Abstract
Factor H is a circulating protein that regulates activation of the alternative pathway (AP) of complement. Mutations and genetic variations of factor H are associated with several AP-mediated diseases, highlighting the critical role of factor H in AP regulation. AP-mediated inflammation is typically triggered by illness or tissue injury, however, and tissue injury can trigger AP activation in individuals with fully functional factor H. This suggests that factor H function is affected by local conditions within tissues. We hypothesized that inducible proteins impair the ability of factor H to locally control the AP, thereby increasing AP activation. We used purified murine factor H to immunoprecipitate binding partners from mouse kidneys. Using immunoaffinity liquid chromatography-mass spectrometry we then identified annexin A2 as a factor H binding partner. Further experiments showed that annexin A2 reduces the binding of factor H to cell surfaces. Recombinant annexin A2 impaired complement regulation by factor H, and increased complement activation on renal cell surfaces in vitro and in vivo. In a murine model of acute pneumococcal otitis media the administration of annexin A2 increased AP-mediated bacterial opsonization and clearance. In conclusion, the local production of annexin A2 within tissues suppresses regulation of the AP by factor H. Annexin A2 can contribute to AP-mediated tissue inflammation by locally impairing factor H function, but annexin A2 can also improve complement-mediated bacterial clearance.
Introduction
A fundamental task of the immune system is to discriminate between host tissue and invasive pathogens. The alternative pathway is spontaneously activated in the fluid phase and on cell surfaces. Regulatory proteins control AP activation on host cells but not on pathogens, thereby enabling the AP to “recognize” invasive pathogens. Factor H is an AP regulatory protein that circulates in plasma, but can control AP activation on the surface of host cells through its interaction with anionic molecules and complement C3 fragments displayed on the cell membrane (1). In the absence of factor H (e.g. in factor H deficient mice), spontaneous complement activation is observed in plasma and in the kidneys (2). The strong association of variations and mutations in the gene for factor H with the development of inflammatory disease demonstrates the importance of factor H in controlling AP activation in humans (3).
Although factor H is present in high concentrations throughout the body, factor H mutations are associated with diseases of specific organs, including the kidneys (4-7) and the eyes (8-11), suggesting that these tissues are particularly dependent upon local factor H function for controlling AP-mediated injury. Even in patients with congenital factor H mutations, AP-mediated injury is usually triggered by systemic illness or by tissue injury (e.g. during transplantation) (12). It is also noteworthy that the AP contributes to disease even in patients who do not have factor H mutations. For example, clinical and experimental evidence indicates that AP activation causes injury after renal ischemia (13, 14), focal segmental glomerulosclerosis (FSGS) (15, 16), ANCA associated vasculitis (17, 18), and lupus nephritis (19). These observations suggest that factor H is crucial for preventing AP-mediated injury to the host, but that factor H function is affected locally within tissues by illness and other stressors.
We hypothesized that inducible factors expressed within tissues can interfere with factor H function. To test this hypothesis we performed immunoprecipitation experiments in which biotinylated factor H was used to capture factor H binding partners from kidney tissue lysates. Tandem mass spectrometry identified one of the co-immunoprecipitated proteins as annexin A2. Annexin A2 has previously been shown to bind factor H, but the functional consequences of this interactions were not investigated (20). We performed in vitro and in vivo experiments to test whether annexin A2 prevents or enhances AP activation through its interaction with factor H and examined whether annexin A2 levels affect the anti-bacterial effects of the AP. The overall goal of these experiments was to identify novel mechanisms by which the AP is controlled or activated within tissues, and to develop new strategies for preventing AP-mediated disease.
Materials and Methods
Study approval
C57BL/6 mice were purchased from Jackson Laboratories. Mice with targeted deletion of the gene for factor H were generated as previously described (2). Mice were housed and maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The renal I/R protocol was approved by the University of Colorado Center for Laboratory Animal Care, and the otitis media model was approved by the Ohio State University Animal Care and Use Committee.
Animal protocols
Renal I/R
Male C57BL/6J mice (Jackson Laboratories) were used for the in vivo experiments. Ten to twelve week old mice weighing 20-25 g were anesthetized with 60 mg/kg ketamine plus 10 mg/kg xylazine (both from Vedco, Inc) injected intraperitoneally (IP). Mice were placed on a heating pad to maintain their body temperature during surgery. Laparotomies were then performed and the renal pedicles were located and isolated by blunt dissection as previously described (13). The pedicles were clamped with surgical clips (Miltex Instrument Company), and occlusion of blood flow was confirmed by visual inspection of the kidneys. The clamps were left in place for 24 minutes and then released. The kidneys were observed for approximately one minute to ensure blood re-flow, then fascia and skin were sutured with 4-0 silk (United States Surgical). Sham surgery was performed in an identical fashion, except that the renal pedicles were not clamped. The mice were volume resuscitated with 0.5 ml of normal saline and kept in an incubator at 29°C for two hours to maintain body temperature during recovery. After 8 or 24 hours the mice were anesthetized, and blood was obtained by cardiac puncture. Laparotomy was performed and the kidneys were harvested. SUN and creatinine were measured using an Alfa Wasserman ACE Chemistry Analyzer.
Factor H deficient mice
Mice with a targeted deletion of the gene for factor H were generated as previously described (2) and were back-crossed nine generations onto a C57BL/6 background. Heterozygous mice fH+/− were used for the in vivo experiments.
Otitis Media model
Mice were anesthetized by intraperitoneal injection with ketamine hydrochloride (20 mg/kg of body weight) and xylazine (5 mg/kg). Acute otitis media was then induced by direct bilateral transtympanical inoculation with approximately 1 × 103 colony forming units (CFU) of opaque or transparent Streptococcus pneumoniae (Spn) in sterile pyrogen-free saline as previously described (21, 22). Mice were anesthetized and sacrificed 24 or 48 hours post inoculation. The middle ear spaces were lavaged to quantitatively determine titers of Spn. The middle ear lavage samples were cultured overnight at 37°C on Columbia CNA agar plates in an incubator supplemented with humidity and 5% CO2. The number of CFU per milliliter was then determined by a standard dilution assay and plate counting.
Protein purification and production
Factor H
An affinity column for factor H was made by ligating polyclonal goat antibody for human factor H (Quidel) to CNBR sepharose (Amersham Biosciences) according to the manufacturer's instructions. Plasma was collected from C57BL/6 mice and was passed over the column. After washing the column with PBS, pH 7.4, factor H was eluted with 5 M LiCl2. The buffer was exchanged with PBS and factor H was then passed through a Hiload Superdex 200 size exclusion column (GE Amersham). The purity and identity of the isolated protein was verified by SDS PAGE and by Western blot analysis. Recombinant murine factor H was generated from the codon-optimized (Homo sapiens) DNA sequence for murine factor H (comprising residues 19-1234; Uniprot identifier: P06909) that was sub-cloned into the pDONR221 entry vector (Life Technologies; construct purchased from GeneArt). The engineered sequences additionally contained DNA encoding an Ig kappa chain leader sequence to facilitate secretion of the target protein, a Gly-Ala-Gly-Ala-Gly-Ala linker region, a hexa-histidine (His6-) fusion tag, a second linker region (Asp-Tyr-Asp-Ile-Pro-Thr-Thr) and a Tobacco Etch Virus nuclear inclusion A endopeptidase (TEV) cleavage site (Glu-Asn-Leu-Tyr-Gln-Gly), all of which were located 5′ prime to the factor H gene. The synthetic gene was then re-combined into a pcDNA3.2/V5-Dest expression vector using Gateway LR clonase II enzyme mix (Life Technologies Inc) according to the manufacturer's instructions.
Recombinant Annexin A2
Total mRNA from the kidney of a C57BL/6 mouse was reverse transcribed to generate cDNA, and the cDNA encoding annexin A2 was amplified by PCR (forward primer 5’ TTGGATCCTCTACTGTCCACGAAATCC 3’; reverse primer 5’ AAGATATCGTCATCCCCACCACACAGGTA 3’). BamHI and EcoRV restriction sites were introduced, and the cDNA was inserted into the pSecTag2 Hygro B vector (Invitrogen) along with sequence for a thrombin cleavage site. The plasmid was produced using BL21 Escherichia coli (Amersham Pharmacia Biotech Inc.) under ampicillin selection. DNA sequencing at the University of Colorado sequencing core confirmed the proper sequence of the amplified plasmid. Chinese hamster ovary (CHO) cells were then transfected with the plasmid using Lipofectamine 2000 (Invitrogen), and protein was produced under Hygromycin B (Corning Cellgro) selection. The cells were grown in Freestyle CHO media (Invitrogen) supplemented with fetal calf serum and Hygromycin B (Corning Cellgro). The protein was purified using a HiTrap His column (GE), and the purity of the protein was confirmed by SDS PAGE and by Western Blot analysis.
Western blot and Far Western blot analysis
For analysis of kidney proteins, kidneys were homogenized in RIPA lysis buffer containing 1% Triton X-100, 0.5% deoxycholic acid, 150 mM NaCl, 20 mM β-glycerophosphate, 20 mM Tris·HCl (pH 8.0), 5 mM EGTA, 3 mM MgCl2, 0.1% SDS, 1mM DTT, 50μM Na3VO4, and one tablet of complete, EDTA-free, protease inhibitor cocktail (Roche Applied Science). Homogenates were centrifuged at 14,000 rpm for 15 min. at 4° C and the supernatant was collected. Protein concentrations for kidney samples were determined using the Bio-rad protein assay (Bio-rad Laboratories). One hundred μg of protein from each kidney was resolved by electrophoresis with a 10% Bis-Tris polyacrylamide gel (Invitrogen) and transferred to a nitrocellulose membrane.
Factor H was detected in tissue lysates or in solution using a polyclonal goat anti-human factor H antibody (Quidel) diluted 1/100. Annexin A2 was detected in kidney lysates or in solution using a polyclonal anti-human annexin A2 antibody (Proteintech) diluted 1/1000. Densitometry was performed using ImageJ software. The relative expression of annexin A2 in each sample was determined by dividing the band density by that for α-actin. Appropriate secondary antibodies from Jackson Immunoresearch Laboratories were used. To detect the binding of factor H to proteins in kidney lysates (Far Western analysis), purified factor H was biotinylated with Sulfo-NHS-Biotin (Pierce) according to the manufacturer's instructions. The sample proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and they were incubated with 1 μg/mL of biotinylated factor H in PBS, pH 7.4. The binding of factor H to protein bands was then detected with Streptavidin-HRP (Zymed) diluted 1/500 in PBS, followed by treatment with ECL.
Immunoprecipitation of factor H binding partners
Purified factor H was biotinylated using sulfo-NHS-Biotin (Pierce). Three μg of factor H was added to 100 μg of kidney protein lysate. Streptavidin Beads (0.5 mg of Dynabeads M-280) were added to the mixture and incubated for 30 minutes at room temperature. A magnet was then used to separate the beads, and the beads were washed four times with PBS. Precipitated proteins were separated on a Bis-Tris gel and stained with Bio-Safe Coomassie Stain (Bio-Rad). The ~39 kD gel band was excised and digested using trypsin (Promega Gold) following reduction with 1.5 mg/mL dithiothreitol and alkylation with 10 mg/mL iodoacetamide. Resultant peptides were extracted with acetonitrile and 1% formic acid.
Protein identification by nanoLC/quadrupole ion trap MS/MS
Tryptic peptides were analysed by reverse-phase nano-scale liquid chromatography nanoelectrospray quadrupole-iontrap mass spectrometry (nanoLC ESI-MS/MS) using an Agilent 1100 HPLC-system equipped with a nanopump coupled to an Agilent LC/MSD Trap XCT Plus (Agilent Technologies) mass spectrometer. Peptides were loaded onto a Zorbax C18 column (75 μm ID × 10 cm, 300Å porosity, 5 μm particles) (Agilent Technologies) for 2 min using a micro-well plate autosampler and a capillary pump delivering a flow of 5 μl/min without split. Peptides were eluted by a gradient of solvent A (0.1 % formic acid) and solvent B (90 % Acetonitrile, 0.1 % formic acid) at a flow rate of 300 nl/min. The gradient was ramped from 3 to 8 % solvent B in 1 minute, from 8 to 45 % solvent B in 85 minutes, and finally to 90 % B in 5 minutes, until the mobile phase was returned to the initial conditions after 10 min.
Spray was established using 8 μm ID emitters (New Objective, Woburn, MA) and a typical capillary voltage of 1600 V. Spectra were collected over 350-1800 m/z. Three fragmentation spectra were collected for the three most abundant m/z values. Subsequently, those m/z were excluded from analysis for 1 minute and the next three most abundant m/z values were selected for fragmentation to enable analysis of lower abundance peptide ions.
The Spectrum Mill database search algorithm (Agilent Technologies) was used to search the UniProt database, employing the taxonomy filter for mouse. Parameters used for the search included the monoisotopic mass, a peptide mass tolerance of 1.2 Da and a fragment ion mass tolerance of 0.6 Da. Furthermore, tryptic peptides were only allowed two missed cleavages, and carbamidomethylation of cysteine was selected as a fixed modification. Post-translational modifications (glycosylations and/or phosphorylations) as possible variable peptide modifications were not included in the search parameters.
Database matches were validated by reverse database scoring using SpectrumMill software. Proteins with SpectrumMill scores above 13, peptide scores above 10, and that scored a percent intensity (SPI) of 70% were used as thresholds for initial “hit” validation. Each identified protein required confirmation with least 2 unique peptides. For the most interesting candidates, peptide determinations used for protein identifications were manually validated, marking observed and theoretically expected mass ions. Water losses, evident when peptide fragments containing a serine (S) or threonine (T) were measured, were used to assist in sequence assignment validation.
Immunofluorescence microscopy and immunohistochemistry
Kidneys were snap frozen in OCT compound (Sakura Finetek). Four μm sections were cut with a cryostat and stored at −70° C. The slides were later fixed with acetone. C3 was detected with a FITC-conjugated anti-mouse C3 antibody (MP Biomedicals) diluted 1:150. A monoclonal antibody that only recognizes the iC3b and C3d fragments of C3 was also used (23). Annexin A2 in kidney sections was detected by immunohistochemistry using an anti-annexin II antibody (clone Z014) and a SuperPicture Polymer Detection Kit (both from Invitrogen).
Annexin A2-Factor H binding assays
Fluid phase binding
Purified murine factor H and recombinant annexin A2 were separated by size exclusion chromatography using a Superdex 200 column (GE Amersham), and the eluate was divided into 5 different fractions. The eluted fractions were concentrated, and factor H and annexin A2 were detected by Western Blot Analysis.
Solid phase binding
To examine solid phase binding of annexin A2 and factor H, 800 ng of recombinant annexin A2 was adsorbed overnight to round bottom plates (Immulon). The plates were then blocked with 3% milk in TBS, ph7.5 for two hours. Factor H (100 μL at 20 μg/mL in TBS with 2 mM Ca) was added to the wells and the plates were incubated for 3 hours on a rocker at room temperature, and the plates were then washed three times with TBS. Bound factor H was then detected by incubating the plates with 100 μl/well of goat anti-factor H (Quidel) diluted 1:10 in 3% milk blocking buffer for 1 hour with rocking. The plates were washed five times, and 100 μl per well 1:1000 anti goat-HRP in 3% milk was added to each well. After incubating the plates for one hour the plates were again washed, and then developed with 1-Step Ultra TMB-ELISA Substrate (Pierce). After ~20 minutes the reaction was stopped with 100 μl 2M sulfuric acid and absorbance was read at 450nm.
To study the effect of annexin A2 on the surface of sheep erythrocytes, antibody-sensitized erythrocytes were purchased from CompTech. C3bBbP was generated on the cell surface, and the effects of annexin A2 on decay of the the convertase by factor H were studied as previously described (23).
The interaction between annexin A2 and factor H was also examined using a BIAcore 3000 (Biacore) at the University of Colorado Biophysics Core. Recombinant factor H was immobilized on a carboxymethyl-dextran (CM5) chip using random amine coupling with 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride/N-hydroxysulfosuccinimide as the activating reagent and at a concentration of 50 ug/mL in 100 mM sodium acetate, pH 5.0. Approximately 7000 RU of factor H was immobilized, and remaining activated groups on the surface of the chip were blocked with a 1 M Ethanolamine solution, pH 8.5. Binding experiments were conducted in 10mM HEPES, 150mM NaCl, 0.005% P20, pH 7.4, and the chip was regenerated between analyte injections with two 10uL injections of 10mM NaOH. Recombinant annexin A2 was injected at concentrations of 3 uM, 1 uM, 333 nM, 111 nM, and 37 nM for 3min at a flow rate of 25 uL/min, and the dissociation of the resulting annexin A2-factor H complexes was monitored for ten minutes. All injections were performed in triplicate to verify reproducibility, and all data was double referenced using a blank flowcell and a blank injection of buffer to account for non-specific binding and baseline drift, respectively. Data was fit using a 1:1 Langmuir binding model and data analysis was performed using SCRUBBER-2 software (distributed by Dr. David Myszka of the University of Utah Center for Biomolecular Interactions).
Suppression of Annexin A2 expression with siRNA
To suppress annexin A2 expression, cells were treated with three siRNA plasmids or with a control plasmid (FI514942, FI514943, FI514944, FI514945) according to the manufacturer's instructions (Origene). Immortalized murine tubular epithelial cells (24) were grown in culture until ~80% confluent and were then transfected with the siRNA using Lipofectamine 2000 and OPTI MEM media (Life Technologies) under selection with puromycin for seven days. Suppression of annexin A2 expression was confirmed by Western Blot analysis and by flow cytometry, and clones with reduced annexin A2 expression were chosen for further analysis.
Quantitative Reverse-Transcriptase Polymerase Chain Reaction
RNA was isolated from tissue using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. cDNA was generated from 1 μg of RNA using a High Capacity cDNA Reverse Transcriptase kit from Applied Biosystems. The cDNA abundance for annexin A2 and actin (as a loading control) were then measured using Power SYBR Green Master Mix (Applied Biosystems) and a Roche light cycler machine. The primers used to detect annexin A2 were: CCCAAGTGCCTACGGGTCAG (forward) and GCTGTCTCAATGTTCAGAGCA (reverse). The primers used to detect actin were: GGCTGTATTCCCCTCCATCG (forward) and CCAGTTGGTAACAATGCCATGT (reverse).
Flow cytometry
Flow cytometry was performed using a FACSCalibur flow cytometer (BD Biosciences). Cells analyzed included murine TECs, murine microvascular endothelial cells (MS1 cells, ATCC), murine mesangial cells (SV40 MES 13, ATCC), and Jurkat cells. C3 deposition on cells using a FITC-labeled goat anti-mouse C3 antibody (MP Biomedicals; 12 μg/ml). Annexin A2 expression on cell surfaces was detected using Invitrogen clone Z014 diluted 1:50 followed by Cy-5 labeled donkey anti-mouse IgG (Jackson Immunoresearch) diluted 1:200. For some binding experiments recombinant factor H or recombinant annexin A2 were fluorescently labeled with DyLight 650 (ThermoFisher Scientific). For some experiments the populations of necrotic cells were identified by staining the cells with propidium iodide.
Enzyme-linked immunosorbent assays (ELISAs)
C3a levels were measured by ELISA using monoclonal antibodies and standards from BD Biosciences according to the manufacturer's instructions. IL-6 and KC were measured using kits from R&D Systems according to the manufacturer's instructions.
Statistics
Multiple group comparisons were performed using ANOVA with post-test using Tukey's multiple comparison test. Comparisons between two groups were performed with Student's T tests. A P value of less than 0.05 was considered statistically significant. Results are reported as mean ± SEM.
Results
Cofactor activity is lost in the injured kidney
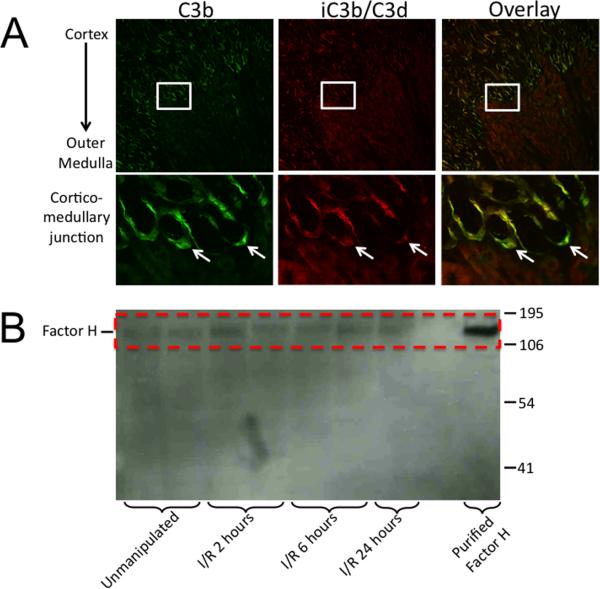
The AP is activated in the renal tubulointerstitium after ischemia/reperfusion (I/R) (13). During complement activation the protein C3 is converted to C3b. C3b is subsequently cleaved (inactivated) by factor I, generating iC3b. To perform this cleavage factor I requires a “cofactor” protein. In the mouse, the proteins Crry (25) and factor H (26) can serve as cofactors. We stained tissues from sham treated and post-ischemic mice using an antibody that recognizes C3b and a monoclonal antibody that only recognizes iC3b and/or C3d (23) (Figure 1A). C3b is deposited in renal tubules after I/R reflecting complement activation in the tubulointerstitium. The C3b is not completely converted to iC3b/C3d, however, indicating limited cofactor activity in the renal tubulointerstitium.
Figure 1. Factor H does not prevent complement activation in the kidney after ischemia/reperfusion.
Mice were subjected to renal ischemia/reperfusion. A) Kidney sections were immunostained for C3b and iC3b/C3d. C3 fragments were deposited on tubules at the cortic-medullary junction. Enlarged views of C3 fragment deposition at the cortico-medullary junction reveal that in some locations the C3b is incompletely converted to C3d (arrows). Original magnification 100X. B) Lysates of the outer medullae of kidneys were probed for factor H by Western blot analysis. Factor H was detected in the kidneys of unmanipulated C57BL/6 mice, and in kidneys subjected to ischemia and variable times of reperfusion.
We have previously shown that expression of Crry decreases on tubular epithelial cells after I/R and that complement activation begins within 6 hours of reperfusion (27). Because factor H can also serve as a cofactor, we performed Western blot analysis for factor H in lysates made from the outer medullae of kidneys subjected to I/R. Factor H was present in unmanipulated ischemic kidneys, and levels persisted during reperfusion (Figure 1B). Thus, complement activation occurs in the renal tubulointerstitium even in the presence of factor H. Factor H localizes to anionic surfaces enriched in anionic markers, such as glycosaminoglycans (GAGs), sialic acid glycans, or C3b (1, 28). Factor H was also detected within the kidneys of factor B knockout mice subjected to renal I/R (data not shown), even though C3b is not deposited on the tubules after I/R in this mouse strain (13). This indicates that binding partners for factor H other than C3b are present within the post-ischemic kidney.
Factor H binds to the protein annexin A2 in the post-ischemic kidney
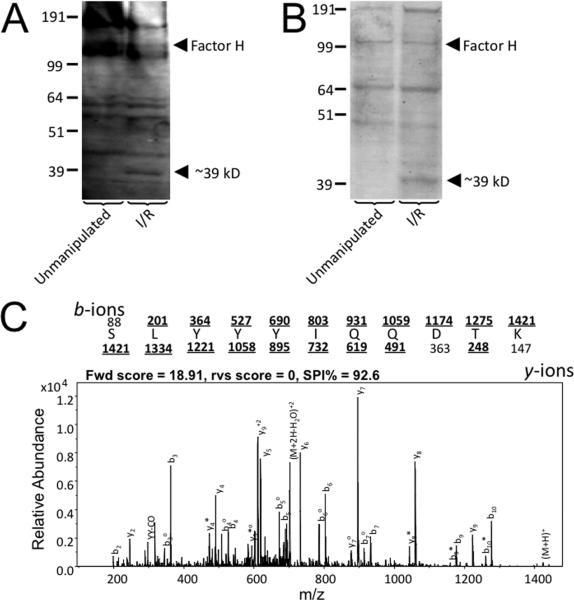
To identify potential binding ligands for factor H in the post-ischemic kidney, we performed Far-Western blots in which kidney lysates were probed with biotinylated factor H. Factor H bound to several proteins within the lysates, and binding to some bands was stronger in lysates from post-ischemic kidneys (Figure 2A). Biotinylated factor H was also incubated with kidney lysates, and factor H was then isolated using streptavidin beads to co-immunoprecipitate binding partners. The isolated proteins were examined by gel electrophoresis and multiple protein bands were visualized (Figure 2B). Several bands appeared stronger in lysates made from post-ischemic kidneys, including a band at ~39kD that was also seen in the Far-Western blot.
Figure 2. Factor H binds to annexin A2 expressed within the kidney after ischemia/reperfusion.
Factor H was purified from mouse plasma and biotinylated. Protein lysates were made from unmanipulated kidneys and kidneys subjected to ischemia/reperfusion. A) Far-Western Blot Analysis was performed using biotinylated factor H as a probe, and the factor H bound to multiple proteins within the lysates. It bound to some proteins in the lysates from post-ischemic kidneys that were not seen in lysates from unmanipulated kidneys, including a notably different band at ~39 kD. B) Factor H was used to immunoprecipitate proteins from kidney lysates. The proteins were separated by SDS-PAGE and stained with Coomassie. Again, a prominent band near ~39 kD was present only in the lysates from post-ischemic kidneys. C) Mass spectrometry of the ~39 kD protein identified it as annexin A2. A tandem mass spectrum of a peptide from annexin A2 (SLYYYIQQDTK) is shown. The protonated mass was m/z 1422.05 amu and the doubly-charged ion at m/z 711.53 was selected for fragmentation. The amino acid sequence is shown above the spectrum. The observed fragment ions are indicated in bold and are marked by a line placed over or under the letter representing the amino acid.
The ~39kD band from the immunoprecipitation reaction was excised from the gel and digested using trypsin. Resultant peptides were analyzed by liquid chromatography tandem mass spectrometry and spectra identified by database searching. This band yielded 8 peptides, 6 of which were unique, that mapped to annexin A2; the protein search score was 75.13 and these peptides accounted for 17% of the protein amino acid sequence. Identifications for three of the peptides were manually confirmed using their tandem mass spectra. The tandem mass spectrum for one of the peptides is shown (Figure 2C). The peptide score was 18.91 and the SPI% (a measure of how much of the ion current is accounted for by theoretically expected peaks) for this spectrum was 92.6, suggesting that the dominant ions conform to those theoretically expected for this sequence (29). Thirteen other proteins were identified from this analysis, but all of the other proteins were either intracellular proteins or likely contaminants (e.g. keratin).
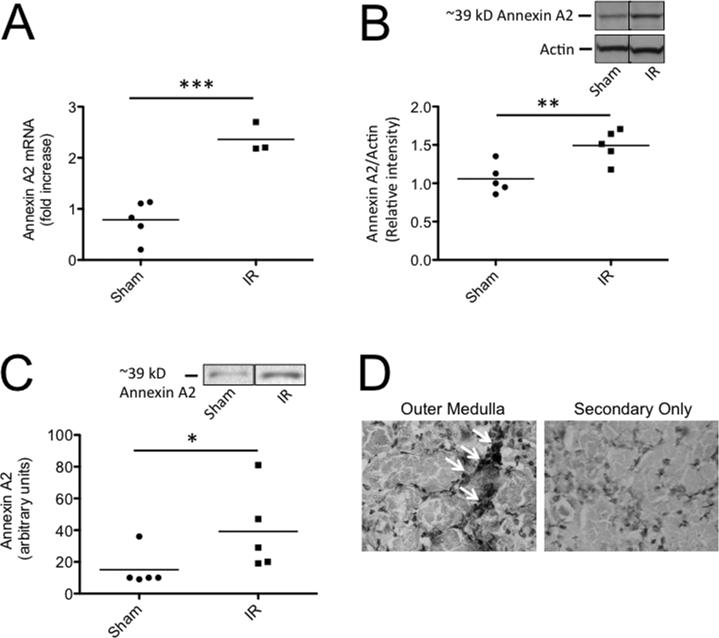
Factor H has previously been shown to bind Annexin A2 on the surface of apoptotic cells (20), and the expression of annexin A2 by tubular epithelial cells has been shown to increase after renal I/R (30). We performed quantitative reverse-transcriptase PCR (RT-PCR) and found that mRNA levels for annexin A2 increased after I/R in our model (Figure 3A), and Western blot analysis confirmed that levels of annexin A2 protein in the kidney increased after I/R (Figure 3B). Immunoprecipitation experiments in which biotinylated factor H was used to pull-down annexin A2 demonstrated that factor H bound to more annexin A2 in lysates from post-ischemic kidneys than in sham treated controls (Figure 3C). Annexin A2 was detected in the outer medulla of post-ischemic mice by immunohistochemistry (Figure 3D), the location in which complement activation occurs after I/R (Figure 1A).
Figure 3. Annexin A2 is expressed in the kidney after ischemia/reperfusion.
C57BL/6 mice were subjected to renal ischemia and 24 hours of reperfusion. A) RNA was isolated from kidneys and the expression of annexin A2 was measured by RT-PCR. Annexin A2 expression was significantly increased after ischemia/reperfusion. B) Annexin A2 was detected in kidney lysates by Western blot analysis and was normalized to levels of actin. The relative intensity of annexin A2 protein in the two groups was compared by Student's T test, and annexin A2 protein levels were significantly increased after ischemia/reperfusion. Bands from two non-adjacent lanes are shown. C) Biotinylated mouse factor H was used to immunoprecipitate binding partners from kidney lysates, and the immunoprecipitated proteins were examined by Western blot analysis with an antibody for annexin A2. The abundance of annexin A2 immunoprecipitated from the kidneys was greater in post-ischemic kidneys than in sham treated kidneys. Bands from two non-adjacent lanes are shown. The groups were compared with Student's T tests. D) Immunohistochemistry of kidney sections for annexin A2 demonstrated peri-tubular localization of the protein in the outer medullae of post-ischemic mice. Original magnification 400X.
Annexin A2 binds to factor H in the fluid phase and on surfaces
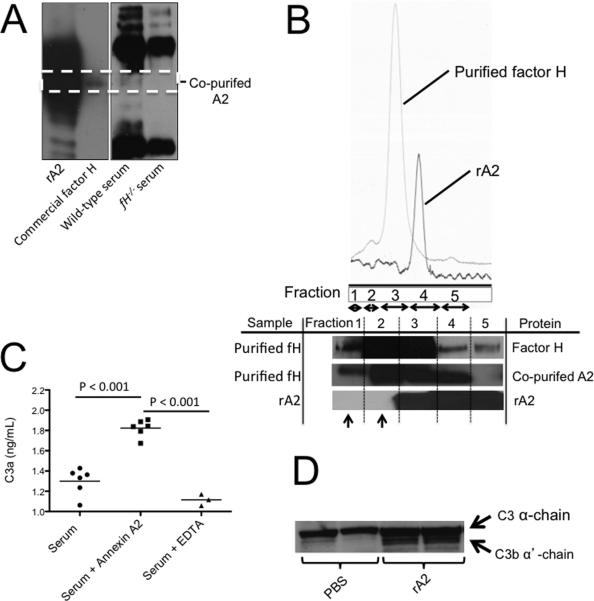
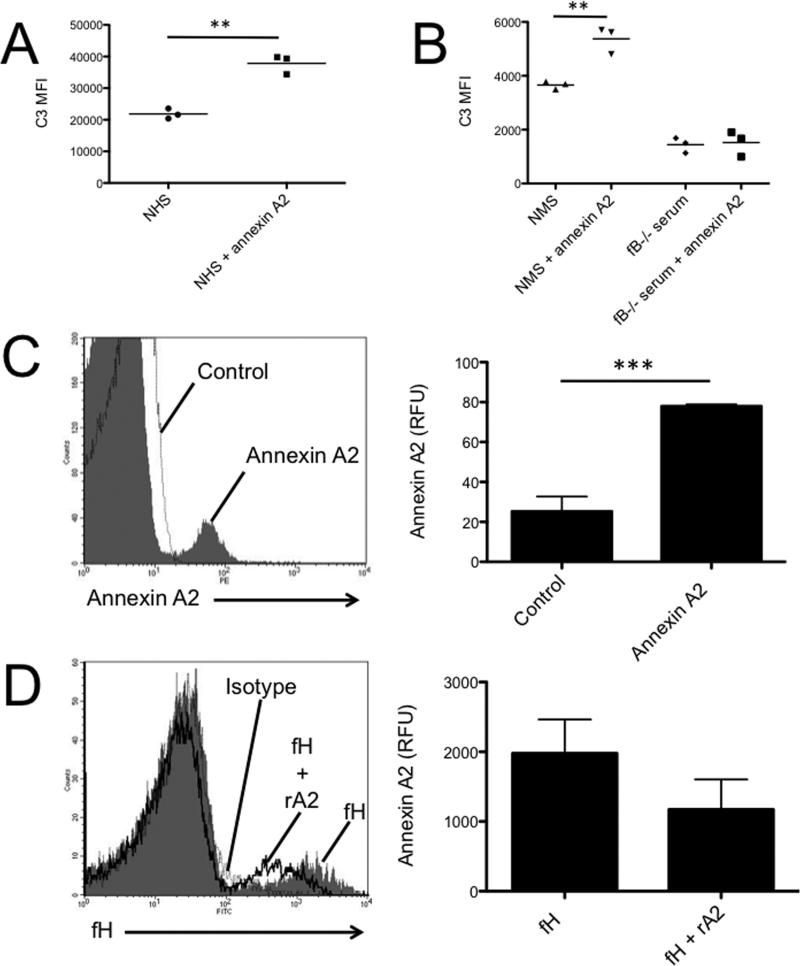
We purified mouse factor H from normal mouse serum using heparin chromatography followed by size exclusion chromatography. We then performed Western blot analysis of the purified factor H with an antibody for annexin A2 and found that annexin A2 was present in the sample. However, when factor H deficient serum was subjected to the same heparin chromatography and size exclusion chromatography protocols, annexin A2 was not purified (Figure 4A). This demonstrates that annexin A2 and factor H in serum bind, and isolation of factor H from normal mouse serum also captures annexin A2. Annexin A2 was also detected in a commercially available sample of human factor H (Figure 4A).
Figure 4. Annexin A2 binds factor H and increases complement activation in the fluid phase.
A) Factor H was purified from mouse plasma by heparin chromatography and Annexin A2 was detected in samples generated from wild-type serum and in a commercial preparation of factor H, but it was not seen in a sample generated from factor H-deficient (fH−/−) serum. Bands from two different blots are shown. B) Factor H purified from plasma and recombinant annexin A2 were passed over a size exclusion column. Annexin A2 in the fractions was detected by Western Blot Analysis. Co-purified annexin A2 was detected in all of fractions of factor H purified from serum. When recombinant annexin A2 (rA2) was passed over the column in the absence of factor H, it did not elute in the higher molecular weight fractions (fractions 1 and 2; indicated with arrows). C) Purified annexin A2 was added to normal mouse serum and incubated at 37° C for 30 minutes, and C3a in the supernatant was measured by ELISA. The addition of annexin A2 to the reaction increased generation of C3a. The groups were compared by ANOVA. D) Western blot analysis of plasma from mice injected with recombinant annexin A2 revealed greater cleavage of plasma C3 compared to mice injected with PBS as a vehicle control.
Factor H from normal mouse serum was purified using heparin chromatography and subsequently applied to a size exclusion column. Eluted proteins were collected, pooled into five separate fractions, and then analyzed by Western blot analysis (Figure 4B). Factor H was detected in all of the pooled fractions after gel filtration (molecular weight range of approximately 25-2000 kDa). Annexin A2 was co-purified from serum during the heparin chromatography, and it too was detected in high molecular weight fractions collected when the purified factor H was separated on the size exclusion column. Recombinant annexin A2 (in which no factor H is present) was passed through the size exclusion gel under the same conditions. This pure annexin A2 sample was only detected in fractions 3-5, corresponding a molecular weight range of approximately 25-120 kD.
To determine whether annexin A2 has a detectable functional effect on complement activation, 3.5 μg of recombinant annexin A2 was added to normal mouse serum (200 μl of 10% serum in PBS), representing approximately a 3-fold molar excess of annexin A2 to factor H. The mixture was incubated at 37° C and C3a was measured as a marker of complement activation in the serum. The addition of annexin A2 increased C3a production in the serum indicating that annexin A2 promotes complement activation (Figure 4C). In addition, 75 μg of recombinant annexin A2 was injected intravenously into wild-type mice and plasma was collected after 24 hours. Western blot analysis of the plasma for C3 demonstrated conversion of C3 to C3b (Figure 4D).
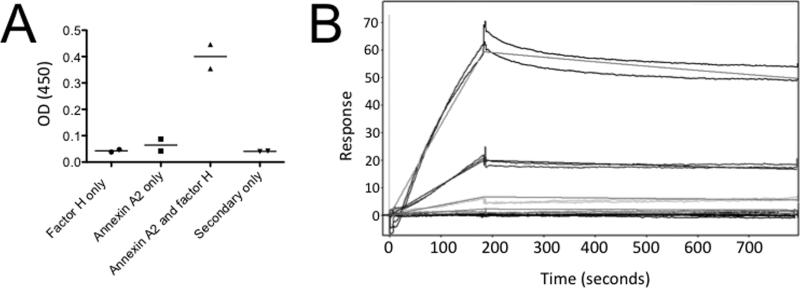
To determine whether factor H binds to annexin A2 on solid surfaces, we immobilized annexin A2 on an ELISA plate. Recombinant factor H bound to annexin A2 in this assay (Figure 5A). We also examined the interaction of the proteins using surface plasmon resonance. Factor H was immobilized on a CM5 chip, and varying concentrations of annexin A2 were injected. Annexin A2 bound to the immobilized factor H with a dissociation constant of approximately 7 μM (Figure 5B).
Figure 5. Annexin A2 binds factor H on solid surfaces.
A) Annexin A2 was immobilized on an ELISA plate and incubated with factor H. Factor H bound to the immobilized annexin A2. The experiment was repeated three times, and representative results are shown. B) Surface plasmon resonance was performed. Factor H was immobilized on a CM5 chip and the binding of annexin A2 was evaluated at several different concentrations (3 μM, 1 μM, 333 nM, 111 nM, and 37 nM). Annexin A2 bound to the factor H with a dissociation constant of approximately 7 μM.
Expression of annexin A2 by renal cells increases complement activation on the cell surface
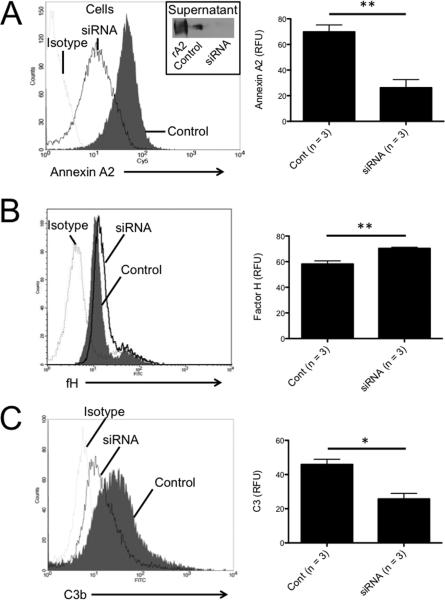
C3b is deposited on the surface of murine renal tubular epithelial cells (TECs) grown in culture after exposure to normal mouse serum, but we have previously found that antagonism of factor H causes a slight increase in complement activation on the cell surface (31). We treated TECs with siRNA in order to suppress annexin A2 expression. The level of annexin A2 on the cell surface and in the cell supernatant decreased after treatment with the siRNA (Figure 6A). Decreased expression of annexin A2 was associated with increased binding of factor H to the cell surface (Figure 6B), indicating that expression of annexin A2 by the cells is associated with reduced binding of factor H to the cell surface. We then exposed the cells to normal mouse serum and measured C3b deposition on the cell surface by flow cytometry (Figure 6C). Suppression of annexin A2 expression with siRNA decreased the amount of C3 deposited on the cell surface, demonstrating that annexin A2 produced by TECs increases complement activation on the cells.
Figure 6. Silencing of annexin A2 reduces complement activation on tubular epithelial cells.
The expression of annexin A2 by tubular epithelial cells was knocked-down by treating the cells with siRNA for annexin A2 or with control siRNA. A) Expression of annexin A2 was examined by flow cytometry. Treatment of the cells with siRNA for annexin A2 significantly reduced surface annexin A2. Annexin A2 in the supernatant was examined by Western blot analysis (inset). Annexin A2 was detected in the supernatant of cells treated with control siRNA, but levels were lower in the supernatant of cells treated with siRNA for annexin A2. B) Clones with low annexin A2 expression were exposed to normal mouse serum, and the level of factor H bound to the cells was examined by flow cytometry. The amount of factor H on the cell surface was significantly higher when annexin A2 expression was reduced by treatment with siRNA. C) Clones with low annexin A2 expression were exposed to normal mouse serum, and C3b deposition on the cells was measured by flow cytometry as a marker of complement activation. C3b deposition on the cell surface was lower on cells with reduced expression of annexin A2 than on cells treated with control siRNA.
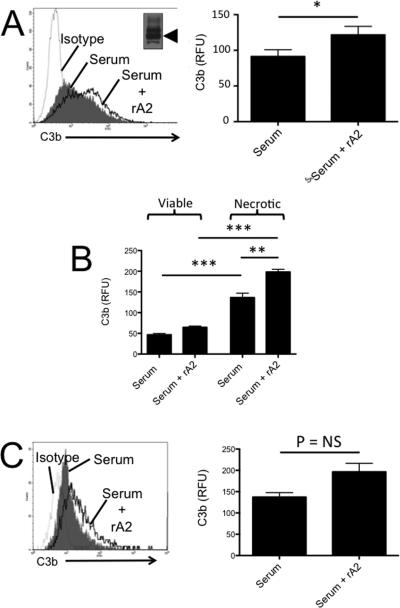
In another set of experiments, cells were incubated with normal mouse serum in the presence or absence of additional recombinant murine annexin A2 (rA2). When murine tubular epithelial cells were incubated with recombinant A2, C3b deposition on cells exposed to serum was increased (Figure 7A). If we replaced the supernatant prior to adding serum, however, no change in C3b deposition was seen, suggesting that unbound annexin A2 in the supernatant mediated the increase in C3b deposition. Increased C3b was seen on both viable and necrotic TECs, but the C3b deposition was greatest on necrotic TECs treated with annexin A2 (Figure 7B). The addition of rA2 to the reaction also increased C3b deposition on murine microvascular endothelial cells (Figure 7C). Use of commercial purified bovine annexin A2 gave results with TECs that were similar to those obtained with the recombinant murine protein (results not shown).
Figure 7. Annexin A2 increases complement activation on the surface of tubular epithelial cells and endothelial cells.
A) Murine tubular epithelial cells were incubated with normal mouse serum, and recombinant annexin A2 (inset) was added to the reaction. C3b deposition on the cells was measured by flow cytometry. The addition of annexin A2 increased C3b deposition on the cells (n = 6). B) Viable and necrotic cell populations were identified using propidium iodide, and C3b deposition on these populations was examined by flow cytometry. More C3b was detected on the surface of necrotic cells than viable cells. The addition of annexin A2 increased C3b deposition on both the viable and the necrotic cell populations. C) We also tested whether annexin A2 affects complement activation on endothelial cells. There was a trend towards greater C3b deposition when the cells were exposed to serum in the presence of annexin A2 (n = 3). *P < 0.05, **P<0.01, ***P< 0.001.
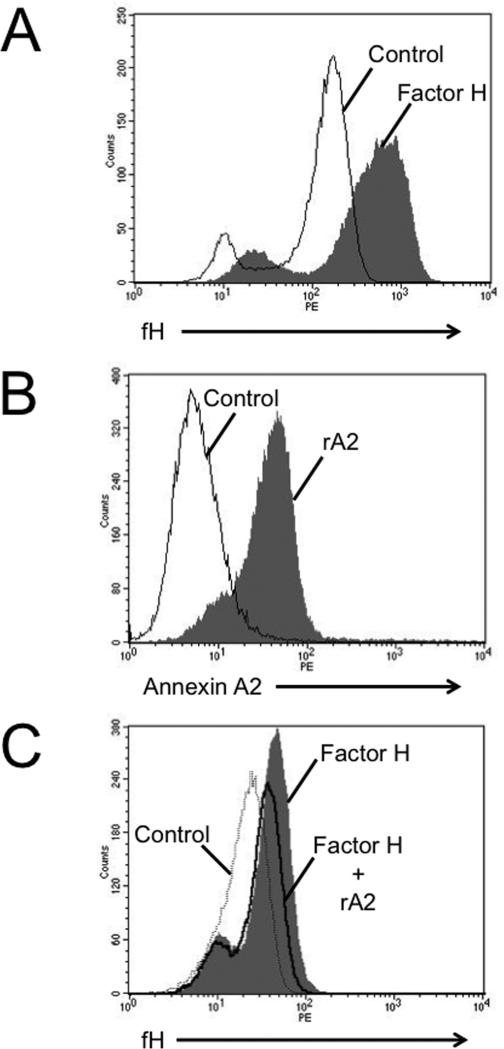
Factor H and annexin A2 both bind to the surface of TECs (Figures 8A-B). Yet, in the presence of annexin A2 less factor H bound to the cells. Thus, annexin A2 produced by the TECs (Figure 6) or added directly to the cell supernatants (Figure 8C) inhibits binding of factor H to the cells and increases AP activation on the cell surface. Although it is possible that some of the annexin A2 bound to the cell surface mediates binding of factor H to the cell membrane, the net affect of annexin A2 in the reactions is to reduce the amount of factor H bound to the cells.
Figure 8. Annexin A2 reduces the binding of factor H to tubular epithelial cells.
A) Tubular epithelial cells were incubated with fluorescently labeled recombinant factor H and were examined by flow cytometry. Factor H bound to the surface of the cells. B) Fluorescently labeled recombinant annexin A2 also bound to tubular epithelial cells C) The addition of annexin A2 reduced binding of purified factor H to the surface of tubular epithelial cells. Each of these experiments was repeated two times.
The effect of annexin A2 on complement regulation appears to be cell specific. Recombinant annexin A2 did not increase complement activation on the surface of Jurkat cells (an immortalized human T cell line) but did increase complement activation on the surface of ARPE cells (a human retinal epithelial cell line) when both cell types were exposed to human serum (data not shown). Similarly, we performed an assay in which sheep erythrocytes are lysed by the alternative pathway when exposed to human serum. Purified factor H decreases cell lysis in this assay by accelerating the decay of the C3bBbP complexes on the sheep erythrocytes. However, we did not detect an effect of recombinant annexin A2 on factor H function in this assay (data not shown).
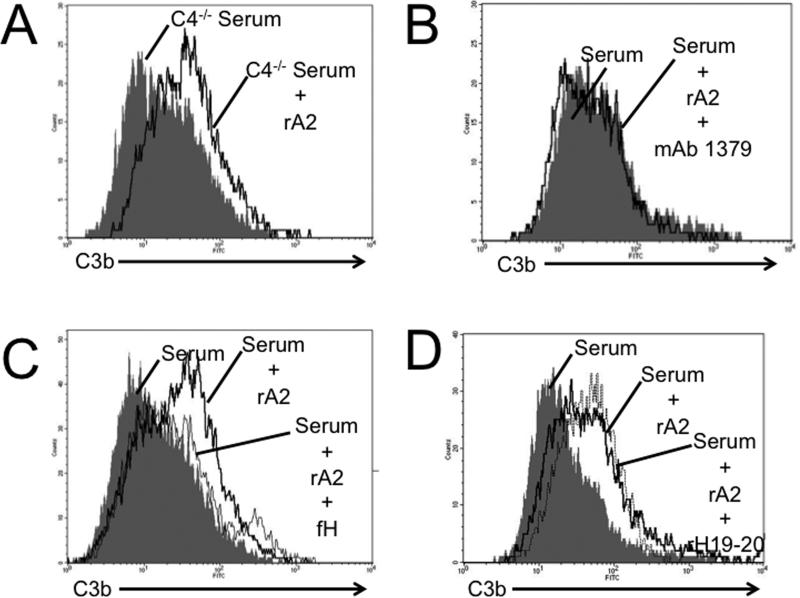
To further explore the mechanism by which annexin A2 promotes complement activation on the TEC surface, we incubated TECs with recombinant annexin A2 and serum from mice deficient in C4 (C4−/− mice; deficient in classical pathway activity). Annexin A2 increased C3 deposition on TECs exposed to serum from C4−/− mice (Figure 9A), indicating that increased complement activation in the presence of annexin A2 does not require the classical pathway. However, treatment with a monoclonal antibody to factor B that prevents AP activation [mAb 1379, (32)] prevented the increase in C3 deposition in cells exposed to annexin A2 (Figure 9B). Furthermore, the addition of 100 μg/mL of purified murine factor H to the reaction prevented the rA2-induced increase in C3 deposition (Figure 9C). On the other hand, when we added a peptide that prevents factor H from binding to cell surfaces without affecting its complement regulatory function [rH19-20 (33)], no additional C3b deposition was seen (Figure 9D). These results demonstrate that annexin A2 promotes AP activation on nearby cell surfaces. This effect is likely mediated through its interaction with factor H as the addition of supraphysiologic concentrations of factor H reverses the effect yet the addition of an antagonist of factor H binding (rH19-20) has no further effect on complement activation.
Figure 9. Annexin A2 triggers alternative pathway activation on tubular epithelial cells by antagonizing factor H.
Tubular epithelial cells were incubated with mouse serum and recombinant annexin A2, and C3b deposition on the cells was measured by flow cytometry as a marker of complement activation. A) When serum from C4-deficient mice (C4−/−; classical pathway deficient) was used, the annexin A2 increased C3b deposition on the cells. B) When mAb 1379 (an inhibitor of the alternative pathway) was added to wild-type serum it prevented an increase in C3b deposition by annexin A2. C) The addition of additional murine factor H reversed the effect of annexin A2 on C3b deposition. The above experiments were each repeated three times. D) The addition of rH19-20 (an antagonist of murine factor H binding) did not have any additional effect in the presence of annexin A2. These experiments were repeated two times, and a representative result is shown.
Annexin A2 increases intra-renal complement activation
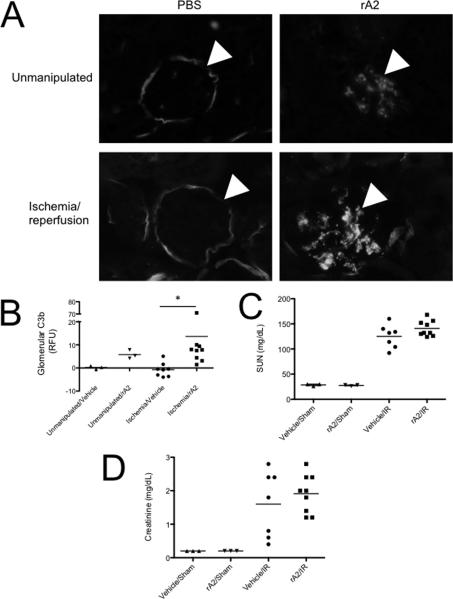
To determine whether annexin A2 promotes complement activation in vivo, we subjected mice to sham treatment or renal I/R. We then injected mice from each group with 75 μg of recombinant annexin A2. The mice were sacrificed after 24 hours and serum and tissues were examined. Both sham and IR treated mice demonstrated increased glomerular C3 deposition (Figures 10A and B). There was a trend towards higher serum urea nitrogen (SUN) and creatinine values in mice subjected to renal I/R and annexin A2 injection compared to mice treated with renal I/R and vehicle, although this did not achieve statistical significance (Figures 10C and D).
Figure 10. Injection of mice with annexin A2 causes glomerular complement activation.
Unmanipulated mice and mice subjected to renal ischemia/reperfusion were injected with 75 μg of recombinant annexin A2 and sacrificed after 24 hours. A) Immunofluorescence microscopy demonstrated glomerular C3b deposition. Original magnification 400X. B) Glomerular C3b deposits were significantly greater in mice injected with recombinant annexin A2 after renal ischemia/reperfusion than in those injected with a vehicle control. The C) serum urea nitrogen and D) serum creatinine levels were not significantly different in mice injected with recombinant annexin A2 compared with vehicle controls.
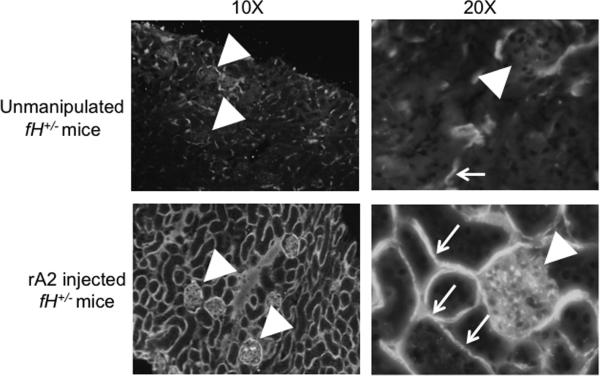
To further evaluate the interaction of annexin A2 with factor H in vivo, we also injected mice with heterozygous deficiency of factor H (fH+/− mice) with 75 μg of recombinant annexin A2. Exogenous annexin A2 caused widespread C3b deposition in the kidneys of fH+/− mice (Figure 11). In addition to C3b deposition in the mesangium, C3b was deposited continuously along the renal tubules throughout the tubulointerstitium. The effects of annexin A2 were much more extensive in fH+/− mice than in wild-type mice, demonstrating that these proteins have opposing roles in controlling complement activation in vivo.
Figure 11. Injection of factor H-deficient mice with annexin A2 causes glomerular and tubulointerstitial complement activation.
Mice with targeted deletion of one of the genes for factor H (fH+/− mice) were injected with 75 μg of recombinant annexin A2 and sacrificed after 24 hours. C3b deposition was examined by immunofluorescence microscopy. In unmanipulated fH+/− mice C3b was detected sporadically throughout the tubulointerstitium and Bowman's capsule (arrowhead), similar to what is seen in wild-type mice. In fH+/− mice injected with annexin A2, C3b deposits were seen in the glomeruli (arrowheads) and on the basolateral surface of tubules throughout the tubulointerstitium (small arrows). This experiment was performed three times, and representative images are shown.
Annexin A2 improves clearance of Streptococcus pneumoniae (S. pneumoniae) during acute otitis media
We have previously shown that AP activation mediates opsonophagocytosis of the bacteria in this model (22). In vitro, more C3b was deposited on S. pneumoniae incubated with either normal human or murine serum when annexin A2 was added to the reaction (Figures 12A and B), demonstrating that annexin A2 can also increase complement activation on the surface of bacteria. Annexin A2 did not increase C3b deposition on the bacteria when serum from factor B deficient mice was used. Purified annexin A2 and factor H both bound to a subset of the bacteria (Figures 12C and D). Similar to what was seen with TECs, however, the addition of annexin A2 reduced binding of factor H to the surface of the bacteria.
Figure 12. Annexin A2 increases clearance of S. pneumonia in acute otitis media.
A) S. pneumonia (Spn 6A) was incubated with 50% normal human serum (NHS) with or without the addition of annexin A2 (2 μg/ml). C3b deposition on the bacteria was measured by flow cytometry and was significantly greater when annexin a2 was added to the reaction. B) Spn 6A was incubated with 50% normal mouse serum (NMS) or serum from factor B deficient mice (fB−/−). C3b deposition on the bacteria was measured by flow cytometry and was significantly greater when annexin a2 was added to NMS. The addition of annexin A2 did not increase C3b deposition on the bacteria when incubated with serum from fB−/− mice. C) Spn 6A was incubated with fluorescently labeled annexin A2 which bound to a subset of the cells. D) Spn 6A was incubated with fluorescently labeled factor H and recombinant annexin A2. The addition of annexin A2 reduced binding of factor H to the bacteria. Each experiment was performed three times. **P<0.01, ***P< 0.001.
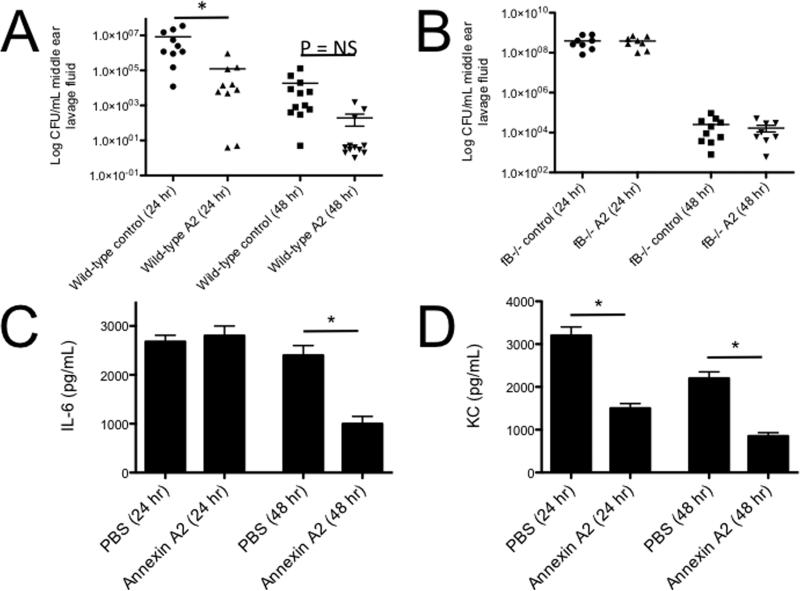
To test whether annexin A2 affects bacterial clearance by the complement system in vivo, we injected annexin A2 (2.5 μg per ear) with S. pneumoniae into the middle ears of mice. Injection of annexin A2 improved clearance of the bacteria (Figure 13A). Clearance of the bacteria was not improved in fB−/− mice injected with annexin A2 (Figure 13B), confirming that improved bacterial clearance in mice injected with annexin A2 is mediated by the alternative pathway. Although annexin A2 promotes complement activation, it decreased production of the inflammatory cytokines IL-6 (Figure 13C) and keratinocyte chemoattractant (KC or CXCL1; Figure 13D) in mice with otitis media.
Figure 13. Annexin A2 increases clearance of S. pneumonia in acute otitis media.
Survival of Spn type 6A in the middle ear of A) WT and B) fB−/− mice with or without annexin A2 was examined. Each data point represents the mean of colony forming units (CFU) of Spn per milliliter of the middle ear lavage fluid samples. Concentrations of C) IL-6 and D) KC in the middle ear lavage samples at 24 and 48 h post-infection with S. pneumoniae type 6A with or without annexin A2. Results are the mean concentrations of IL-6 and KC (±SEM) in the middle ear lavage samples from two duplicate wells from two separate experiments. *P<0.05.
Discussion
Factor H is the most important regulator of the AP, and it is present at high concentrations in the plasma of healthy individuals (approximately 250 μg/mL) (20, 34). Infections and aseptic tissue injury trigger AP activation, however, suggesting that AP regulation by factor H is impaired or overwhelmed in injured tissues. In the current study we report that production of the protein annexin A2 locally blocks regulation of the AP by factor H and increases complement activation.
The AP is activated in the kidneys of mice (13) and humans (14) after I/R, yet factor H is present in kidneys during the period of complement activation. Proteomic analysis revealed that the protein annexin A2 was present in the ischemic kidney and bound to factor H. Further experiments demonstrated that annexin A2 interfered with AP regulation by factor H and promoted AP activation on the surface of certain cell types. TECs treated with siRNA expressed reduced annexin A2 on the cell surface and in the supernatant. Decreased expression of annexin A2 was associated with increased binding of factor H to the cell surface and reduced C3b deposition on the cell. In contrast, the addition of recombinant annexin A2 to the cell supernatant decreased binding of factor H to the cell surface and increased C3b deposition on the cells. However, increased complement activation was not observed when the media containing recombinant Annexin A2 was replaced prior to addition of the serum. Therefore, although annexin A2 is expressed on cell surfaces and can bind to cell surfaces, its effect on AP activation is primarily mediated by binding to factor H in solution and preventing factor H from binding to cells. The effect of annexin A2 on complement activation proceeded via the AP, and supraphysiologic factor H was able to reverse the process.
The injection of mice with annexin A2 increased complement activation within the glomeruli. Complement activation was even more extensive when annexin A2 was injected into mice with partial deficiency of factor H (fH+/− mice). In these mice C3b deposition was seen in the glomeruli and along the tubules throughout the kidney. We also found, however, that injection of annexin A2 into the inner ears of mice improved the clearance of S. pneumoniae and reduced production of pro-inflammatory cytokines in a model of acute pneumococcal otitis media. Opsonization of bacteria with C3 was greater in the presence annexin A2. No effect was seen, however, when annexin A2 was injected into fB−/− mice during acute pneumococcal otitis media, confirming that the anti-bacterial effect of annexin A2 requires an intact alternative pathway. Although annexin A2 may also trigger pathologic AP-activation in the middle ear in other contexts, our in vitro and in vivo results suggest that increased eradication of bacteria facilitated by annexin A2 is associated with reduced production of inflammatory mediators during acute otitis media.
Annexin A2 is a Ca2+ regulated phospholipid binding protein that has numerous intracellular and extracellular functions (35, 36). Extracellular Annexin A2 functions as a surface bound receptor for several different molecules, including factor H (20), C1q (37), plasminogen (38), tissue plasminogen activator (39), and some pathogens (40, 41). Annexin A2 is also the target of autoantibodies in several diseases. Anti-phospholipid antibodies recognize A2 and block its fibrinolytic function (42), and anti-double stranded DNA antibodies in patients with lupus react to annexin A2 (43). Interestingly, annexin A2 has been identified within the lesions of several complement-mediated diseases, including age-related macular degeneration (44), dense deposit disease (45), and lupus nephritis (43). By binding C1q, annexin A2 may trigger classical pathway activation (37), and simultaneous inactivation of factor H would cause amplification through the AP. Whether endogenous annexin A2 plays a pathogenic role in tissue inflammation through its interaction with the complement system remains to be determined.
In recent years, significant progress has been made in our understanding of the role of the AP in diseases such as atypical hemolytic uremic syndrome (aHUS) (46) and C3 glomerulopathy (47). Nevertheless, several features of these AP-mediated diseases remain unexplained. Although a large number of complement defects have been identified in patients with aHUS, no predisposing conditions are identified in up to 40% of patients (12, 46). This suggests that other factors that are not traditionally regarded as part of the complement cascade can affect AP activation. It is also not clear why specific tissues are susceptible to AP mediated injury in patients with systemic defects in AP regulation. Furthermore, disease flares are frequently triggered by infections (12) or tissue ischemia (48, 49). Finally, it is noteworthy that patients with active disease do not always respond to treatment with factor H-sufficient plasma, even if the primary risk of aHUS is a deficiency of factor H (12). These clinical observations indicate that local factors influence activation of the AP by factor H, and the results presented in the current study provide a potential explanation for these findings.
The results in this study expand our general understanding of how the complement system is regulated. The ability of factor H to discriminate between host cells and invasive pathogens has been attributed to binding of the molecule to negatively charged molecules such as sialic acid moieties and GAGs that are displayed on the surface of host cells (1). In diseases such as AMD and aHUS the anatomic restriction of disease may be due to tissue specific distribution of these binding ligands or to differences among tissues in sulfation of the expressed GAGs (50). A set of proteins that are closely related to factor H, known as the factor H-related proteins (in humans designated Factor H-related proteins 1-5) have recently been identified as possible antagonists of FH (51-53). Each of these proteins possesses varying degrees of sequence identity with regions of CFH that are involved in GAG/sialic acid-binding and with C3b-binding, and may additionally bind to other ligands recognized by factor H (28, 54-60). Our data demonstrates that regulation of the AP by factor H on specific surfaces is also affected by expression of annexin A2. Multiple proteins can likely, therefore, dysregulate the AP through their interactions or competition with factor H. It is also likely that the balance of these molecules determines the overall AP activity on a given surface. The Factor H-related proteins are primarily produced in the liver (61), but tissues can actively regulate their expression of annexin A2 thereby affecting local AP activity.
A limitation of our study is that we have not yet identified the region of factor H that interacts with annexin A2. A previous study reported that the SCR 6-8 region of factor H binds to annexin A2 (20). This region of factor H mediates binding to tissue surfaces, but does not directly mediate complement regulation. Our results indicate that annexin A2 disrupts fluid phase regulation (Figures 4C and 4D) and cell surface complement regulation (Figures 6 and 7) by factor H. A direct interaction of annexin A2 with the first four SCRs could impair regulation in the fluid phase and on surfaces. It is also possible that binding of annexin A2 to other regions of the protein (e.g. SCR 6-8) changes the conformation of factor H, thereby affecting fluid phase complement regulation by the protein.
Our data indicate that therapies that block the interaction between annexin A2 and factor H could ameliorate complement-mediated inflammation. Such a therapeutic strategy could theoretically reduce inflammation in patients with diseases associated with increased annexin A2 expression, but such an agent would have less effect in healthy individuals or in unaffected tissues. This approach would be preferable to untargeted immunomodulatory drugs that completely block immune functions throughout the body. Based on our data, however, agents that interfere with the annexin A2-factor H interaction could still increase a patient's risk of infection. In addition, annexin A2 influences hemostasis on the surface of endothelial cells (62), and biologic agents that target annexin A2 could be thrombophilic (62). Ideally, therapies that target the annexin A2-factor H interaction would not disrupt the other functions of annexin A2.
In conclusion, we have found that the protein annexin A2 is up-regulated in the kidney after I/R and binds to factor H. Annexin A2 reduces the binding of factor H to cell surfaces, increasing AP activation on the cells. This novel mechanism of complement activation can improve bacterial clearance by the AP, but it also increases complement activation on host tissue surfaces. In patients with infections or tissue ischemia the increased production of proteins, such as annexin A2, that interfere with factor H function could trigger complement activation. Local production of these proteins could also explain why systemic defects in AP-regulation frequently lead to tissue specific disease. Therapies that block the annexin A2-factor H interaction may increase complement regulation by endogenous factor H and reduce tissue injury in AP-mediated diseases.
Acknowledgements
We thank Viviana P. Ferreira for providing recombinant factor H 19-20 (rH19-20), and Shaun Bevers at the University of Colorado Biophysics Core for technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Grants R01 DK076690 (JMT), R01 AR51749 (VMH), R01 AI051436 (DH), and a Novel Research Project in Lupus grant from the Lupus Research Institute (JMT).
References
- 1.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nature genetics. 2002:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 3.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clinical and experimental immunology. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465–473. doi: 10.1038/ki.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servais A, Noel LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grunfeld JP, Niaudet P, Lesavre P, Fremeaux-Bacchi V. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 6.Fremeaux-Bacchi V, Kemp EJ, Goodship JA, Dragon-Durey MA, Strain L, Loirat C, Deng HW, Goodship TH. The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: evidence from two independent cohorts. Journal of medical genetics. 2005;42:852–856. doi: 10.1136/jmg.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noris M, Ruggenenti P, Perna A, Orisio S, Caprioli J, Skerka C, Vasile B, Zipfel PF, Remuzzi G. Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: role of factor H abnormalities. Italian Registry of Familial and Recurrent Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura. J Am Soc Nephrol. 1999;10:281–293. doi: 10.1681/ASN.V102281. [DOI] [PubMed] [Google Scholar]
- 8.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 9.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 10.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 14.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67:524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 15.Lenderink AM, Liegel K, Ljubanovic D, Coleman KE, Gilkeson GS, Holers VM, Thurman JM. The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. American journal of physiology. Renal physiology. 2007;293:F555–564. doi: 10.1152/ajprenal.00403.2006. [DOI] [PubMed] [Google Scholar]
- 16.Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT. Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol. 2006;177:4094–4102. doi: 10.4049/jimmunol.177.6.4094. [DOI] [PubMed] [Google Scholar]
- 17.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. The American journal of pathology. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, E J, Kallenberg CG, Zhao MH. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. Journal of clinical immunology. 2009;29:282–291. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164:786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 20.Leffler J, Herbert AP, Norstrom E, Schmidt CQ, Barlow PN, Blom AM, Martin M. Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells. The Journal of biological chemistry. 2010;285:3766–3776. doi: 10.1074/jbc.M109.045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Li YX, Douthitt K, Stahl GL, Thurman JM, Tong HH. Role of the alternative and classical complement activation pathway in complement mediated killing against Streptococcus pneumoniae colony opacity variants during acute pneumococcal otitis media in mice. Microbes and infection / Institut Pasteur. 2012;14:1308–1318. doi: 10.1016/j.micinf.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Li YX, Stahl GL, Thurman JM, He Y, Tong HH. Essential role of factor B of the alternative complement pathway in complement activation and opsonophagocytosis during acute pneumococcal otitis media in mice. Infection and immunity. 2011;79:2578–2585. doi: 10.1128/IAI.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM. Detection of complement activation using monoclonal antibodies against C3d. The Journal of clinical investigation. 2013;123:2218–2230. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz N, Hosford M, Sandoval RM, Wagner MC, Atkinson SJ, Bamburg J, Molitoris BA. Ischemia activates actin depolymerizing factor: role in proximal tubule microvillar actin alterations. The American journal of physiology. 1999;276:F544–551. doi: 10.1152/ajprenal.1999.276.4.F544. [DOI] [PubMed] [Google Scholar]
- 25.Kim YU, Kinoshita T, Molina H, Hourcade D, Seya T, Wagner LM, Holers VM. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. The Journal of experimental medicine. 1995;181:151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natsuume-Sakai S, Okada M, Seya T, Nonaka M, Harada Y, Moriwaki K. The mouse factor H allotypes with multiple amino acid replacement, H.1 and H.2 show indistinguishable co-factor activity for factor I. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics. 1991;18:399–403. doi: 10.1111/j.1744-313x.1991.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 27.Thurman JM, Ljubanovic D, Royer PA, Kraus DM, Molina H, Barry NP, Proctor G, Levi M, Holers VM. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. The Journal of clinical investigation. 2006;116:357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pangburn MK, Pangburn KL, Koistinen V, Meri S, Sharma AK. Molecular mechanisms of target recognition in an innate immune system: interactions among factor H, C3b, and target in the alternative pathway of human complement. J Immunol. 2000;164:4742–4751. doi: 10.4049/jimmunol.164.9.4742. [DOI] [PubMed] [Google Scholar]
- 29.Hunt DF, Yates JR, 3rd, Shabanowitz J, Winston S, Hauer CR. Protein sequencing by tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng CW, Rifai A, Ka SM, Shui HA, Lin YF, Lee WH, Chen A. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int. 2005;68:2694–2703. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 31.Renner B, Coleman K, Goldberg R, Amura C, Holland-Neidermyer A, Pierce K, Orth HN, Molina H, Ferreira VP, Cortes C, Pangburn MK, Holers VM, Thurman JM. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J Immunol. 2010;185:3086–3094. doi: 10.4049/jimmunol.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Molecular immunology. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor h in regulating complement activation at cell surfaces. J Immunol. 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 34.Hakobyan S, Tortajada A, Harris CL, de Cordoba SR, Morgan BP. Variant-specific quantification of factor H in plasma identifies null alleles associated with atypical hemolytic uremic syndrome. Kidney Int. 2010;78:782–788. doi: 10.1038/ki.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Seminars in thrombosis and hemostasis. 2013;39:338–346. doi: 10.1055/s-0033-1334143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Leffler J, Blom AM. Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. The Journal of biological chemistry. 2012;287:33733–33744. doi: 10.1074/jbc.M112.341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjar KA, Guevara CA, Lev E, Dowling K, Chacko J. Interaction of the fibrinolytic receptor, annexin II, with the endothelial cell surface. Essential role of endonexin repeat 2. The Journal of biological chemistry. 1996;271:21652–21659. doi: 10.1074/jbc.271.35.21652. [DOI] [PubMed] [Google Scholar]
- 39.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. The Journal of biological chemistry. 1994;269:21191–21197. [PubMed] [Google Scholar]
- 40.Malhotra R, Ward M, Bright H, Priest R, Foster MR, Hurle M, Blair E, Bird M. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes and infection / Institut Pasteur. 2003;5:123–133. doi: 10.1016/s1286-4579(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 41.Kirschnek S, Adams C, Gulbins E. Annexin II is a novel receptor for Pseudomonas aeruginosa. Biochemical and biophysical research communications. 2005;327:900–906. doi: 10.1016/j.bbrc.2004.12.089. [DOI] [PubMed] [Google Scholar]
- 42.Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, Cravioto Mdel C, Alarcon-Segovia D, Sanchez-Guerrero J, Hajjar KA. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood. 2006;107:4375–4382. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol. 2010;21:1912–1927. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis). FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- 45.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, 3rd, Zipfel PF, Dogan A, Smith RJ. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 47.Pickering MC, D'Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Fremeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodriguez de Cordoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Quintrec M, Zuber J, Moulin B, Kamar N, Jablonski M, Lionet A, Chatelet V, Mousson C, Mourad G, Bridoux F, Cassuto E, Loirat C, Rondeau E, Delahousse M, Fremeaux-Bacchi V. Complement Genes Strongly Predict Recurrence and Graft Outcome in Adult Renal Transplant Recipients with Atypical Hemolytic and Uremic Syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 doi: 10.1111/ajt.12077. [DOI] [PubMed] [Google Scholar]
- 49.Noris M, Remuzzi G. Thrombotic microangiopathy after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1517–1523. doi: 10.1111/j.1600-6143.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 50.Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Wurzner R, Morgan BP, Uhrin D, Bishop PN, Day AJ. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J Immunol. 2013;190:2049–2057. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tortajada A, Yebenes H, Abarrategui-Garrido C, Anter J, Garcia-Fernandez JM, Martinez-Barricarte R, Alba-Dominguez M, Malik TH, Bedoya R, Cabrera Perez R, Lopez Trascasa M, Pickering MC, Harris CL, Sanchez-Corral P, Llorca O, Rodriguez de Cordoba S. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. The Journal of clinical investigation. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q, Wiesener M, Eberhardt HU, Hartmann A, Uzonyi B, Kirschfink M, Amann K, Buettner M, Goodship T, Hugo C, Skerka C, Zipfel PF. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. The Journal of clinical investigation. 2014;124:145–155. doi: 10.1172/JCI71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrin D, Barlow PN, Sim RB, Day AJ, Lea SM. Structural basis for complement factor H linked age-related macular degeneration. The Journal of experimental medicine. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma AK, Pangburn MK. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10996–11001. doi: 10.1073/pnas.93.20.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn S, Zipfel PF. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. European journal of immunology. 1996;26:2383–2387. doi: 10.1002/eji.1830261017. [DOI] [PubMed] [Google Scholar]
- 58.Blackmore TK, Hellwage J, Sadlon TA, Higgs N, Zipfel PF, Ward HM, Gordon DL. Identification of the second heparin-binding domain in human complement factor H. J Immunol. 1998;160:3342–3348. [PubMed] [Google Scholar]
- 59.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, Stehle T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nature chemical biology. 2015;11:77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- 60.Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HD, Svergun DI, Johansson CM, Uhrin D, Barlow PN, Hannan JP. Structural basis for engagement by complement factor H of C3b on a self surface. Nature structural & molecular biology. 2011;18:463–470. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends in immunology. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. The Journal of clinical investigation. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]