Abstract
Objective
To elucidate the association of a functional catechol-O-methyltransferase (COMT) genotype (rs4680) with recovery of executive functions up to 18 months after early childhood traumatic brain injury (TBI) compared to an orthopedic injury (OI) group.
Setting
Outpatient
Participants
134 children with a moderate to severe TBI (n=63) or OI (n=71) between the ages of 3-6 years who were followed 18 months post-injury
Design
Case-comparison, longitudinal cohort
Main Measures
The Behavior Rating Inventory of Executive Function (BRIEF), developmental neuropsychological assessment (NEPSY) of verbal fluency (VF), and a modified Stroop Test for young children (Shape School)
Results
The low activity COMT enzyme genotype (AA) was associated with better scores on the NEPSY VF (F=3.80, p=.02) and the Shape School (F=2.89, p =.06) in all participants when controlling for injury type (TBI vs. OI) over the first 18 months after injury. Injury type (TBI vs. OI) did not significantly moderate the effect of the COMT genotypes on executive function recovery.
Conclusion
This study provides preliminary evidence for a role of COMT genotypes in long-term recovery of executive function after pediatric TBI and OI. Larger studies are needed to determine the exact link between genetic variation in the COMT gene and TBI recovery in children.
Keywords: Brain Injury, Genetics, Pediatrics, Child, Executive Function, Recovery
Introduction
Traumatic Brain Injury (TBI) is a leading cause of morbidity and mortality for children.1 TBI commonly results in physical and cognitive impairments. Common cognitive impairments include memory deficits, executive dysfunction, decreased concentration and attention, and language impairment.2-13 The prognosis after TBI varies among individuals, even those with similar injuries. Such variability is likely due to environmental, injury-related, and other individual factors, including genetics. To date, a paucity of research has evaluated the association of genetics with outcomes after pediatric TBI.14 Genetic variants associated with inflammatory cascades, neuroplasticity, cognitive functioning, and neurologic signaling pathways may influence initial biologic response and longer-term recovery after TBI.15,16 Catecholaminergic systems are of particular interest because these systems are sensitive to TBI and play roles in neural plasticity and repair, as well as attention and memory function.16-21
Dopamine, epinephrine, and norepinephrine are catecholamines that are thought to play a role in recovery and functioning after TBI, given that variation in the metabolism of catechoamines after injury may influence cognitive function and recovery.16 Because the enzyme Catechol-O-methyltransferase (COMT) primarily functions to degrade catecholinergic neurotransmitters, variations in the functioning of the enzyme may affect recovery after TBI.22 The COMT enzyme is coded by a gene located on the long arm of chromosome 22q11.21.22 The gene contains a common site of genetic variation that results in a methionine to valine substitution at codon 158 and leads to differing enzyme activity.23,24 The AA genotype codes for methionine homozygotes that are associated with lower enzyme activity and the GG genotype codes for valine homozygotes that are associated with higher enzyme activity. Low COMT enzyme activity (i.e., decreased degradation of catecholamines) is associated with potentially higher levels of neurotransmitters while high activity of COMT (i.e., increased degradation of catecholamines) is associated with potentially lower levels of neurotransmitters.23,24 Because of the biologic implications of this COMT genetic variation on catecholamine metabolism, genotypes associated with lower or higher levels of catecholamines may influence cognitive and behavioral recovery after injury.
The COMT genoyptes have been associated with performance on cognitive tasks in several populations. In healthy participants, Blasi et al. showed that the low activity COMT genotype (AA) was associated with superior attention when compared to the high activity COMT genotype (GG).25 Kramer et al. showed that university student participants homozygous for the high activity COMT genotype (GG) had greater prefrontal processing related to inhibitory functions.26 Fossela et al. reported that adult subjects homozygous for the high activity COMT genotype (GG) had poorer executive attention scores than those homozygous for the low activity genotype (AA).27 Additionally, amphetamine, a stimulant known to increase dopamine signaling, enhanced working memory task performance and efficiency of prefrontal cortex function in healthy individuals homozygous for the high activity COMT genotype (GG).28 In adults with TBI, homozygotes for the higher activity COMT genotype (GG) showed more executive dysfunction on the Wisconsin Card Sorting Test compared to homozygotes for the lower COMT genotype (AA).29 COMT polymorphisms have also been linked to emotion, thinking, and self-regulatory functions.30 Specifically, they are involved in the mediation of cognitive functions related to executive functioning and self-regulating behavior.30 In summary, this prior work indicates that these COMT genotypes may have implications for cognitive and behavioral functioning in several conditions associated with attention and executive functions; however, the association of this genetic variation with cognitive and behavioral recovery after pediatric TBI has not been explored. The aim of this study was to broaden the evaluation of the association of genetic variants with cognitive and behavioral recovery after pediatric TBI to COMT variants. Specifically, our goal was to better understand the association of the functional COMT variants (rs4680) with executive function and behavioral recovery longitudinally, up to 18 months after early childhood TBI compared to an orthopedic injury group. We predicted that the high enzyme activity genotype (GG) of the rs4680 COMT genetic variant would be associated with poorer executive functioning and that TBI would amplify these adverse effects over time.
Methods
Design
This was a prospective, longitudinal observational, case-comparison study of the association of a functional COMT variant (rs4680) with behavioral and executive function outcomes of young children with TBI and orthopedic injuries (OI).
Participants
Participants were recruited from an ongoing, prospective, long-term descriptive study evaluating children who sustained a TBI between age 3 and 7 years and a comparison group of age-matched children with orthopedic injury (OI). 213 participants who were enrolled in the original study were eligible for the current genetic study. Recruitment was from 3 children’s hospitals and 1 general hospital in Ohio. Participants underwent assessments at multiple time points, including the immediate post-acute period (0-3 months after injury) and 6, 12, and 18 months post-injury. Inclusion criteria included hospitalization overnight for traumatic injury (TBI or OI) sustained between the ages of 36 and 83 months, no evidence of child abuse as the cause of the injury, no history of documented neurological problems or developmental delays pre-injury, and English as the primary language in the home. The severity of injury was characterized using the Glasgow Coma Scale (GCS). 31 Severe TBI was defined as a GCS score less than or equal to 8 as the lowest post resuscitation score. The moderate TBI group had a GCS score of 9-12 or a GCS score of 13-15 in association with abnormal brain imaging. Children with mild TBI had a GCS of 13-15 without evidence of abnormal brain imaging. The OI group included children who sustained a bone fracture (not including skull fractures), had an overnight stay in the hospital, and did not exhibit alterations in consciousness or other signs or symptoms of head trauma or brain injury.
DNA Collection
DNA was collected from saliva samples from the participants, purified using the Oragene (DNA Genotek, Ottawa, Ontario, Canada) OG-500 self-collection tubes, and analyzed using TaqMan (Applied Biosystems) assay protocols to identify the COMT rs4680 genotypes. Genotypes were AA (methionine/methionine homozygote; low COMT enzyme activity; higher catecholamine levels), GA (valine/methionine heterozygote; intermediate COMT enzyme activity, intermediate catecholamine levels) and GG (valine/valine homozygote; high COMT enzyme activity; lower catecholamine levels)
Measures
Several measures were used to ensure comprehensive assessment of behavior and executive function skills. The Behavior Rating Inventory of Executive Function (BRIEF) provides a parent rating of child executive function skills.32-34 A developmental neuropsychological assessment (NEPSY) verbal fluency (VF) subtest measures mental flexibility or the ability to shift from one conceptual set to another.35 The Shape School is a modified Stroop test designed to evaluate executive function skills, specifically inhibition, attentional control, and the ability to shift from one set of rules to another in pre-school aged children.36,37 Because the BRIEF is a parent rating of executive function skills, initial ratings were based on the parent’s interpretation of the child’s executive function skills prior to the injury. The 6, 12, and 18 month evaluations represent the parent’s ratings of the child’s current executive function skills. Since the NEPSY and Shape School are measures completed by the child, they assess functioning at the time of evaluation. We used the global executive composite score on the BRIEF (BRIEF GEC) and the total score on the NEPSY VF to assess global executive function behaviors and mental flexibility respectively. For the Shape School, we used efficiency subtest scores (#correct responses minus incorrect responses/time) on tasks of inhibition, switching, and a task combining inhibition and switching as our main outcome measures. This measure evaluates selective attention, cognitive flexibility, and processing speed skills.
Analysis
Simple statistics such as means, standard deviations, and frequencies were used to summarize the data. Group comparisons (TBI and OI) were conducted using independent T-tests, Chi-Square tests, and analysis of variance (ANOVA) when appropriate. COMT genotypes were tested for Hardy-Weinberg equilibrium using JMP genomics software as part of the SAS program. Mixed model linear regression was used to analyze the relationship between executive functioning and the COMT genotypes over time, with behavior and executive functioning as the dependent variable and COMT genotypes as the independent variable. The three outcome measures or dependent variables were: BRIEF GEC, Verbal Fluency, and a measure of executive efficiency on the Shape School. Baseline BRIEF GEC was included as a covariate in the models because it was a retrospective pre-injury rating; for verbal fluency and shape school, baseline outcomes were post-injury and were treated as dependent variable in the modeling. We included genotypes as a categorical variable. To examine the moderating effect of injury type (TBI vs. OI) on the association of genotype with the executive function outcomes over time, the triple interaction of genotype with injury type and time since injury, as well as the lower-level interaction terms were included in the model. The models also controlled for age at injury, gender, race (white versus non-white), and socioeconomic status (defined as Z-score that combined parental education and median census track income by zip code). Because of potential confounding of outcomes with race, race was retained as a covariate in all models. Additionally, because of the potential effects of socioeconomic status on outcomes, socioeconomic status was also included as a covariate. We examined multicollinearity and dropped gender from the models due to substantial collinearity with age at injury (Variance Inflation Factor ~ 10). Because standard scores for the BRIEF-GEC and VF were used, age at injury was not a significant predictor (p>.05) for the Brief GEC or VF total, and was trimmed from these models. However, because standard scores are not available for the Shape School, raw scores were used for the Shape School model and age at injury was significant in the Shape School model and was retained in this model. The interaction of injury group × genotype × time was the primary association evaluated in interaction models. Given the exploratory nature of the study, a p value of less than 0.05 was considered statistically significant and p values less than 0.1 were considered as trending toward significance. All statistical analysis was conducted using SAS 9.3©.
Results
Genetic data was collected for 134 participants. The TBI (n=63) group was 57% (n=36) male with an average age at injury of 5.2 ± 1.1 years, 30% (n=19) nonwhite, and 14% (n=9), 64% (n=40), and 22% (n=14) with mild, moderate, severe TBI, respectively. The OI group (n=71) was 52% (n=37) male with an average age at injury of 5.1±1.1 years and 24% (n=17) nonwhite. There was no difference in age, race, or gender between the TBI and OI groups (Table 1). The TBI group had significantly longer times since injury at baseline (0.12±0.07 versus 0.09±0.04 years, p < 0.001). There were 79 potential participants from the original cohort that were unable to be contacted or declined participation. There was no difference between groups with and without genetic data collected in terms of injury type (44.3% versus 47.0% TBI, p = .70), gender (64.6% versus 54.5% male, p = .15), race (31.7% versus 26.9% nonwhite, p = .46), and age at injury (4.9 versus 5.1 years, p = .22)
Table 1.
Demographics of participants and outcome measures
| Demographics | OI (n=71) | TBI (n=63) |
|---|---|---|
| Gender, n (%) | ||
| Male | 37 (52.1) | 36 (57.1) |
| Female | 34 (47.9) | 27 (42.9) |
| Race, n (%) | ||
| White | 54 (76.1) | 44 (69.8) |
| Non-white | 11 (15.5) | 14 (22.2) |
| Age at injury in years, mean (stdv) | 5.1 (1.1) | 5.2 (1.1) |
| Time since injury at baseline in years, mean (stdv) |
0.09 (0.04)* | 0.12 (0.07)* |
| Median family income, mean (stdv) | $60,712 (21,964) | $59,647 (23,047) |
| Highest Educational Attainment, n (%) | ||
| Less than 2 years of high school | 1 (1.4) | 3 (4.8) |
| Two years of high school | 5 (7.0) | 7 (11.1) |
| High school degree | 24 (33.8) | 27 (42.9) |
| Two years of college | 15 (21.1) | 12 (19.1) |
| Four years of college | 18 (25.4) | 10 (15.9) |
| Graduate degree | 8 (11.3) | 4 (6.4) |
| Outcome Measures | ||
| BRIEF-GEC, mean (stdv) | 47.7 (12.8) | 50.8 (15.2) |
| NEPSY: VF, mean (stdv) | 9.5 (3) | 8.6 (2.8) |
| Shape School, mean (stdv) | 0.4 (0.2) | 0.3 (0.2) |
indicates significant difference at p-value < .05
No bias was identified in the baseline data. Hardy Weinberg Equilibrium was not violated. There were no significant differences in genotype frequency between the TBI (AA = 8, GA = 32, and GG = 23) and OI (AA = 16, GA = 34, and GG = 21) groups. There were no significant differences in genotype frequency among the mild (AA = 1, GA = 4, GG = 4), moderate (AA = 4, GA = 21, GG = 15), and severe (AA = 3, GA = 7, GG = 4) TBI groups. There was no significant difference in mean injury severity score (ISS) within the OI group among the genotypes: ISS (stdv) = 6.50 (2.58), 7.21(3.04), 6.14(2.54) for AA, GA, and GG genotypes, respectively.
Main effect analyses
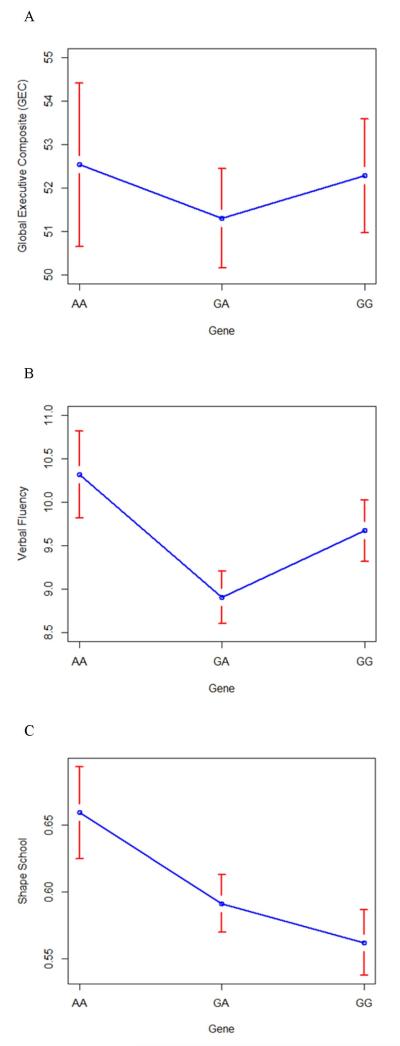
There was a main effect association of the low activity COMT genotype (AA) with improved scores on the NEPSY VF (F=3.80, p = .02, Figure 1B) and trend for an association with the Shape School (F=2.89, p = .06, Figure 1C) over time. There was not a main effect association of COMT genotypes with the BRIEF GEC (F=.27, p = .76, Figure 1A) over time.
Figure 1.
Mixed model analysis and least square means for the main effect association of COMT genitive variants with executive function outcomes in all participants: (A) GEC, (B) NEPSY VF, and (C) Shape School
The primary independent variable was genetic variant (AA, GA, GG). Covariates included in the models were baseline global executive composite (GEC) score (GEC outcome only), age at injury (Shape School outcome only), race, socioeconomic status, group (TBI versus OI), time since injury. Error bars indicate standard error.
Moderation analyses
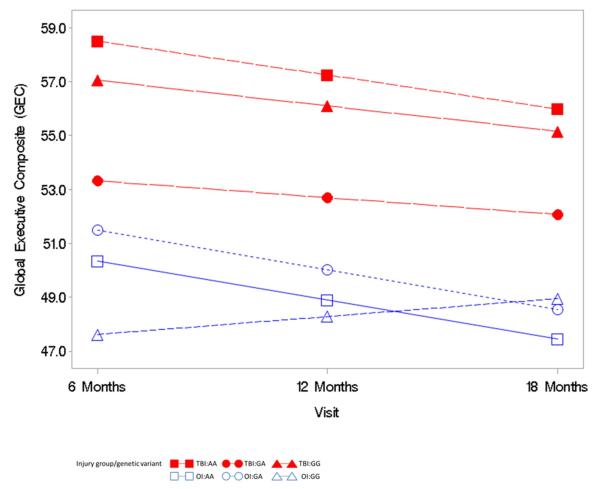
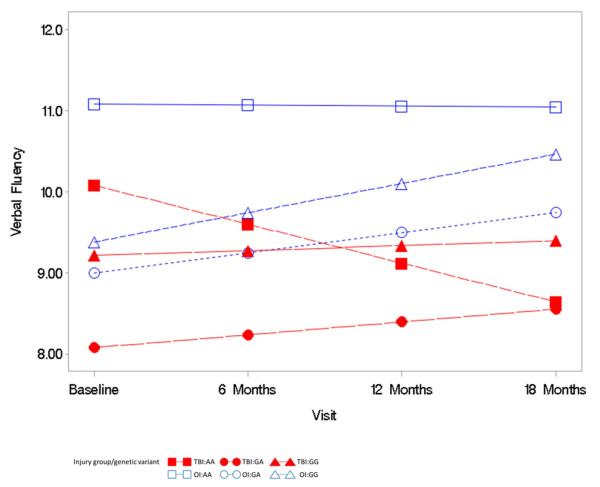
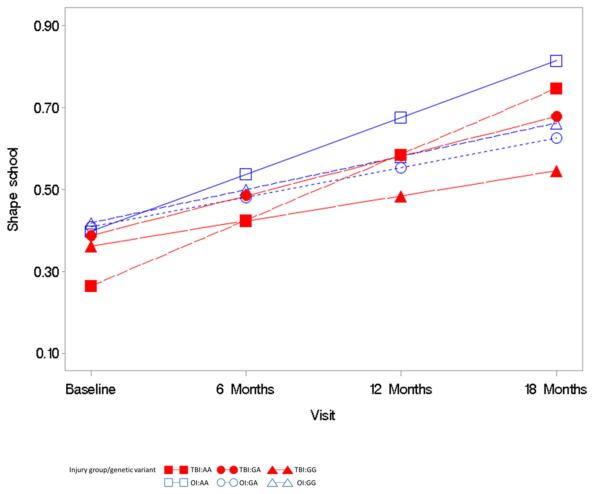
The triple interaction term of time since injury by group by genotype was not significantly predictive of the BRIEF GEC (F= 0.83, p = 0.44, Figure 2), NEPSY VF (F=.32, p = .72, Figure 2), or Shape School (F=.92, p = .40, Figure 3) over time. These moderation analyses did not support the hypothesis that the high activity genotype (GG) would further exacerbate executive dysfunction, as measured by the BRIEF-GEC, NEPSY VF, and Shape School, over time after TBI. This finding is demonstrated in Figure 2 by the overall parallel nature of the plotted lines rather than a widening of a difference in outcomes over time for the BREF-GEC. In the NEPSY VF analysis (Figure 3), the plotted lines demonstrate a trend for low activity genotype (AA) in the TBI group to be associated with poorer performance over time. In the Shape School analysis (Figure 4), the plotted lines demonstrate a comparable rate of improvement over time across all groups, with the lower activity genotype (AA) in the TBI group showing the most improvement and the high activity genotype (GG) in the TBI group showing the least improvement.
Figure 2.
Mixed model analysis of the relationship of the COMT variants with the Global Executive Composite (GEC) ratings, evaluation of the interaction of group by genetic variant by time.
The primary independent variable was genetic variant (AA, GA, GG). Co-variates included in the model were baseline (i.e., pre-injury rating) GEC, race, socioeconomic status, group (TBI versus OI), time since injury, interaction terms of group by genetic variant, time since injury by genetic variant, time since injry by group, and time since injury by group by genetic variant.
Figure 3.
Mixed model analysis of the relationship of the COMT variants with the Verbal Fluency outcome, evaluation of the interaction of group by genetic variant by time
The primary independent variable was genetic variant (AA, GA, GG). Co-variates included in the model were race, socioeconomic status, group (TBI versus OI), time since injury, interaction terms of group by genetic variant, time since injury by genetic variant, time since injry by group, and time since injury by group by genetic variant.
Figure 4.
Mixed model analysis of the relationship of the COMT variants with Shape School outcome, evaluation of the interaction of group by genetic variant by time
The primary independent variable was genetic variant (AA, GA, GG). Co-variates included in the model were age at injury, race, socioeconomic status, group (TBI versus OI), time since injury, interaction terms of group by genetic variant, time since injury by genetic variant, time since injry by group, and time since injury by group by genetic variant.
Discussion
Our findings indicate that there is likely a complex association of the COMT genotypes evaluated in this study with executive function recovery after pediatric TBI and OI. When controlling for injury type (TBI versus OI), main effect analyses of the entire cohort indicated that the low activity COMT genotype (AA) was associated with improved lab-based measures of executive function, but not parent ratings of executive function as manifest in everyday behavior. The effect was not limited to the TBI group as the interaction of group by time by genotype did not achieve significance. Thus these analyses did not support the hypothesis that the high enzyme activity genotype (GG) would exacerbate executive dysfunction in the TBI group. Further work is needed to better understand the association of these functional COMT genotypes with recovery after pediatric TBI.
To our knowledge, this is the first study to evaluate the association of a functional COMT genotype with longer-term recovery after pediatric TBI and OI. The study builds on prior work that has evaluated the association of COMT and other catecholamine-related genetic variants after TBI in adults.17,29,38,39 In prior cross-sectional work, the low COMT activity enzyme genotype (AA) was associated with better executive function.29 In agreement with this prior work, our results indicate that the low activity COMT genotype (AA) was associated with overall better function on lab-based measures of executive functioning in the entire cohort (TBI and OI). In contrast, our study did not demonstrate a protective association of the low activity genotype (AA) with executive function in the TBI group compared to an OI group. This finding should be interpreted as exploratory, although several potential explanations exist for these seemingly conflicting findings. First, because an individual genetic variant is likely to have a relatively small influence on outcome, larger studies are needed to definitively evaluate the relationship of these COMT genotypes with executive function outcomes. Additionally, executive function development occurs at a variable pace in children;40,41 thus it is difficult to measure executive function with precision, especially in younger children. Furthermore, it is possible that COMT enzyme functioning may have differential effects in a population of children with pediatric TBI compared to non-injured children or adults. Finally, prior work did not evaluate association of the COMT genotypes over time and it is possible that the relationship of these COMT genotypes with executive function outcomes may be dynamic and change over time after injury. In conclusion, results of this study did not conclusively demonstrate a role of COMT genotypes in long-term recovery of executive function after pediatric TBI. However, these results raise the possibility of gene-dependent moderation of injury effects that may vary across different measures of outcome and time. Further work is needed to better understand the role of the COMT genotypes evaluated in this study and other genetic variants in moderating the effects of injury on executive functioning.
Multiple factors likely influence recovery after TBI. In pediatric TBI, the child’s environment, specifically, family environment and parenting styles, is associated with neurocognitive and behavioral recovery.42-46 There are also several examples of the interaction of environmental and genetic factors in determining disease phenotypes.47 Specifically, dopamine receptor and dopamine pathway, serotonin transporter, and COMT genetic variants interact with environmental factors to influence cognitive and behavioral functioning in various childhood populations.18,48-58 Although the higher activity enzyme genotype (GG) is generally associated with a reduction in executive cognition, it is also associated with better stress resiliency, indicating that stressful environmental factors may overwhelm the direct effects the COMT genotype has on cognition59 Because a TBI often leads to increased family burden42,60,61, the potential stress resiliency effects of the high activity genotype (GG) may outweigh the effects on executive cognition. Future studies should evaluate the interaction of genetics and environmental and other factors on recovery.
Although several previous studies have demonstrated an association of the COMT genotypes evaluated in this manuscript with cognitive and behavioral functioning, with most reporting an association of the low COMT enzyme activity genotype with improved cognitive function25-27,29, there may be a within-gene explanation of the conflicting associations in the TBI group. One study suggests that several genetic variants within the COMT gene may contribute to the final overall activity of the enzyme;62,63 therefore, interaction of several within-gene genotypes (i.e., a haplotype) may be better associated with overall activity. Additionally, there may be a complex interaction across several genes that may explain the genetic association with outcomes. Genes involved in other neuro-signaling, inflammatory, or neuroplasticity pathways may interact with the COMT genotypes evaluated in this manuscript to determine cognitive and behavioral recovery after pediatric TBI.
Limitations
Although this study is large relative to other studies of pediatric TBI, much larger studies and replication are needed to provide a firm understanding of the influence of COMT genotypes on cognitive and behavioral recovery after pediatric TBI. This study consisted primarily of subjects with moderate TBI, limiting the ability to perform subpopulation analyses to understand whether the COMT genotype effects are more pronounced among individuals with milder and severe TBI. Larger studies that include a broad range of injury severity are needed to better elucidate the interaction between severity and genotype. There was no examination of the interaction between environment and genotype. Future studies should take environment into consideration. Additionally, other functional polymorphisms within the COMT gene or genetic variation in other genes may influence recovery after pediatric TBI. Thus, larger studies examining the contribution of a set of genetic variants within and across genes are needed in the future.
Conclusions
Limited research has examined the effects of genetics on outcomes after pediatric TBI. This current study expands the knowledge base with a relative large sample that has been followed prospectively. The study provides preliminary evidence that genetic variation in the COMT gene may influence long-term recovery of certain executive function domains. The results indicate that the COMT genotypes evaluated may have different effects on executive function depending on domain and type of measure used to assess executive functioning. Larger studies and replication are needed to determine the exact link between genetic variation in the COMT gene and other genes with recovery after TBI in children, and how this information can be used to inform prognosis and develop individualized treatment protocols. These preliminary findings indicate that there are potential genetic influences on outcomes after pediatric TBI that warrant further investigation.
Acknowledgements
This Funding for this study was supported in part by the Rehabilitation Medicine Scientist Training Program (RMSTP) K-12 HD001097-16, National Institute for Child Health and Human Development K23HD074683-01A1 and R01 HD42729, and Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other supporting agencies.
References
- 1.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): [Accessed September 9, 2013]. 2010. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. [Google Scholar]
- 2.Anderson V, Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain injury : [BI] 2005;19(6):459–470. doi: 10.1080/02699050400004823. [DOI] [PubMed] [Google Scholar]
- 3.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Recovery of intellectual ability following traumatic brain injury in childhood: impact of injury severity and age at injury. Pediatr Neurosurg. 2000;32(6):282–290. doi: 10.1159/000028956. [DOI] [PubMed] [Google Scholar]
- 4.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Injury: [BI] 2005;19(9):699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp M, Catroppa C, Godfrey C, Morse S, Rosenfeld JV, Anderson V. Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Dev Neuropsychol. 2011;36(5):578–595. doi: 10.1080/87565641.2011.555572. [DOI] [PubMed] [Google Scholar]
- 6.Catroppa C, Anderson V. Recovery and predictors of language skills two years following pediatric traumatic brain injury. Brain Lang. 2004;88(1):68–78. doi: 10.1016/s0093-934x(03)00159-7. [DOI] [PubMed] [Google Scholar]
- 7.Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Children’s attentional skills 5 years post-TBI. Journal of pediatric psychology. 2007;32(3):354–369. doi: 10.1093/jpepsy/jsl019. [DOI] [PubMed] [Google Scholar]
- 8.Nadebaum C, Anderson V, Catroppa C. Executive function outcomes following traumatic brain injury in young children: a five year follow-up. Dev Neuropsychol. 2007;32(2):703–728. doi: 10.1080/87565640701376086. [DOI] [PubMed] [Google Scholar]
- 9.Backeljauw B, Kurowski BG. Interventions for Attention Problems After Pediatric Traumatic Brain Injury: What Is the Evidence? PM & R : the journal of injury, function, and rehabilitation. 2014 doi: 10.1016/j.pmrj.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bussing R, Mason DM, Bell L, Porter P, Garvan C. Adolescent outcomes of childhood attention-deficit/hyperactivity disorder in a diverse community sample. J Am Acad Child Adolesc Psychiatry. 2010;49(6):595–605. doi: 10.1016/j.jaac.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Max JE, Lansing AE, Koele SL, et al. Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev Neuropsychol. 2004;25(1-2):159–177. doi: 10.1080/87565641.2004.9651926. [DOI] [PubMed] [Google Scholar]
- 12.Max JE, Schachar RJ, Levin HS, et al. Predictors of attention-deficit/hyperactivity disorder within 6 months after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(10):1032–1040. doi: 10.1097/01.chi.0000173293.05817.b1. [DOI] [PubMed] [Google Scholar]
- 13.Max JE, Schachar RJ, Levin HS, et al. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2005;44(10):1041–1049. doi: 10.1097/01.chi.0000173292.05817.f8. [DOI] [PubMed] [Google Scholar]
- 14.Kurowski B, Martin LJ, Wade SL. Genetics and outcomes after traumatic brain injury (TBI): What do we know about pediatric TBI? J Pediatr Rehabil Med. 2012;5(3):217–231. doi: 10.3233/PRM-2012-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson J, Cusimano MD, Bendena WG. Post-Traumatic Brain Injury: Genetic Susceptibility to Outcome. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014 doi: 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]
- 16.McAllister TW. Genetic Factors Modulating Outcome After Neurotrauma. PM&R. 2010;2(12, Supplement 2):S241–252. doi: 10.1016/j.pmrj.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 17.McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment -- a review. Brain injury : [BI] 2004;18(4):331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Chen C, Moyzis R, et al. Contributions of dopamine-related genes and environmental factors to highly sensitive personality: a multi-step neuronal system-level approach. PloS one. 2011;6(7):e21636. doi: 10.1371/journal.pone.0021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAllister TW. Polymorphisms in genes modulating the dopamine system: do they influence outcome and response to medication after traumatic brain injury? J Head Trauma Rehabil. 2009;24(1):65–68. doi: 10.1097/HTR.0b013e3181996e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neuroscience and biobehavioral reviews. 2009;33(7):981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23(7):1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- 22.COMT catechol-O-methyltransferase [Accessed April, 7, 2009]; http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1312&ordinalpos=1&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum.
- 23.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 25.Blasi G, Mattay VS, Bertolino A, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer UM, Cunillera T, Camara E, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci. 2007;27(51):14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossella J, Sommer T, Fan J, et al. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsky RH, Sparling MB, Ryan LM, et al. Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(4):465–471. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- 30.Williams LM, Gatt JM, Hatch A, et al. The integrate model of emotion, thinking and self regulation: an application to the “paradox of aging”. J Integr Neurosci. 2008;7(3):367–404. doi: 10.1142/s0219635208001939. [DOI] [PubMed] [Google Scholar]
- 31.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 32.Gioia GA, Isquith PK. Ecological assessment of executive function in traumatic brain injury. Dev Neuropsychol. 2004;25(1-2):135–158. doi: 10.1080/87565641.2004.9651925. [DOI] [PubMed] [Google Scholar]
- 33.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 34.Derogatis L, Spencer P. The Brief Symptom Inventory: Administration, Scoring, and Procedures Manual-I. Clinical Psychology Research; Baltimore, MD: 1982. [Google Scholar]
- 35.Korkman M, Kirk U, Kemp S. NEPSY A Developmental Neuropsychological Assessment: Manual. The Psychological Corporation; San Antonio: 1998. [Google Scholar]
- 36.Espy KA, Bull R, Martin J, Stroup W. Measuring the development of executive control with the shape school. Psychol Assessment. 2006;18(4):373–381. doi: 10.1037/1040-3590.18.4.373. [DOI] [PubMed] [Google Scholar]
- 37.Espy KA. The shape school: Assessing executive function in preschool children. Dev Neuropsychol. 1997;13:495–499. [Google Scholar]
- 38.McAllister TW, Flashman LA, Harker Rhodes C, et al. Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: a replication and extension study. Brain injury : [BI] 2008;22(9):705–714. doi: 10.1080/02699050802263019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23(10):1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 40.Wasserman T, Wasserman LD. Toward an integrated model of executive functioning in children. Applied neuropsychology. Child. 2013;2(2):88–96. doi: 10.1080/21622965.2013.748394. [DOI] [PubMed] [Google Scholar]
- 41.Best JR, Miller PH. A developmental perspective on executive function. Child development. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsych Soc. 2001;7(6):755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 43.Wade SL, Cassedy A, Walz NC, Taylor HG, Stancin T, Yeates KO. The Relationship of Parental Warm Responsiveness and Negativity to Emerging Behavior Problems Following Traumatic Brain Injury in Young Children. Dev Psychol. 2011;47(1):119–133. doi: 10.1037/a0021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeates KO, Taylor HG, Drotar D, et al. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society : JINS. 1997;3(6):617–630. [PubMed] [Google Scholar]
- 45.Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–356. doi: 10.1037/a0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM & R : the journal of injury, function, and rehabilitation. 2011;3(9):836–845. doi: 10.1016/j.pmrj.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nussbaum R, McInnes R, Huntington W, editors. Thompson & Thompson Genetics in Medicine. Saunders Elsevier; Philadelphia, PA: 2007. Genetics of common disorders with complex inheritance; pp. 151–174. [Google Scholar]
- 48.Lavigne JV, Herzing LB, Cook EH, et al. Gene × environment effects of serotonin transporter, dopamine receptor D4, and monoamine oxidase A genes with contextual and parenting risk factors on symptoms of oppositional defiant disorder, anxiety, and depression in a community sample of 4-year-old children. Dev Psychopathol. 2013;25(2):555–575. doi: 10.1017/S0954579412001241. [DOI] [PubMed] [Google Scholar]
- 49.Berry D, Deater-Deckard K, McCartney K, Wang Z, Petrill SA. Gene-environment interaction between dopamine receptor D4 7-repeat polymorphism and early maternal sensitivity predicts inattention trajectories across middle childhood. Dev Psychopathol. 2013;25(2):291–306. doi: 10.1017/S095457941200106X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry D, McCartney K, Petrill S, Deater-Deckard K, Blair C. Gene-environment interaction between DRD4 7-repeat VNTR and early child-care experiences predicts self-regulation abilities in prekindergarten. Developmental psychobiology. 2014;56(3):373–391. doi: 10.1002/dev.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cicchetti D, Rogosch FA. Gene × Environment interaction and resilience: effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Dev Psychopathol. 2012;24(2):411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schellekens AF, Franke B, Ellenbroek B, et al. COMT Val158Met modulates the effect of childhood adverse experiences on the risk of alcohol dependence. Addiction biology. 2013;18(2):344–356. doi: 10.1111/j.1369-1600.2012.00438.x. [DOI] [PubMed] [Google Scholar]
- 53.Sweitzer MM, Halder I, Flory JD, et al. Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: evidence for differential susceptibility. Social cognitive and affective neuroscience. 2013;8(5):499–508. doi: 10.1093/scan/nss020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Roekel E, Goossens L, Scholte RH, Engels RC, Verhagen M. The dopamine D2 receptor gene, perceived parental support, and adolescent loneliness: longitudinal evidence for gene-environment interactions. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(10):1044–1051. doi: 10.1111/j.1469-7610.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- 55.Das D, Tan X, Easteal S. Effect of model choice in genetic association studies: DRD4 exon III VNTR and cigarette use in young adults. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B(3):346–351. doi: 10.1002/ajmg.b.31169. [DOI] [PubMed] [Google Scholar]
- 56.Beaver KM, Belsky J. Gene-environment interaction and the intergenerational transmission of parenting: testing the differential-susceptibility hypothesis. The Psychiatric quarterly. 2012;83(1):29–40. doi: 10.1007/s11126-011-9180-4. [DOI] [PubMed] [Google Scholar]
- 57.Martel MM, Nikolas M, Jernigan K, Friderici K, Waldman I, Nigg JT. The dopamine receptor D4 gene (DRD4) moderates family environmental effects on ADHD. Journal of abnormal child psychology. 2011;39(1):1–10. doi: 10.1007/s10802-010-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayden EP, Klein DN, Dougherty LR, et al. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatr Genet. 2010;20(6):304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. [Accessed November 17, 2014];SNPedia: Rs4680. http://www.snpedia.com/index.php/Rs4680.
- 60.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO. Family burden and adaptation during the initial year after traumatic brain injury in children. Pediatrics. 1998;102(1):110–116. doi: 10.1542/peds.102.1.110. [DOI] [PubMed] [Google Scholar]
- 61.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO, Minich NM. Interpersonal stressors and resources as predictors, of parental adaptation following pediatric traumatic injury. Journal of consulting and clinical psychology. 2004;72(5):776–784. doi: 10.1037/0022-006X.72.5.776. [DOI] [PubMed] [Google Scholar]
- 62.Halleland H, Lundervold AJ, Halmoy A, Haavik J, Johansson S. Association between catechol O-methyltransferase (COMT) haplotypes and severity of hyperactivity symptoms in adults. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B(3):403–410. doi: 10.1002/ajmg.b.30831. [DOI] [PubMed] [Google Scholar]
- 63.Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314(5807):1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]