Abstract
Retinoic acids (RAs), which are metabolites of vitamin A, have been shown to be involved in multiple T cell effector responses through their binding to the retinoic acid receptor, a ligand-activated transcription factor. Since the molecular mechanism of regulation by RA is still not fully uncovered, we investigated the gene expression profile of all-trans retinoic acid (ATRA)–treated human CD4+ T cells. Leucine zipper transcription factor-like 1 (LZTFL1) was upregulated by ATRA in a dose- and time-dependent manner. The expression of LZTFL1 depended on both ATRA and TCR signaling. LZTFL1 accumulated in the plasma membrane compartment of human CD4+ T cells, and during immunological synapse (IS) formation, it transiently redistributed to the T cell and APC contact zone, indicating its role in T cell activation. Live cell imaging demonstrates that at the initial stage of IS formation, LZTFL1 is concentrated at the APC contact site, and during later stages, it relocates to the distal pole. Knockdown of LZTFL1 reduced the basal- and ATRA-induced levels of IL-5 in CD4+ T cells, and overexpression of LZTFL1 enhanced the TCR-mediated NFAT signaling, suggesting that LZTFL1 is an important regulator of ATRA-induced T cell response. Together, these data indicate that LZTFL1 modulates T cell activation and IL-5 levels.
Introduction
Retinoic acids (RAs), especially all-trans retinoic acid (ATRA), the active metabolite of vitamin A, are known to regulate cell differentiation, proliferation, and apoptosis in a variety of cell types through their binding to the retinoic acid receptor (RAR), a ligand-activated transcription factor (1, 2). Vitamin A and RAs influence T cell function in many ways, including peripheral T cell differentiation, gut-homing capacity, and effector T cell activity (3-8). RAs are known to favor Th2 cell development (8-14). Vitamin A deficiency causes immune dysfunction, including IFN-γ overproduction and impaired antibody responses, which is the result of excess Th1 and insufficient Th2 function (15, 16). Vitamin A–deficient mice showed reduced Th2 cytokine production and bone marrow eosinophilia with parasitic helminth infection (17), and mice given a vitamin A or RA supplement showed decreased production of the Th1 cytokine IFN-γ and increased production of Th2 cytokines IL-4, -IL-5, and -IL-13 (15). Even though the mechanism of RAs’ impact on Th2 cell development is still not fully understood, the direct and indirect effects of RAs have been suggested. By inhibiting IL-12 production in activated macrophages, RA pretreatment of macrophages reduced IFN-γ production and increased IL-4 production in antigen-primed CD4+ T cells (18), and stimulating Ab-primed human PBMCs and purified T cells with RAs in vitro directly increased the mRNA and protein levels of IL-4, IL-5, and IL-13, and decreased the levels of IFN-γ, IL-2, IL-12, and TNF-α (8, 11).
The differentiation of naïve CD4+ T cells into Th2 cells is induced by antigen-presenting cells and also requires TCR-mediated signaling (19, 20). In vivo, gut dendritic cells (DCs) and macrophages process vitamin A to generate RAs and present them to T cells during antigen presentation and T cell activation (16, 21), indicating the important role of RAs in T cell activation and differentiation. Even though little is known about the mechanism of RAs’ regulation in this process, their influence on T cell activation is suggested. T cell activation markers CD69 and CD38 are upregulated by ATRA, indicating the engagement of RA-RAR signaling in T cell activation (10). Moreover, RAs also upregulate transcriptional factors for Th2 differentiation, including cMAF, GATA-3, and STAT-6, with a concomitant downregulation of the Th1 factor T-bet (11). All these observations indicate that RA-RAR signaling is engaged in the induction of Th2 differentiation by RA.
Leucine zipper transcription factor-like 1 (LZTFL1) was first identified as a tumor suppressor. The gene encoding LZTFL1 is located on human chromosome 3p21.3 and is found to be deleted in several types of cancer (22). LZTFL1 overexpression in cervical cancer cell line HeLa cells inhibited anchorage-independent cell growth and cell migration in vitro, and repressed tumor growth in vivo (23). Recently, a deletion mutant of LZTFL1 was also found in a family with Bardet-Biedl syndrome (BBS), which suggests that LZTFL1 is involved in BBS (24). Seo et al. (25) further showed that LZTFL1 interacts with a BBS protein complex, known as the BBSome, and regulates its primary ciliary trafficking. A role for LZTFL1 in hedgehog signaling is also suggested (24, 25).
In our effort to understand the role that ATRA plays in CD4+ T cell development, we have found that both LZTFL1 mRNA and protein production are upregulated by ATRA treatment in human CD4+ T cells. During CD4+ T cell activation in contact with APC, LZTFL1 transiently localizes to the immunological synapse (IS). Overexpression of LZTFL1 in CD4+ T cells further enhanced the T cell activation signal, as indicated by increased NFAT activity. Moreover, LZTFL1 knockdown decreased Th2 cytokine production, especially IL-5 mRNA and protein production, and further suppressed ATRA-induced IL-5 production. Our data suggest that LZTFL1 is involved in ATRA-regulated Th2 cytokine production, possibly through LZTFL1-induced TCR-NFAT signaling.
Material and Methods
Reagents
ATRA, Actinomycin D and Latrunculin B were purchased from Sigma-Aldrich (St. Louis, MO). The following Abs were used: mouse anti-LZTFL1 and rabbit anti-TCRβ (Santa Cruz Biotechnology, CA); mouse anti-Flag M2 (Sigma-Aldrich, St. Louis, MO); and mouse anti-GAPDH (Abcam, Cambridge, MA).
Plasmids
NFAT and NFkB luciferase reporter plasmids were described earlier (26) pRL-TK and pHTN HaloTag® CMV-neo plasmids were purchased from Promega (Promega, Madison, WI). The vector encoding Myc-DDK-tagged human LZTFL1 (Accession NM 020347) was purchased from Origene (Rockville, MD). LZTFL1 was cloned in-frame downstream of the HaloTag sequence of pHTN HaloTag plasmid and a fragment containing HaloTag-LZTFL1 from the resulting plasmid was cloned into pENTR vector (Life Technologies, Grand Island, NY) to obtain pENTRHalo-LZTFL1. A termination codon after the HaloTag coding sequence was introduced to obtain pENTRHalo-LZSTOP. Lentiviral vectors containing either Halo-LZTFL1 or Halo-LZSTOP were generated by transfecting HEK-293 cells as suggested by manufacturer (Life Technologies, Grand Island, NY).
Cell culture
Primary human CD4+ T cells were isolated from PBMCs of healthy donors using Dynabeads Untouched Human CD4 T cells isolation kit (Life Technologies) following the manufacturer's instruction. Cells were cultured in RPMI 1640 supplemented with 10% dialyzed FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 50 U/ml IL-2 (PeproTech, Rocky Hill, NJ). To activate CD4+ T cells, cells were primed with anti-CD3 and anti-CD28 Abs using Dynabeads CD3/CD28 T cell expander (Life Technologies). Jurkat E6.1 cell line, a CD4+ human T cell lymphoblast-like cell line was cultured as described (27). D10.G4.1 cell line, a mouse Th2 lymphoblast was purchased from ATCC and cultured in RPMI 1640 medium supplemented with 10% T-STIM with Con A (Becton Dickinson, Franklin Lakes, NJ), 10% fetal bovine serum, 0.05 mM 2-mercaptoethanol and 10 pg/ml mouse IL-1 alpha (R&D Systems, Minneapolis, MN). Jurkat T cells stably expressing Halo-LZTFL1 (Halo-LZTFL1) or Halo-LZSTOP were generated by infecting Jurkat cells with lentivirus Halo-LZTFL1 or Halo-LZSTOP, and growing the cells positive for Halo-Tag expression by fluorescence-activated cell sorting (FACS) analysis.
For latrunculin B treatment, Halo-LZTFL1 Jurkat T cells were plated on poly-l-lysine-coated chamber slides and immediately treated with 1uM of latrunculin B (Sigma) for 30 min at 37°C and 5% CO2. Cells were then fixed and incubated with phalloidin conjugated Alexa 488 at room temperature for 1 h and then with anti-Halo Ab (Promega) at 4°C for overnight. After washing, cells were incubated with goat anti-rabbit at room temperature for 1 h. The ratio of fluorescent intensities of membrane localized LZTFL1 to that of total cellular LZTFL1 was calculated by Matlab software (Mathwork, MA, USA) after imaging with a confocal microscope.
Reverse transcription and Real-time PCR
Total cellular RNA was extracted using RNAqueous®-4PCR Kit (Life Technologies). To quantitatively analyze gene expression, 200 ng of total RNA was used to synthesize the first strand DNA with random primers (SuperScript II Reverse Transcriptase, Life Technologies). The real-time PCR was performed by using SYBR Green Master Mix (Qiagen, Valencia, CA) and the following primers: LZTFL1-forward 5′- GGCCTAAATGAGCACCATCA -3′ and reverse 5′- ATCCACTTCTCAGCTTGTGC -3′; pre-developed FAM- and TAMRA-labeled internal oligonucleotide probes and primers for IL-5 and GAPDH (Life Technologies). The quantity of LZTFL1 and IL-5 mRNAs were normalized by the levels of GAPDH mRNA.
Western blot for LZTFL1
Whole cell proteins were extracted using M-PER mammalian protein extraction reagent with protease inhibitor cocktails (Thermo Scientific, Waltham, MA). Protein extracts were electrophoresed in a 4-12% gradient NuPAGE Bis Tris Gel (Life Technologies), and transferred to PVDF membrane and detected with fluorophore-labeled secondary Ab using Odyssey Infrared Imaging System (LI-COR Biotechnology, Lincoln, NE).
Northern blotting
Total RNA from activated CD4+ T cells treated with DMSO or ATRA was purified using RNAqueous®-4PCR Kit (Life Technologies) according to the manufacturer’s protocol and treated with DNase to remove residual contaminations with DNA. Digoxigenin (DIG) -labeled probes to LZTFL1 coding region was generated using the DIG Northern Starter Kit (Roche Diagnostics Corporation, Indianapolis, IN). For Northern blot analysis, 20 μg of total RNA was separated on a denaturing formaldehyde 1% agarose gel. The RNA was transferred onto a positively charged nylon filter by capillary transfer and hybridized with DIG-labeled DNA probes directed against human LZTFL1 and β-Actin according to the manufacturer’s protocol (DIG Northern Starter Kit, Roche Diagnostics Corporation, Indianapolis, IN). The hybridization signal was detected by chemiluminescence with an anti-digoxigenin alkaline phosphatase conjugate and CDP-Star.
Nuclear run-on assay
A nuclear run-on assay was performed following the method reported previously with some modification (28). Briefly, primed CD4+ T cells were treated with DMSO or ATRA for 24 h. Cells were washed with cold PBS and lysed in lysis buffer (10 mm Tris-HCl, pH 7.4; 3 mm MgCl2; 10 mm NaCl; and 0.5% Nonidet P-40) for 5 min on ice. Nuclei were pelleted and washed once with lysis buffer without Nonidet P-40 and resuspended in freezing buffer (50 mM Tris-HCl, pH 8.3; 40% glycerol; 5 mM MgCl2; and 0.1 mM EDTA) at −80 °C until use. For in vitro transcription, isolated nuclei were incubated in the transcription buffer (100 mM KCl, 10 mM Tris–HCl, pH 8.0, 2.5 mM MgCl2, 2 mM DTT, 2 μM each of ATP, GTP and CTP, 100 mM sucrose, and 10% glycerol) in the presence of UTP or biotin-16-UTP (Roche Molecular Biochemicals) for 30 min at 29°C. In vitro transcription was stopped by DNase I treatment at 37°C for 20 min. Synthesized RNAs were extracted using Master Pure RNA purification kit (Epicentre biotechnology, Madison, WI). Biotin-labeled RNA was isolated with M-280 streptavidin magnetic beads (Life Technologies) according to the manufacturer's recommendations, and used for reverse transcription and real-time PCR.
Confocal microscopy
For LZTFL1 staining, cells were allowed to rest in 0.1% poly-l-lysine-coated 8-well chamber slide for 5 min before a short spin, fixed with 4% formaldehyde (Thermo Scientific) for 30 min at room temperature and permeabilized for 5 min at room temperature with 0.2% Triton X-100 in PBS. Cells were then stained with indicated primary Ab and visualized by Alexa-Fluor 488 or 568-labeled secondary Ab. Lipid raft was stained with Alexa-Fluor 594-labeled Cholera toxin b. Filamentous actin (F-actin) was stained with Alexa Fluor 594 phalloidin (Life Technologies). Cover slips were mounted onto the slides with Prolong Gold Antifade reagent with DAPI (Life Technologies) to stain the nucleus and fluorescent images were captured by confocal microscope (FV-1000; Olympus).
For conjugation analysis, Raji B cells were stained with CellTracker violet BMQC (Life Technologies) and pulsed with or without 5 μg/ml staphylococcal enterotoxin E (SEE; Toxin Technology, Sarasota, FL) for 30 min at 37°C. Then, Raji B cells were mixed with an equal number of Jurkat T cells and plated onto poly-l-lysine-precoated slide. After brief centrifugation, cells were incubated for 1.5, 5 and 15 min at 37°C and fixed for 30 min at room temperature with 4% formaldehyde in PBS and permeabilized for 5 min at room temperature with 0.2% Triton X-100 in PBS, then stained with indicated primary Ab and visualized by Alexa-Fluor 568- or Alexa-Fluor 488-labeled secondary Ab. Cover slips were mounted onto the slides with Prolong Gold Antifade reagent (Life Technologies) and fluorescent images were captured by confocal microscope (FV-1000; Olympus). Conjugates formed between Jurkat T cells and Raji B cells were scored in 10 fields chosen at random at indicated time points. The percentage of conjugates was calculated as T cells forming interface with Raji B cells to total T cells in the field. The percentage of conjugates with accumulation of LZTFL1 at the T cell-B cell contact site - IS and distal pole (DP) was also quantified.
Live cell imaging for LZTFL1 localization at the IS
Halo-LZTFL1 or Halo-LZSTOP expressing Jurkat cells were stained with TMR ligand (Promega, Madison, WI) at 37°C and 5% CO2 for 40 min and washed with complete media for 4 times followed by incubation at 37°C and 5% CO2 for 1 h. After washing cells once with complete media, cells were then incubated with 5 μg/ml SEE superantigen-loaded Raji B cells prestained with Hoechst as APCs on poly-L-lysine coated glass-bottom dishes. Cells were maintained at 37°C and 5% CO2 during the entire period of imaging. Halo-tagged T cell images were acquired by fast wide field imaging using an Olympus TIRF 3 system with a 60x oil objective (1.49 NA), under non-TIRF mode. The wide field illumination was achieved by focusing the laser beams into the back focal plane of the objective. Samples labeled with HaloTag and Hoechst for nucleus were excited with 561 nm and 405 nm lasers respectively and the fluorescence signals were extracted with filters LF561-A-OMF for red (Semrock, Rochester, New York) and LF405-A-OMF for blue then recorded with an EMCCD camera (Hamamatsu, New Jersey, USA). Images of the two color channels were acquired in a time course of 10-second interval without interruption. The Acquired images were first processed to remove background and then combined into a movie with home Matlab codes on a Matlab programming environment (Mathwork, MA, USA).
Transfection
For luciferase assay, Jurkat T cells were washed twice with serum-free RPMI 1640 and 1.0 × 107 cells were resuspended in 250 μl of serum-free RPMI 1640 containing LZTFL1 expression plasmid, NFAT and NFkB reporter luciferase plasmid, and pRL-TK (Renilla luciferase control plasmid for normalization). Cells were electroporated at 750V and 1000 μF in a 0.4-cm-gap cuvette using Gene Pulser (Bio-Rad Laboratories, Hercules, CA) and allowed to recover for 24 h before stimulation. To transfect CD4+ T cells from healthy donors and D10.G4.1 cells, cells were suspended in the buffer for T cells and electroporated with Neon Electroporation System (Life Technologies).
RNA synthesis and transfection
A plasmid DNA with N-terminal flag-tagged LZTFL1 cDNA cloned downstream of T7 promoter was linearized and used for in vitro synthesis of LZTFL1 mRNA with the T7 mMessage mMachine Kit (Ambion, Austin, TX). CD4+ T cells were isolated from healthy donor, activated, and expanded 10 to 20 fold over a week using T cell expander beads (Life Technologies) in presence of IL-2 and dialyzed fetal calf serum.
For mRNA transfection, 30 to 40 × 106 expanded CD4 T cells were mixed with 40 μg of flag-tagged LZTFL1 mRNA and electroporated as described by Li et al (29). Electroporated cells were treated with DMSO or ATRA for 72 h and cellular RNA and proteins were analyzed.
RNA interference
Small interfering RNA (siRNA) SMARTpools targeting human and mouse LZTFL1 were from predesigned siGenome collection from Dharmacon, Inc. (Lafayette, CO). siGenome Non-Targeting siRNA was used as control. For siRNA transfection, 200 pmol of siRNA were mixed with 2.0 × 106 cells, and electroporated with either Nuleofector (Lonza) for Jurkat cells or Neon for primary CD4+ T cells and D10.G4.1 cells according to the manufacturer’s instruction. Cells were allowed to recover for 24 h before stimulation.
Luciferase reporter assays
Cells were transfected and stimulated with or without anti-CD3/CD28 coated beads (Life technologies) for 10 h. Luciferase activities were analyzed using the Promega dual luciferase assay system and measured in a luminometer. Renilla luciferase activities were used to normalize transfection efficiency. Normalized luciferase activities were determined in triplicate and expressed as fold increase relative to the basal activity obtained in unstimulated mock-transfected cells.
Cytokine ELISA
Human Th1 / Th2 / Th17 Cytokines Multi-Analyte ELISArray™ Kits (SABiosciences, Frederick, MD) were utilized to examine the following cytokines: IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17A, IFN-γ, TNF-α, G-CSF, and TGF-β1. The IL-4 and IL-5 ELISA was obtained from eBioscience (San Diego, CA). All of the ELISAs and multiplex assays were performed according to the manufacturer's instructions. The results are expressed as fold increase relative to the basal activity obtained in un-stimulated mock-transfected cells. All assays were run in triplicates.
Statistical analysis
Statistical analysis was assessed by Student’s t test. Results are shown as mean ± SD. A value of p < 0.05 was considered statistically significant.
Results
ATRA upregulates LZTFL1 in human CD4+ T cells
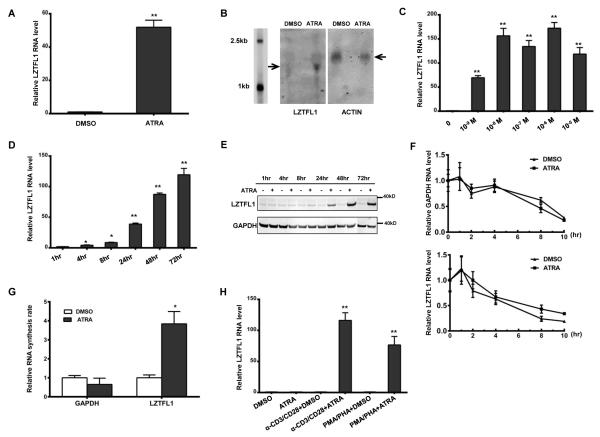
RAs have been shown to influence the function of T cells, although their effects on T cells are not fully understood. To assess the effect of ATRA on gene expressions in CD4+ T cells, we treated anti-CD3/CD28 Ab-primed CD4+ T cells from healthy donors with or without ATRA, and we isolated RNAs from the CD4+ T cells and used them for gene array analysis (30). LZTFL1 is one of the genes that are most upregulated by ATRA treatment (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74280). To confirm this result, LZTFL1 mRNA levels, in response to ATRA treatment, were assessed using quantitative real-time PCR, following reverse transcription. Consistent with the gene array result, LZTFL1 mRNA levels were dramatically upregulated by ATRA treatment in cells from all donors tested (Fig. 1A). Northern blotting also indicated the elevation of the LZTFL1 transcript upon ATRA treatment (Fig. 1B). The induction of LZTFL1 expression was both time- and dose-dependent. ATRA upregulated LZTFL1 RNA expression at concentrations as low as 1 nM, and at physiological concentrations of 10 nM, it could induce LZTFL1 RNA expression over 100 folds (Fig. 1C). As early as 4 h after ATRA treatment at concentration of 1μM, LZTFL1 mRNA expression increased by almost three folds, and by 72 h of treatment, the stimulation of LZTFL1 expression reached the maximum level (Fig. 1D). The effect of ATRA on LZTFL1 protein expression was also analyzed by Western blotting. As shown in Fig. 1E, the basal expression of LZTFL1 is barely detectable in primary human CD4+ T cells. In response to stimulation with ATRA, LZTFL1 level significantly increased after 8 h of treatment and reached maximum by 48h, which is parallel to the finding of induced LZTFL1 mRNA production. Alternative mechanisms could contribute to elevated LZTFL1 mRNA levels during ATRA treatment, including transcription initiation rate and RNA degradation rate. To evaluate whether the ATRA-induced increase in LZTFL1 mRNA levels resulted from increased mRNA stability, we incubated the DMSO- or ATRA-treated CD4+ T cells with actinomycin D (5 μg/ml) for 10 h. Treatment with actinomycin D inhibits RNA synthesis by blocking the function of the transcriptional machinery. As shown in Fig. 1F, ATRA treatment did not change the LZTFL1 mRNA turnover rate significantly. Next, we analyzed the effect of ATRA on LZTFL1 transcription initiation using the nuclear run-on assay. Newly synthesized RNAs were labeled with biotin, captured by magnetic beads, and analyzed by RT-PCR as described in the Materials and Methods section. This semiquantitative technique revealed that newly synthesized LZTFL1 mRNA was markedly increased in ATRA-treated CD4+ T cells (Fig. 1G). These data clearly indicate that ATRA treatment increased LZTFL1 transcription.
FIGURE 1.
ATRA induces LZTFL1 expression in human primary CD4+ T cells. (A) Human CD4+ T cells isolated from healthy donors were primed with anti-CD3 and anti-CD28 Abs (α-CD3/CD28) and treated with DMSO or 1 μM of ATRA for 3 d. RNAs were extracted from these cells, and LZTFL1 RNA was quantitated by reverse transcription, followed by real-time PCR. LZTFL1 RNA expression was normalized to GAPDH RNA expression, and the relative RNA fold changes compared to those from DMSO treatment were plotted (mean ± SD). (B) LZTFL1 and actin mRNAs (indicated by arrows) were detected by Northern blotting. (C–D) LZTFL1 RNA expression levels in response to different concentrations of ATRA for 72 h (C), and at 1μM ATRA for various lengths of time (D), were analyzed. (E) LZTFL1 and GAPDH proteins were detected by Western blotting. (F) CD4+ T cells treated with DMSO or ATRA for 24 h were incubated with or without actinomycin D (5 μg/ml) for 10 h and the levels of LZTFL1 and GAPDH RNAs were analyzed by real-time PCR. (G) Nuclear run-on assay was used to analyze the effect of ATRA on LZTFL1 transcription initiation. CD4+ T cells were treated with DMSO or ATRA for 24 h. Nuclei were isolated, and biotinylated transcripts were synthesized in vitro, captured, and analyzed by real-time PCR. (H) The effect of different T cell activators on LZTFL1 RNA expression was analyzed. Results are representative of three experiments with cells from three different donors. *, p < 0.05; **, p < 0.01.
LZTFL1 induction by ATRA is dependent on TCR signaling
ATRA has no effect on LZTFL1 expression in resting CD4+ T cells. Upon T cell activation with anti-CD3 and CD28 Abs, the expression of LZTFL1 increased around 100-fold in response to ATRA treatment. PMA and PHA treatment, together with ATRA, also dramatically increased LZTFL1 expression (Fig. 1H), whereas, without ATRA, T cell activation alone had no effect. These results indicate that both ATRA and TCR signaling are required for LZTFL1 expression, and TCR signaling is essential for ATRA’s effect on LZTFL1 upregulation. Together, with the result that ATRA upregulates LZTFL1 expression at physiological concentrations (Fig. 1D), our data suggest a possible function of LZTFL1 in ATRA-induced T cell response.
LZTFL1 localizes to the T cell membrane in an F-actin–dependent manner
To investigate the function of LZTFL1, we first analyzed its localization in CD4+ T cells. Cells expressing Flag-tagged LZTFL1 were fixed for immunological staining. We detected the enrichment of LZTFL1 in the CD4+ T cell membrane compartment, with some distribution in the cytoplasm (Fig. 2A). To further confirm the membrane localization of LZTFL1, cholera toxin B was used to stain the lipid raft. The staining for LZTFL1 and the lipid raft showed some overlap between the two, even though most of the LZTFL1 were located outside the lipid raft. We also stained endogenous LZTFL1 in Jurkat T cells using anti-LZTFL1 Ab, and the results confirmed membrane localization of LZTFL1 (Fig. 2B). In a previous publication, it was suggested that LZTFL1 binds to actin in vitro (23). To verify the effect of actin on LZTFL1 membrane distribution, Jurkat cells stably expressing Halo-tagged LZTFL1 were treated with latrunculin B to disturb the polymerization of actin. Cells were fixed and stained for LZTFL1 and F-actin. As expected, latrunculin B–treated cells had very low levels of F-actin, causing LZTFL1 to lose most of its membrane localization and relocate to the cytoplasmic compartment (Fig. 2C). This result indicates that LZTFL1 depends on F-actin for membrane localization.
FIGURE 2.
LZTFL1 localizes on the cell membrane in an F-actin-dependent manner. (A) Human CD4+ T cells isolated from healthy donors were transiently transfected with LZTFL1-Flag expression DNA. Cells were fixed and stained with anti-Flag Ab for LZTFL1-Flag (green). Cholera toxin B and DAPI were used to stain the cytoplasmic membrane marker GM1 ganglioside (red) and the nucleus (blue), respectively, and the staining was analyzed by confocal microscopy. (B) Fluorescent images of Jurkat T cells stained with anti-LZTFL1 Ab (green) and DAPI (blue). (C) Fluorescent images of Halo-LZTFL1-expressing Jurkat T cells treated with or without latrunculin B (1 μM) for 30 min. Cells were stained with phalloidin for F-actin (green), anti-halo Ab for LZTFL1 (red), and DAPI for the nucleus (blue).
LZTFL1 is transiently recruited to the T cell–APC contact site during T cell activation
Next, we performed immunoprecipitation, followed by mass spectrometry, to identify LZTFL1-interacting proteins. HEK-293 cells were used. Our results showed that LZTFL1 interacts with BBS proteins BBS-2 and BBS-7. Similarly, in recent publications, LZTFL1 is shown to be involved in BBS (24, 25). A subset of LZTFL1 interacts with the BBS-9 through its C-terminal half. Even though there is no enrichment of LZTFL1 in cilia or basal bodies, it influenced BBSome trafficking in primary cilium and hedgehog signaling (25). Primary cilium is a specialized cell-surface projection in almost all vertebrate cells. It plays important roles in sight, smell, mechanosensation, and intercellular signaling (31). Lymphocytes are among the very few types of cells that do not form cilium. Instead, during T cell activation, lymphocytes and APCs form a highly organized interface in their interaction area: IS (32, 33). The IS is considered to be a homolog of primary cilium based on the common features between IS and primary cilium (34, 35). The IS plays an important role in lymphocyte activation and allows for the polarized delivery of cytokines or lytic granules to target cells. To investigate whether LZTFL1 has a role in IS formation or T cell activation, superantigen-pulsed Raji B cells were used. Raji B cells were labeled with cell tracker violet (BMQC) to distinguish them from T cells. Raji B cells pulsed with superantigen SEE were incubated with Jurkat T cells for the indicated time to allow for the formation of conjugates (Fig. 3A). The distribution of LZTFL1 was detected by immunostaining, followed by confocal microscope analysis. TCRβ was used as an indicator for the formation of the IS. During T cell activation, the TCR cluster at the center of the T cell/antigen-presenting cell interface formed a key component of the IS (36). After 15 min of incubation with SEE-pulsed Raji cells, about 40% of Jurkat T cells formed conjugates with Raji B cells (Fig. 3B). In the absence of the superantigen, LZTFL1 was homogeneously distributed in the membrane of Jurkat cells (Fig. 3A). Following superantigen stimulation, a time-dependent relocalization of LZTFL1 was observed. At the early stages of conjugate formation, LZTFL1 was relocated to the Jurkat/Raji cell contact zone in 90% of the conjugates. With the maturation of the IS, as indicated by TCRβ aggregation to the center of the contact zone, the percentage of cells containing LZTFL1 in the contact zone decreased. After 15 min of incubation, LZTFL1 was excluded from the center of the contact zone to the distal pole of Jurkat T cells in 60% of the conjugates (Fig. 3A, 3C).
FIGURE 3.
LZTFL1 transiently redistributes to the contact zone between T cells and APCs. (A) Jurkat T cells (denoted by T) were copelleted with BMQC-stained, SEE-loaded Raji B cells (blue; denoted by B) and incubated at 37°C for 1.5-, 5-, and 15-min increments to induce IS formation. Cell conjugates were fixed and co-stained with Abs for LZTFL1 (green) and TCRβ (red). SEE-, no SEE-loading control, incubated for 15 min. Arrows indicate the T cell and B cell contact zone. (B) The percentage of conjugated T cells was calculated for all T cells in 10 fields chosen at random at indicated time points (mean ± SD). Results are representative of three independent experiments. *, p-value < 0.05. (C) LZTFL1 accumulation at the IS and the distal pole (DP) was quantified, and the percentage in total conjugates was plotted for both (mean ± SD). Results are representative of three independent experiments. *, p-value < 0.05 (comparison to LZTFL1 located at the IS after 1.5 min of incubation); (D and E) Live cell imaging of LZTFL1 localization at the IS. Jurkat T cells (red; denoted by T) stably expressing Halo-STOP (D) or Halo-LZTFL1 (E) interacting with SEE superantigen–loaded Raji B cells (blue; denoted by B) and forming the IS at indicated times on live cell imaging, as analyzed by wide field microscope (Supplemental video1). Data are representative of two independent experiments. (Scale bar, 10 μm.)
Similar to fixed cells, live cell imaging of Jurkat T cells stably expressing a halo-tagged LZTFL1 also showed the movement of LZTFL1 towards the site of the IS as a result of SEE-pulsed Raji B cells (Fig. 3D, 3E). Jurkat T cells stably expressing Halo-LZTFL1 were imaged during migration and interaction with SEE superantigen–loaded Raji B cells. Before contact with the APC, Halo-LZTFL1 in the motile T cells was evenly distributed in the cytoplasm and cell membrane. After contact with the APC, Halo-LZTFL1 first moves toward, and concentrates at, the IS, and then it moves to the distal pole; the localization of Halo-LZSTOP does not change (Supplemental video1.mov). These data demonstrate that LZTFL1 undergoes dynamic trafficking during IS formation, raising the possibility that LZTFL1 plays a role in T cell activation.
RNA interference of LZTFL1 inhibits ATRA’s function on Th2 cytokine production
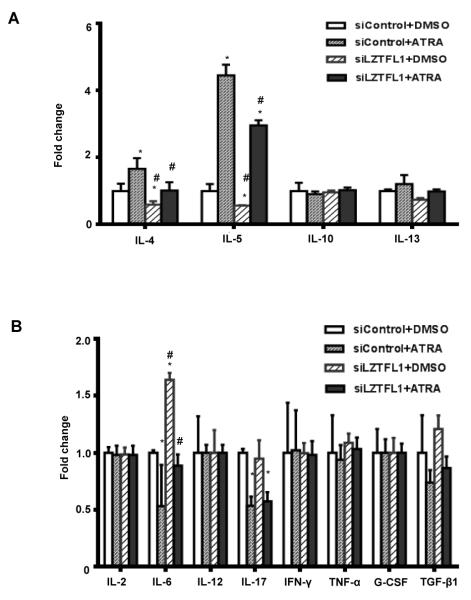
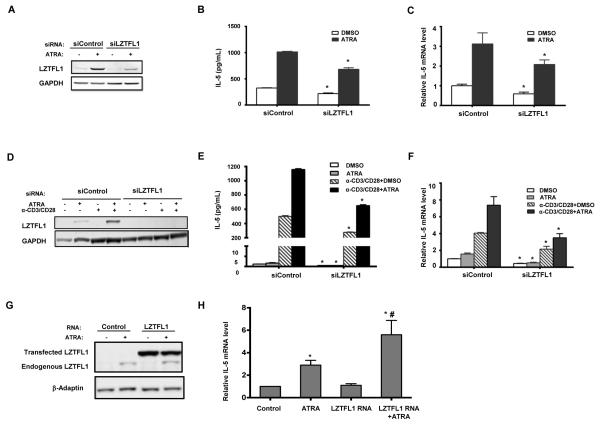
RAs are known to play a role in Th2 T cell differentiation and Th2 cytokine production (9-11). Because ATRA induced a dramatic increase in LZTFL1 production in primed human CD4+ T cells, we reasoned that the expression of LZTFL1 could have a role in Th2 cytokine production. To test this hypothesis, we screened for cytokine expression in primed and ATRA treated CD4+ T cells from three healthy donors. As shown in Fig. 4, ATRA specifically stimulated the production of Th2 cytokines, including IL-4 and IL-5 in all three donors, with minor stimulation of IL-13 production. IL-10 was found induced in one of the donors. The production of IL-5 was affected the most by ATRA. Negative effects of ATRA on IL-6 and IL-17 were also detected in all the donors. ATRA has little effect on other cytokines tested. Knockdown of LZTFL1 with RNA interference significantly suppressed IL-4 and IL-5 production. IL-13 production was also inhibited to a lesser degree. Contrary to the Th2 cytokines, there was a small increase in the production of IL-6. No effect was detected for other cytokines tested. Next, we investigated the involvement of LZTFL1 and ATRA in the regulation of Th2 cytokine production. Due to a stronger IL-5 production response to ATRA treatment, IL-5 was used as an indicator of ATRA’s effect. Human CD4+ T cells transfected with LZTFL1 siRNA were primed with bead-bound anti-CD3/CD28 Ab and incubated in the medium with 1 μM of ATRA. The knockdown of LZTFL1 was first confirmed by Western blotting (Fig. 5A). Knockdown of LZTFL1 significantly inhibited the upregulation of IL-5 production by ATRA (Fig. 5B). We also tested the effect of LZTFL1 in the D10.G4.1 cells of a mouse Th2 cell line. ATRA upregulated LZTFL1 protein expression in D10.G4.1 cells, just as it did in human CD4+ T cells from PBMCs (Fig. 5D). Activation of these cells with bead-bound anti-CD3/CD28 Ab further increased the level of LZTFL1. To investigate the effect of LZTFL1, D10.G4.1 cells were transfected with LZTFL1 siRNA, followed by activation with bead-bound anti-CD3/CD28 Ab and ATRA treatment. Western blot analysis shows that siRNA against LZTFL1 mRNA reduced the basal and ATRA-induced LZTFL1 levels (Fig. 5D). Results in Fig. 5E show that ATRA induced IL-5 production in both resting and activated D10.G4.1 cells. As expected, LZTFL1 knockdown significantly suppressed IL-5 production in both resting and activated D10.G4.1 cells. More interestingly, in activated D10.G4.1 cells transfected with LZTFL1 siRNA, compared to the DMSO control, ATRA failed to further induce IL-5 production. All these data indicate that LZTFL1 plays an important role in ATRA-induced IL-5 production.
FIGURE 4.
Involvement of LZTFL1 in ATRA-induced Th2 cytokine expression. Human CD4+ T cells isolated from healthy donors were transfected with control siRNA (siControl) or siRNA targeting human LZTFL1 (siLZTFL1). Cells were then activated with anti-CD3 and anti-CD28 Ab-bound beads (α-CD3/CD28) and treated with DMSO or 1 μM of ATRA for 3 d. Th2 (A), Th1, and Th17 (B) cytokine expressions in the culture supernatants were quantitated by ELISA. n = 3. *, p-value < 0.05 (comparison to cells transfected with siControl and treated with DMSO); #, p-value < 0.05 (comparison to cells transfected with siControl and treated with ATRA).
FIGURE 5.
LZTFL1 enhances ATRA induced IL-5 protein and RNA levels. (A) Human CD4+ T cells isolated from healthy donors were transfected with control siRNA (siControl) or siRNA targeting human LZTFL1 (siLZTFL1). Cells were then activated with anti-CD3/CD28 and treated with DMSO or 1 μM of ATRA for 1 d. LZTFL1 expression was detected by Western blotting. (B) The level of the IL-5 protein in the culture supernatants was quantitated by ELISA. (C) IL-5 RNA expression was detected by reverse transcription, followed by real-time PCR, as described in the Materials and Methods. IL-5 RNA expression was normalized to GAPDH RNA expression, and relative RNA fold changes compared to those from DMSO treatment were plotted (mean ± SD). (D) D10.G4.1 cells, a mouse Th2 cell line, were transfected with siControl or siLZTFL1 targeting mouse LZTFL1. Cells were activated and treated with 1 μM of ATRA for 1 d. LZTFL1 expression was detected by Western blotting. (E) Expression of the IL-5 protein was quantitated by ELISA. (F) IL-5 RNA expression was analyzed. (G-H) CD3/CD28 activation beads expanded human CD4+ T cells were transfected with LZTFL1 mRNA and 72 h later cells were used to prepare protein lysates for western blot analysis (G) and RNA for real-time PCR measurement of GAPDH and IL-5 RNA (H). Results are representatives of five independent experiments for A to F and three for G and H. *, p-value <0.05 (comparison with each corresponding cells transfected with siControl or control RNA); #, p-value <0.05 (comparison with control and cells transfected with LZTFL1 mRNA and treated with ATRA).
LZTFL1 stimulates IL-5 RNA expression
Th2 cytokines were regulated at the promoter level by Th2 cell differentiation transcriptional factors during T cell activation. IL-4, IL-5, and IL-13 are coordinately expressed in Th2 cells (37). The genes of these cytokines are clustered on chromosomal locus 5q31 in humans and chromosome 11 in mice. The genes encoding IL-4 and IL-13 are linked and transcribed in the same direction, whereas the gene encoding IL-5 is separated by the rad50 gene and is transcribed in the opposite direction (37). Next, we asked whether LZTFL1 regulates Th2 cytokine gene expression. To answer this question, CD4+ T cells from healthy donors were transfected with LZTFL1 siRNA, followed by T cell activation with bead-bound anti-CD3/CD28 Ab priming. Changes in IL-5 mRNA levels in response to LZTFL1 knockdown were assessed by quantitative real-time PCR. As shown in Fig. 5C, in line with the upregulation of IL-5 protein levels by ATRA, IL-5 mRNA expression increased about three-fold in response to ATRA treatment. LZTFL1 knockdown decreased IL-5 mRNA levels by 40% and ATRA-induced IL-5 mRNA expression by 30%. Similar results were also observed in D10.G4.1 cells (Fig. 5F). IL-5 mRNA levels increased about three-fold following T cell activation, and ATRA treatment further increased IL-5 RNA levels. LZTFL1 knockdown decreased IL-5 RNA levels by more than 50% in both resting and activated D10.G4.1 cells. Also, in cells transfected with LZTFL1 siRNA, compared to the DMSO control, ATRA treatment did not increase the IL-5 RNA as much as it did in control siRNA–treated cells. Furthermore, when LZTFL1 was ectopically expressed in expanded CD4+ T cells, it significantly enhanced ATRA induced IL-5 mRNA expression, even though over expressed LZTFL1 by itself has little effect (Fig. 5 G and H). These results confirm a role for LZTFL1 in ATRA mediated IL-5 expression.
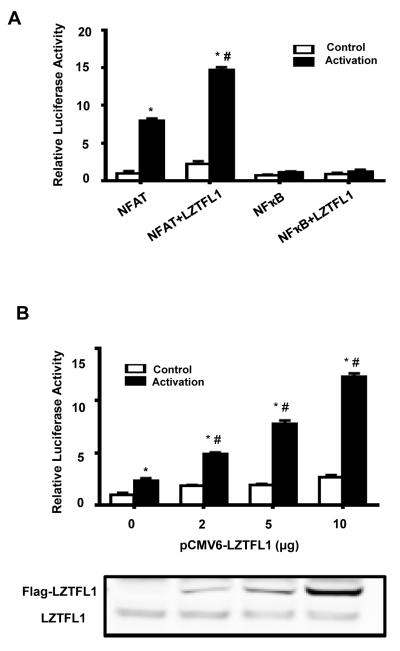
LZTFL1 enhances the TCR signal to NFAT
TCR engagement activates a series of proximal signaling molecule cascades, leading to transcriptional activation of cytokine genes. There are three major signaling pathways downstream of TCR signaling that are responsible for TCR-mediated gene expression: the calcium-NFAT pathway, NF-κB pathway, and Ras-MAPK-AP-1 pathway (38). These pathways are important for TCR activation–driven cytokine gene transcription. NFATc1, a downstream signal of the TCR-signaling pathway, plays an essential role in regulating Th2 cytokine production and Th2 cell differentiation, and NFATc1−/− mice showed impaired Th2 responses (39). NFAT regulates Th2 cytokine expression by binding to the NFAT response elements on Th2 cytokine gene promoter. To investigate the role of LZTFL1 on these signaling pathways, Jurkat T cells were transfected with reporter vectors containing the responsive elements to NFAT, NF-κB, and AP-1 upstream of the firefly luciferase gene, along with a plasmid expressing LZTFL1. Cells were activated by incubation with bead-bound anti-CD3/CD28 Abs, and luciferase activity was estimated to quantitate the promoter activity. As shown in Fig. 6A, TCR engagement in Jurkat cells induced both NFAT and NF-κB reporter activities, with a preference to NFAT. LZTFL1 further enhanced NFAT reporter activity by about two-fold and did not have any effect on NF-κB activity. LZTFL1 did not show any effect on AP-1 reporter activity (data not shown). The regulation of LZTFL1 on NFAT reporter activity is dose dependent, indicating that this effect is specific (Fig. 6B). Taken together, all these data suggest that LZTFL1 is an important regulator of ATRA induction on Th2 cytokine production. Increased TCR-NFAT signaling may contribute to this effect.
FIGURE 6.
LZTFL1 enhances T cell activation by specifically upregulating NFAT activity. (A) Jurkat cells were cotransfected with DNAs encoding LZTFL1-Flag and NFAT-Luc or LZTFL1-Flag and NFkB-Luc for 2 d. Renilla luciferase, under the control of the thymidine kinase promoter, was used as a transfection control. Cells were activated with anti-CD3 and anti-CD28 Abs-bound beads for 10 h. The firefly luciferase to Renilla luciferase ratio was calculated, and levels relative to those of the pCMV6 empty vector–transfected and unactivated cells were plotted (mean ± SD). n = 5. *, p value < 0.05 (comparison to cells transfected with pCMV6 empty vector and unactivated); #, p < 0.05 (comparison to cells transfected with pCMV6 empty vector and activated). (B) Jurkat cells cotransfected with reporter NFAT-Luc and an increasing amount of LZTFL1 expression DNA. Cells were activated, and the relative firefly luciferase to Renilla luciferase ratio was plotted as in (A) (mean ± SD). n = 5. *, p value < 0.05 (comparison to cells transfected with pCMV6 empty vector and unactivated); #, p value < 0.05 (comparison to cells transfected with pCMV6 empty vector and activated). The expression of endogenous and transfected LZTFL1 was analyzed by Western blotting.
Discussion
RAs favor Th2 cell differentiation and could directly induce the production of Th2 cytokines IL-4, IL-5, and IL-13 in Ab-primed human PBMCs (11). In this study, we identified LZTFL1 as an essential regulator of ATRA-induced Th2 cytokine expression, especially IL-5 expression in CD4+ T cells. LZTFL1 was markedly and rapidly upregulated at physiological concentrations of ATRA in primed CD4+ T cells (Fig. 1D). LZTFL1 knockdown using siRNA particularly reduced IL-5 production in primed human CD4+ T cells from PBMCs and in a mouse Th2 cell line, and it significantly suppressed ATRA-induced IL-5 production in these cells (Fig. 5). Given the fact that LZTFL1 is upregulated by ATRA in activated T cells, we reason that LZTFL1 is a positive regulator of ATRA-induced T cell response.
How does LZTFL1 regulate IL-5 expression? LZTFL1 knockdown using siRNA suppressed ATRA-induced IL-5 expression at both protein and RNA levels (Figs. 4, 5). So far, no RAR or retinoid X receptor response element has been located in the 5′ promoter region of the Th2 cytokine genes. Unlike Th1 cells, Th2 polarization requires prolonged TCR stimulation (40). Th2 cytokine genes are regulated in two steps (41-44). In the first step, naïve T cells were differentiated to mature effector Th2 cells triggered by antigen presenting, IL-4 and STAT6. In this stage, DNase I hypersensitive regions are detected in the IL-4/IL-5/IL-13 gene cluster. DNA methylation status is also changed. The second step is the stage that Th2 cytokine gene expression is induced. Many transcription factors are involved in this stage, including NFAT, AP-1, Est-1, Maf, and GATA3. Among them, Maf and GATA3 are Th2 specific (45, 46). NFAT, a downstream signal of the TCR-signaling pathway, though not specific for Th2 cells, plays an essential role in the regulation of Th2 cytokine production and Th2 cell differentiation (39). Mice with either NFATc1 or NFATc2 knockout showed impaired Th2 responses. NFAT binds the IL-4 and IL-5 promoters only in stimulated Th2 cells not Th1 cells (47, 48). NFATc1 is also required to recruit GATA-3 to IL-5 promoter in cAMP mediated IL-5 expression in activated Th2 cells (48). RAs affect Th2 differentiation through regulating the expression of Th2 specific transcription factors, including Ets-1, cMAF, GATA-3, and STAT-6 (11, 49, 50). RA also participates in T cell activation, supported by upregulation of the expression of T cell activation markers CD38 and CD69 (10). Here, we show a link between LZTFL1 and TCR-NFAT signaling. LZTFL1 accumulates in the plasma membrane of human CD4+ T cells. During T cell activation, it transiently redistributed to the T cell and APC contact zone at the beginning of IS formation, and excluded to the distal pole of T cells when the IS matured, and overexpression of LZTFL1 in CD4+ T cells enhanced TCR-NFAT signaling (Fig. 6). These data indicate that LZTFL1 plays a role in immune response. LZTFL1 was induced by T cell activation and its protein expression could be detected after 8 h. It is possible LZTFL1 could be induced during the prolonged period of APC-T cell interaction (51), which in turn could enhance TCR signaling and facilitate ATRA induced IL-5 production.
During T cell activation, a rearrangement of membrane and cytosolic molecules occurs at the T cell and APC contact zone, resulting in the formation of a highly organized interface known as the IS. The IS provides a platform for signal molecule assembly and accumulation at the interface, and promotes polarized exocytosis (32, 33). Similar to the IS, primary cilium is also a highly organized structure. It is characterized as the site for directional movement of structural and regulatory molecules (31). Both the IS and primary cilium show reorientation of the microtubule-organizing center. Most of the vertebrate cells have primary cilium. Lymphocytes are among a very few types of cells which do not form cilium. Based on the molecular similarity, the IS is considered to be a homolog to primary cilium (33-35). Besides the reorientation of the microtubule-organizing center in both processes, some proteins functioning in the primary cilium are also found to play a role in the IS. GTPase rat brain 11 (Rab11), an essential protein for primary ciliogenesis (52), is involved in Lck trafficking to the IS (53). SNARE proteins regulate cilia exocytosis as well as TCR recycling to the IS (54, 55). Intraflagellar transport 20 (IFT20), an IFT component essential for ciliary assembly, was also found to be expressed in lymphocytes (56) and was required for polarized recycling of TCR to the IS. LZTFL1 was recently identified as a member of the BBsome (24). It affects BBsome ciliary traffic and hedgehog signaling (25). In the present study, we have shown that LZTFL1 is upregulated by ATRA in activated T cells. It transiently located to the IS and enhanced NFAT activity. LZTFL1 regulation by RA was also shown by Kang et al. (57).
The mechanism by which LZTFL1 relocates during IS formation and impacts NFAT activity remains to be elucidated. One possibility is that LZTFL1 associates with polymerized actin or F-actin. Actin is particularly important for T cell activation. Actin or myosin interference affects surface protein trafficking in the IS more in T cell side than in the B cell side (58-60). Upon TCR engagement, the actin cytoskeleton in T cells starts to reorganize. It polymerizes beneath the area of the T cell and APC cell contact zone. When TCR microclusters move to the center of the contact, and the central supramolecular activation cluster (cSMAC) forms, F-actin forms a ring structure around the cSMAC and stabilizes integrin-dependent adhesive interactions between T cells and APCs (61). F-actin also relocates to the distal pole, the site that is opposite to the IS, and facilitates the formation of a distal pole complex (61, 62), although the exact picture of the dynamic change of F-actin localization is not fully established yet. The IS is a fine-tuned process; like F-actin, actin-binding proteins also traffic in T cells in a bipolar manner during IS formation. Actin-binding protein 1, HIP55, has been found to translocate to the IS and regulate TCR internalization (63), while ezrin-radixin-moesin proteins, from a protein family that binds to F-actin through the C-terminal domain, are excluded from the mature IS (64). Upon TCR engagement, moesin is depleted from the IS, while ezrin transit locates to the IS and then redistributes to the distal pole complex. Ezrin-radixin-moesin proteins mediate the exclusion of CD43 from the IS, which is believed to be important for T cell activation (64-66). Wei et al. have reported that LZTFL1 binds to actin in vitro (23), and since both actin and LZTFL1 show similar relocation patterns during IS formation, it is possible that LZTFL1 relocates along the actin cytoskeleton. Blocking the polymerization of F-actin with latrunculin B abolished LZTFL1 membrane localization, providing additional support for this hypothesis (Fig. 2C). However, we cannot rule out other possibilities. Alternatively, LZTFL1 may be required to sequester a negative regulator from the cSMAC. In primary cilia, LZTFL1 was found to interact with BBS-9 and to sequester it from its ciliary localization, which negatively regulates BBSome ciliary trafficking and hedgehog signaling (25). The plasma membrane and cytoplasmic localization of LZTFL1 in T cells makes it a good candidate as an adaptor. Precisely how LZTFL1 regulates TCR-NFAT signaling is under further investigation.
Supplementary Material
Acknowledgements
We thank Drs. Jiangsong Jiang, M. Ishaq, and Howard Young for helpful discussions.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract [HHSN261200800001E]. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported by the National Institute of Allergy and Infectious Diseases.
Abbreviations
- ATRA
all trans retinoic acid
- RA
retinoic acid
- LZTFL1
Leucine-zipper transcription factor-like 1
- IS
immunological synapse
- siRNA
small interfering RNA
- BBS
Bardet–Biedl syndrome proteins
- BBsome
complex of Bardet–Biedl syndrome proteins
- SEE
staphylococcal enterotoxin E
References
- 1.De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 2.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 3.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 8.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 9.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol. 2002;168:4495–4503. doi: 10.4049/jimmunol.168.9.4495. [DOI] [PubMed] [Google Scholar]
- 10.Dawson HD, Collins G, Pyle R, Key M, Taub DD. The Retinoic Acid Receptor-alpha mediates human T-cell activation and Th2 cytokine and chemokine production. BMC Immunol. 2008;9:16. doi: 10.1186/1471-2172-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pino-Lagos K, Guo Y, Brown C, Alexander MP, Elgueta R, Bennett KA, De Vries V, Nowak E, Blomhoff R, Sockanathan S, Chandraratna RA, Dmitrovsky E, Noelle RJ. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. The Journal of experimental medicine. 2011;208:1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spilianakis CG, Lee GR, Flavell RA. Twisting the Th1/Th2 immune response via the retinoid X receptor: lessons from a genetic approach. Eur J Immunol. 2005;35:3400–3404. doi: 10.1002/eji.200535588. [DOI] [PubMed] [Google Scholar]
- 14.Tilley SL, Jaradat M, Stapleton C, Dixon D, Hua X, Erikson CJ, McCaskill JG, Chason KD, Liao G, Jania L, Koller BH, Jetten AM. Retinoid-related orphan receptor gamma controls immunoglobulin production and Th1/Th2 cytokine balance in the adaptive immune response to allergen. J Immunol. 2007;178:3208–3218. doi: 10.4049/jimmunol.178.5.3208. [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- 16.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Seminars in immunology. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Carman JA, Pond L, Nashold F, Wassom DL, Hayes CE. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. The Journal of experimental medicine. 1992;175:111–120. doi: 10.1084/jem.175.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang BY, Chung SW, Kim SH, Kang SN, Choe YK, Kim TS. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine profile in CD4(+) T cells. British journal of pharmacology. 2000;130:581–586. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunological reviews. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nature reviews. Immunology. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 21.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiss H, Kedra D, Kiss C, Kost-Alimova M, Yang Y, Klein G, Imreh S, Dumanski JP. The LZTFL1 gene is a part of a transcriptional map covering 250 kb within the common eliminated region 1 (C3CER1) in 3p21.3. Genomics. 2001;73:10–19. doi: 10.1006/geno.2000.6498. [DOI] [PubMed] [Google Scholar]
- 23.Wei Q, Zhou W, Wang W, Gao B, Wang L, Cao J, Liu ZP. Tumor-suppressive functions of leucine zipper transcription factor-like 1. Cancer research. 2010;70:2942–2950. doi: 10.1158/0008-5472.CAN-09-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marion V, Stutzmann F, Gerard M, De Melo C, Schaefer E, Claussmann A, Helle S, Delague V, Souied E, Barrey C, Verloes A, Stoetzel C, Dollfus H. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet--Biedl syndrome with situs inversus and insertional polydactyly. Journal of medical genetics. 2012;49:317–321. doi: 10.1136/jmedgenet-2012-100737. [DOI] [PubMed] [Google Scholar]
- 25.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS genetics. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishaq M, DeGray G, Natarajan V. Protein kinase C0 modulates nuclear receptor-corepressor interaction during T cell activation. Journal of Biological Chemistry. 2003;278:39296–39302. doi: 10.1074/jbc.M302767200. [DOI] [PubMed] [Google Scholar]
- 27.Ishaq M, Lin BR, Bosche M, Zheng X, Yang J, Huang D, Lempicki RA, Natarajan V. LIM kinase 1 - dependent cofilin 1 pathway and actin dynamics mediate nuclear retinoid receptor function in T lymphocytes. BMC molecular biology. 2011;12:41. doi: 10.1186/1471-2199-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrone G, Puppo F, Cusano R, Scaranari M, Ceccherini I, Puliti A, Ravazzolo R. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. BioTechniques. 2000;29:1012–1014. 1016–1017. doi: 10.2144/00295st02. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Liu LN, Feller S, Allen C, Shivakumar R, Fratantoni J, Wolfraim LA, Fujisaki H, Campana D, Chopas N, Dzekunov S, Peshwa M. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer gene therapy. 2010;17:147–154. doi: 10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H, Badralmaa Y, Yang J, Lempicki R, Hazen A, Natarajan V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids in health and disease. 2012;11:69. doi: 10.1186/1476-511X-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 32.Cemerski S, Shaw A. Immune synapses in T-cell activation. Current opinion in immunology. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Current opinion in immunology. 2006;18:512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Finetti F, Paccani SR, Rosenbaum J, Baldari CT. Intraflagellar transport: a new player at the immune synapse. Trends in immunology. 2011;32:139–145. doi: 10.1016/j.it.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Current opinion in cell biology. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 37.Avni O, Rao A. T cell differentiation: a mechanistic view. Current opinion in immunology. 2000;12:654–659. doi: 10.1016/s0952-7915(00)00158-8. [DOI] [PubMed] [Google Scholar]
- 38.Huse M. The T-cell-receptor signaling network. Journal of cell science. 2009;122:1269–1273. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida H, Nishina H, Takimoto H, Marengere LE, Wakeham AC, Bouchard D, Kong YY, Ohteki T, Shahinian A, Bachmann M, Ohashi PS, Penninger JM, Crabtree GR, Mak TW. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 40.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 42.Rincon M, Flavell RA. T-cell subsets: transcriptional control in the Th1/Th2 decision. Current biology : CB. 1997;7:R729–732. doi: 10.1016/s0960-9822(06)00368-x. [DOI] [PubMed] [Google Scholar]
- 43.Zeng WP. 'All things considered': transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation. Immunology. 2013;140:31–38. doi: 10.1111/imm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amsen D, Spilianakis CG, Flavell RA. How are T(H)1 and T(H)2 effector cells made? Current opinion in immunology. 2009;21:153–160. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. The Journal of experimental medicine. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–652. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 48.Klein-Hessling S, Bopp T, Jha MK, Schmidt A, Miyatake S, Schmitt E, Serfling E. Cyclic AMP-induced chromatin changes support the NFATc-mediated recruitment of GATA-3 to the interleukin 5 promoter. J Biol Chem. 2008;283:31030–31037. doi: 10.1074/jbc.M805929200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raouf A, Li V, Kola I, Watson DK, Seth A. The Ets1 proto-oncogene is upregulated by retinoic acid: characterization of a functional retinoic acid response element in the Ets1 promoter. Oncogene. 2000;19:1969–1974. doi: 10.1038/sj.onc.1203505. [DOI] [PubMed] [Google Scholar]
- 50.Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH, Lanotte M, Waxman S, Chen SJ, Mao M, Hu GX, Zhu L, Chen Z. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood. 2000;96:1496–1504. [PubMed] [Google Scholar]
- 51.Liwski RS, Chase JC, Baldridge WH, Sadek I, Rowden G, West KA. Prolonged costimulation is required for naive T cell activation. Immunology letters. 2006;106:135–143. doi: 10.1016/j.imlet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorska MM, Liang Q, Karim Z, Alam R. Uncoordinated 119 protein controls trafficking of Lck via the Rab11 endosome and is critical for immunological synapse formation. J Immunol. 2009;183:1675–1684. doi: 10.4049/jimmunol.0900792. [DOI] [PubMed] [Google Scholar]
- 54.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annual review of cell and developmental biology. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–588. doi: 10.1016/s1074-7613(04)00106-2. [DOI] [PubMed] [Google Scholar]
- 56.Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature cell biology. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang SG, Park J, Cho JY, Ulrich B, Kim CH. Complementary roles of retinoic acid and TGF-beta1 in coordinated expression of mucosal integrins by T cells. Mucosal immunology. 2011;4:66–82. doi: 10.1038/mi.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delon J, Bercovici N, Liblau R, Trautmann A. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur J Immunol. 1998;28:716–729. doi: 10.1002/(SICI)1521-4141(199802)28:02<716::AID-IMMU716>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 59.Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6302–6307. doi: 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 61.Piragyte I, Jun CD. Actin engine in immunological synapse. Immune network. 2012;12:71–83. doi: 10.4110/in.2012.12.3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunological reviews. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 63.Han J, Shui JW, Zhang X, Zheng B, Han S, Tan TH. HIP-55 is important for T-cell proliferation, cytokine production, and immune responses. Molecular and cellular biology. 2005;25:6869–6878. doi: 10.1128/MCB.25.16.6869-6878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, Freedman BD, Burkhardt JK. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182:1021–1032. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 66.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. The Journal of cell biology. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.