the WHI Core Biomarker Studies, including 8,505 African American and 3,503 Hispanic participants who constituted the WHI Single Nucleotide Polymorphism (SNP) Health Association Resource (SHARe) cohort and 10,300 participants of European ancestry who had participated in the WHI Memory Study,(16) an ancillary study of the WHI hormone trials, or whose samples were analyzed in another WHI ancillary study, the Genomic and Randomized Trials Network (GARNET) study (Figure 1).(17) The African American and Hispanic participants in the SHARe cohort were drawn from the observational study and the clinical trial components. The participants of European ancestry were drawn from the hormone therapy components. The participants in these trials provided baseline data on various CV disease biomarkers including creatinine, cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, insulin, glucose, and C-reactive protein. After excluding participants whose serum creatinine measurements were not available (n=197) and/or those with missing data on covariates of interest (n=2,700), the final cohort included 19,411 participants. There were no clear patterns of missingness in our covariates and none of the variables we excluded had missing rates greater than 5%.
Figure 1. Flow Diagram of WHI CVD Biomarker Study Cohort.
Our analysis was restricted to a subset of WHI participants who participated in the WHI Core Biomarker Studies, including 8,505 African American and 3,503 Hispanic participants who comprised the WHI Single Nucleotide Polymorphism (SNP) Health Association Resource (SHARe) cohort and 10,300 participants of European ancestry who had participated in the WHI Memory Study or whose samples were analyzed in another WHI ancillary study, the Genomic and Randomized Trials Network (GARNET) study.(17) The African American and Hispanic participants in the SHARe cohort were drawn from the observational study and the clinical trial components. The participants of European ancestry were drawn from the hormone therapy components. The participants in these trials provided baseline data on various cardiovascular disease biomarkers including creatinine. After excluding participants whose serum creatinine measurements were not available (n=197) and/or those with missing data on covariates of interest (n=2,700), the final cohort included 19,411 participants. Abbreviations: SHARe, WHI SNP Health Association Resource; GWAS, Genome-Wide Association Study; WHIMS, WHI Memory study; GARNET, Genomic and Randomized Trials Network; CVD, cardiovascular disease.
Primary Exposure of Interest
The exposure of interest was eGFR at baseline. Stored frozen serum specimens were sent to the University of Minnesota–Fairview laboratory for measurement of baseline creatinine concentrations and other CV risk markers (see below). Serum creatinine was measured by the Roche enzymatic method on a Roche Modular P Chemistry Analyzer (Roche Diagnostic Corporation) and calibrated against an isotope-dilution mass spectroscopic standard.(18) Each patient’s GFR was then estimated using the CKD-EPI (CKD Epidemiology Collaboration) equation from her serum creatinine concentration, age, sex, and African American versus non-African American race. The CKD-EPI creatinine equation has been shown to provide a more accurate estimation of measured GFR compared with previously established creatinine-based equations.(19, 20) The eGFR was the exposure of interest in this study and, due to a small number of participants with eGFR <30 ml/min/1.73m2 (n=46; 0.24%), participants were divided into the following six eGFR strata: <45, 45–<60, 60–<75, 75–<90, 90–<105, and ≥105 ml/min/1.73m2.
Ascertainment of Outcomes
The primary outcome of interest was the composite of three CV events: MI, stroke, or death related to a CV event. Secondary outcomes of interest were the individual events that constituted the composite CV outcome. The diagnosis and adjudication of these outcomes have been previously described and outlined in established protocols (see Item S1 for CV adjudication information).(14)
Covariate of Interest: Effect Modifier
We sought to test for effect modification of the association between eGFR and CV events by self-identified race/ethnicity; participating women had the opportunity to select one of the following categories: non-Hispanic White, African American, Asian or Pacific Islander, Hispanic/Latino, American Indian or Alaskan Native, and other. It was not recorded in the WHI of what race self-identified Hispanic participants considered themselves. For the purpose of this report, we will refer to the categories as non-Hispanic white, African American, and Hispanic with the understanding that most African American women were likely non-Hispanic and most Hispanic women were white. Analyses were restricted to participants who reported one of these three categories because the participants from the SHARe, WHI Memory Study, and GARNET cohorts with data on CVD biomarkers only include women from these three categories.
Other Covariates: Demographic and Health Characteristics
Details of baseline self- or interview-administered study questionnaires, physical measurements, and blood measurement have been previously described.(14, 15) Standardized questionnaires administered at study entry ascertained sociodemographic characteristics including age, presence of diabetes, history of MI or stroke, drinking status (none, past, current), and smoking status (current, past, non-smoker). Medical history was updated annually for women in the observational study or semiannually for women in the clinical trials by mail and/or telephone questionnaires.
Trained clinical center staff obtained anthropometric measurements at the baseline clinic visit. Height was measured with a calibrated stadiometer and weight with a calibrated scale, with participants in light clothing, without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Based on their BMI, individuals were classified as being underweight (BMI of <18.5 kg/m2), normal weight (BMI of 18.5–24.9 kg/m2), overweight (BMI of 25.0 to 29.9 kg/m2) or obese (BMI of ≥30 kg/m2). Waist circumference was measured at the narrowest part of the torso and hip circumference at the maximal circumference. Seated systolic blood pressures were measured in the right arm using a conventional mercury sphygmomanometer after 5 minutes of rest, with an appropriate cuff size based on arm circumference measurement. Two blood pressure measurements were taken at least 30 seconds apart, and were averaged for the current analyses.
Physical activity (PA) at baseline was assessed using a validated 9-item measure of physical activity from the Personal Habits Questionnaire and was expressed in metabolic equivalent task (MET)-minutes per week.(21) Participants were categorized as sedentary, low, moderate, or high (0, 0.1–<5, 5–11.9, or ≥12 MET hours per week).
A quality of life measure characterizing physical function was taken from the results of a 36-Item Short Form Health Survey (SF-36), which was administered at baseline. This construct ranges from 0 to 100, with higher values corresponding to better health.(22)
In addition to creatinine concentrations, C-reactive protein, total and HDL cholesterol, and triglyceride concentrations were also measured from stored biospecimens. The Friedewald equation(23) was used to calculate LDL concentration from total cholesterol, HDL cholesterol, and triglyceride concentrations.
Statistical Analyses
The goal of our analysis was to determine the relationships between baseline eGFR and composite and individual CV events in this multiethnic cohort of postmenopausal women with particular focus on examining racial/ethnic heterogeneity in all analyses. The relationships among eGFR categories and CV events were modeled using Cox proportional hazard regression, overall as well as stratified by racial/ethnic group. Participants who did not have a CV event or who died from non-CV causes were censored at the time of death or end of study period, whichever occurred first. The eGFR category with the lowest rate of CV events was chosen to be the referent group. We fit four models to estimate the hazard ratios (HRs) for the individual and composite CV outcomes: model 1 estimated unadjusted HRs; model 2 adjusted for age (categorical); model 3 additionally adjusted for presence of diabetes, history of stroke, and history of MI; model 4 additionally adjusted for BMI, lifestyle factors (physical activity, history of smoking, and antihypertensive and alcohol use), laboratory measurements (blood pressure, C-reactive protein, and LDL and HDL cholesterol), and SF-36 physical function score. All models were stratified by cohort (i.e. observational study or clinical trial) to allow different baseline hazards for each cohort. Analyses were explored for violation of proportional hazards by stratification and inspection of the Kaplan-Meier plots for all outcomes and no apparent violations were observed. We examined log-log plots and calculated Schoenfeld residual p-values, and found no significant evidence of departure from the proportional hazards assumption. In the multivariable-adjusted Cox proportional hazards model 4, we formally tested for effect modification between race/ethnicity and eGFR category by using a Wald test to obtain an omnibus P-value for interaction between the eGFR categories and the 3 categories of race/ethnicity. Within the interaction model, we also examined the individual contrasts between African Americans versus non-Hispanic whites and between Hispanics versus non-Hispanic whites. P-values for these interaction tests were assessed at the α <0.05 level of significance.
In order to assess the robustness of our results from a complete case analytic approach, missing data were imputed using SAS’s PROC IMPUTE, and fit using the primary analysis models in a sensitivity analysis.
Participants provided written informed consent, and study procedures and protocols were approved by the institutional review boards at the National Institutes of Health and at all 40 participating institutions. Analyses were performed with SAS software, Version 9.4 (SAS Institute Inc., Cary, NC) and R 3.1.0 software (The R Foundation for Statistical Computing).
Results
Baseline Characteristics
From the WHI biomarker cohort, we identified 22,111 women aged 50–79 years who enrolled in the WHI study between 1993 and 1998 and had a valid serum creatinine concentration measured. Participants with missing covariates were excluded (n=2,700). Excluded participants were similar in age to those included (mean age, 63.5 ±7.4 (standard deviation) versus 63.9 ±7.3 years) but more likely to be non-Hispanic white (46.4 % versus 45.8%). The eGFRs were similar for excluded and included participants (86.9 ±17.3) versus 87.6 ±16.6 ml/min/1.73 m2). Excluded participants had a higher rate of CV events (9.5 vs. 7.1 per 1000 years of follow-up).
The final analytic cohort of 19,411 women included 8921 (46.0%) non-Hispanic white, 7436 (38.3%) African American, and 3054 (15.7%) Hispanic women (Figure 1). Baseline characteristics of the study participants stratified by eGFR categories and race/ethnicity are shown in Table 1. Given the large cohort, statistically significant differences were observed across racial/ethnic groups for all variables (all P<0.05). In general, participants with a lower eGFR were more likely to be older, have diabetes, and have a history of MI and stroke, and participants with lower eGFR were more likely to have dyslipidemia and higher C-reactive protein levels, irrespective of race and Hispanic ethnicity.
Table 1.
Baseline Characteristics of 19,411 Participants Stratified by eGFR Category and Racial/Ethnic Group
| Characteristic | eGFR ≥105 (n = 2702 [13.9%]) |
eGFR 90 – <105 (n = 6529 [33.6%]) |
eGFR 75 – <90 (n = 5953 [30.7%]) |
eGFR 60 – <75 (n = 3127 [16.1%]) |
eGFR 45 – <60 (n = 904 [4.7%]) |
eGFR <45 (n = 196 [1.0%]) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | H | W | AA | H | W | AA | H | W | AA | H | W | AA | H | W | AA | H | W | |
| No. of participants | 2241 | 313 | 148 | 1962 | 1569 | 2998 | 1800 | 687 | 3466 | 999 | 361 | 1767 | 335 | 106 | 463 | 99 | 18 | 79 |
| Age category | ||||||||||||||||||
| 50–59 y | 59.9 | 94.6 | 77.7 | 41.0 | 56.0 | 24.2 | 35.8 | 34.9 | 8.7 | 24.6 | 30.5 | 5.4 | 18.5 | 19.8 | 1.9 | 18 | 39 | 5 |
| 60–69 y | 37.8 | 5.4 | 20.9 | 44.3 | 40.0 | 60.5 | 46.3 | 47.3 | 43.3 | 47.8 | 47.4 | 41.9 | 45.7 | 51.9 | 34.1 | 35 | 33 | 30 |
| 70–79 y | 2.3 | 0.0 | 1.4 | 14.7 | 4.0 | 15.3 | 17.9 | 17.8 | 48.0 | 27.5 | 22.2 | 52.6 | 35.8 | 28.3 | 63.9 | 47 | 28 | 65 |
| Diabetes | 15.7 | 14.7 | 17.6 | 11.5 | 7.6 | 7.3 | 10.3 | 5.7 | 5.9 | 13.0 | 5.5 | 5.6 | 20.3 | 15.1 | 6.7 | 42 | 11 | 18 |
| History of MI | 2.2 | 0.6 | 2.7 | 2.4 | 1.1 | 2.5 | 3.6 | 1.9 | 2.9 | 4.8 | 2.5 | 3.8 | 8.4 | 3.8 | 4.8 | 11 | 6 | 10 |
| History of stroke | 1.6 | 0.6 | 0.0 | 1.9 | 1.2 | 0.8 | 2.2 | 1.5 | 1.3 | 3.0 | 2.5 | 1.7 | 3.9 | 4.7 | 1.3 | 17 | 6 | 10 |
| BMI | ||||||||||||||||||
| Underweight | 0.6 | 0.3 | 0.0 | 0.3 | 0.6 | 0.8 | 0.4 | 0.3 | 0.6 | 0.8 | 0.8 | 0.5 | 0.6 | 0.0 | 0.4 | 1 | 0 | 0 |
| Normal | 16.7 | 22.4 | 19.6 | 16.1 | 25.3 | 27.1 | 15.6 | 26.1 | 29.4 | 16.2 | 24.9 | 28.1 | 14.0 | 22.6 | 23.3 | 10 | 6 | 17 |
| Overweight | 32.3 | 33.5 | 25.0 | 33.5 | 38.8 | 32.9 | 34.4 | 41.6 | 35.8 | 33.6 | 41.0 | 36.7 | 34.6 | 29.2 | 39.1 | 36 | 44 | 29 |
| Obese | 50.4 | 43.8 | 55.4 | 50.2 | 35.3 | 39.3 | 49.6 | 32.0 | 34.5 | 49.3 | 33.2 | 34.6 | 50.7 | 48.1 | 37.1 | 53 | 50 | 54 |
| Alcohol use | ||||||||||||||||||
| Non drinker | 15.3 | 19.5 | 8.8 | 14.6 | 16.3 | 10.3 | 15.4 | 16.3 | 11.5 | 18.4 | 18.3 | 10.1 | 21.8 | 20.8 | 13.0 | 23 | 33 | 15 |
| Past drinker | 31.5 | 20.4 | 21.6 | 33.5 | 20.7 | 18.88 | 31.3 | 21.8 | 16.6 | 33.1 | 21.1 | 17.8 | 39.4 | 26.4 | 20.5 | 47 | 22 | 30 |
| Current drinker | 53.2 | 60.1 | 69.6 | 51.9 | 63.0 | 70.9 | 53.3 | 61.9 | 71.9 | 48.4 | 60.7 | 72.1 | 38.8 | 52.8 | 66.5 | 30 | 44 | 54 |
| Smoking status | ||||||||||||||||||
| Never Smoked | 44.8 | 60.4 | 39.9 | 48.4 | 62.7 | 46.2 | 49.5 | 61.1 | 52.5 | 51.2 | 68.4 | 56.1 | 55.2 | 63.2 | 55.1 | 48 | 56 | 46 |
| Past Smoker | 40.8 | 30.0 | 46.6 | 40.8 | 30.0 | 41.1 | 39.5 | 33.2 | 40.5 | 40.1 | 28.0 | 38.0 | 64.9 | 32.1 | 37.8 | 39 | 33 | 43 |
| Current Smoker | 14.3 | 9.6 | 13.5 | 10.8 | 7.4 | 12.8 | 11.0 | 5.7 | 7.0 | 8.7 | 3.6 | 5.9 | 9.9 | 4.7 | 7.1 | 13 | 11 | 11 |
| Physical activity | ||||||||||||||||||
| Sedentary: 0 MET-h/wk | 25.4 | 25.9 | 28.4 | 21.6 | 19.4 | 18.5 | 21.2 | 19.5 | 17.5 | 22.9 | 22.7 | 16.0 | 23.3 | 20.8 | 27.0 | 29 | 33 | 27 |
| Low: 0.1–<5 MET-h/wk | 24.9 | 26.2 | 28.4 | 24.3 | 24.7 | 23.7 | 25.8 | 22.9 | 22.6 | 24.0 | 21.9 | 23.1 | 29.6 | 24.5 | 22.9 | 25 | 33 | 30 |
| Moderate: 5–11.9 MET-h/wk | 22.8 | 24.6 | 27.7 | 23.2 | 24.0 | 23.8 | 23.3 | 22.6 | 24.2 | 24.4 | 22.4 | 25.0 | 20.3 | 17.9 | 18.8 | 22 | 11 | 20 |
| High: ≥12 MET-h/wk | 26.9 | 23.3 | 15.5 | 30.9 | 32.0 | 34.0 | 29.7 | 35.1 | 35.7 | 28.6 | 33.0 | 35.9 | 26.9 | 36.8 | 31.3 | 23 | 22 | 23 |
| SBP (mm Hg) | 130 (21) | 122 (24) | 126 (20) | 130 (21) | 122 (23) | 128 (23) | 130 (22) | 124 (25) | 130 (22) | 131 (25) | 124 (23) | 129 (22) | 135 (29) | 131 (24) | 133 (23) | 142 (30) | 132 (22) | 134 (24) |
| Cholesterol | ||||||||||||||||||
| Total (mg/dL) | 218 (53) | 218 (53) | 234 (56) | 220 (49) | 222 (51) | 230 (51) | 222 (52) | 222 (52) | 233 (51) | 230 (57) | 226 (59) | 234 (50) | 237 (63) | 237 (69) | 236 (54) | 254 (69) | 230 (122) | 243 (63) |
| HDL (mg/dL) | 55 (19) | 49 (16) | 47 (17) | 56 (18) | 51 (17) | 51 (15) | 54 (19) | 52 (16) | 51 (16) | 54 (19) | 51 (17) | 51 (15) | 57 (24) | 51 (17) | 50 (16) | 56 (20) | 47 (18) | 48 (15) |
| LDL (mg/dL) | 139 (51) | 137 (46) | 150 (49) | 143 (49) | 139 (46) | 148 (47) | 145 (50) | 140 (49) | 152 (46) | 152 (53) | 144 (50) | 152 (46) | 154 (61) | 159 (57) | 153 (51) | 165 (53) | 140 (90) | 157 (59) |
| TG (mg/dL) | 94 (60) | 133 (83) | 130 (105) | 95 (61) | 135 (89) | 126 (91) | 96 (59) | 129 (88) | 127 (84) | 102 (59) | 135 (70) | 130 (79) | 109 (78) | 140 (91) | 141 (96) | 130 (77) | 199 (108) | 155 (121) |
| CRP (mg/L) | 3.8 (6.6) | 4.0 (5.4) | 4.1 (7.4) | 3.5 (6.0) | 3.1 (4.7) | 2.4 (3.9) | 3.5 (6.0) | 2.7 (4.4) | 2.1 (3.3) | 3.6 (6.0) | 2.8 (4.0) | 2.2 (3.1) | 4.0 (7.1) | 3.9 (4.6) | 2.6 (3.9) | 6.3 (7.7) | 4.5 (10.7) | 4.3 (6.4) |
| Physical function | 85 (30) | 90 (20) | 80 (35) | 85 (34) | 90 (20) | 85 (20) | 85 (35) | 85 (25) | 85 (25) | 80 (40) | 85 (30) | 85 (25) | 70 (40) | 75 (40) | 80 (30) | 60 (35) | 78 (43) | 70 (50) |
Note: eGFR categories expressed in mL/min/1.73 m2. Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range]. Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586.
Abbreviations: AA, African American; BMI, body mass index; eGFR, estimated glomerular filtration rate; H, Hispanic; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent task; MI, myocardial infarction; SBP, systolic blood pressure; W, non-Hispanic white; TG, triglycerides; CRP, C-reactive protein
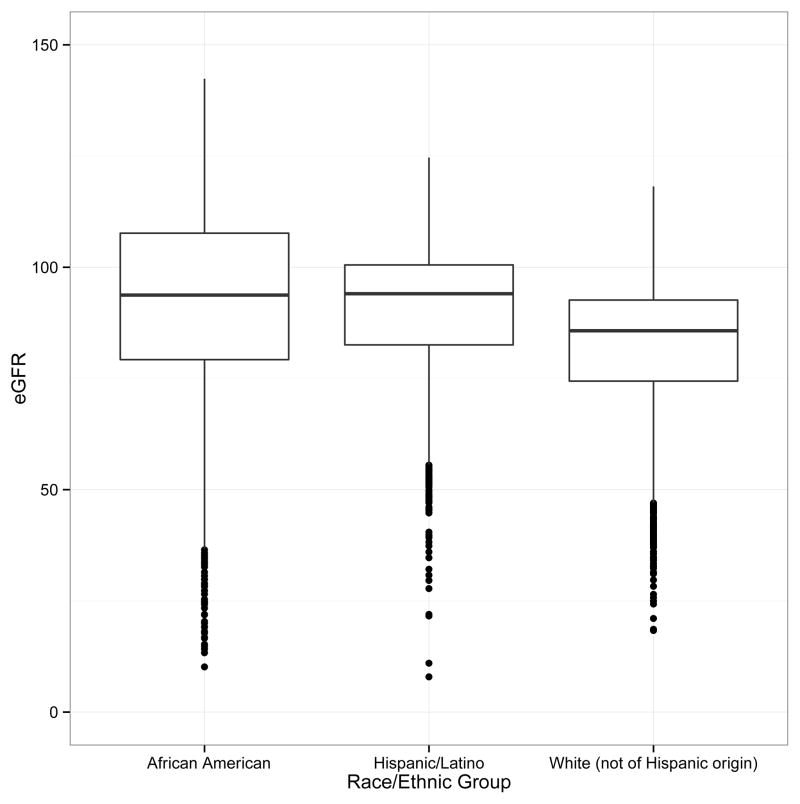
The distribution of eGFR, stratified by self-reported race/ethnicity, is shown in Figure 2. Women self-identifying as Hispanic had a higher median eGFR (93.9 [IQR, 82.5–100.5] ml/min/1.73m2) than that of non-Hispanic whites (85.7 [IQR, 74.4–92.6] ml/min/1.73m2) and eGFR comparable to that of African Americans (93.7 [IQR, 79.3–107.5] ml/min/1.73 m2). The range for eGFR values was widest for the African American participants and most narrow for the non-Hispanic white participants.
Figure 2. Distribution of Estimated Glomerular Filtration Rate by Racial/Ethnic Group.
This figure shows the distribution of eGFR, stratified by self-reported race and ethnicity. Racial and ethnic groups were in mutually exclusive categories: African American, Hispanic, and non-Hispanic white. Women self-identifying as Hispanic had a higher median eGFR (93.9 [IQR, 82.5–100.5] ml/min/1.73m2) compared to non-Hispanic whites (85.7 [IQR, 74.4–92.6] ml/min/1.73m2) and eGFR comparable to African American (93.7 [IQR, 79.3–107.5] ml/min/1.73m2) participants. The range for eGFR values was widest for the African American participants and most narrow for the non-Hispanic white participants. Abbreviations: eGFR, estimated glomerular filtration rate (in mL/min/1.73m2).
Study Outcomes
Over a mean follow-up period of 7.6 ±1.8 years, a total of 1392 women had one or more CV events of interest, including 622 MIs, 597 strokes, and 286 CV deaths (Table 2). For the composite outcome, we observed 1,360 events across 147,744 person-years of follow-up. Of note, within the highest eGFR category (≥105 mg/min/1.73 m2), no CV events were observed among self-reported Hispanic participants.
Table 2.
Cardiovascular Event Counts in 19,411 Participants Stratified by eGFR Category and Racial/Ethnic Group
| eGFR ≥105 | eGFR 90 – <105 | eGFR 75 – <90 | eGFR 60 – <75 | eGFR 45 – <60 | eGFR <45 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | H | W | AA | H | W | AA | H | W | AA | H | W | AA | H | W | AA | H | W | |
| No. of participants | 2241 | 313 | 148 | 1962 | 1569 | 2998 | 1800 | 687 | 3466 | 999 | 361 | 1767 | 335 | 106 | 463 | 99 | 18 | 79 |
| Composite CV End point | 79 (4) | 0 (0) | 19 (13) | 101 (5) | 35 (2) | 260 (9) | 107 (6) | 24 (4) | 313 (9) | 68 (7) | 16 (4) | 183 (10) | 41 (12) | 8 (8) | 59 (13) | 28 (28) | 2 (11) | 18 (23) |
| MI | 30 (1) | 0 (0) | 11 (7) | 38 (2) | 15 (1) | 129 (4) | 43 (2) | 8 (1) | 149 (4) | 25 (3) | 9 (3) | 99 (6) | 13 (4) | 4 (4) | 32 (7) | 10 (10) | 2 (11) | 5 (6) |
| Stroke | 37 (2) | 0 (0) | 5 (3) | 41 (2) | 17 (1) | 113 (4) | 51 (3) | 11 (2) | 143 (4) | 36 (4) | 6 (2) | 70 (4) | 17 (5) | 5 (5) | 25 (5) | 11 (11) | 0 (0) | 9 (11) |
| Death | 19 (1) | 0 (0) | 3 (2) | 38 (2) | 6 (0) | 31 (1) | 26 (1) | 6 (1) | 53 (2) | 13 (1) | 3 (1) | 36 (2) | 17 (5) | 2 (2) | 11 (2) | 14 (14) | 0 (0) | 8 (10) |
Note: eGFR categories expressed in mL/min/1.73 m2. Unless otherwise indicated, values are given as number [percentage].. The composite CV event may not equal the total number of individual CV events as participants could have had more than one CV event.
Abbreviations: AA, African American; BMI, body mass index; eGFR, estimated glomerular filtration rate; H, Hispanic; MET, metabolic equivalent of task; MI, myocardial infarction; SBP, systolic blood pressure; W, non-Hispanic white.
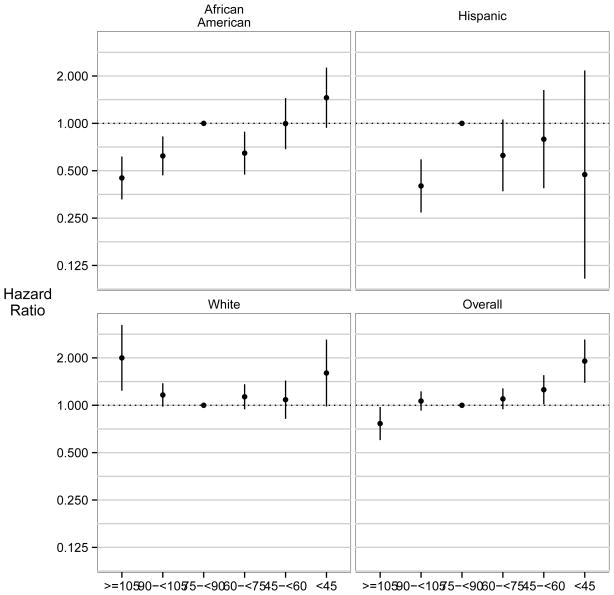
In Figure 3, we show the adjusted HRs of the composite CV event across categories of eGFR stratified by race and ethnicity. After adjustment for socio-demographic characteristics, comorbid conditions, lifestyle factors, and biometric/laboratory data, the association between eGFR and the composite CV end point was curvilinear in the overall cohort, but modified by race/ethnicity (P for interaction (omnibus test) =0.003). Stratum specific effect modification was found for race (African Americans vs. non-Hispanic whites, P=0.001), but not for ethnicity (Hispanics vs. non-Hispanic whites, P=0.4). Compared to the referent group with an eGFR of 75–<90 ml/min/1.73m2, non-Hispanic white participants had a U-shaped relationship between eGFR and CV events: both higher (≥105 ml/min/1.73m2) and lower (<45 ml/min/1.73m2) eGFR were associated with higher event rates (HRs of 2.00 [95% CI, 1.24–3.24] and 1.60 [95% CI, 1.01–2.94], respectively) (Table S1). Among African American participants, however, a rather curvilinear relationship was observed; for the composite CV outcome, compared with participants with eGFR in the 75–<90 ml/min/1.73m2 reference group, an eGFR of 90–<105 ml/min/1.73m2 was associated with a lower event rate (HR, 0.62; 95% CI, 0.47–0.83), and eGFR ≥105 ml/min/1.73m2 was associated with an even lower event rate (HR, 0.45; 95% CI, 0.33–0.62). On the other end of the eGFR spectrum, in the eGFR <45 ml/min/1.73m2 category, African American women had similar rates of composite CV events (HR, 1.46; 95% CI, 0.94–2.26) as those of non-Hispanic white women, but the association was not significant. For the Hispanic participants, the relative rates of CV events could not be determined in the highest eGFR category (no events) and were not significantly different from the referent for eGFR categories ≤60 ml/min/1.73m2. However, Hispanic women had lower rates of composite CV events in the category of eGFR 90–<105 ml/min/1.73m2 (HR, 0.40; 95% CI, 0.27–0.59).
Figure 3. Associations between Estimated Glomerular Filtration Rate and Time to Cardiovascular Events (Myocardial Infarction, Stroke, Cardiovascular Death); Overall and by Racial/Ethnic Group.
In the overall cohort, the association between eGFR and CV events curvilinear; effect modification by race and ethnicity was present in an omnibus test (P=0.006) as well as in a stratum-specific test for interaction by non-Hispanic white versus African American racial/ethnic group membership (P=0.001). Stratum-specific interaction was not found between Hispanic versus non-Hispanic white racial/ethnic group membership (P=0.4). In stratified analyses, we observed that the U-shaped association was present in non-Hispanic whites, whereas black participants had a rather curvilinear relationship with lower eGFR being associated with reduced (CV risk) and higher eGFR with increased CV risk. Analyses among Hispanic women were inconclusive owing to few Hispanic women having very low or high eGFR and very few events occurring in these categories. Multivariable adjustment for HRs included age, presence of diabetes, history of stroke, history of myocardial infarction, body mass index, physical activity, physical function, history of smoking, history of alcohol use, C- reactive protein, blood pressure, use of antihypertensives, and LDL and HDL. Reference group was defined as eGFR 75–90 ml/min/1.73m2. Abbreviations: eGFR, estimated glomerular filtration rate (in mL/min/1.73m2).
Analyses of individual CV events were naturally limited by fewer events and reduced power. The association between eGFR and MI in the full study sample was J-shaped and the omnibus test for interaction by race/ethnicity was of borderline significance (P=0.09). Stratum specific interaction was present for African American race vs. non-Hispanic whites (P=0.03) but not for self-reported Hispanic women vs. non-Hispanic whites (P=0.4). In a multivariable model adjusted for patient characteristics and CV risk factors, non-Hispanic whites with high eGFR (≥105 ml/min/1.73m2) had increased rates of MI (HR, 2.04; 95% CI, 1.07–3.87), whereas both African Americans and Hispanics had reduced MI rates with more preserved eGFR compared to the reference group. No associations were found among the eGFR categories below 60 ml/min/1.73m2 for any of the racial and ethnic groups (Table S2).
Non-Hispanic whites had higher adjusted rates of stroke at an eGFR <45 ml/min/1.73m2, compared to those in the referent eGFR category (HR, 2.09; 95% CI, 1.05–4.18) (Table S3). No significant associations were found in any of the other eGFR categories for whites. Stroke rates were lower for African Americans and Hispanics at an eGFR of 90 ml/min/1.73m2 or higher.
For the outcome of CV death, an increased risk was observed at an eGFR <45 ml/min/1.73m2 among non-Hispanic whites and <60 ml/min/1.73m2 among African Americans. Hispanic participants had very few events (n=17), leading to rather unstable estimates (Table S4).
The results of the sensitivity analyses using imputation methods did not differ meaningfully from the results of our main complete case analyses.
Discussion
In this large, multiethnic study of postmenopausal women, we confirmed associations between eGFR, a kidney disease marker, and CV risk. However, the patterns of association between eGFR and the composite CV outcome varied significantly between non-Hispanic whites and African Americans, with a U-shaped association for non-Hispanic whites and a rather curvilinear association for African Americans. The association between eGFR and CV events among Hispanics could not be evaluated in sufficient detail since there were only few Hispanic participants and even fewer CV events in the extreme eGFR categories. Analyses of individual CV events were naturally limited by reduced precision due to their small number, but found to be broadly compatible with the findings of the composite CV end point.
The strong relationship between CKD and CV risk is well established. In a landmark study using data from the Kaiser Permanente of Northern California, Go and colleagues described the detailed trajectory of CV risk across the spectrum of kidney function in adults 20 years of age or older. Compared with individuals with normal kidney function (eGFR ≥60 ml/min/1.73m2, using the 4-variable MDRD [Modification of Diet in Renal Disease] Study equation), an independent, monotonic increase in the risk of CV events was observed for each successive stratum of GFR decline.(3) Moreover, recent studies have shown that the association between eGFR and CV risk is even stronger when using the more accurate CKD-EPI (CKD Epidemiology Collaboration) equation, or using equations based on measurements of cystatin C instead of or additional to serum creatinine concentrations.(19, 20, 24)
Racial and ethnic disparities in the CV risk among individuals with CKD have previously been described.(7, 25) Peralta et al. found that among patients with eGFR <60 ml/min/1.73m2, Hispanic individuals had 18% lower rates of CV events compared with otherwise similar non-Hispanic whites.(7) In a pooled analysis of community-based cohorts, Weiner et al. found effect modification by African American versus white race of the association between eGFR <60 ml/min/1.73m2 and a composite outcome of nonfatal CV events and all-cause mortality.(8) However, the reference group included all individuals with eGFR ≥60 ml/min/1.73m2 and did not use any finer categorization of eGFR, especially within the relatively normal range of eGFR where we have found significant racial differences.
The largest study on the subject to date was a recent investigation by the CKD Prognosis Consortium, where effect modification by race (white, African American, Asian) of the associations between kidney markers (eGFR, ACR) with mortality, CV mortality, and ESRD was evaluated.(26) African Americans constituted a small minority of the 1.1 million study subjects, but still numbered almost 50,000 individuals. Despite the large sample size, the only CV event studied (CV mortality) was relatively rare and led to wide confidence limits for African Americans, especially in the extreme ranges of eGFR. No effect modification was found between whites and African Americans; however, due to the imprecise estimates in the higher eGFR range, their confidence bands appear compatible with our finding of a curvilinear rather than U-shaped association between eGFR and CV events.
More recently, Gutierrez et al. identified racial differences in the association between ACR, the other marker of kidney disease, and CV events in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Ultimately, higher urinary albumin excretion was more strongly associated with incident coronary heart disease risk among African American individuals as compared to whites.(4) A similar analysis using data from the Atherosclerosis Risk in Communities (ARIC) study did not identify any racial differences in the association of ACR with CV risk. Interestingly, among study participants with ACR > 30 mg/g, women had increased CV risk compared to men (1.3- to 1.8-fold higher HR).(27) This sex-albuminuria interaction on CV risk had previously been described in other cohorts.(13, 28)
In the general population, the risk of CVD among women increases after menopause, which is believed to result from loss of the protective effect of estrogen on lipids and vascular function or a consequence of older age.(29–31) Previous studies using the WHI cohort have investigated racial/ethnic differences in CVD risk and mortality—interestingly, no racial/ethnic disparities were observed in the context of diabetes(32) and severe obesity.(33) Few studies have described the relationship of CVD among women burdened with CKD in the United States. In 2001, Shlipak and colleagues identified 2,763 postmenopausal women with established CV heart disease using data from the Heart and Estrogen/Progestin Replacement Study (HERS). After multivariable adjustment, both mild (1.2–1.4 mg/dl) and moderate (>1.4 mg/dl) elevations of serum creatinine were independently associated with a 60%–80% increased risk of CV events compared with in women with normal kidney function.(34) However, potential effect modification by race and ethnicity on the relationship between kidney and CV risk was not explored.
The paradoxically increased CV risk in patients with higher eGFR has been previously reported and attributed to frailty or muscle wasting secondary to illness, leading to decreased creatinine generation rather than high level of kidney function.(35) Our observation that this elevated CV risk with high eGFR concentrations is found in non-Hispanic whites, but not African Americans, is novel. While it is possible that African American WHI participants were less frail and had more preserved muscle mass, we adjusted for other markers of frailty or poor health and nutritional status including BMI, waist circumference, physical activity level, and SF-36 Physical Function score. Although cystatin C has not been proven to be a superior biomarker compared to creatinine, it has a more linear relationship with CV outcomes in older adults, compared with creatinine, which may have a J- or U-shaped relationship.(36, 37) Future studies should examine whether the accuracy of creatinine-based (or cystatin C-based) estimation equations among individuals with seemingly preserved kidney function differs among racial and ethnic groups.
This study had several limitations. First, urinary albumin measurements were not available in WHI to better discriminate kidney disease burden. This could introduce probable confounding; however, eGFR and ACR have been shown to be independent risk factors for CV events.(3) In addition, only single measurements of creatinine were available and so we were unable to account for error associated with random intra-individual variability, or to analyze kidney and CV disease progression over time. While race and ethnicity was collected prospectively, misclassification may have occurred. Furthermore, as with other studies using interval monitoring of participants’ health events, under-reporting of CV events may have occurred. However, recall bias was limited as CV outcomes were adjudicated by physicians following participant report.(38, 39) In addition, although the CKD-EPI equation is more accurate compared to the MDRD Study equation in estimating GFR and predicting CV risk, a lack of precision in GFR estimates > 90 mL/min/1.73m2 has been reported among the Black population.(40) Moreover, although we adjusted for various covariates at baseline, we cannot rule out the possibility of residual confounding by unmeasured confounders. In order to account for covariates, the analytic cohort differed from the eligible participants in that participants excluded from the analysis were more likely to be non-Hispanic white, thus introducing potential selection bias. Moreover, generalizability of our findings may be limited because the study only consisted of relatively healthy postmenopausal women. Finally, lack of recruitment of Hispanics with eGFR values in the lower and higher spectrum of kidney disease and small number of cases in several eGFR categories limited the power to detect effect modification for this ethnic group. This narrow eGFR range observed among Hispanic participants may have been the result of selection bias during recruitment and/or differential participant nonresponse (in particular a potentially lower response among sicker Hispanic women).
In conclusion, using a large, national, and prospectively assembled cohort of diverse, postmenopausal women, we found that the patterns of association between eGFR and CV outcomes varied by race/ethnicity—the association was U-shaped for non-Hispanic white women and more curvilinear for African American women. These findings add to the growing body of evidence on the racial differences in CV risk with reduced kidney function. Future studies, including ones using cystatin C-based estimations of glomerular filtration rate, need to confirm our findings of racially distinct patterns of CV risk from decreased kidney function.
Supplementary Material
Women’s Health Initiative clinical trial and observational study design and adjudication of cardiovascular events.
Hazard ratios for the composite CV outcome by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for myocardial infarction by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for stroke events by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for cardiovascular deaths by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Acknowledgments
A short list of the WHI Investigators follows. Program Office, National Heart, Lung and Blood Institute, Bethesda, MD: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC), Marcia L. Stefanick (Stanford Prevention Research Center, CA), Rebecca Jackson (The Ohio State University, Columbus), Cynthia A. Thomson (University of Arizona, Tucson/Phoenix), Jean Wactawski-Wende (University at Buffalo, NY), Marian Limacher (University of Florida, Gainesville/Jacksonville), Robert Wallace (University of Iowa, Iowa City/Davenport), Lewis Kuller (University of Pittsburgh, PA), and Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC). WHI Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC). A list of all the investigators who have contributed to WHI science is available at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf Support: Dr. Arce was supported by an underrepresented minority supplement to T32DK007357. Dr. Rhee was supported by T32DK007357 and F32DK103473. Dr. Winkelmayer receives salary and research support through the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine. The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, US Department of Health and Human Services, through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Because an author of this article is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Luxia Xhang, MD, MPH) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: CMA, JJR, WCW; data acquisition: MLS; data analysis/interpretation: all authors; statistical analysis: HH, KK, MD; supervision or mentorship: WCW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. WCW takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Table S1: HRs for composite CV outcome by eGFR category and race/ethnicity.
Table S2: HRs for MI by eGFR category and race/ethnicity.
Table S3: HRs for stroke events by eGFR category and race/ethnicity.
Table S4: HRs for CV deaths by eGFR category and race/ethnicity.
Item S1: WHI clinical trial and observational study design and adjudication of CV events.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA : the journal of the American Medical Association. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Grams ME, Juraschek SP, Selvin E, et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:253–260. doi: 10.1053/j.ajkd.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez OM, Khodneva YA, Muntner P, et al. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA : the journal of the American Medical Association. 2013;310:706–714. doi: 10.1001/jama.2013.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peralta CA, Vittinghoff E, Bansal N, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:261–266. doi: 10.1053/j.ajkd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Renal Data System. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease. Bethesda, MD: 2012. [Google Scholar]
- 7.Peralta CA, Shlipak MG, Fan D, et al. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2006;17:2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE, Tabatabai S, Tighiouart H, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Roderick PJ, Atkins RJ, Smeeth L, et al. CKD and mortality risk in older people: a community-based population study in the United Kingdom. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:950–960. doi: 10.1053/j.ajkd.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. Journal of the American Society of Nephrology : JASN. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 11.Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. Bmj. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:691–702. doi: 10.1053/j.ajkd.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jassal SK, Langenberg C, von Muhlen D, Bergstrom J, Barrett-Connor E. Usefulness of microalbuminuria versus the metabolic syndrome as a predictor of cardiovascular disease in women and men>40 years of age (from the Rancho Bernardo Study) The American journal of cardiology. 2008;101:1275–1280. doi: 10.1016/j.amjcard.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Annals of epidemiology. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan L, Espeland MA, Snively B, et al. The rationale, design, and baseline characteristics of the Women's Health Initiative Memory Study of Younger Women (WHIMS-Y) Brain research. 2013;1514:3–11. doi: 10.1016/j.brainres.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genomics and Randomized Trials Network (GARNET) Study. National Institutes of Health, National Human Genome Research Institute; Seattle, WA: 2011. [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Annals of epidemiology. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 22.Bohannon RW, DePasquale L. Physical Functioning Scale of the Short-Form (SF) 36: internal consistency and validity with older adults. Journal of geriatric physical therapy. 2010;33:16–18. [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of lowdensity lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly SE, Burrows NR, Chen SC, et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clinical journal of the American Society of Nephrology : CJASN. 2011;6:1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen CP, Matsushita K, Coresh J, et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney international. 2014 doi: 10.1038/ki.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui X, Matsushita K, Sang Y, Ballew SH, Fulop T, Coresh J. CKD and Cardiovascular Disease in the Atherosclerosis Risk in Communities (ARIC) Study: Interactions With Age, Sex, and Race. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 doi: 10.1053/j.ajkd.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muntner P, Garrett E, Klag MJ, Coresh J. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. Stroke; a journal of cerebral circulation. 2002;33:1209–1213. doi: 10.1161/01.str.0000015031.57955.d1. [DOI] [PubMed] [Google Scholar]
- 29.Kaushik M, Sontineni SP, Hunter C. Cardiovascular disease and androgens: a review. International journal of cardiology. 2010;142:8–14. doi: 10.1016/j.ijcard.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Leuzzi C, Marzullo R, Modena MG. Is menopause a risk factor for ischemic heart disease in women? Giornale italiano di cardiologia. 2012;13:401–406. doi: 10.1714/1073.11757. [DOI] [PubMed] [Google Scholar]
- 31.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. Journal of the American College of Cardiology. 2001;37:1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Hebert JR, Balasubramanian R, et al. All-cause, cardiovascular, and cancer mortality rates in postmenopausal white, black, Hispanic, and Asian women with and without diabetes in the United States: the Women's Health Initiative, 1993–2009. American journal of epidemiology. 2013;178:1533–1541. doi: 10.1093/aje/kwt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McTigue KM, Chang YF, Eaton C, et al. Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women. Obesity. 2014;22:801–810. doi: 10.1002/oby.20224. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. Journal of the American College of Cardiology. 2001;38:705–711. doi: 10.1016/s0735-1097(01)01450-4. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waheed S, Matsushita K, Astor BC, Hoogeveen RC, Ballantyne C, Coresh J. Combined Association of Creatinine, Albuminuria, and Cystatin C with All-Cause Mortality and Cardiovascular and Kidney Outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:434–442. doi: 10.2215/CJN.04960512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England journal of medicine. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 38.Hlatky MA, Ray RM, Burwen DR, et al. Use of Medicare data to identify coronary heart disease outcomes in the Women's Health Initiative. Circulation Cardiovascular quality and outcomes. 2014;7:157–162. doi: 10.1161/CIRCOUTCOMES.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare claims versus physician adjudication for identifying stroke outcomes in the Women's Health Initiative. Stroke; a journal of cerebral circulation. 2014;45:815–821. doi: 10.1161/STROKEAHA.113.003408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens LA, Schmid CH, Greene T, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56:486–495. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Women’s Health Initiative clinical trial and observational study design and adjudication of cardiovascular events.
Hazard ratios for the composite CV outcome by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for myocardial infarction by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for stroke events by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.
Hazard ratios for cardiovascular deaths by eGFR category and race/ethnicity
Abbreviations: CVD, cardiovascular; eGFR, estimated glomerular filtration rate; N.E., non-estimable.
aP-value for interaction among the three racial/ethnic groups (omnibus test).
bP-value for stratum specific interaction, African American versus non-Hispanic white.
cP-value for stratum specific interaction, Hispanic versus non-Hispanic white.