Abstract
Background
Retinal nerve fiber and ganglion cell+inner plexiform (GCIP) layer thinning following multiple sclerosis-related acute optic neuritis (AON) is well-described. However, whether AON results in changes in the inner nuclear (INL), outer plexiform (OPL), outer nuclear (ONL) and/or photoreceptor segment (PS) layers remains undetermined.
Objectives
To determine if INL+OPL and/or ONL+PS changes occur following AON.
Methods
33 AON patients underwent serial optical coherence tomography (OCT) and visual function testing (mean follow-up: 25 months). Longitudinal changes in retinal layer thickness were analyzed using mixed-effects linear regression.
Results
Four months following AON, the mean decrease in GCIP thickness relative to baseline was 11.4% (p<0.001). At 4-months, a concomitant 3.4% increase in average ONL+PS thickness was observed (p<0.001). The percentage decrease in GCIP thickness and increase in ONL+PS thickness were strongly correlated (r = -0.70; p<0.001). Between months 4 to 12, ONL+PS thickness declined and, at 12-months, was no longer significantly different from baseline (mean change: 0.5%; p = 0.37). Similar, albeit less robust, changes in the INL+OPL were observed.
Conclusions
Following AON, dynamic changes occur in the deep retinal layers, which are proportional to GCIP thinning. These novel findings help further our understanding of the biological and/or anatomical sequelae resulting from AON.
Keywords: Multiple sclerosis, optic neuritis, optical coherence tomography, retinal ganglion cells, retinal photoreceptors, optic nerve injuries
Introduction
Multiple sclerosis (MS) is an immune-mediated disorder of the central nervous system (CNS) in which neuroaxonal degeneration following inflammatory demyelination is thought to represent the principal pathological correlate of neurologic impairment1. MS has a strong predilection to affect the optic nerves. Acute optic neuritis (AON) is the presenting feature of MS in 20% of cases, with 30-70% of MS patients experiencing AON at some point during the course of illness2. Therefore, the anterior visual pathway represents an ideal model to study neurodegeneration following AON and in MS overall. The retina can be imaged using optical coherence tomography (OCT), a reproducible, non-invasive imaging technique that enables generation of high resolution, cross-sectional images allowing precise quantification of retinal layer thickness3.
Prior OCT studies demonstrate peripapillary retinal nerve fiber layer (RNFL) swelling related to edema, early in the course of AON4,5. As this edema resolves, thinning of the RNFL ensues6–8. Retrograde degeneration of RNFL axons following AON culminates in death of retinal ganglion cells, from which these axons are derived. Longitudinal OCT studies following AON demonstrate thinning of the composite ganglion cell+inner plexiform layers (GCIP). Unlike the RNFL, edema is not thought to occur in the GCIP during the initial phase of AON.
Whether AON directly results in inner nuclear (INL) and/or outer nuclear layer (ONL) changes and the temporal pattern of such changes, if any, remains undetermined. Although cross-sectional studies suggest an inverse relationship between INL and GCIP thicknesses in eyes with AON history9, it remains unclear whether this association is a direct consequence of AON, or alternatively is related to a greater degree of inflammatory activity that may be reflected by increased INL thickness10. Although there is some suggestion of photoreceptor changes in longstanding optic neuropathies11, longitudinal assessments of ONL changes following AON are lacking.
The primary objectives of this longitudinal study were (1) to determine whether INL and ONL changes occur following AON, (2) how these changes relate to GCIP thinning and visual function recovery.
Methods
Patients
Study participants were recruited from the Johns Hopkins MS center. The study protocol was approved by the Johns Hopkins University institutional review board and all participants provided written informed consent prior to study enrollment. Patients diagnosed with clinically isolated syndrome (CIS) or relapsing-remitting MS (RRMS) who presented with unilateral AON were recruited by convenience sampling between October 2008 and July 2012. AON patients with symptom onset (defined as onset of visual loss consistent with AON) 35 days or more from baseline assessment were not included. NMO-IgG testing was performed in 11 AON patients, all of whom tested negative. A comparison cohort of patients with MS, who did not develop AON during follow-up, were matched 1:1 to the AON cohort at a subject level based on age, gender, and duration of OCT follow-up. MS diagnosis was confirmed by the treating neurologists (SS, PAC, and SDN) according to the 2010 revised McDonald criteria12. Exclusion criteria included a known history of diabetes, uncontrolled hypertension, glaucoma, refractive errors of more or less than six diopters, or other ophthalmological and/or neurological disorders.
Thirty-three patients with unilateral AON participated in the study. All recruited AON patients underwent a study evaluation acutely, with a mean delay from the onset of AON symptoms to first evaluation of 14 days (SD 8.7, range: 1-33 days), as well as serial follow-up assessments for a mean duration of 25.2 months (median 22.8 months, range 6-55, SD 13.2). Compared to the control cohort of 33 MS patients (who did not have AON at study entry or develop AON during follow-up) gender, age, and OCT follow-up duration were similar between both groups (Table 1). The AON cohort comprised 14 (42%) patients with previously diagnosed RRMS, 12 patients (36%) with newly diagnosed RRMS at the time of AON, and 7 patients (21%) with CIS. The majority of CIS patients (n=6) had high-risk CIS due to the presence of lesions characteristic of demyelination on baseline brain MRI. Thirty patients were treated with corticosteroids alone, one patient received plasmapheresis following inadequate response to steroid therapy, and one patient was treated with plasmapheresis alone. Only one AON patient did not receive any treatment. None of the participants in the AON cohort had further episodes of optic neuritis during follow-up.
Table 1. Baseline demographic and clinical characteristics.
| AON cohort | MS Control Cohort* | P-value | |
|---|---|---|---|
|
| |||
| Patients (eyes) | 33 (65) | 33 (66) | - |
|
| |||
| Age, y, mean (SD) | 36.3 (9.5) | 35.9 (9.1) | 0.85a |
|
| |||
| Female, n (%) | 29 (88) | 29 (88) | 1.00b |
|
| |||
| Race, n (%) ‡ | 0.31b | ||
| White | 22 (67) | 28 (85) | |
| Black | 7 (21) | 4 (12) | |
| Hispanic | 2 (6) | 0 (0) | |
| Other | 2 (6) | 1 (3) | |
|
| |||
| Disease duration, years, median (IQR) | 1.25 (0.5-8) | 7 (3-11) | 0.009c |
|
| |||
| Eyes with a previous history of AON, n (%) | 12 (18.2) | 19 (28.8) | 0.15d |
|
| |||
| Follow-up duration, months, median (IQR) | 22.8 (12.6-34.2) | 23.6 (14.2-36.1) | 0.68c |
Abbreviations: AON = Acute optic neuritis; MS = multiple sclerosis; SD = standard deviation; CIS = clinically isolated syndrome; RRMS = relapsing-remitting multiple sclerosis; IQR = interquartile range.
No patients in this cohort had AON at study entry or developed AON during follow-up.
Race was self-reported.
Two-sample Student's t-test.
Fisher's exact test.
Mann–Whitney U test.
Chi-squared test.
Optical coherence tomography
Study participants underwent OCT imaging using spectral-domain Cirrus HD-OCT (Model 4000, Software version 5.0; Carl Zeiss Meditec, Dublin, CA), as described in detail elsewhere13,14. Briefly, peripapillary and macular scans were obtained using the Optic Disc Cube 200×200 and Macular Cube 512×128 protocols, respectively. Scans with signal strength less than 7, out of a maximum of 10, were excluded from the analysis. All macular cube scans were reviewed to ensure proper fixation and adequate quality, in accordance with the OSCARIB criteria15. Macular scans were assessed for the presence of apparent macular pathology, including microcysts and traction, by a single rater blinded to clinical status (OAA). One contralateral eye in an AON patient was excluded from the analyses due to the presence of an epiretinal membrane (ERM). Otherwise, qualitative assessment of OCT scans did not reveal gross macular pathology in either the AON or MS control cohorts at any point during the study.
Macular cube scans were processed in a blinded fashion as has been described previously 10,13,14,16. Briefly, macular cube scans were analyzed using an automated 3D segmentation algorithm that computes macular layer thicknesses within an annulus of inner radius 0.54mm and outer radius 2.4mm (Figure 1). This segmentation method yields the thicknesses of the following retinal layers: 1) macular RNFL; 2) GCIP; 3) INL+outer plexiform layer (INL+OPL) 4) ONL+photoreceptor segments (ONL+PS). Segmentation results obtained using this protocol have been shown to be reproducible in MS patients and healthy controls (inter-rater intra-class correlation coefficients: 0.91-0.99 for all segmentation measures)13. A detailed description of the scan-rescan reliability of the segmentation technique in the AON setting (categorized by time interval after AON onset) is presented in supplementary Table 1.
Figure 1.
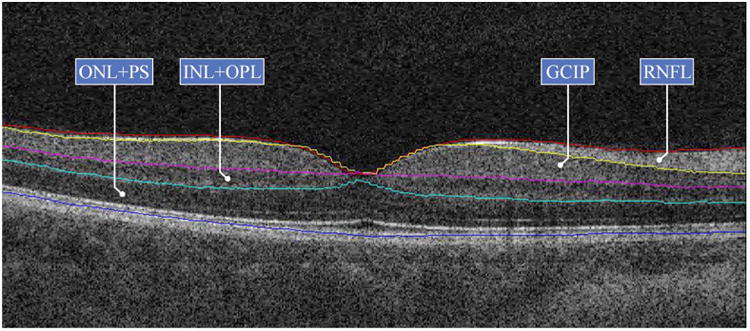
A cross-sectional view of a Cirrus HD-OCT B-scan demonstrating the macular segmentation utilized. The close similarity in tissue reflectivity between the ganglion cell (GCL) and inner plexiform layers (IPL) precludes accurate separate assessment of either layer. As a consequence, the composite of both the GCL and IPL (GCIP) is assessed. The segmentation technique employed also assesses the composite thickness of the inner nuclear layer and outer plexiform layer (INL + OPL), as well as the composite thickness of the outer nuclear layer and the photoreceptor segments (ONL + PS)
Abbreviations: GCIP = ganglion cell and inner plexiform layers; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; PS = photoreceptor segments; RNFL = retinal nerve fiber layer.
Visual function testing
Standardized visual function testing was performed at each study visit using a retro-illuminated high-contrast ETDRS chart (at 4m) and low-contrast Sloan letter charts (2.5% and 1.25% contrast; at 2m). Testing was performed monocularly, with habitual distance spectacles or contact lenses being used as needed. Accordingly, 100% high-contrast, 2.5% low-contrast, and 1.25% low-contrast letter-acuity scores were recorded based on the number of letters correctly read out of a maximum of 70. The 12-month visual acuity recovery was defined as the difference between the number of letters correctly identified at the 12 month visit compared to baseline.
Statistical analysis
Statistical analysis was conducted using Stata version 12 (StataCorp, College Station, Texas, USA). The Shapiro-Wilk test was used to assess the normality of distributions. Comparisons between groups were performed using Student's t test (for age), chi-squared test (for AON history), fisher's exact test (for gender and race), and Mann–Whitney U test (for disease and follow-up durations). For the MS controls, a single eye per subject was randomly sampled and then all the longitudinal visits for that eye were included in the analysis.
In order to analyze the course of retinal layer thickness changes over the study duration, time was taken as a continuous covariate (starting at the onset of visual loss attributed to AON). To account for data clustering due to multiple study visits for each eye of participants, multilevel mixed-effects linear spline regression models were used. Rates of change in retinal layer thickness were calculated by regressing retinal layer thickness values over follow-up time. Linear spline breakpoints (allowing for a change in slope to occur) were positioned according to the best fit to the data; at 4 and 8 months for GCIP and INL+OPL thicknesses, and at 4, 8, and 12 months for ONL+PS thickness (Figure 2). Interaction terms with time were used for the purpose of comparisons between AON eyes, fellow eyes, and MS control eyes. Models were adjusted for age, gender, disease duration, and previous history of AON in analyses between groups. Pearson correlation and multivariate linear regression were undertaken to assess the relationship between continuous responses of both the changes in different retinal layers and visual acuity measures at set time points. Statistical significance was defined as p<0.05.
Figure 2.
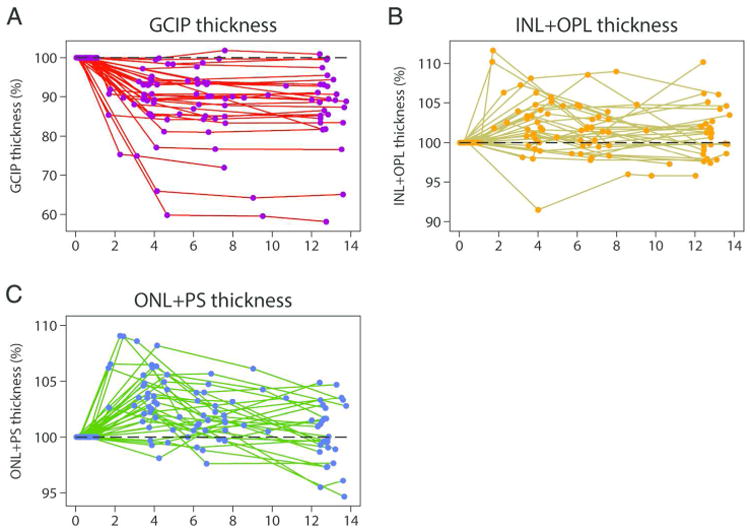
Line graphs depicting changes in composite ganglion cell and inner plexiform layers (GCIP; Panel A), inner nuclear layer, including the outer plexiform layer (INL+OPL; Panel B) and outer nuclear layer, including the inner and outer photoreceptor segments (ONL+PS; Panel C) thicknesses for subjects followed in the study over follow-up time after AON expressed as percentages relative to baseline (dashed lines).
Abbreviations: AON = acute optic neuritis; GCIP = ganglion cell + inner plexiform layers; INL+OPL = inner nuclear layer, including the outer plexiform layer; ONL+PS = outer nuclear layer, including the inner and outer photoreceptor segments.
Results
Baseline retinal measures in the AON group
Baseline OCT-derived retinal measures from clinically affected AON eyes and their fellow unaffected eyes are illustrated in Table 2 and supplementary Table 2 respectively. Follow-up OCT examinations were categorized into 3 time intervals relative to AON onset: 1-4 months (n=24), 4-8 months (n=31), and 8-16 months (n=31). A sub-group of patients (n=15) who underwent additional scanning beyond 16 months were designated as a long-term follow-up group in order to assess the longevity of outer retinal changes following AON. Peripapillary RNFL thickness at baseline in AON eyes was, on average, 18.4μm greater than in fellow eyes (95% CI: 3.0, 33.8; p =0.02) and 21.3μm greater than in MS control eyes (95% CI: 4.6, 38.1; p =0.01), indicative of RNFL edema. All macular segmentation measures were similar at baseline between clinically-affected AON eyes, their fellow contralateral eyes, and MS control eyes.
Table 2.
Summary of retinal thickness and visual function measures in clinically-affected AON eyes at study time points.
| Baseline n=33 | 1-4 months n=24 | 4-8 months n=31 | 8-16 months n=31 | ||
|---|---|---|---|---|---|
| Days since AON symptom onset, median (range) | 14 (1-33) | 103 (34-119) | 191 (120-240) | 382 (257-476) | |
| Average peripapillary RNFL thickness, μm, mean (SD) | 111.6 (44.2) | 88.0 (11.8) | 82.8 (10.7) | 82.9 (11.0) | |
| Average macular thickness, μm, mean (SD) | 278.5 (12.3) | 275.4 (14.4) | 269.9 (13.6) | 268.3 (13.0) | |
| Average GCIP thickness, μm, mean (SD) | 75.9 (6.1) | 68.0 (7.4) | 66.7 (8.7) | 67.0 (9.0) | |
| Average INL+OPL thickness, μm, mean (SD) | 64.9 (3.0) | 67.0 (3.4) | 65.9 (3.4) | 65.7 (3.1) | |
| Average ONL+PS thickness, μm, mean (SD) | 119.8 (7.3) | 124.5 (8.5) | 122.0 (7.7) | 120.3 (7.4) | |
| Visual function, letter acuity score*, mean (SD) | 100% contrast | 41.1 (21.0) | 53.9 (10.8) | 56.6 (6.1) | 55.4 (13.4) |
| 2.5% contrast | 6.5 (9.5) | 15.2 (14.5) | 21.5 (10.8) | 23.2 (12.8) | |
| 1.25% contrast | 2.2 (3.8) | 6.7 (8.9) | 11.8 (9.9) | 11.0 (10.9) | |
Available at baseline for 29 eyes.
Abbreviations: AON = acute optic neuritis; GCIP = ganglion cell + inner plexiform layers; INL = inner nuclear layer; OPL = outer plexiform layer; ONL = outer nuclear layer; PS =photoreceptor segments; RNFL = retinal nerve fiber layer; SD = standard deviation.
Longitudinal changes in GCIP thickness
Similar to previous studies9,14,17, GCIP thinning was pronounced in clinically-affected AON eyes during the first 4 months after the event compared to fellow and MS control eyes (p <0.001 for both comparisons). Between 4-8 months, no statistically significant loss of GCIP thickness was observed in clinically-affected AON eyes, fellow or MS control eyes (Table 3). Relative to baseline, GCIP thickness was reduced in affected eyes by an average of 11.4% and 12.3% at 4 and 12 months respectively (p <0.01 for both comparisons).
Table 3.
Estimated rates of change in GCIP thickness over the first 8 months of follow-up after AON.
| Time interval after AON | Group | Rate of change (μm/month) | 95% CI | P-value | P-value comparing rates in affected vs. fellow eyes | P-value comparing rates in affected vs. control eyes |
|---|---|---|---|---|---|---|
| Baseline to 4 months | Affected eyes | -2.52 | -2.9, -2.2 | <0.001 | <0.001 | <0.001 |
| Fellow eyes | -0.15 | -0.5, 0.2 | 0.411 | |||
| Control eyes* | -0.004 | -0.6, 0.6 | 0.988 | - | ||
| 4 to 8 months | Affected eyes | -0.004 | -0.3, 0.3 | 0.978 | 0.928 | 0.955 |
| Fellow eyes | -0.02 | -0.3, 0.3 | 0.878 | |||
| Control eyes* | 0.02 | -0.6, 0.7 | 0.946 | - |
Randomly-selected, single eye per control.
Abbreviations: AON = acute optic neuritis; GCIP = ganglion cell + inner plexiform layers; CI = confidence interval.
Longitudinal changes in INL+OPL thickness
A small but statistically significant increase was observed in INL+OPL thickness at 4 months after AON in the affected eyes (mean change: 1.9%; 95% CI: 0.7, 3.2; p=0.005). Notably, a less pronounced increase in INL+OPL thickness also occurred in the fellow eyes at 4 months (mean change: 1.0%; 95% CI: 0.02, 2.0; p=0.047). The rate of INL+OPL change in affected eyes during the first 4 months after AON, however, was not significantly different from the rates observed during the same period in fellow and MS control eyes (p = 0.24 and p = 0.34 respectively). A corresponding trend towards a decline in INL+OPL thickness for the affected eyes was observed after this time period, between 4 to 8 months, but did not reach statistical significance (rate: -0.17 μm/month, p=0.07). No significant change occurred in INL+OPL thickness between 4 to 8 months after AON onset for the fellow and control eyes (Table 4) or between 8 to 12 months for the affected, fellow, or control eyes (results not shown).
Table 4.
Estimated rates of change in INL+OPL thickness over the first 8 months of follow-up after AON.
| Time interval after AON | Group | Rate of change (μm/month) | 95% CI | P-value | P-value comparing rates in affected vs. fellow eyes | P-value comparing rates in affected vs. control eyes |
|---|---|---|---|---|---|---|
| Baseline to 4 months | Affected eyes | 0.42 | 0.2, 0.6 | <0.001 | 0.243 | 0.341 |
| Fellow eyes | 0.24 | 0.03, 0.5 | 0.027 | |||
| Control eyes* | 0.21 | -0.2, 0.6 | 0.269 | - | ||
| 4 to 8 months | Affected eyes | -0.17 | -0.4, 0.01 | 0.067 | 0.550 | 0.847 |
| Fellow eyes | -0.09 | -0.3, 0.1 | 0.339 | |||
| Control eyes* | -0.13 | -0.5, 0.3 | 0.513 | - |
Randomly-selected, single eye per control.
Abbreviations: AON = acute optic neuritis; INL = inner nuclear layer; OPL = outer plexiform layer; CI = confidence interval.
Longitudinal changes in ONL+PS thickness
Relative to baseline, ONL+PS thickness at 4 months increased by an average of 3.4% in affected eyes (p <0.001) and 0.2% in fellow eyes (p = 0.65). The average rate of increase in ONL+PS thickness in AON eyes during the first 4 months was 1.16 μm/month (p<0.001; 95% CI: 0.8, 1.5) (Table 5). This rate of increase in ONL+PS thickness was significantly greater than the rate of change in fellow (0.08μm/month) and MS control eyes (-0.11μm/month) during the same time period (p<0.001 for both comparisons). A reciprocal reduction in ONL+PS thickness was observed in the affected eyes between 4-8 months (rate: -0.79 μm/month; p<0.001) but no significant change occurred in the fellow and control eyes during the same time period. At 12 months, ONL+PS thickness was not significantly different from baseline in either the affected or fellow eyes (mean change: 0.47% and -0.2%, p = 0.37 and 0.66 respectively).
Table 5.
Estimated rates of change in ONL+PS thickness over the first 12 months of follow-up after AON.
| Time interval after AON | Group | Rate of change (μm/month) | 95% CI | P-value | P-value comparing rates in affected vs. fellow eyes | P-value comparing rates in affected vs. control eyes |
|---|---|---|---|---|---|---|
| Baseline to 4 months | Affected eyes | 1.16 | 0.8, 1.5 | <0.001 | <0.001 | <0.001 |
| Fellow eyes | 0.08 | -0.2, 0.4 | 0.609 | |||
| Control eyes* | -0.11 | -0.7, 0.5 | 0.714 | - | ||
| 4 to 8 months | Affected eyes | -0.79 | -1.2, -0.4 | <0.001 | 0.002 | 0.040 |
| Fellow eyes | 0.04 | -0.3, 0.4 | 0.857 | |||
| Control eyes* | 0.23 | -0.7, 1.1 | 0.617 | - | ||
| 8 to 12 months | Affected eyes | -0.17 | -0.5, 0.1 | 0.293 | 0.587 | 0.705 |
| Fellow eyes | -0.04 | -0.4, 0.3 | 0.786 | |||
| Control eyes* | -0.04 | -0.6, 0.5 | 0.873 | - |
Randomly-selected, single eye per control.
Abbreviations: AON = acute optic neuritis; ONL = outer nuclear layer; PS = photoreceptor segments; CI = confidence interval.
Relationships between changes in retinal layer thicknesses
The approximate peak of ONL+PS thickening detected in AON eyes occurred during the 1-4 month time interval (Figure 2). In AON eyes, there was no relationship between baseline peripapillary RNFL thickness and the percentage change in ONL+PS thickness at 4 months relative to baseline (r = 0.06, p = 0.76). There was no correlation between the percentage change in peripapillary RNFL and ONL+PS thicknesses at 4 months relative to baseline (r = -0.23, p = 0.25). However, a highly significant negative correlation was observed between the percentage change in GCIP and ONL+PS thicknesses at 4 months relative to baseline (i.e. eyes with a greater degree of GCIP thinning being associated with a greater increase in ONL+PS thickness during this period: r = -0.70, p<0.001). Likewise, there was a non-significant trend between the magnitude of GCIP thinning and INL+OPL thickening at 4 months (r = -0.36, p=0.06) (Figure 3). A similar association was not seen in the fellow eyes of the AON group for either ONL+PS (r = -0.28, p = 0.16) or INL+OPL thickness changes (r = 0.31, p = 0.12).
Figure 3.
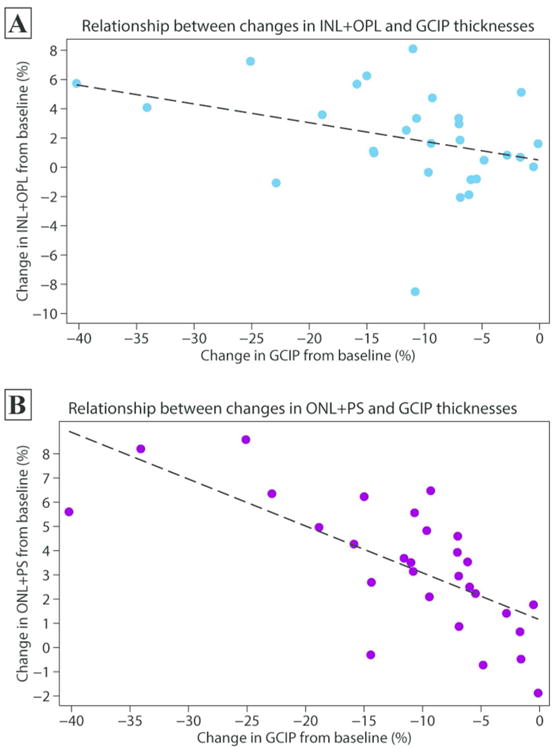
Scatter plots depicting the association between the percentage change in GCIP thickness in relation to changes in INL+OPL (panel A) and ONL+PS (panel B) thicknesses for AON eyes at 4 months after the event relative to baseline.
Abbreviations: AON = acute optic neuritis; GCIP = ganglion cell + inner plexiform layers; INL+OPL = inner nuclear layer, including the outer plexiform layer; ONL+PS = outer nuclear layer, including the inner and outer photoreceptor segments.
Analysis of the long-term follow-up subgroup of AON patients scanned beyond 16 months following the onset of AON (mean follow-up duration = 34.4 months; SD = 11.1; range 20-55) revealed that the INL+OPL thickening in the affected eyes was most pronounced 1-4 months following AON (+3.5% increase relative to baseline in this cohort, p = 0.001) before it gradually declined. INL+OPL thickness remained statistically significantly different from baseline 16-55 months beyond AON onset (+2.7%, p = 0.006). Similarly, the average increase in ONL+PS thickness was most marked 1-4 months following AON (+4.3% relative to baseline in this cohort, p < 0.001) before it gradually declined. In contrast, however, ONL+PS thickness of the affected eyes 16-55 months following AON onset no longer remained significantly different from its baseline value (+0.6%, p = 0.314). No statistically significant INL+OPL or ONL+PS thinning was detected at any time point during the course of this study.
As a sensitivity analysis, we assessed the effects of the changing cohort at each follow-up time interval by restricting our analysis to the group of patients examined during all of the designated time intervals (n=20). Consistent with the effect observed in the entire cohort, INL+OPL thickness increased in the affected eyes of this patient subgroup by an average of +3.2% (p <0.001) between 1-4 months, +2.3% (p =0.001) between 4-8 months, and +1.9% (p =0.01) between 8-16 months. Similarly, in this sub-group of patients, the change in ONL+PS thickness was estimated as +4.4% (p<0.001) between 1-4 months, +2.1% (p<0.001) between 4-8 months, and +0.3% (p=0.561) between 8-16 months.
Relationships with visual acuity
Visual acuity measures in AON eyes at different study time-points are summarized in Table 2. Eyes with lower baseline 100% high-contrast letter-acuity scores demonstrated a significantly greater increase in ONL+PS thickness at 4 months (n = 27; r = -0.53, p = 0.005) but not INL+OPL thickness (r = -0.35, p = 0.07). Using linear regression analysis, it is estimated that for each 5-letter decline in 100% contrast visual acuity letter score at baseline, the ONL+PS thickness increased by 0.26% at 4 months after adjusting for age, gender, and baseline GCIP thickness (partial r= -0.49, p = 0.02). Low-contrast (2.5% and 1.25%) letter-acuity scores at baseline were not correlated with increased ONL+PS thickness at 4 months (p = 0.49 and p = 0.63 respectively). However, 18 (62%) and 20 (69%) AON eyes had letter acuity scores of zero at baseline with 2.5% and 1.25% low contrast testing respectively, indicating the presence of a floor effect, which could mask potential associations.
Twelve months after AON, lower GCIP thickness was associated with lower 100%, 2.5%, and 1.25% contrast letter-acuity scores (p <0.01 for all comparisons). The degree of ONL+PS thickening at 4 months was not significantly associated with 100%, 2.5%, or 1.25% contrast letter-acuity scores at 12-months, but did correlate with 12-month 100% high-contrast visual recovery (p = 0.02). Nevertheless, the relationship lost significance after adjusting for baseline 100% high-contrast letter-acuity score (p = 0.77), indicating that eyes with worse baseline high-contrast visual function had greater degrees of ONL+PS thickening at 4 months and, at the same time, had a greater numerical potential for high-contrast visual recovery. Similar associations with INL+OPL thickening were not found to be significant.
Discussion
Results of this study confirm that GCIP thinning occurs rapidly following AON, predominantly within the first 4 months, as previously reported9,14,18,17. Importantly, our findings also suggest that during the same time-frame that the GCIP is thinning following AON, there is concomitant thickening of the ONL+PS, and less markedly the INL+OPL. These results are in accordance with a previous study showing a cross-sectional inverse relationship between GCIP and INL thicknesses in eyes with a previous history of AON9. Our findings expand upon previous work by demonstrating that the INL+OPL thickening is maximal during the first 1-4 months after the AON episode then gradually declines thereafter, but nonetheless continues to remain elevated. Prior studies have also suggested cross-sectional aberrations in the ONL after AON. Selective attenuation of the b-wave of the flash electroretinogram in the early period following demyelinating AON has been proposed, and thought to be independent of optic nerve pathology and suggestive of underlying retinal alterations19,20. Moreover, it has been suggested that photoreceptor pathology may occur as a result of optic neuropathy11. In this study, the transient increase in ONL+PS thickness during the first 4 months after AON appeared strongly related to the degree of GCIP thinning during the same time period. These findings raise several possibilities regarding the potential mechanism(s) underlying these changes.
First, it is possible that this increase in thickness of the retinal nuclear layers may be mediated through trans-synaptic mechanisms or represent a reaction to trans-synaptic retrograde degeneration in those layers. The occurrence of trans-synaptic retinal neuronal degeneration in animal models of optic nerve transection has been controversial. Earlier studies reported that optic nerve transection did not alter amacrine cell body count or distribution in the rat retina21,22, nor did it affect the pattern of cell death observed in the INL and ONL of the normal postnatal rat retina23. More recent studies however report that ganglion cell death results in modulation of some amacrine cell subtypes in the rat and ferret retinae24,25 and may result in increased cell death within the INL of the developing ferret26. It is worth noting that a study examining an experimental autoimmune encephalomyelitis (EAE) model of MS associated with optic neuritis in rats demonstrated positive TUNEL labeling (indicative of DNA fragmentation and apoptosis) within the INL and ONL, as well as breakdown of the blood retinal barrier following ganglion cell degeneration27. Lymphocytic infiltration and blood-retinal barrier disruption in EAE-associated optic neuritis might provide an inflammatory milieu that additionally promotes retinal neuronal death, as compared to other models involving optic nerve transection. This suggests that the underlying pathobiology driving optic nerve damage might be relevant to whether and how retinal trans-synaptic retrograde degeneration occurs. Post-mortem studies of MS patients clearly demonstrate neuronal loss within the INL, with focal reductions in cell density28. However, whether such pathology is attributable to trans-synaptic retrograde degeneration or a primary retinal process still remains to be fully elucidated. It is worthwhile to note that in this study no evidence of INL+OPL or ONL+PS thinning occurred at any time point, raising the question of whether alternative mechanisms occurring secondary to cell death, such as neuronal swelling, glial hypertrophy, or extracellular fluid accumulation, could instigate the observed increase in thickness, ultimately masking any eventual neuronal loss.
Alternatively, our findings may reflect a traction phenomenon in which the abrupt volume loss in the RNFL and GCIP over the early months following AON results in a subsequent rise in INL+OPL and ONL+PS thicknesses or morphometric changes in the retina occurring independently of any traction. A similar theory involving vitreous traction has been proposed as a possible explanation for the occurrence of macular microcysts within the INL in patients with optic atrophy29,30. Nevertheless, a recent study utilized predictions from a mechanical model to show that macular microcyst formation was convincingly independent and unrelated to vitreous traction31. Several factors suggest that the observed increase in ONL+PS thickness, and less markedly the INL+OPL thickness, following AON observed in this study is not due to traction: (1) the increases were transient, peaking at approximately 4 months, and then declining thereafter, in contrast to GCIP thinning which persisted (2) traction from volume loss in the RNFL and GCIP might be expected to affect the more adjacent INL+OPL to an even greater degree; however the increase in INL+OPL thickness was less robust than the increase in ONL+PS thickness (3) no evidence of morphologic macular pathology or vitreous traction was detected during qualitative scan review. Conceptually, it is possible for retinal morphometric changes to occur independently of vitreal traction and the question of whether a retinal anatomical accommodation to GCIP thinning is at play after AON remains to be elucidated. Another plausible mechanism explaining the observed increase in ONL+PS thickness (as well as the less pronounced increase in INL+OPL thickness) is the presence of retinal inflammation within these layers. Although no gross evidence of intraretinal or subretinal fluid accumulation has been observed in this investigation to suggest an inflammatory process, previous studies have described the presence of retinal inflammation, as illustrated by perivenous sheathing and/or fluorescein leakage, in 24% of eyes with isolated AON within 8 weeks of symptom onset (including subclinical fluorescein leakage of fellow eyes in 36% of affected patients). While this retinal inflammation may relate to opening of the blood-retinal-barrier concomitant with opening of the blood-brain-barrier, it is not mutually exclusive with the possibility of an inflammatory glial reaction in response to ganglion cell degeneration. Indeed, INL+OPL thickness was noted to increase over the first 4 months of follow-up in contralateral eyes in this study raising the possibility of concomitant retinal inflammation in fellow eyes.
Our study has a number of limitations. The majority of patients enrolled in this study underwent routine ophthalmological examinations during their diagnostic work up for AON. However, a comprehensive ophthalmological assessment was not performed as part of the study protocol. It is plausible that differences in follow-up timing and failure to follow-up may have influenced our findings. Yet, in order to minimize both possibilities, we used time since the AON event as a continuous covariate in our models and performed a sensitivity analysis that showed consistent effects among the sub-group of patients examined during all of the relevant time intervals. Current OCT acquisition techniques offer limited capability to analyze the cellular composition within different retinal layers which, by extension, limits our capacity to confirm the exact mechanistic underpinnings of our findings. As a result, it remains to be clarified whether the alterations observed in the nuclear layers in this study are driven by changes in the extracellular matrix, hypertrophy of neuronal cell bodies, direct glial cell (including Muller cell) activation in response to ganglion cell death, or changes in their capacity to handle interstitial retinal fluid, or a combination of these factors. Given that the focus of this study was on AON cases that were related to MS, this may limit the generalizability of our findings to optic neuropathy of all etiologies.
In summary, we have identified novel retinal changes characterized by a thickening of the ONL+PS, and less markedly the INL+OPL, in the early months following AON. The magnitude of this change in the ONL+PS, and more weakly in the INL+OPL, is proportional to the degree of GCIP thinning, suggesting it may be the derivative of either trans-synaptic alterations, or concomitant retinal inflammation. Future studies with a more rigorous, protocol-driven follow-up are warranted to confirm these findings, as well as to identify and characterize the retinal processes underlying these changes. Moreover, it remains to be determined whether similar patterns of INL and ONL change occur in acute optic neuropathies from other causes. Our novel findings provide convincing evidence that the effects of AON are not limited to the inner retina, but that dynamic changes occur in the deeper retinal neuronal layers as well.
Supplementary Material
Supplementary Table 1: Summary of test-re-test reliability for macular segmentation measures derived from affected eyes of the sub-group of patients scanned two or more times at the same visit.
Supplementary Table 2: Summary of retinal thickness measures in the fellow eyes of AON patients at study time points
Acknowledgments
We thank the patients who participated in this study. Race to Erase MS grant (to SS) and National Institutes of Health (5R01NS082347-02 to PAC) funded the study.
Disclosures: PB receives research support from the National Multiple Sclerosis Society. SDN has received personal compensation for consulting from Biogen-Idec and Genzyme and has received research funding from Biogen-Idec and Novartis. LJB has received speaking and consulting honoraria from Biogen Idec, Bayer, and Novartis. EMF has received speaker and consulting fees from Novartis, Genzyme, Acorda, and TEVA. PAC has received personal compensation for consulting and serving on scientific advisory boards from: Vertex, Vaccinex, Prothena, and Abbvie and has received research funding from Biogen-IDEC, MedImmune, and Novartis. SS has received consulting fees from Medical Logix for the development of CME programs in neurology, consulting fees from Axon Advisors LLC, Educational Grant Support from Novartis & Teva Neurosciences, speaking honoraria from the National Association of Managed Care Physicians, Family Medicine Foundation of West Virginia, and Advanced Studies in Medicine and served on a scientific advisory board for Biogen-Idec. OAL and CC have no disclosures.
Footnotes
Contributions: OAA, PAC, and SS conceptualized and designed the study. OAA and SS gathered the data. OAA, CMC, PAC, and SS analyzed and interpreted the data. All authors drafted, revised, and critically reviewed the manuscript. SS and PAC supervised the study.
Conflicts of Interest: The authors report no conflicts of interest
References
- 1.Friese MA, Schattling B, Fugger L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat Rev Neurol. 2014;10(4):225–238. doi: 10.1038/nrneurol.2014.37. [DOI] [PubMed] [Google Scholar]
- 2.Balcer LJ. Optic Neuritis. N Engl J Med. 2006;354:1273–1280. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- 3.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–839. doi: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
- 4.Pro MJ, Pons ME, Liebmann JM, et al. Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritis. J Neurol Sci. 2006;250(1-2):114–119. doi: 10.1016/j.jns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Kallenbach K, Simonsen H, Sander B, et al. Retinal nerve fiber layer thickness is associated with lesion length in acute optic neuritis. Neurology. 2010;74(3):252–258. doi: 10.1212/WNL.0b013e3181ca0135. [DOI] [PubMed] [Google Scholar]
- 6.Parisi V, Manni G, Spadaro M, et al. Correlation between Morphological and Functional Retinal Impairment in Multiple Sclerosis Patients. 1999;40(11):2520–2527. [PubMed] [Google Scholar]
- 7.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58(3):383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 8.Klistorner A, Arvind H, Nguyen T, et al. Axonal loss and myelin in early ON loss in postacute optic neuritis. Ann Neurol. 2008;64(3):325–331. doi: 10.1002/ana.21474. [DOI] [PubMed] [Google Scholar]
- 9.Kaushik M, Wang CY, Barnett MH, et al. Inner nuclear layer thickening is inversley proportional to retinal ganglion cell loss in optic neuritis. PLoS One. 2013;8(10):e78341. doi: 10.1371/journal.pone.0078341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saidha S, Sotirchos ES, Ibrahim Ma, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner JS, Keltner JL, Zawadzki RJ, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye (Lond) 2011;25(3):279–289. doi: 10.1038/eye.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saidha S, Syc SB, Ibrahim Ma, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 2):518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 14.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(Pt 2):521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. doi: 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology. 2013;80(15):1406–1414. doi: 10.1212/WNL.0b013e31828c2f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One. 2013;8(8):e71145. doi: 10.1371/journal.pone.0071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papakostopoulos D, Fotiou F, Hart JC, Banerji NK. The electroretinogram in multiple sclerosis and demyelinating optic neuritis. Electroencephalogr Clin Neurophysiol. 1989;74(1):1–10. doi: 10.1016/0168-5597(89)90045-2. http://www.ncbi.nlm.nih.gov/pubmed/2463143. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda H, Tremain KE, Sanders MD. Neurophysiological investigation in optic nerve disease: combined assessment of the visual evoked response and electroretinogram. Br J Ophthalmol. 1978;62(4):227–239. doi: 10.1136/bjo.62.4.227. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1043194&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne NN, Perry VH. Effect of neonatal optic nerve transection on some classes of amacrine cells in the rat retina. Brain Res. 1985;343(2):230–235. doi: 10.1016/0006-8993(85)90739-5. http://www.ncbi.nlm.nih.gov/pubmed/2413958. [DOI] [PubMed] [Google Scholar]
- 22.Wu DK, Cepko CL. Development of dopaminergic neurons is insensitive to optic nerve section in the neonatal rat retina. Brain Res Dev Brain Res. 1993;74(2):253–260. doi: 10.1016/0165-3806(93)90011-x. http://www.ncbi.nlm.nih.gov/pubmed/8104745. [DOI] [PubMed] [Google Scholar]
- 23.Beazley LD, Perry VH, Baker B, Darby JE. An investigation into the role of ganglion cells in the regulation of division and death of other retinal cells. Brain Res. 1987;430(2):169–184. doi: 10.1016/0165-3806(87)90151-9. http://www.ncbi.nlm.nih.gov/pubmed/3607511. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki EN, Andrade da Costa BL, Barbosa VD, Hokoç JN. Retinal ganglion cell depletion alters the phenotypic expression of GABA and GAD in the rat retina. Eur J Neurosci. 1997;9(9):1885–1890. doi: 10.1111/j.1460-9568.1997.tb00755.x. http://www.ncbi.nlm.nih.gov/pubmed/9383211. [DOI] [PubMed] [Google Scholar]
- 25.Williams RR, Cusato K, Raven Ma, Reese BE. Organization of the inner retina following early elimination of the retinal ganglion cell population: effects on cell numbers and stratification patterns. Vis Neurosci. 2001;18(2):233–244. doi: 10.1017/s0952523801182088. http://www.ncbi.nlm.nih.gov/pubmed/11417798. [DOI] [PubMed] [Google Scholar]
- 26.Cusato K, Stagg SB, Reese BE. Two Phases of Increased Cell Death in the Inner Retina Following Early Cell Population. 2001;449(May):440–449. doi: 10.1002/cne.1361. [DOI] [PubMed] [Google Scholar]
- 27.Fairless R, Williams SK, Hoffmann DB, et al. Preclinical retinal neurodegeneration in a model of multiple sclerosis. J Neurosci. 2012;32(16):5585–5597. doi: 10.1523/JNEUROSCI.5705-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(Pt 6):1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lujan BJ, Horton JC. Microcysts in the inner nuclear layer from optic atrophy are caused by retrograde trans-synaptic degeneration combined with vitreous traction on the retinal surface. Brain. 2013;136(Pt 11):e260. doi: 10.1093/brain/awt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barboni P, Carelli V, Savini G, Carbonelli M, La Morgia C, Sadun Aa. Microcystic macular degeneration from optic neuropathy: not inflammatory, not trans-synaptic degeneration. Brain. 2013;136(Pt 7):e239. doi: 10.1093/brain/awt014. [DOI] [PubMed] [Google Scholar]
- 31.Brandt AU, Oberwahrenbrock T, Kadas EM, Lagrèze Wa, Paul F. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology. 2014 doi: 10.1212/WNL.0000000000000545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Summary of test-re-test reliability for macular segmentation measures derived from affected eyes of the sub-group of patients scanned two or more times at the same visit.
Supplementary Table 2: Summary of retinal thickness measures in the fellow eyes of AON patients at study time points


