Abstract
Endothelial E- and P-selectins mediate lymphocyte trafficking in inflammatory processes by interacting with lymphocyte selectin ligands. These are differentially expressed among different T cell subsets and function alone or in cooperation to mediate T cell adhesion interactions. Here we characterize the expression and functionality of E-selectin ligands in T helper type 17 lymphocytes (Th17 cells) and report that CD43 functions as a Th17 cell E-selectin ligand in vitro that mediates Th17 cell rolling on the vascular endothelium and recruitment in vivo. We demonstrate Th17 cells express CD44, P-selectin glycoprotein ligand (PSGL-1) and CD43. Few PSGL-1−/−CD43−/− Th17 cells accumulated on E-selectin under shear flow conditions compared with WT cells. CD43−/− Th17 cells accumulation on E-selectin was impaired as compared to WT and PSGL-1−/−, and similar to that observed for PSGL-1−/−CD43−/− Th17 cells, indicating that CD43 alone is a dominant ligand for E-selectin. Notably, this finding is Th17 cell subset specific because CD43 requires cooperation with PSGL-1 in Th1 cells for binding to E-selectin. In vivo, Th17 cell recruitment into the air pouch was reduced in CD43−/− mice in response to CCL20 or TNF-α, and intravital microscopy studies demonstrated that CD43−/− Th17 cells had impaired rolling on TNF-α treated microvessels. Furthermore, CD43−/− mice were protected from Experimental Autoimmune Encephalomyelitis and had impaired recruitment of Th17 cells in the spinal cord. Our findings demonstrate CD43 is a major E-selectin ligand in Th17 cells that functions independent of PSGL-1, and suggest CD43 may hold promise as a therapeutic target to modulate Th17 cell recruitment.
Introduction
T helper type lymphocytes (Th17 cells) secrete IL-17A, IL-17F, IL-22 and IL-21, play a dominant role during the immune response against bacteria and fungi pathogens, and participate in several human immune/inflammatory diseases strongly associated with chronic inflammation and organ specific autoimmunity (1-4). The selective recruitment of T helper cell subsets into tissues is a highly regulated process that requires adhesive interactions between specific ligands expressed on the T cell surface with their respective adhesion molecule expressed on the vascular endothelium, in combination with appropriate chemokines (5,6). Endothelial selectins, E-selectin and P-selectin, mediate the initial contact of Th cells with the activated endothelium resulting in rapid and transient interactions that initiate rolling on the surface of endothelial cells under shear flow conditions (7). We have previously published that Th17 cells adhesion to the vascular endothelium is highly dependent on their interactions with E-selectin in vitro and in vivo (8) and that IL-17A can further induce E-selectin and promote subsequent leukocyte adhesion (9), but the E-selectin ligands expressed on Th17 cells have not been identified to date. There are several ligands for selectins, but only P-selectin glycoprotein ligand 1 (PSGL-1), which binds all three selectins in vivo, has been characterized at the molecular, cellular and functional level (10,11). Two other glycoproteins, the sialomucin CD43, and the hyaluronan receptor CD44, have been shown to mediate adhesion of Th1 cells to E-selectin in vitro and in vivo, but only in cooperation with PSGL-1 as deduced by studies using single and double deficient mice (12-14). CD43 was also identified as an E-selectin ligand in skin homing CLA+ human cells (15). These findings were reported before the identification of Th17 cells, and therefore the role of E-selectin ligands on Th17 cells remains unknown. CD43 is a highly glycosylated surface protein expressed by most hematopoietic cells existing in two isoforms, a 115 kDa from expressed on resting cells and a 130kDa form produced by changes in O-glycosylation due in part to upregulation of core 2 N-acetylglucosaminyltransferase (16). CD43 has multiple functions including regulation of T cell activation, proliferation and trafficking to the lymph nodes (17-19). In addition, studies in CD43−/−, CD44−/−, and PSGL-1−/− mice have proven useful in determining the role of these molecules in autoimmune diseases (20-22). These studies, however, occurred prior to the discovery of Th17 cells, and therefore did not address whether specific Th17 cell recruitment to sites of inflammation or immune reactions were affected. Moreover, the dominance of Th17 cells in certain organ-specific autoimmune diseases occurs (1,3,4) and we previously demonstrated that Th17 and Th1 cells differ in their interactions with the vascular endothelium in an E-selectin dependent manner (8). Given this evidence, we hypothesized that E-selectin ligand-endothelial selectin interactions determine selective recruitment of Th17 cells to sites of inflammation.
In the current study, we have determined the functionality of the three characterized T cell E-selectin ligands CD44, PSGL-1 and CD43 on Th17 cells using a combination of biochemical and biofunctional blot rolling assays. Using Th17 cells generated from mice deficient in these molecules and real time videomicroscopy under shear flow conditions in vitro that simulate flow conditions in postcapillary venules we report that both PSGL-1 and CD43, but not CD44, function as E-selectin ligands for Th17 cells. Moreover, our results indicate that CD43 functions as a major E-selectin ligand for Th17 cells independent of PSGL-1 in vitro, and uniquely participates in Th17 cell recruitment to the dermal air pouch model and to the spinal cord in Experimental Autoimmune Encephalomyelitis (EAE), contrary to Th1 cells. Using competitive rolling assays and confocal intravital microscopy, we provide compelling evidence that CD43 mediates Th17 cell rolling to the activated vascular endothelium in vivo in an E-selectin dependent manner. Further examination of triple knock out (TKO) CD43−/−PSGL-1−/−CD44−/− mice suggest that there are most likely no additional glycoprotein ligands that function as E-selectin ligands in Th17 cells. Our data position CD43 as the major E-selectin ligand responsible for Th17 cell rolling on activated vasculature and recruitment during inflammation and autoimmunity.
Materials and Methods
Reagents
Recombinant mouse IL-23, E-selectin and P-selectin Fc-chimeras were from R&D Systems (Minneapolis, MN). Recombinant mouse IL-12, IL-2, IL-6, TNF-α, recombinant human TGF-β, and the following antibodies to mouse cytokines and adhesion molecules: IL-4 (clone 11B11), IFNγ (clone XMG 1.2), IL-2 (clone JES6-1A12), CD4 (clone GK 1.5), CD3 (clone145-2C11), CD28 (clone 37.51), IL-17A (clone 2C11-18H10.1), CD43 activation-associated glycoform (clone 1B11), and CD44 (clone IM7) are all from Biolegend (San Diego, CA). Anti- PSGL-1 and mouse TNF-α were purchased from BD-Pharmingen (San Jose, CA), and carrier free CCL20 from Peprotech (Rocky Hill, NJ). PMA and ionomycin were from SIGMA (St. Louis, MO). Secondary Abs coupled to alkaline phosphatase were from Promega (Madison, WI). Vibrant CFSE and Alexa 680 were from Life Technologies (Carlsbad, CA). Myelin Oligodendrocyte glycoprotein was purchased from Anaspec (Fremont, CA) and Pertussis Toxin was purchased from List Biological Laboratories (Campbell, CA). Anti-E-selectin (clone 9A9) antibody was generously provided by Dr. F. William Luscinskas (Brigham and Women's Hospital, Boston, MA) and IgG control was from Biolegend (San Diego, CA).
Mice
All mice used were bred in the pathogen free facility at Tufts University School of Medicine, Ziskind Building, in accordance with the guidelines of the committee of Animal research at Tufts University School of Medicine, Tufts Medical Center and the NIH Animal research guidelines. C57Bl/6 (WT) mice were purchased from Jackson Laboratory (Bar Harbor, Maine) or used as littermates from heterozygous crosses. Double knock out (DKO) PSGL-1−/−CD44−/− and PSGL-1−/−CD43−/− mice were obtained from Dr. Rodger McEver (OMRF, Oklahoma City, OK). CD43 (CD43−/−) and PSGL-1 (PSGL-1−/−) were generated from inter-crosses of PSGL-1−/−CD43−/− DKO and PSGL-1−/−CD44−/− DKO mice with C57Bl/6 (WT) mice. Triple knock out (TKO) PSGL-1−/−CD43−/−CD44−/− were generated by crossing PSGL-1−/−CD43−/− DKO mice with PSGL-1−/−CD44−/− DKO mice. CD44−/− mice were purchased from Jackson Laboratories. Mice were sacrificed at 7-12 weeks of age for harvest of naïve T cells, or used between 8-10 weeks of age for air pouch and intravital microscopy experiments. The genotypes were determined by PCR, and null mutations were also confirmed by FACS analysis of spleen cells. All deficient mice in this study were viable and fertile as previously described (13,14,23).
Preparation of effector T cells
CD4+ cells were isolated from spleen and lymph node cell suspensions of WT or genetically deficient mice using positive selection by immunomagnetic beads (Invitrogen, Carlsbad, CA). Th1 cells were derived from the naïve T cells by anti-CD3 and anti-CD28 stimulation in the presence of IL-12 and IFN-γ, as previously described (8). To achieve Th17 differentiation, naïve T cell were stimulated with anti-CD3 in the presence of human TGF-β (3ng/ml), mouse IL-6 (30ng/ml), mouse IL-23 (20ng/ml), plus anti-IFN-γ (10ug/ml), anti-IL-4 (10μg ml), and anti-IL-2 (10μg/ml) mAb. On day 3, Th1 and Th17 cultures were diluted 1:1 with fresh medium containing IL-2 (25U/ml) and IL-23 (20ng/ml), respectively. Cells were harvested on day 4 and immediately used in experiments. No significant differences were observed in Th17 cell differentiation among the different mice strains. Note that studies of T cell subsets in many laboratories use populations that do not uniformly produce the detectable defining cytokines by flow cytometric assays, and usually the signature cytokine- producing cells are <40% of the population (8,13,17,21,24), reflecting the nature of the assays not inducing synchronized cytokine expression in all cells rather than the indicated percent positive undergoing appropriate differentiation.
Measurement of interactions of T cells with selectins under defined flow conditions in vitro
T cell interactions with immobilized E-selectin and P-selectin were observed by videomicroscopy (20x objective) under defined laminar flow conditions in a parallel plate apparatus using a Nikon Elements NIS software. Accumulation of T cells on immobilized adhesion molecules was measured as determined in 8 different fields of view after the initial minute of each flow rate (shear stress 0.8 dynes/cm2 and 1 dyne/ cm2) as previously described (8,14). Where indicated, the E-selectin coated coverslips were incubated with anti-E-selectin function blocking Ab (clone 9A9) or IgG at 20μg/ml during 20 minutes before Th17 cells were perfused in the flow chamber.
Flow cytometry and cell sorting
Flow cytometry was performed to corroborate the differentiation of Th17 cells as described, the expression levels of E-selectin ligands on Th1 and Th17 cells (8), and the infiltration of Th17 cells and Th1 cells into the air pouch and spinal cord in vivo. To detect intracellular IL-17A and IFNγ, Th cells or leukocytes harvested from the sites of inflammation were stimulated during 4h with PMA, ionomycin, brefeldin and monensin, stained for CD4, fixed, permeabilized using the Fix/Perm buffer from Biolegend (San Diego, CA), and stained for the indicated cytokine. For the sorting of Th17 and Th1 cells, cells were stained with the CD43 activation associated glycoform antibody (clone 1B11) from Biolegend (San Diego, CA) and separated based on expression of CD43 based on histogram analysis using a MoFlo Astrios EQ (Beckman Coulter). The data was acquired on a FACS Canto flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Ashland, OR).
Western Blots, Immunoprecipitation, and blot rolling assays
The preparation of cell lysates with 1% N-octylglucoside (Roche, Indianapolis, IN), SDS-PAGE, and immunoprecipitation and western blotting techniques were all performed as previously described (14,15). Protein concentrations were determined with the BCA kit (Thermofisher Scientific, Rockford, IL). Immunoprecipitation reactions used anti-PSGL-1 antibody (2PH1), anti-CD43 glycoform antibody (1B11) plus anti-CD43 antibody (sc-7054), or antiCD44 (clone IM7). Briefly, aliquots of lysate from Th17 cells (300–450 μg) were incubated with 6 μg of the indicated mAb or with isotype control mAb and 30–45 μl of protein A-G PLUS Agarose beads (Santa Cruz Biotechnology) with rotation overnight at 4°C. Agarose bead immune complexes were collected by centrifugation, washed with 1% N-octylglucoside lysis buffer and the process repeated once more. Each sample was subjected to SDS-PAGE and western blotting under standard conditions. Where indicated, WT or TKO Th17 lysates (100ug) were loaded in the SDS-PAGE. The blot rolling assay allows for real-time observation of selectin-mediated interactions with cellular glycoproteins separated by molecular mass or immunoprecipitated from total cellular lysates and immobilized on membrane used in Western blot. The parallel flow chamber system, maintenance and processing of E- or P-selectin transfected CHO cells and use of the blot rolling assay have been described in detail (14,25). Studies were performed at shear stress 0.6 dynes/cm2 to allow quantification of rolling interactions on the membrane substrate without turbulence interference, as described (25).
In vivo air pouch model of leukocyte recruitment
Air pouches were created in the dorsal side of the back of aged matched WT and the indicated selectin ligand deficient mice as previously described (8,26). PBS, TNF-α (500ng/mouse) or CCL20 (400ng/mouse) were injected in the air pouch, and 24h later, cell infiltrates were harvested by repeated washes with PBS. Single cell suspensions were permeabilized and stained for intracellular IL-17A or IFNγ and analyzed by flow cytometry.
Competitive rolling assay for T cell adhesion in vivo in the cremaster muscle
Intravital microscopy studies of the mouse cremaster muscle microcirculation were performed as described (8,27). Mouse recombinant TNF-α (500 μg in 200 μl saline/mouse), was injected intrascrotally 1.5h prior to cremaster exteriorization. Mice were anesthetized and a microcatheter was introduced to the right femoral artery to enable retrograde injection of fluorescently labeled Th17 cells. Where indicated, 90 μg of IgG or anti-E-selectin Ab (clone 9A9) were injected via the jugular vein right before injection of the T cells. Transmitted light and fluorescence cremaster imaging was performed with an Olympus FV1000 confocal intravital microscope using a 20x water immersion objective (Olympus). Fluorescence imaging was done sequentially at 473 and 635nM to reduce the potential for channel crosstalk. CFSE and Alexa 680 labeled Th17 WT and CD43−/− Th17 cells or Th1 cells were suspended at 33×107 cells/ml and small boluses (3 × 106 of each type) of a mixture of both were injected retrograde into the femoral artery catheter to visualize their adhesion in the postcapillary venules. Microvessel images were analyzed off-line using Imaris software (Imaris, South Windson, CT).
Experimental Autoimmune Encephalomyelitis
WT and CD43−/− mice were immunized using 100μg of Myelin Oligodendrocyte Glycoprotein (MOG) emulsified 1:1 in CFA. Mice were also given 200ng of Pertussis Toxin to permeabilize the blood brain barrier. Mice were clinically scored using the following criteria: 0. no disease; 1. flaccid tail; 2. hind limb weakness; 3. hind limb paralysis; 4. forelimb weakness; 5 moribund. The spinal cords were harvested at day 16 post-injection, cells were isolated using ficoll gradient and stained for CD4, IL-17A and IFNγ by flow cytometry. Lymph nodes were harvested at day 8 post immunization, passed through a cell strainer and the cells were cultured ex vivo with MOG (10μg/ml), IL-2 (25U/ml) and IL-23 (25ng/ml). Cells were harvested 72 hours later and stained for Flow Cytometry.
Results
PSGL-1 and CD43 immunoprecipitated from Th17 cell lysates support major E-selectin dependent rolling under flow conditions in vitro
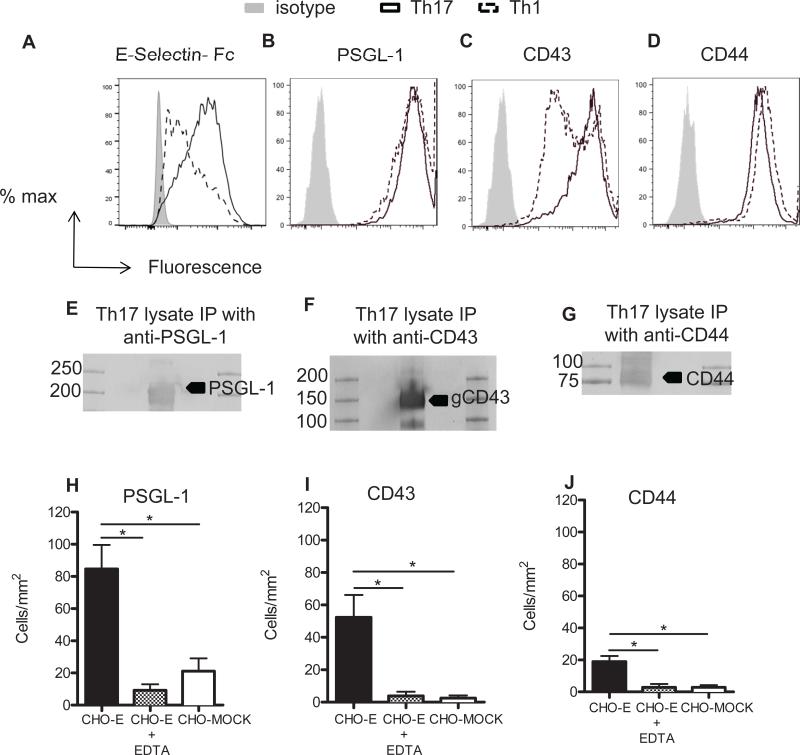
While we have previously shown that Th17 cells adhere to E-selectin in higher numbers than Th1 cells under shear flow conditions, the functional E-selectin ligands that mediate these interactions have yet to be determined. We first examined the expression levels of the three candidate scaffold proteins known to be functional E-selectin ligands expressed on CD4+ T cells, PSGL-1, CD43 and CD44 (7). An E-selectin chimeric protein bound to Th17 cells with higher affinity than to Th1 cells, suggesting higher expression of E-selectin ligands in Th17 cells (Fig. 1A). By flow cytometry we found that Th17 cells express PSGL-1, CD44 and the highly glycosylated form of CD43. Whereas PSGL-1 and CD44 were expressed at similar levels in Th17 and Th1 cells, Th17 cells showed a more robust expression of CD43 and a loss of the typical bimodal expression of highly glycosylated CD43 observed in Th1 cells (28,29) (Fig. 1B-D). We next evaluated the functionality of these molecules as Th17 cell E-selectin ligands under flow conditions using blot rolling assays. This assay can detect E-selectin dependent rolling on CD43, CD44 and PSGL-1 immunoprecipitated from WT in vitro generated Th17 cell lysates and immobilized on western blots. As expected, PSGL-1 migrated at a band of ~220Kda, CD43 at a band of ~130kDa, and CD44 at a band of ~90KDa (Fig. 1E-G). PSGL-1 and CD43 immunopurified from Th17 cells supported significant rolling of CHO cells overexpressing E-selectin (CHO-E cells). Moreover, the observed rolling activity was E-selectin specific because the immunoprecipitated proteins did not support rolling of control CHO-MOCK cells under the same conditions, and the rolling activity was removed in the presence of the Ca2+ chelator EDTA (Fig. 1H-J). CD44, a neutrophil and Th1 cell expressed E-selectin ligand (13,30,31) supported very little E-selectin dependent rolling as compared with PSGL-1 and CD43, however, rolling was not observed with CHO-MOCK or in the presence of EDTA (Fig. 1J). Rolling was also absent in other regions of the gels (data not shown). Taken together, our findings indicate that PSGL-1, CD43 and CD44 are all expressed at high levels on Th17 cells, but when isolated from Th17 cells and immobilized on western blots in large quantities, only PSGL-1 and CD43 support major E-selectin mediated rolling in vitro, whereas the contribution of CD44 to Th17 cell rolling on E-selectin is minor.
Figure 1. Expression of candidate scaffold molecules and their functionality as E-selectin ligands in Th17 cells.
A-D. Th17 cells were stained for total expression of E-selectin ligands using an E-selectin chimeric protein (A) or the indicated candidate scaffold molecules and analyzed by flow cytometry (B-D). E-J. Aliquots of lysate from Th17 cells were immunoprecipitated with the indicated Abs or the isotype control (data not shown) using A-G plus agarose beads. Immuno-complexes were subjected to SDS-PAGE and western blotting for the indicated Abs under standard non reduced conditions as described in methods. Molecular mass markers are included in the flanking lanes of the blot. A representative western blot is shown for each ligand. CHO-MOCK and CHO-E cells were drawn in the flow chamber at a shear stress of 0.6 dynes/cm2 in the presence or absence of EDTA across Th17 cells PSGL-1 (E), CD43 (F) and CD44 (G). Data show the mean ± SD values. *p<0.05.
Absence of PSGL-1, CD43 or both molecules results in severe impairment of Th17 cell adhesion to E-selectin under flow conditions in vitro
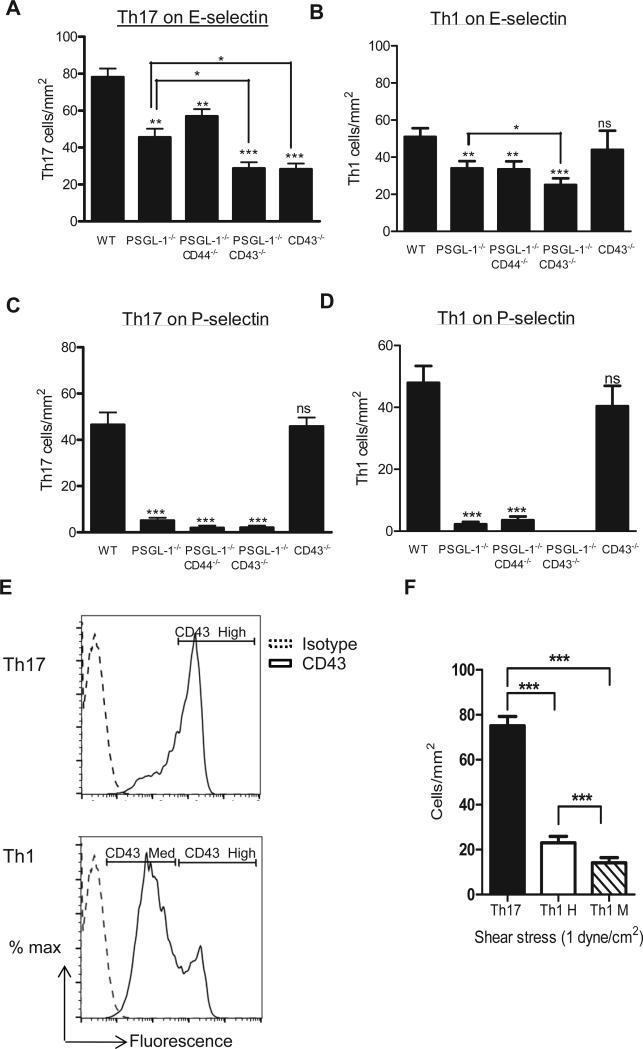
We next evaluated the ability of Th17 cells generated in vitro from WT or PSGL-1−/− mice to accumulate on E-selectin under flow conditions. Because both CD43 and CD44 have been reported to function as E-selectin ligands on Th1 cells only in cooperation with PSGL-1 (12-14), we also evaluated the ability of Th17 cells generated from PSGL-1−/−CD44−/− and PSGL-1−/−CD43−/− double knock out (DKO) mice to accumulate on E-selectin. CD4+ cells isolated from each of the mice under study differentiated similarly into Th17 cells as CD4+ cells isolated from WT mice, indicating that the lack of these molecules does not result in impaired Th17 cell differentiation in vitro (Supplemental Fig. 1). Removal of PSGL-1 alone resulted in a 42% decrease in Th17 cells accumulation on E-selectin as compared with WT Th17 cells. The removal of CD43 in combination with PSGL-1 (PSGL-1−/−CD43−/− DKO Th17 cells) resulted in significantly reduced E-selectin interactions as compared with both WT and single PSGL-1−/− mice (66% reduction PSGL-1−/−CD43−/− vs WT, and 41.5% reduction on PSGL-1−/−CD43−/− vs PSGL-1−/−). In contrast, Th17 cells lacking CD44 and PSGL-1 (PSGL-1−/−CD44−/−) showed no further significant decrease in binding to E-selectin compared to PSGL-1−/−, suggesting that the reduction in E-selectin binding compared to WT cells was attributable to the absence of PSGL-1 (Fig. 2A). The absence of CD44 alone did not impact the adhesion of either Th17 cells or Th1 cells to E-selectin under the same conditions (Supplemental Fig. 2). Our findings so far indicated a unique expression of CD43 in Th17 cells vs Th1 cells (Fig. 1A), functionality of CD43 isolated from Th17 cells in mediating E-selectin dependent rolling (Fig. 1C) and decreased adhesion of PSGL-1−/−CD43−/− vs PSGL-1−/− Th17 cells to E-selectin under flow conditions. Therefore we hypothesized that CD43 functions as an E-selectin ligand for Th17 cells that is sufficient to mediate E-selectin adhesion and does not require cooperation with PSGL-1. Interestingly, CD43−/− Th17 cells showed significant impairment in binding to E-selectin (63.4% reduction vs WT), similar to PSGL-1−/−CD43−/− DKO Th17 cells and was further decreased as compared to PSGL-1−/− Th17 cells (Fig. 2A). This was a specific and unique characteristic of Th17 cells, since CD43−/− Th1 cells adhered to E-selectin in comparable numbers as WT cells (Fig. 2B), as we and others have previously described (14). We observed that PSGL-1−/− Th17 and Th1 cells alone or in combination with other ligands (DKO) did not interact with P-selectin under flow conditions, whereas Th17 and Th1 cells from WT and CD43−/− did interact (Fig. 2C, D). Taken together, our results indicate that CD43 functions as a major ligand for E-selectin on Th17 cells but not on Th1 cells under shear flow conditions in vitro.
Figure 2. CD43, PSGL-1, but not CD44, function as Th17 cell E-selectin ligands under shear flow conditions in vitro.
Th17 (A, C) and Th1 (B, D) cells (1 × 106 cells/ml) generated in vitro from the indicated deficient mice were perfused at 1 dyne/cm2 over glass coverslips coated with recombinant E-selectin (A, B) or P-selectin (C, D). n=8 independent Th17 and Th1 preparations for WT mice; n= 5 independent cell preparations for PSGL1−/−CD43−/− DKO and PSGL1−/−CD44−/− DKO; n= 4 independent cell preparations for CD43−/−. All groups were studied side by side in at least n=3 independent experiments. Each group was studied side by side with WT cells in n= 4 or n= 5 independent experiments. E. Th17 and Th1 cells were sorted based on the level of CD43 expression in three groups: CD43high for Th17 cells, and CD43 intermediate (CD43M) or CD43high (CD43H) for Th1 cells. F. Th17 cells, Th1 CD43M and Th1 CD43H were perfused at a shear stress of 1 dyne/ cm2 over E-selectin coated coverslips. n= 3 independent experiments with 3 different T cell subset preparations. Data show the mean ± SD values. *p<0.05, **p<0.01, ***p<0.001 as compared with WT or as indicated.
Adhesion to E-selectin of Th17 and Th1 cells expressing different levels of glycosylated CD43
We next tested whether the difference in adhesion to E-selectin observed between Th17 cells and Th1 cells in WT cells was due to the different level of expression of glycosylated CD43 (Fig. 1A) or the mechanism by which CD43 is functioning in both cell types (Fig. 2A, B). Th17 and Th1 cells were sorted based on the levels of expression of CD43: Th17 cells (uniformly expressing high levels of CD43), Th1 cells expressing intermediate levels of CD43 (CD43M) and Th1 cells expressing high levels of CD43 (CD43H) (Fig. 2E), and perfused into the flow chamber to evaluate their adhesion to E-selectin. CD43M Th1 cells showed significantly decreased adhesion to E-selectin as compared to Th17 cells, indicating that the difference in expression of CD43 contributes to decreased adhesion to E-selectin. Moreover, CD43H Th1 cells also had impaired adhesion to E-selectin as compared to Th17 cells, suggesting that CD43 can also function differently in both cell subsets (Fig. 2F). Some Th17 and Th1 cells were perfused into the flow chamber before being sorted based on the expression of CD43 to control that the sorting process was not altering the ability of Th17 and Th1 cells to adhere to E-selectin. We observed that 91±7 Th17 cells/mm2 adhered to E-selectin under flow conditions, whereas 80± 4 Th17 cells/mm2 adhered after sorting. For Th1 cells we observed that 50± 6 cells/mm2 (including a mixed population of CD43H and CD43M before sorting) adhered to E-selectin, whereas 25± 2.8 CD43H cells/mm2 and 19± 2.3 CD43M cells/mm2 did so after sorting. These suggest that sorting of Th1 and Th17 cells does not impair cell adhesion to E-selectin under physiological flow conditions.
Screening of alternative novel functional glycoprotein E-selectin ligands on Th17 cells
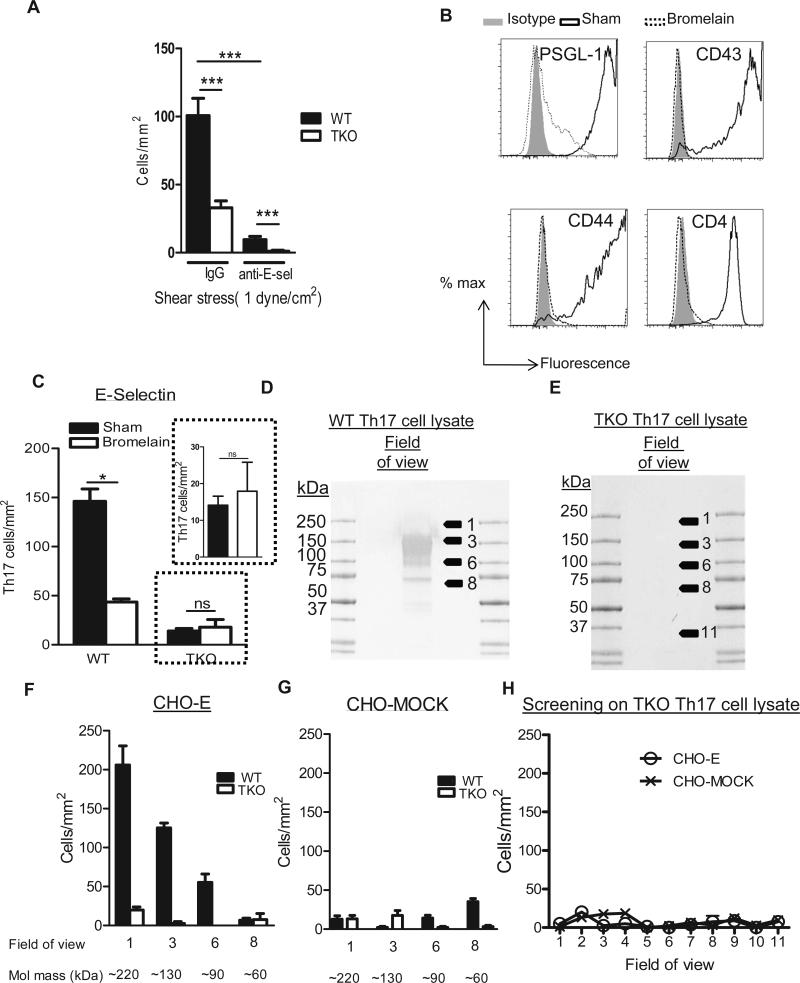
Our results demonstrate that depletion of PSGL-1 or CD43 results in severe impairment in Th17 cell adhesion to E-selectin. However, the lack of both ligands (PSGL-1−/−CD43−/−) did not completely abolish Th17 cell adhesion to E-selectin. In addition, CD44 supported low levels of E-selectin dependent rolling (Fig. 1J), but it did not mediate Th17 cell adhesion to E-selectin in our assays on immobilized E-selectin (Fig. 2A and Supplemental Fig. 2). To evaluate the possibility that Th17 cells express novel functional E-selectin ligands besides CD43, and PSGL-1, we generated Th17 cells in vitro from WT and PSGL1−/−CD43−/−CD44−/− (TKO) mice and performed adhesion assays on immobilized E-selectin under flow conditions. TKO CD4+ cells differentiated in vitro to Th17 cells similarly than WT cells (Supplemental Fig. 1). To our surprise, Th17 cells generated from TKO mice showed residual interactions with E-selectin, and these were abolished in the presence of an anti-E-selectin function blocking Ab, but not in the presence of an IgG control Ab demonstrating that these residual interactions are E-selectin dependent (Fig. 3A). These data suggested the existence of potential novel functional E-selectin ligands to be identified in Th17 cells. Because E-selectin ligands are generally proteins that require post translational glycosylation to be functional, we next evaluated whether the residual E-selectin binding activity observed in TKO Th17 cells (Fig. 3A) was mediated by a glycoprotein by treating TKO cells with the cystein-protease bromelain (32). Treatment of WT Th17 cells with bromelain resulted in complete cleavage of PSGL-1, CD43 and CD44 from the cell surface and of other Th17 cell surface proteins that are not E-selectin ligands, such as CD4, also completely removed from the cell surface (Fig. 3B). As expected, bromelain treatment of WT Th17 cells resulted in abrogated adhesion to E-selectin, even when double amount of Th17 cells (2×106 cells/ ml) were perfused. In contrast, bromelain treatment of TKO Th17 cells did not result in abrogation of Th17 cells adhesion to E-selectin, with the number of Th17 cells that accumulated on E-selectin being similar between vehicle and bromelain treated TKO Th17 (Fig. 3C). Because TKO mice lack PSGL-1, CD43 and CD44, and bromelain cleaves additional surface proteins in TKO Th17 cells, this result suggests that the observed remaining Th17 cell adhesion to E-selectin is not mediated by glycoproteins and may likely be due to glycolipids acting as E-selectin ligands, as it has previously been postulated for human T cells (33). To confirm this observation, we prepared lysates of WT and TKO Th17 cells and performed blot rolling assays to screen for potential glycoproteins that support Th17 cell E-selectin rolling. As expected, CHO-E cells, but not CHO-MOCK cells, rolled on the regions corresponding to PSGL-1 (220kDa) and to CD43 (130 kDa) in WT Th17 cell lysates (Fig. 3D, F-G). In contrast and consistent with our prediction, we did not observe any E-selectin dependent cell rolling activity in lysates of TKO Th17 cells (Fig. 3E, G-H). Taken together, these data support the idea that Th17 cells most likely do not express alternative glycoproteins to PSGL-1 and CD43 as E-selectin ligands. The residual adhesion to E-selectin observed in TKO (Fig. 3A) is most likely due to glycolipids, which run with the dye front in an SDS-PAGE gel and cannot be detected by blot rolling assays.
Figure 3. Th17 cells do not express detectable glycoproteins different than PSGL-1, CD43 and CD44 that function as E-selectin ligands.
A. Th17 cells generated from WT and PSGL-1−/−CD44−/−CD43−/− triple knockout mice (TKO) (A, C) were perfused at 1 dyne/cm2 over glass coverslips coated with recombinant E-selectin (A-C). A. Coverslips were treated with anti-E-selectin antibody (clone 9A9) or Rat IgG Isotype Control (20μg/ml) for 20 minutes prior to the perfusion of the cells. B. WT Th17 cells were treated with the protease Bromelain (1U/ml) 30 minutes at 37 °C, fixed, stained for the indicated markers and analyzed by flow cytometry. Histograms from one representative experiment are shown from 3 separate experiments performed. C. WT and TKO Th17 cells were treated with bromelain as in B and 2×106 cells/ml were perfused at 1 dyne/cm2 over glass coverslips coated with recombinant E-selectin on A. Data show the mean ± SD values from 3 different experiments. *p<0.05, ***p<0.001. D-H. WT (D) or TKO (E) Th17 cell lysates (50μg) were separated by molecular mass under non-reducing conditions and immobilized on western blots. The blot was assayed for functional E-selectin ligand activity by blot rolling analysis perfusing CHO-MOCK (G, H) or CHO-E (F, H) cells at the necessary shear of 0.6 dynes/cm2 to avoid the turbulence created by the membrane surface at higher shears. Data show the mean ± SD values from 3 independent experiments, in which 6-10 fields were recorded at each indicated molecular mass.
CD43 mediates Th17 cell recruitment into the air pouch in response to CCL20 and TNF-α in vivo
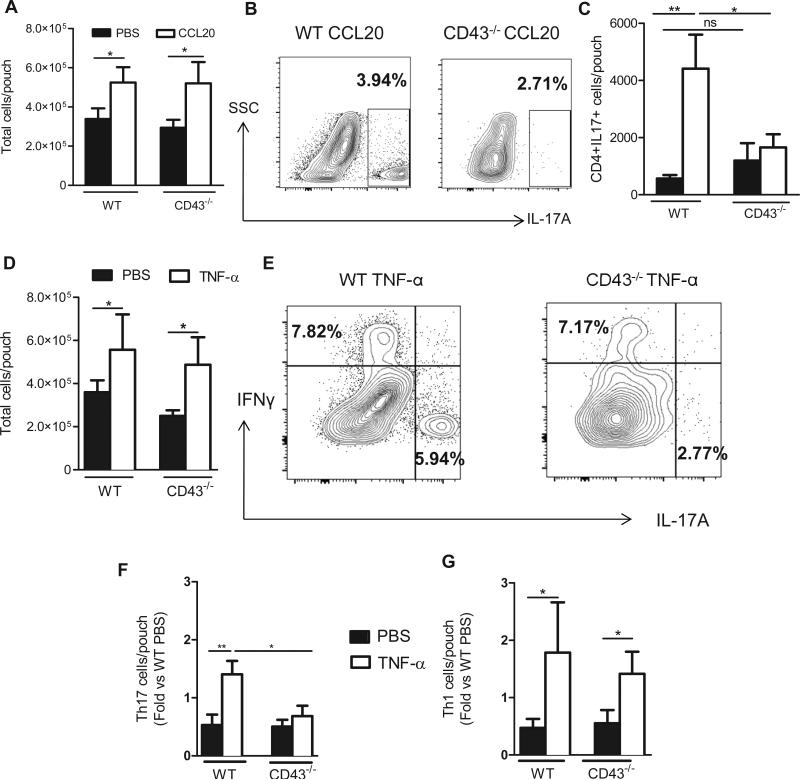
Both CD43 and PSGL-1 isolated from Th17 cells mediated functional E-selectin rolling (Fig. 1), but only CD43- E-selectin dependent adhesion seemed to be specific for Th17 cells, in contrast to PSGL-1 which functioned as an E-selectin ligand, and also as a P-selectin ligand on both Th17 and Th1 cells (Fig. 2). We therefore focused on CD43 to next investigate its role in Th17 cell recruitment in vivo using the air pouch model of leukocyte recruitment. We created a dermal air pouch model in WT and CD43−/− mice and injected CCL20 as an inflammatory stimulus that triggers CCR6+ cells, the receptor for CCL20, which efficiently recruits Th17 cells in vitro and in vivo (8,24,34). Blood leukocyte counts were normal in CD43−/− mice as previously described (35). We found that CCL20 recruited leukocytes in WT and CD43−/− mice as compared to PBS control (Fig. 4A). The recruitment of CD4+ T cells into the air pouch was significantly impaired in CD43−/− mice vs WT mice, suggesting that CD43 mediates recruitment of CD4+ T cells in vivo (Supplemental Fig. 3A-B). Because CCL20 injection into the air pouch results in robust recruitment of Th17 cells but not Th1 cells, as we have previously published (8), we next investigated the presence of Th17 cells within the CD4+ population and whether their recruitment was dependent upon CD43. As expected, injection of CCL20 into the WT air pouch resulted in enhanced Th17 cell recruitment as compared with PBS. In contrast, CCL20 did not induce Th17 cell recruitment in CD43−/− mice, and the frequency and number of Th17 cells recovered in CD43−/− mice was severely impaired vs WT mice (Fig. 4B-C). We further explored whether CD43 mediated specific Th17 cell recruitment in vivo using the air pouch model but this time injecting the pro-inflammatory stimulus TNF-α, which results in recruitment of mainly Gr1+ neutrophils as well as both Th17 cells and Th1 cell subsets (8,36). TNF-α injection into the air pouch resulted in increased leukocyte recruitment as compared to PBS in both WT and CD43−/− mice (Fig. 4D), however, CD43−/− mice showed significantly reduced recruitment of total CD4+ T cells as compared with WT mice (Supplemental Fig. 3C-D). From those CD4+ T cells that were recovered in the CD43−/− air pouch, only Th1 cells were identified in the air pouch, with Th17 cell recruitment being significantly impaired. Both Th1 and Th17 cells were identified in the lavage from WT mice (Fig. 4D-G). Taken together these data demonstrate that CD43 is essential for Th17 cell recruitment in vivo in response to 24h CCL20 and TNF-α induced inflammation in the air pouch.
Figure 4. CD43−/− mice show impaired recruitment of Th17 cells in response to CCL20 and TNF-α in vivo.
A-G. The indicated mice (5 per group in three independent experiments) received PBS or 400ng of CCL20 or TNF-α (4- 7 mice per group in three independent experiments) into the air pouch, and recruited cells were harvested 24h post injection and analyzed by cell counting (A, D) and flow cytometry (B, C, E-G). Representative dot plots indicate IL-17A and/or IFNγ staining in CCL20 (B) or TNF-α (E) air pouches. C. The number of Th17 cells in the PBS or CCL20 injected air pouch was calculated based on the percent CD4+ cells referred to the total number of cells harvested per air pouch. The number of Th17 cells (F) and Th1 cells (G) infiltrated in response to PBS or TNF-α in each mouse was divided by the average number of Th17 or Th1 cells infiltrated in the PBS mice, for WT and CD43−/− groups, and in each of the 3 independent experiments performed. The values of each experiment were averaged to determine the Fold vs PBS.*p<0.05**p<0.01, ns = not significant.
CD43 regulates specific Th17 cells rolling on the cremaster microvasculature in vivo
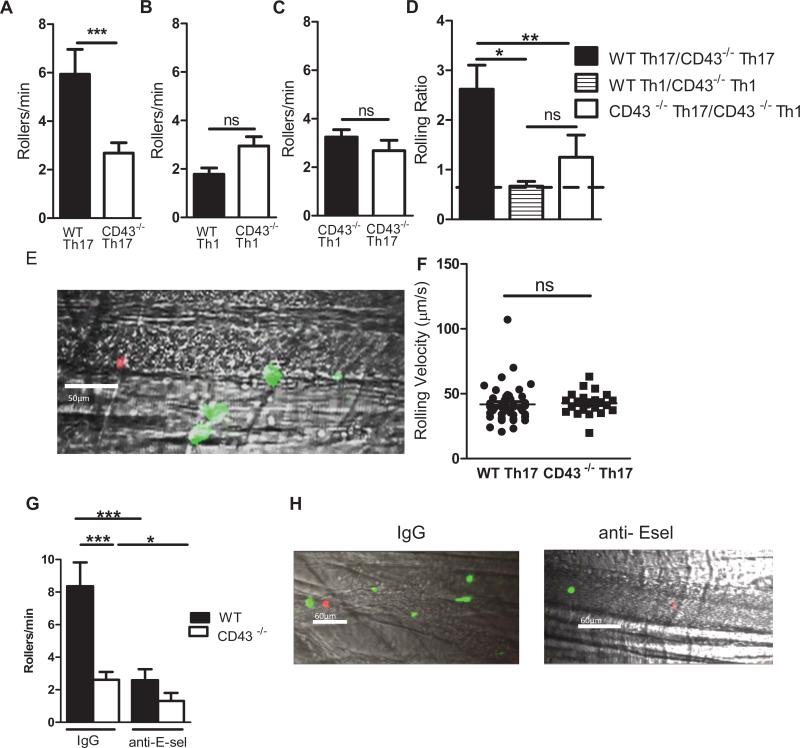
Our air pouch data positions CD43 as a regulator of Th17 cell recruitment in vivo, however, the observed defect cannot exclusively be attributed to CD43 regulating Th17 cell rolling interactions with E-selectin given the broad expression of CD43 in hematopoietic cells and its role in T cell function, signaling, and neutrophil emigration (18,19). We therefore sought to evaluate directly the interactions of Th17 cells from WT and CD43−/− mice with the activated vascular endothelium using confocal intravital microscopy. When mouse cremaster muscle is exposed to the mild trauma induced by surgical tissue preparation, leukocytes undergo rolling interactions on the vessel wall that are mediated by P-selectin and L-selectin (37). Pre-exposure of the cremaster muscle to TNF-α for 2 h additionally induces local E-selectin expression and further elevates leukocyte adhesion (8,27,37). Hence, this model is ideal to study whether CD43 mediate Th17 cell rolling interactions with the vascular endothelium in vivo in an E-selectin dependent manner. WT and CD43−/− Th17 cells were similarly differentiated in vitro (Supplemental Fig. 4A-C). Equal numbers of cells, verified by cell counting, were labeled with different fluorescent dyes, mixed in a 1:1 ratio and injected via the femoral artery after exteriorization of the TNF-α-stimulated cremaster muscle, thus enabling comparison of WT and CD43−/− Th17 cell interactions in the same vessels by competitive rolling analysis. WT Th17 cells interacted with a significantly higher frequency compared to CD43−/− Th17 cells (5.95 ± 1.03 Rollers/min WT, 2.68± 0.42 Rollers/min CD43−/−) (Fig. 5A, E, and Video 1). In contrast, this effect was not observed in similar studies comparing WT and CD43−/− Th1 cells (1.79 ± 0.26 Rollers/min WT, 2.95 ± 0.38 Rollers/min CD43−/−) (Fig. 5B), or CD43−/− Th1 cells (3.22± 0.31 Rollers/min) vs CD43−/− Th17 cells (2.68± 0.42 Rollers/min) (Fig. 5C). The rolling ratio between WT and CD43−/− Th17 cells was significantly higher than the rolling ratio between WT and CD43−/− Th1 cells and between CD43−/− Th17 and CD43−/− Th1, which both remained close to 1 (Fig. 5D). Although less numbers of CD43 Th17 cells rolled on the vascular endothelium as compared to WT Th17 cells, the average rolling velocities of those cells rolling were not different between CD43−/− (42.63 ± 1.75 μm/s) and WT mice (41.80± 2.16 μm/s) (Fig. 5F), and were in the range of what has been described for T cells before (8). Similar results were obtained regardless of whether WT Th17 or CD43−/− Th17 cells were stained with a CFSE or with an Alexa 680 dye (Supplemental Fig. 4D, E). To further prove that the difference between WT Th17 cells and CD43−/− Th17 cells rolling interactions with the endothelium was due to E-selectin, we performed similar assays in the presence of an anti-E-selectin function blocking Ab or an isotype control. As expected, anti-E-selectin treatment resulted in a significant decrease of WT Th17 cells. Moreover, the number of WT and CD43−/− Th17 cells rolling on the vasculature was similar in the presence of anti-E-selectin, but significantly more WT Th17 cells rolled in the presence of an IgG control Ab. The remaining rolling interactions observed in the presence of anti-E-selectin are probably due to Th17 cells interaction with other endothelial cell adhesion molecules such as P-selectin (Fig 5G-H and Supplemental videos 2 and 3). These data confirms the role of CD43 as a critical mediator of Th17 cell rolling with the vascular endothelium in vivo in an E-selectin dependent manner.
Figure 5. CD43−/− Th17 cells have impaired rolling interactions with the cremaster microvasculature as compared to WT Th17 cells.
Equal numbers of WT and CD43−/− Th17 cells (A, D- F), WT and CD43−/− Th1 cells (B, D) or CD43−/− Th17 and CD43−/− Th1 cells (C, D) were injected into WT mice 2 hours after 500ng of TNF-α intra-scrotal injection in competitive rolling assays. The number of rollers/minute in several independent vessel studies are shown (8 mice, 28 vessels, 3 independent T cell preparations) D. Rolling ratio of the indicated T cell types that roll on each vessel. E. Representative caption of WT Th17 cells (green) vs CD43−/− Th17 cells (red) rolling on cremaster vessels (grey). Recordings of each vessel were analyzed for 120-180 seconds and rolling T cells were identified as visible cells passing through a plane perpendicular to the vessel axis. F. The rolling velocity of high velocity cell (Vcell) as calculated to ensure that these qualified as rolling leukocytes, defined Vcell < critical velocity (Vcrit). Velocities of WT Th17 cells and CD43−/− Th17 cells from 3 independent preparations (44 WT cells and 25 CD43−/− cells were analyzed). G. WT and CD43−/− Th17 cells Rollers/min on the cremaster microvasculature in mice treated with anti-E-selectin or Rat Isotype IgG at 90 μg/ml. H. Representative captions of microvasculature treated with anti-E-sel or IgG. (6 mice, 15 vessels, and 3 independent T cell preparations) *p<0.05, **p<0.01, ***p<0.005.
CD43 regulates Th17 cell recruitment to the spinal cord in Experimental Autoimmune Encephalomyelitis (EAE)
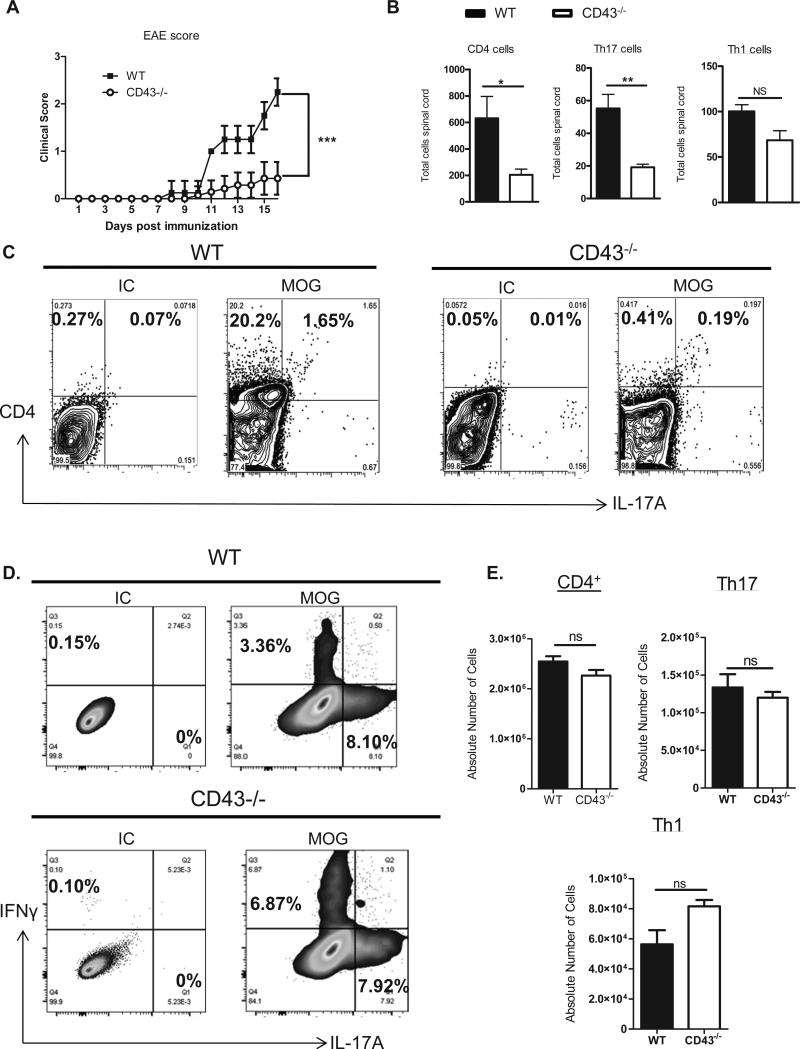
To further study the role of CD43 in Th17 cell recruitment in vivo, we evaluated the role of CD43 in EAE, a model in which Th17 cells are generated in vivo in response to Myelin Oligodendrocyte Glycoprotein (MOG) peptide immunization and are known to be recruited into the spinal cord at the peak of disease (14-16 days post immunization) and induce multiple sclerosis like symptoms (38). Thus, this model allows us to test whether CD43 impairs recruitment of antigen specific Th17 cells. We found that CD43−/− mice were protected from EAE at the peak of disease as compared to WT mice indicated by the significantly decreased clinical score (Fig. 6A). This correlated with impaired infiltration of CD4+ T cells in the spinal cord, and more specifically Th17 cells in CD43−/− mice as compared to WT mice. The number of Th1 cells recruited in the spinal cord at this time point was similar between WT and CD43−/− mice (Fig. 6B-C). Importantly, the lack of CD43 did not impair Th17 cell differentiation in the lymph nodes in response to MOG immunization, suggesting that CD43−/− T cells respond to MOG, but have a defect in being recruited into the spinal cord (Fig 6D-E). This data confirms that CD43 is a critical regulator for antigen specific Th17 cell recruitment in vivo.
Figure 6. CD43−/− mice are protected from EAE and have decreased Th17 cell recruitment in the spinal cord as compared to WT mice.
A. WT mice and CD43−/− mice (n=7 mice/ group) were injected with 100 μg of MOG peptide and 200 ng of PT and graded daily for clinical score. B. Mice were sacrificed 16 days post immunization, the spinal cord was harvested, stained for CD4, IL-17A and IFNγ, each with their isotype controls, and analyzed for leukocyte infiltrates by flow cytometry. C. Representative FACS plots of the spinal cords analyzed in B. D-E. WT and CD43−/− mice (3-5) were immunized as in A and sacrificed 8 days post immunization, lymph nodes were harvested and stained and analyzed by flow cytometry. Representative FACS plots and quantification of Th17 and Th1 cells within the CD4+ gate. *p<0.05, **p<0.01, ***p<0.005.
Discussion
In this study, we have investigated the contribution of E-selectin ligands expressed by T cells to adhesion of murine Th17 cells to E-selectin in vitro and identified the glycoprotein CD43 as a major functional Th17 cells E-selectin ligand in vitro that also regulates Th17 cell recruitment and rolling interactions with the vascular endothelium in vivo. We report for the first time a detailed description of the expression and functionality of T cell expressed E-selectin ligands in murine Th17 cells, and we suggest that there are most likely no additional glycoproteins that function as E-selectin ligands expressed on Th17 cells. Moreover, we present for the first time CD43 as a major E-selectin ligand with the unique feature of mediating Th17 cell adhesion to E-selectin and the activated vascular endothelium without requiring the cooperation of PSGL-1. We suggest that this specificity of E-selectin ligand expression contributes to the differences in T cell subset recruitment during inflammation.
Multiple studies over the years have identified glycoconjugates that function as E-selectin ligands. Most molecules were characterized in neutrophils (7,13,30) or activated T cells, including murine Th1 cells (12,14) and human Cutaneous Lymphocyte Antigen (CLA+) T cells (15), before the pro-inflammatory Th17 cell T cell subset emerged in 2008. In addition, except for the best characterized and initially described E-selectin ligand, PSGL-1, which is also a ligand for P-selectin and functional in neutrophils and T cells (7), the other two T cell described E-selectin ligands, CD43 and CD44, were shown to be functional only in cooperation with PSGL-1(12-14), with CD44 acting as an E-selectin ligand in neutrophils (31) and T cells (13), and CD43 doing so in T cells exclusively (12,14). Our data confirms the expression of all previously described T cell E-selectin ligands on Th17 cells, and reveals that among the three, the highly glycosylated form of CD43 has a unique expression pattern by FACS different than the classic bimodal expression described for Th1 cells (28), (Fig. 1). This may reflect a difference in the glycoconjugates decorating CD43 in Th17 and Th1 cells, possibly sialyl Lewis X (sLx), the minimal E-selectin ligand (39). A lack of antibodies to detect sLx in the mouse prevents us from testing this hypothesis. Furthermore, our findings indicate for the first time, to our knowledge, that CD43 is on its own a uniquely important E-selectin ligand and does not require cooperation with PSGL-1 to mediate adhesion to E-selectin, and this is T cell subset specific, as it is not observed on Th1 cells. Moreover, our data demonstrates that sorted Th1 cells expressing high CD43 or medium CD43 both adhere to E-selectin in lower numbers than Th17 cells, suggesting that CD43 functions differently in both T cell subsets. A glycomics analysis of the two Th subsets would define whether CD43 glycosylation in Th17 and Th1 cells can definitively explain this subset specificity. Our conclusion is supported by our studies evaluating E-selectin ligand functionality under shear flow conditions using two different approaches. First by showing that each ligand directly purified from Th17 cells supports rolling interactions with E-selectin (Fig. 1), and second by generating Th17 cells from different E-selectin ligand deficient mice and evaluating their interactions with E-selectin immobilized on glass coverslips (Fig. 2). Our blot rolling studies indicate quantitative differences in the number of E-selectin dependent rolling events mediated by Th17 cell expressed PSGL-1, CD43 and CD44: PSGL-1 and CD43 support significantly more E-selectin dependent rolling than CD44 that was abrogated in the presence of EDTA and not observed with CHO-MOCK cells that do not express E-selectin. Our second approach further supports our findings indicating that PSGL-1−/− Th17 cells have decreased adhesion to E-selectin, and PSGL-1−/−CD43−/− an even greater decrease in adhesion with CD43−/− Th17 cells binding in similar numbers to PSGL-1−/−CD43−/− Th17 cells. In contrast, Th17 cells that lack CD44 in addition to PSGL-1 (PSGL-1−/−CD44−/−) do not show further decreased adhesion to E-selectin as compared with PSGL-1−/−, indicating that CD44 is not a functional E-selectin ligand for Th17 cells in vitro. This differs somewhat from what we observed in the blot rolling assays where CD44 played a minor, but functional role on E-selectin dependent adhesion. We believe this discrepancy between the two approaches is likely due to our striking finding that CD43 plays a major role in Th17 cell adhesion to E-selectin: the PSGL-1−/− CD44−/− Th17 cells express CD43, which may be obscuring the minor role for CD44 observed in the blot rolling assays performed on immunopurified CD44 from Th17 cells and therefore in the absence of CD43. Our data using PSGL-1−/−CD44−/− Th1 cells to control for Th17 cells specificity slightly differs from a recent publication demonstrating that PSGL-1−/−CD44−/− Th1 cells have impaired adhesion to E-selectin in vitro as compared with PSGL-1−/− Th1 cells (13). We suggest this discrepancy is based on the different in vitro adhesion assays used by Nacher et al (static) vs ours (flow conditions on immobilized E-selectin). Our combined results using both approaches as well as highly expressing sorted CD43 Th17 and Th1 cells support the idea that CD43 is a major prominent ligand that mediates Th17 cells, but not Th1 cells, adhesion to E-selectin, and that its different role in T cell subsets is not exclusively due to different expression levels, but also potentially to different functionality of CD43 itself in Th17 cells.
Our rationale to study potential novel E-selectin ligands expressed on Th17 cells comes from our results indicating that TKO Th17 cells showed residual adhesion to immobilized E-selectin implying that an uncharacterized and potentially novel E-selectin ligands may exist (Fig 3A). However, the two approaches we used suggested that this is not the case. First, treatment of TKO Th17 cells with the cysteine-protease bromelain, which should remove all surface proteins (32), did not completely eliminate TKO Th17 cell E-selectin activity. Second, using blot rolling assays we could not detect any area that supported E-selectin dependent rolling on whole Th17 cells lysates generated from TKO mice. These studies cannot rule out the possibility of glycolipids acting as E-selectin ligands, as previously postulated in T cells (33,40,41), since glycolipids migrate with the dye front when loaded on SDS-PAGE gel, and hence are lost from the gel. If other glycoprotein ligands exist besides PSGL-1 and CD43 that mediate Th17 cell adhesion to E-selectin, they are below detectable levels using our two approaches (Fig 3).
We used the dermal air pouch model to study Th17 cell recruitment in response to defined stimuli in vivo, CCL20 being more specific to chemoattract CCR6+ cells such as Th17 cells (Fig 4), and TNF-α which recruits several leukocytes and T cell subsets besides Th17 cells (8,9,24,34,42) (Fig 4). Our studies support the role for CD43 in Th17 cell recruitment, and also exemplify the complexity of addressing the relevance of functional E-selectin ligands- E-selectin interactions in vivo. This is due primarily to an existing redundancy between E-selectin and P-selectin in mediating leukocyte recruitment, as demonstrated in several studies, including the air pouch model, employing E-selectin−/− mice (8,43-45) and PSGL-1−/− mice, the ligand for both E- and P-selectin (14). Secondly, the redundant repertoire of functional E-selectin ligands expressed on leukocytes (7) together with the involvement of several leukocyte types recruited expressing this repertoire. Our data using CCL20 as a chemoattractant supports a non-redundant role of CD43 as an E-selectin ligand in Th17 cells. Using TNF-α as a pro-inflammatory stimulus we find this role is T cell subset specific in vivo, since both Th1 and Th17 cells are recruited in WT mice whereas impaired Th17 cell recruitment is observed in CD43−/− mice. Our results are in line with previous studies demonstrating that CD43−/− and WT mice have similar inflammation and leukocyte recruitment in response to delayed type hypersensitivity, implying that CD43 alone does not regulate recruitment (12,14) in this model mediated mainly by Th1 cells and neutrophils (46,47), leukocytes where CD43 does not itself function as an E-selectin ligand. Noteworthy, our observations using this model cannot be attributed to Th17 cell- E-selectin mediated interactions, given that T cell recruitment into the air pouch does not exclusively depend on the adhesion molecule E-selectin (8). A likely possibility is that CD43 can adhere to other adhesion putative ligands. Among these other adhesion molecules we discard CD43 regulating Th17 cell interactions with P-selectin based on our in vitro data demonstrating that CD43−/− and WT Th17 cells adhere similarly to P-selectin (Fig 2B). We can however speculate that CD43 may regulate adhesion to ICAM-1 as an alternative mechanism that explain the decreased recruitment to the air pouch, as CD43 expressed by human T cells was shown to bind soluble ICAM-1 (48). Further studies are necessary to determine whether this mechanism takes place, and if it is specific for Th17 cells.
It has previously been demonstrated that CD43−/− mice have impaired neutrophil emigration in a mouse model of recruitment to the peritoneum (49). Because neutrophils are recruited into the air pouch, our studies cannot conclude whether the observed lack of recruitment is exclusively mediated by Th17 cell CD43-E-selectin interactions with the activated endothelium. Our competitive rolling assays using a model that is highly dependent on E-selectin (8,27) combined with an anti-E-selectin function blocking antibody specific approach, addressed directly whether CD43 regulates Th17 cell rolling on the vascular endothelium in vivo in a T cell subset specific and E-selectin specific way. Our data indicates a unique Th17 cell dependency on CD43 in their interactions with the vascular endothelium via E-selectin (Fig 5). It has been demonstrated previously that CD43−/− activated T cells adhered to post-capillary venules similarly than WT in competitive migration assays using intravital microscopy (50). However, this study involved ConA activated lymphoblasts and not specific T cell subsets, including Th17 cells, which had not been described at the time. Our in vivo studies specifically evaluate CD43−/− T cell subsets and support a role for CD43 mediating rolling of Th17 cells to post capillary venules. Moreover, we believe our results using these models of recruitment are relevant in a broad sense to inflammation in many tissues and shed new light into understanding more complex inflammation contexts. One example is that CD43−/− mice are protected from experimental autoimmune encephalomyelitis (EAE) (20), a disease where Th17 cell recruitment into the central nervous system plays a critical pathogenic role (3,4). This study, however, was performed before Th17 cells emerged and therefore did not address whether the protection could be attributable to specific Th17 cell recruitment defect. We have now investigated this directly and our data indicates that Th17 cells, do not infiltrate in the spinal cord of mice with EAE at the peak of disease, suggesting that this is one additional reason of the observed disease protection. Our data also indicates that unlike in certain mouse strains such as Balb/c where CD43 regulates T cell activation, signaling and differentiation in response to immunization with OVA peptide (17,19), this is not the case in C57/BL6 in response to MOG. In fact, we demonstrate that CD43 does not play a role in antigen specific Th17 cell differentiation, but does alter specific Th17 cell recruitment into the spinal cord. Further studies would be required to conclude whether the mechanisms we are proposing take place in Th17 cell mediated autoimmune disease.
In summary our work contributes to understanding the role of E-selectin ligands on Th17 cells, identifies CD43 as a unique E-selectin ligand for Th17 cells in vitro that also specifically regulates Th17 cell interactions with the vascular endothelium and recruitment to the air pouch and to the spinal cord in vivo, and contributes to our understanding of the processes regulating Th17 cell recruitment, which takes place in various inflammatory diseases. Our results suggest the intriguing possibility that different T cell subsets utilize the repertoire of E-selectin ligands differently to migrate into tissues during inflammation, therefore opening the possibility of interfering with T cell specific subset recruitment to treat T cell mediated diseases.
Supplementary Material
Acknowledgements
We thank Dr. Francis William Luscinskas for the helpful scientific discussions of this manuscript, Dr. Sandy King and Dr. Robert Fuhlbrigge for providing CHO-E, CHO-P and CHO-MOCK cells, and members of the Molecular Cardiology Research Institute for helpful suggestions and the flow core facilities at Tufts Sackler Graduate School of Biomedical Sciences.
Footnotes
These studies were supported by NIH grants, K99-HL097406 and HL123658 (PA), AHA undergrad fellowship (AMS), GIA 13GRNT 14560068 (PA), T32 HL 69770 (TN).
References
- 1.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park EJ, Mora JR, Carman CV, Chen J, Sasaki Y, Cheng G, von Andrian UH, Shimaoka M. Aberrant activation of integrin alpha4beta7 suppresses lymphocyte migration to the gut. J. Clin. Invest. 2007;117:2526–2538. doi: 10.1172/JCI31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 6.Lichtman AH, Ding H, Henault L, Vachino G, Camphausen R, Cumming D, Luscinskas FW. CD45RA-RO+ (memory) but not CD45RA+RO− (naive) T cells roll efficiently on E- and P-selectin and vascular cell adhesion molecule-1 under flow. J. Immunol. 1997;158:3640–3650. [PubMed] [Google Scholar]
- 7.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, Luscinskas FW, Lichtman AH. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J. Immunol. 2012;188:1421–1430. doi: 10.4049/jimmunol.1101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, Lichtman AH. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J. Immunol. 2007;178:2499–2506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- 13.Nacher M, Blazquez AB, Shao B, Matesanz A, Prophete C, Berin MC, Frenette PS, Hidalgo A. Physiological contribution of CD44 as a ligand for E-Selectin during inflammatory T-cell recruitment. Am. J. Pathol. 2011;178:2437–2446. doi: 10.1016/j.ajpath.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcaide P, King SL, Dimitroff CJ, Lim YC, Fuhlbrigge RC, Luscinskas FW. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J. Invest Dermatol. 2007;127:1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 15.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellies LG, Jones AT, Williams MJ, Ziltener HJ. Differential regulation of CD43 glycoforms on CD4+ and CD8+ T lymphocytes in graft-versus-host disease. Glycobiology. 1994;4:885–893. doi: 10.1093/glycob/4.6.885. [DOI] [PubMed] [Google Scholar]
- 17.Cannon JL, Collins A, Mody PD, Balachandran D, Henriksen KJ, Smith CE, Tong J, Clay BS, Miller SD, Sperling AI. CD43 regulates Th2 differentiation and inflammation. J. Immunol. 2008;180:7385–7393. doi: 10.4049/jimmunol.180.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions. Immunol. Res. 1999;20:89–99. doi: 10.1007/BF02786465. [DOI] [PubMed] [Google Scholar]
- 19.Pedraza-Alva G, Rosenstein Y. CD43 – One molecule, many tales to recount. (7 ed.) 2011:372–385. [Google Scholar]
- 20.Ford ML, Onami TM, Sperling AI, Ahmed R, Evavold BD. CD43 modulates severity and onset of experimental autoimmune encephalomyelitis. J. Immunol. 2003;171:6527–6533. doi: 10.4049/jimmunol.171.12.6527. [DOI] [PubMed] [Google Scholar]
- 21.Flynn KM, Michaud M, Madri JA. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. Am. J. Pathol. 2013;182:1322–1336. doi: 10.1016/j.ajpath.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osmers I, Bullard DC, Barnum SR. PSGL-1 is not required for development of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;166:193–196. doi: 10.1016/j.jneuroim.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Yago T, Fu J, McDaniel JM, Miner JJ, McEver RP, Xia L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9204–9209. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J. Immunol. 2002;168:5645–5651. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JC, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J. Exp. Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabie N, Delfs MW, Lim YC, Westrich JR, Luscinskas FW, Lichtman AH. Beta-galactoside alpha2,3-sialyltransferase-I gene expression during Th2 but not Th1 differentiation: implications for core2-glycan formation on cell surface proteins. Eur. J. Immunol. 2002;32:2766–2772. doi: 10.1002/1521-4141(2002010)32:10<2766::AID-IMMU2766>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J. Exp. Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritonja A, Rowan AD, Buttle DJ, Rawlings ND, Turk V, Barrett AJ. Stem bromelain: amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989;247:419–424. doi: 10.1016/0014-5793(89)81383-3. [DOI] [PubMed] [Google Scholar]
- 33.Alon R, Feizi T, Yuen CT, Fuhlbrigge RC, Springer TA. Glycolipid ligands for selectins support leukocyte tethering and rolling under physiologic flow conditions. J. Immunol. 1995;154:5356–5366. [PubMed] [Google Scholar]
- 34.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal. Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manjunath N, Correa M, Ardman M, Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995;377:535–538. doi: 10.1038/377535a0. [DOI] [PubMed] [Google Scholar]
- 36.Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, Smith CW, Ballantyne CM. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 37.Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuccillo FM, de LA, Palmieri C, Fiume G, Bonelli P, Borrelli A, Tassone P, Scala I, Buonaguro FM, Quinto I, Scala G. Aberrant glycosylation as biomarker for cancer: focus on CD43. Biomed. Res. Int. 2014;2014:742831. doi: 10.1155/2014/742831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang MC, Laskowska A, Vestweber D, Wild MK. The alpha (1,3)-fucosyltransferase Fuc-TIV, but not Fuc-TVII, generates sialyl Lewis X-like epitopes preferentially on glycolipids. J. Biol. Chem. 2002;277:47786–47795. doi: 10.1074/jbc.M208283200. [DOI] [PubMed] [Google Scholar]
- 41.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M, Konstantopoulos K, Schnaar RL. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 43.Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y, Muroi E, Hara T, Ogawa F, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Tedder TF, Sato S. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J. Immunol. 2010;185:2502–2515. doi: 10.4049/jimmunol.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1:709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 45.Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 46.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 47.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- 48.Rosenstein Y, Park JK, Hahn WC, Rosen FS, Bierer BE, Burakoff SJ. CD43, a molecule defective in Wiskott-Aldrich syndrome, binds ICAM-1. Nature. 1991;354:233–235. doi: 10.1038/354233a0. [DOI] [PubMed] [Google Scholar]
- 49.Woodman RC, Johnston B, Hickey MJ, Teoh D, Reinhardt P, Poon BY, Kubes P. The functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J. Exp. Med. 1998;188:2181–2186. doi: 10.1084/jem.188.11.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlow DA, Ziltener HJ. CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J. Immunol. 2006;177:6450–6459. doi: 10.4049/jimmunol.177.9.6450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.