Abstract
Immunosuppression in organ transplantation was revolutionary for its time, but technological and population changes cast new light on its use. First, metabolic syndrome (MS) is increasing as a public health issue, concomitantly increasing as an issue for post-orthotopic liver transplantation patients; yet the medications regularly used for immunosuppression contribute to dysfunctional metabolism. Current mainstay immunosuppression involves the use of calcineurin inhibitors; these are potent, but nonspecifically disrupt intracellular signaling in such a way as to exacerbate the impact of MS on the liver. Second, the impacts of acute cellular rejection and malignancy are reviewed in terms of their severity and possible interactions with immunosuppressive medications. Finally, immunosuppressive agents must be considered in terms of new developments in hepatitis C virus treatment, which undercut what used to be inevitable viral recurrence. Overall, while traditional immunosuppressive agents remain the most used, the specific side-effect profiles of all immunosuppressants must be weighed in light of the individual patient.
Keywords: Immunosuppression, Orthotopic liver transplantation, Metabolic syndrome, Acute cellular rejection, Hepatitis C virus
Core tip: The use of immunosuppressive agents is reviewed in the context of the modern post-orthotopic liver transplantation population. The side effects of mainstay immunosuppressive strategies exacerbate some patient pathologies, and combinations of different immunosuppressants could be more specifically tailored to patient needs. Acute cellular rejection and malignant complications are also discussed with respect to immunosuppressive strategies. Finally, hepatitis C virus and its impact on immunosuppression is re-evaluated in light of recent developments in viral clearance.
INTRODUCTION
The use of immunosuppression in organ transplantation was revolutionary for its time, and its results were quickly embraced for their efficacy at suppressing host rejection of a graft.
However, given the increasing impact of metabolic syndrome (MS) as a public health issue[1], immunosuppression and its side effects may pose a greater risk to patients than rejection of a newly-transplanted organ. In fact, metabolic complications of immunosuppressive therapy were at one point the leading cause of morbidity and mortality for patients following orthotopic liver transplantation (OLT)[2]. Reduction of immunosuppression is a widely-recognized strategy to addressing this issue[3].
Cardiovascular disease (CVD) and renal disease account for 19.3% and 6.8% of nonhepatic causes of death in post-OLT patients, respectively[4]. In patients who survive at least 3 years, non-hepatic cause of death accounts for 58% of all-cause mortality post-OLT[5]. In their evaluation of liver transplantation as a cardiovascular risk factor, Madhwal et al[6] (2012) found that up to two-thirds of patients develop MS after OLT. Clearly, the trend towards post-transplant metabolic disturbances must be addressed; the first place to start is optimizing post-transplant immunosuppressive therapies[7].
Further, with recent advances in the treatment of hepatitis C virus (HCV)[8,9], long-term metabolic complications posed by immunosuppression must be weighed more heavily against the immediate issue of organ rejection. Just as the introduction of immunosuppression made longer-term complications a new focus in transplantation, eradication of this chronic liver infection leaves room to focus on metabolic issues.
BRIEF OVERVIEW OF LIVER IMMUNOLOGY: THE LIVER IS IMMUNOPRIVILEGED
From a physiological standpoint, the liver is one of the first organs exposed to the absorbed contents of the external environment. Handling of newly-acquired blood content immediately after absorption from the external environment necessitates that the liver maintain its own unique balance of immunity vs tolerance. The special status of the liver as immunoprivileged is well-recognized; for example, there is a paucity of hepatic B- and T-cell mediated autoimmune disease, and some autoimmune hepatitis liver markers are found in healthy and ill people alike[10]. Further, transplant tolerance is known to occur at greater frequency for liver transplant recipients, compared to other vascularized organ recipients[11]. At the same time, there remains much to be learned about liver immunology. For example, the role of humoral alloreactivity in ABO-compatible liver transplantation is still being elucidated[12-14].
The liver is rich with parenchymal hepatocytes, but also contains non-parenchymal immune cells that serve as a first barrier to antigens arriving from portal circulation. Hepatic nonparenchymal cells include the largest population of fixed resident macrophages in the body, Kupffer cells, as well as other reticuloendothelial cells[15]. The parenchymal hepatocytes further contribute to immunity by secreting 80%-90% of complement components and pathogen-recognition receptors (PRR), as well as synthesizing membrane-bound PRRs to catch portal antigens[16].
Along with antigens arriving from portal circulation, about 108 peripheral blood lymphocytes pass through the liver every 24 h[17]. These cells are squeezed through fenestrated capillaries that may open a window to T-cell priming[18]. The status of the liver as a major reservoir of immune cells has major implications, then, both as far as catching portal circulation antigens as well as immunomodulation.
For example, the privileged status of the liver might be used in the future to induce complete graft tolerance. As a promising example, animal models have shown a lifetime tolerance to liver grafts: In 20% of outbred pig liver recipients, lifetime tolerance can be achieved without the use of immunosuppressants[19]. The possibility of lifetime tolerance in humans, too, remains optimistic. Ramos et al[20] (1995) review their experience withdrawing immunosuppressive therapy after witnessing patient noncompliance with immunosuppression. They were able to accomplish immunosuppression-free tolerance in 16 patients for 3 to 19 mo, continuing efforts to completely wean 28 patients, but failed in 15 patients without any graft loss or demonstrable loss of function due to rejection.
Not only is the liver self-protective, but there is evidence of immunocompetence conferred to other organs: Simpson et al[21] (2006) showed that patients who receive combined same-donor liver and kidney transplants are immunoprotected compared to patients who receive kidney transplant after liver transplant. The authors speculate that the identical genotypes of the transplanted organs facilitates immunoprotective effects. There is, however, conflicting data: Katznelson et al[22] (1996), comparing incidence of acute rejection between 248 combined liver and kidney transplantations to a control group of 206 kidney-alone transplantations, found that 3-year survival rates are not significantly different.
Despite a key role in immunoregulation generally, human leukocyte antigen (HLA) histocompatibility has little clinical significance to liver allograft outcome[23,24]. On the other hand, HLA compatibility may play a more subtle role in OLT than we see clinically, where immunosuppressive regimens may paint broad enough strokes to obfuscate nuances. Neumann et al[25] (2003) report that HLA compatibility is associated with significantly less acute rejection, but no difference in graft survival. This peculiar behavior of the liver compared to other organs may further reflect the possibility that acute rejection is not as harmful to OLT as previously thought. A better understanding of the mechanisms of liver immunology is necessary to identify how to maximize the utility of histocompatibility[26].
IMMUNOSUPPRESSANT AGENT OVERVIEW
There are three signal pathways targeted by immunosuppressive agents. The first is calcineurin-mediated nuclear factor of activated T-cells activation via the T-cell receptor (TCR) and CD3 meeting an antigen presented on an major histocompatibility complex (MHC) protein; the second is a B7/CD28 costimulatory signal required for TCR-MHC complex synapsing; and the third signal is mediated by interleukin 2 (IL-2) as a ligand to CD25, through adaptor proteins JAK3 and PI-3K to mechanistic target of rapamycin (mTOR) regulation of cyclin-dependent kinases and cyclins to control the cell cycle[27].
Figure 1 depicts the process of an antigen-presenting cell (APC) travelling to a lymphoid organ, where it meets T cells in the paracortex. A native T-cell interacts with the APC, and if a set of several interactions and conditions are met, then the native T-cell replicates many times. This is called T-cell activation, and T-cell clonal expansion, and creates numerous effector T-cells that are specific to the antigen originally presented by the APC. These effector T-cells proceed to leave the lymph nodes and head back to the liver, where they can detect antigen and effect an immune response.
Figure 1.
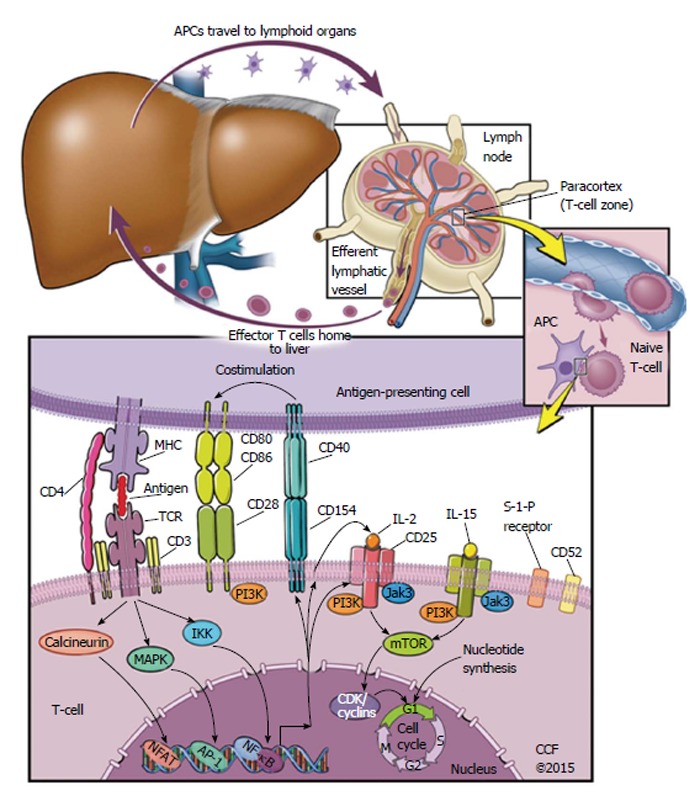
Signaling pathways targeted by modern immunosuppressive agents. Starting from the top left, an antigen-presenting cell (APC) migrates from local tissue to lymphoid organs. In the paracortex of the lymphoid organ, the APC meets a naive T-cell. If the naive T-cell has a T-cell receptor (TCR) that binds the antigen as it is presented by a Major Histocompatibility Complex on the APC, a set of other T-cell-APC interactions are likely to follow. This includes T-cell CD4 binding to the MHC on an APC, as well as costimulatory signals via extracellular receptors CD28 or CD40. After this set of T-cell-APC interactions begins, a set of intracellular signals follow towards the nucleus of the T-cell. The naive T-cell is then activated, and begins to replicate. This replication is called clonal expansion, and produces a population of T-cells that eventually migrate back to the tissue that contains antigen. AP-1: Activator protein 1; CD: Cluster of differentiation; CDK: Cyclin-dependent kinase; IKK: Inhibitor of kappa-B kinase; IL: Interleukin; Jak3: Janus-associated kinase 3; PI3K: Phosphatidyl inositide 3-kinase; MAPK: Mitogen-activated protein kinase; mTOR: Mechanistic target of rapamycin; NFAT: Nuclear factor of activated T-cells; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; TCR: T-cell receptor; MHC: Major histocompatibility complex.
Many current immunosuppressive drugs target either those extracellular interactions or the intracellular signals that are highlighted in Figure 1. Calcineurin inhibitors (CNIs) prevent T-cell activation via an intracellular pathway, for example; on the other hand, the anti-IL-2 receptor antibodies basiliximab and daclizumab prevent IL-2 receptor activation[28].
Immunosuppressant drugs can function as depleting agents, or as non-depleting agents. Depleting immunosuppressive therapies cause destruction of T cells and/or B cells[27], whereas non-depleting agents affect the immune system by preventing immune cell proliferation.
CNIs have been the mainstay of immunosuppression since they were discovered, and increased 1-year patient and graft survival to greater than 80%[29,30]. CNIs serve to prevent transcription of the autocrine factor IL-2, preventing cell proliferation. These drugs halt the phosphatase activity of calcineurin, which is an intracellular signal transduction protein that mediates response to antigenic peptides. CNIs include cyclosporin A (CsA) and tacrolimus (TAC). There is no direct reduction in T or B cell count, so these agents are considered non-depleting.
Second, there is the non-depleting immunosuppressant, mycophenolate mofetil (MMF). MMF is a prodrug of mycophenolic acid (MPA), which inhibits inosine monophosphate dehydrogenase (IMPDH). Because IMPDH catalyzes the rate-limiting step of de novo guanosine synthesis, both genetic replication and transcription are inhibited. Further, MPA is five times more effective at inhibiting the type II isoform of IMDPH - the isoform expressed in activated lymphocytes[31]. Because it is specific to the lymphocyte isoform of IMPDH, MPA prevents lymphocyte proliferation and transcription of activation-associated genes. As far as small molecules go, MMF is one of the more specific immunosuppressant agents. Side effects of MMF include nonimmune issues such as diarrhea and anemia[27], but, more importantly, exacerbation of cytomegalovirus infection[32].
Finally, the third major class of immunosuppressive drugs are called mTOR inhibitors. Found in soil from an Easter Island bacteria called Streptomyces Hygroscopicus, this class blocks IL-2-mediated autocrine leukocyte proliferation via inhibition of an intracellular signal transduction mechanism - thus, mTOR inhibitors are considered non-depleting agents[33,34]. Everolimus (EVR) and sirolimus (SIR) are the two best-known mTOR inhibitors.
Besides their use as immunosuppressants, another interesting property of mTOR inhibitors is their effect on longevity and age-related diseases. It is well-established that one way to increase longevity is through dietary restriction, and this effect is partially mediated by the mTOR pathway. Inhibition of mTORC1 has been associated with protection against neurodegenerative disease, heart disease, metabolic diseases, and a host of other age-related diseases[35]. Rapamycin administration, specifically, has repeatedly been shown to increase both mean and median lifespan in genetically heterogenous mice[36].
Immunosuppressive steroids such as methylprednisone are considered essential to graft tolerance induction, yet their use is highly associated with metabolic complications[37]. Several studies have examined outcomes of steroid-free or reduced-steroid immunosuppressive maintenance regimens[38,39]. In a prospective, randomized, placebo-controlled, double-blinded study, Lerut et al[40] (2014) report that in a cohort of 156 patients, patients treated with minimal or steroid-free immunosuppression displayed excellent outcomes over a period of 5 years. They report 5-year biopsy results, finding that histology presents similarly across both groups. In support of steroid withdrawal, other prospective studies of prednisone withdrawal post-OLT have demonstrated no difference in 2-year and 1-year survival of patients treated with or without prednisone[41,42]. Today, the use of steroids is generally limited to tolerance induction and treatment of acute cellular rejection (ACR); many studies have further investigated the possibility of steroid-free tranplantation[43-45].
Depleting immunosuppressant agents are mostly antibody-based. The first monoclonal antibody to be approved for use in humans was OKT3, an anti-CD3 antibody that modulates T-cell activation[46,47]. There are currently a host of biologics directed against T-cell proliferation, these are specific enough that their metabolic impact is significantly less than that of CNIs[48,49].
Issues associated with biological immunosuppression are unlike those of smaller molecules because they act extracellularly and are specific to their antigen[50], in contrast to small molecules such as tacrolimus that affect intracellular processes across a wider variety of cells. For example, one OKT3 side effect is called cytokine release syndrome, where widespread T-cell activation outweighs the antibody-mediated T-cell destruction that follows it[51]. This complication can become more severe if it leads to a positive-feedback loop, potentially causing a “cytokine storm”[52].
Besides OKT3, biologic agents have shown varying degrees of success and outcomes. For example, basiliximab is an IL-2 receptor antagonist that reduces rates of ACR at the expense of increased disease progression in HCV liver transplant patients[53]. Basiliximab, though, has been found to successfully treat graft-vs-host disease[54]. For those patients with metabolic dysfunction, avoidance of steroid immunosuppression may be enough of a concern to warrant use of basiliximab.
Another biologic used to treat multiple sclerosis and Crohn’s disease, natalizumab, is associated with significant liver injury as a side effect[55]. While the medical field is ripe for biologic development, implementation of biologics requires close evaluation.
On the other hand, some biologics open doors that might otherwise stay shut. Rituximab is an anti-CD20 monoclonal antibody that has been successfully used as immunological prophylaxis for ABO-incompatible (ABOi) liver transplantation[56]. Further, Yoshizawa et al[57] (2005) report that ABOi living-donor liver transplantation is possible without humoral rejection. Their protocol involves hepatic artery infusion and prophylactic use of rituximab, but, unlike previous attempts, does not involve splenectomy. Tanabe et al[58] (2010) later explain that ABOi has progressed to the point that it is as effective as ABO compatible transplantation, in part due to rituximab prophylaxis. Perfection of ABOi OLT will hopefully lead to other organ allocation advancements, such as humanized livers grown in non-human primates[59]. Immunosuppression in this context may become a hot topic in coming years.
ACR: DIAGNOSIS AND TREATMENT
ACR is most likely to occur within the first 6 wk of transplant, and is a very common event; in a study of 762 patients, the incidence of ACR post-transplantation is 64%. Several factors are associated with the occurrence of ACR, such as cold ischemic time of the organ, lower age of recipient, presence of edema, and HLA-DR mismatch[2].
There are three distinguishing features of ACR, each visible on histological examination. The first feature is portal triad inflammation, indicated by mixed inflammatory infiltrate; the second feature is damage to the bile ducts, specifically nonsuppurative cholangitis involving interlobular ductal epithelia; third, venous endotheliitis[60]. Venous endotheliitis is the most reliable diagnostic sign of ACR, but it is worth noting that phasic increase and decrease in lymphocyte aggregation may affect biopsy results[2].
In the clinic, ACR is suspected upon elevated liver function tests preceding jaundice and fever. Unfortunately, blood tests are neither sensitive nor specific for ACR diagnosis[61,62]. It follows, then, that liver biopsy is the gold standard of liver tissue evaluation[63]. To provide a level of standardization to biopsy evaluation, the Banff rejection activity index (RAI) assigns a score between zero and three to each characteristic of ACR[64].
Treatment for ACR includes high-dose steroids, optionally tapering off steroids and/or using biological immunosuppression[65]. Response to steroids is favorable; however, incidence of steroid-resistant rejection has been found to reach up to 14%[66].
ACR MORBIDITY AND MORTALITY
Timing of rejection is of major clinical significance in evaluating the potential impact of ACR[67,68]. While different studies have used different definitions, one commonly accepted cutoff defines early and late ACR as occurring within and after 90 d of transplantation, respectively. Several studies have found that early ACR is common and of lesser significance than late ACR[69]. For example, in a retrospective review of 231 histologically-confirmed cases of early ACR, Höroldt et al[64] (2006) report that neither total RAI score nor any of its components were correlated to steroid treatment response or graft survival. Indeed, Thurairajah et al[70] (2013), in a retrospective review of 970 patients, confirms that early acute rejection cases yield the best 10-year graft survival rates, at 85%.
In contrast to the minimal impact of early ACR, it appears that late ACR is associated with decreased graft survival. Uemura et al[69] (2008) found that of 1604 patients, 19.0% developed ACR later than 6 mo after the transplant; the only predictor of late ACR here was post-transplant lymphoproliferative disease. Thurairajah et al[70] (2013) found that 11% of patients developed late ACR, and that the highest rates of late ACR were found in patients with seronegative hepatitis, primary biliary sclerosis, and primary sclerosing cholangitis. Other studies have found that post-transplant lymphoproliferative disorder, decreased age, and increased medication level variability index are associated with and can predict late ACR[71].
Epidemiological evaluation of ACR is masked by its frequently subclinical character. Bartlett et al[72] (2002) explain that in a retrospective review examining 15 studies with a total of 1566 patients, 32% of standard protocol post-OLT biopsy samples showed evidence of ACR without any biochemical dysfunction. Since ACR is defined according to biopsy but not serum biomarkers, incidence of ACR may be higher than previously accepted. Another study, Tisone et al[42] (1999), explains that 80% of acute rejection episodes in their 45-patient cohort resolved spontaneously. The chances of clinically significant acute rejection, then, must be balanced against the risks of liver biopsy. In the future, metabolomic and other noninvasive studies could shed significant light on incidence of ACR[73-75].
Reduction of immunosuppression in light of ACR is not a new subject: Volpin et al[76] (2002), in a controlled study, evaluated two methylprednisolone regimens in the treatment of acute cellular rejection. They find that a 6-d taper regimen is safer than the higher-dose standard because ACR impact on graft rejection is minor, and the toxic effects of methylprednisolone outweigh the potential benefit of ACR suppression. Goddard et al[77] (2002) reviewed the Volpin study, concluding that immunosuppression therapies should be tailored to the individual patient after careful consideration of the interaction between past medical history and immunosuppression side effects.
More recently, Rodríguez-Perálvarez et al[78] (2013) report that standard TAC trough concentrations are set too high (at 10-15 ng/mL), and that target TAC levels between 7 and 10 ng/mL are associated with longer graft survival while maintaining safety against rejection.
In contrast to the safety of simply reducing TAC, a randomized prospective trial of SIR monotherapy conversion regimen efficacy[79] found that liver transplant patients have no demonstrable benefit at 12 mo. Cumulative rates of graft loss or death were not significantly different, at 6.6% for the SIR group vs 5.6% for the CNI group. However, rates of acute rejection and discontinuation due to side effects were higher for patients treated with SRL. Then, one must consider the characteristics of the individual patient when designing an immunosuppressive regimen. For patients who are at great risk of end-stage renal disease (ESRD), the risk of acute rejection that is posed by conversion to SRL may be outweighed by the nephrotoxicity associated with the use of CNIs. For patients who are more concerned about acute rejection than nephrotoxicity, it makes sense to use CNI therapy.
MALIGNANT COMPLICATIONS POST-OLT
Recurrent and de novo malignancies are the top nonhepatic causes of late death in liver transplant patients, often listed alongside CVD. Some of the increased incidence in de novo malignancies in liver transplant recipients compared to the general population can be attributed to the use of exogenous immunosuppression[80,81].
The greatest incidence of post-transplant malignancies is associated with chronic viral infection. Specifically, Epstein-Barr virus-associated post-transplant lymphoproliferative disease, skin cancers, squamous cell carcinoma, and Kaposi’s sarcoma are associated with status post-OLT[82]. Baccarani et al[82] (2009) find that 42 (12.8%) of patients undergoing OLT, out of 330, developed de novo cancers. Further, these patients had a lower 10-year survival rate than those who did not develop de novo cancer.
In hopes of reducing malignancy, current immunosuppression strategies focus on minimizing TAC with optional use of mTOR inhibitors or MMF. mTOR inhibitors are known for their antineoplastic activity[83], and CNI use can be associated with increased development of malignancy[84], making CNI reduction and replacement with mTOR inhibitors particularly favorable for patients at risk for malignancy.
MS
MS constitutes a number of symptoms that, when occurring simultaneously, indicate a primary clinical outcome of CVD. The criteria for MS is that a patient meet three of five components: Abdominal obesity and visceral fat, increased triglyceride (TG), decreased serum high-density lipoprotein, high blood pressure (HTN), insulin resistance and/or glucose intolerance[85]. Despite the wide range of systemic effects, each of these symptoms converges towards provoking CVD[86].
The Framingham study[87] found that 25% of all new-onset CVD could be predicted by presence of MS. More recently, Watt et al[4] (2010) report that causes of death more than 1 year after OLT have the following etiologies: 28% hepatic, 22% malignancy, 11% cardiovascular, 9% infection, and 6% renal failure. They conclude that modifiable risk factors such as diabetes, hypertension, and renal insufficiency can be used to improve long-term outcomes. Table 1 describes some of the interplay between MS components and immunosuppression. Because immunosuppressive strategies can sometimes be altered, relative risks and benefits should be weighed on a case-by-case basis.
Table 1.
Summary of immunosuppressant effects on metabolic syndrome
| Calcineurin inhibitors | Mycophenolate mofetil | mTOR inhibitors | Steroids | |
| Body mass | Increase[92,103] | No change[105] | Less weight gain than CNIs[37] | Increase[144,145] |
| Dyslipidemia | Increase[104,121] | Less than CNIs[115] | Increase, but anti-atherosclerotic[144] | Increase[145] |
| Hypertension | Increase[107] | Less than CNIs[115,144] | No difference from CNI[146] | Increase[145] |
| Insulin resistance | Increase[128] | Potential benefit[145,146] | Potential benefit[149,150] | Increase[145] |
| Renal damage | Increase[112,113] | Less than both CNIs and mTOR inhibitors[147,151] | Decrease compared to CNIs[152] | Not significant |
| Note | Neoplastic[84] | Leukopenic[147,153,154] | Antineoplastic[155] |
CNIs: Calcineurin inhibitors; mTOR: Mechanistic target of rapamycin.
Obesity: The boss of MS
Abdominal obesity is a prevalent characteristic of MS, and adipocyte dysfunction is hypothesized to underlie many metabolic disorders. Some explanations of adipocyte metabolism focus on the location of fat as a determinant of metabolic properties, such that visceral or abdominal fat might contribute more to MS than subcutaneous fat[88]; other explanations emphasize immunological modulation of adipocyte metabolism[89]. Whatever the underlying cause, obesity is a major public health issue[90], one that may be an environmental hit to a genetic predisposition.
Stegall et al[91] (1995) report that the incidence of obesity for adult liver transplant recipients 1 year after transplant was 41.9% for women, and 39.3% in men. In a more recent article, Richards et al[92] (2005) found that by one and 3 years after liver transplant, 24% and 31% of patients met criteria for obesity as body mass index (BMI) > 30 kg/m2.
Despite clear evidence that obesity reduces graft and patient survival[93,94], there are studies that dispute its independent predictive power. Leonard et al[95] (2008) found that in a cohort of 1313 patients, obesity does not independently correlate to risk of graft or patient survival. Perhaps a measure of obesity is not enough, and there are more specific characteristics of excess adipose tissue that lead to it being a risk factor.
Després et al[96] (2006) explain that the deposition of visceral fat, as opposed to normal subcutaneous fat, can lead to adipose tissue overflow and hormonal imbalance. The net effect of these factors is an increase in ectopic fat to muscle, liver, and epicardial tissue. Compounding this issue, visceral adipocytes are less responsive to insulin, and thus not subject to the antilipolytic effects of insulin[97].
While the anatomical location of an adipocyte may be illuminating, physiologic factors also demonstrate the heterogeneity of metabolic dysfunction: Abnormal fat can accumulate as a result of defects in nuclear receptor genes involved in lipid sensing, synthesis, and oxidation[98]. Supporting the ectopic fat hypothesis, Porter et al[99] (2009) report that analysis of 3000 Framingham study participants indicated that abdominal subcutaneous fat had no corresponding linear increase of obesity-associated risk factors.
Further complicating the issue is what type of lipids are most abundant in a dysfunctional metabolic state. In the context of non-alcoholic steatohepatitis/nonalcoholic fatty liver diseas, it has been suggested that hepatocyte TG accumulation may be protective against free fatty acid (FFA)-induced oxidative lipotoxicity[100]. As far as dietary fats go, there is evidence that unsaturated fats contribute to this lipotoxicity, whereas saturated fats are actually hepatoprotective[101].
Each of these issues - lipid distribution and lipid metabolism - is a qualitative issue that is outside the measure of BMI. Then, a more specific measure of adipose metabolism is necessary to further individualize treatment options for post-OLT patients. Given the heterogeneity of adipose metabolism, some authors suggest adipocyte transplantation as a treatment for metabolic issues such as diabetes, atherosclerosis, and nonalcoholic steatohepatitis[102].
Not only is obesity incident to the population of liver transplant recipients, it is exacerbated by the effects of immunosuppression[92]. A mainstay of immunosuppression, steroids, are well-known for effects on weight gain[103]. CNIs are also associated with weight gain: Ersoy et al[103] (2008) report that weight gain at 12 mo for renal transplant recipients prescribed TAC and CsA was 3.5 and 8.0 kg, respectively. As far as changing immunosuppressive regimens, it appears that using the mTOR inhibitor EVR in combination with a reduced dose of TAC can cause less weight gain than full-dose TAC immunosuppression[104]. If mTOR inhibitors are inappropriate for the specific patient, MMF is a different potential substitute that is not associated with post-transplant weight gain[105].
Hypertension and renal insufficiency
HTN and subsequent renal insufficiency are major concerns for post-OLT patients. Increased blood pressure and systemic vascular resistance is pathological, and can lead to hepatorenal syndrome. The use of immunosuppression, specifically CNIs, compounds the metabolic issues already present in liver transplant recipients[106,107].
The incidence of severe renal dysfunction can reach up to 18.1% at a mean of 13 years post-OLT[108]. Longenecker et al[109] (2015) recently reviewed renal function before and after OLT, finding that the overall rate of progression to ESRD is strongly associated with estimated GFR (eGFR) less than 60 mL/min × 1.73 m2 and diabetes, but find that eGFR at OLT is not associated with 12-mo mortality.
For patients presenting with proteinuria as a result of HTN due to renal-insufficiency, treatment includes standard approaches such as angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers[86,110].
Aside from the standard of care for MS, the specific treatment of post-OLT patients with renal insufficiency should include reduction or withdrawal of CNI therapy as soon as possible because of the nephrotoxicity associated with CNIs[111,112]. Masetti et al[113] (2010) evaluated whether early withdrawal of CsA followed by initiation of EVR monotherapy preserves kidney function compared to their standard CsA regimen. The study found that incidence of stage 3 chronic kidney disease (< 60 mL/min GFR) at 1 year was significantly higher in the standard CsA group (55%) than in the group treated with EVR monotherapy (15.4%). Further, there was no difference in patient survival between the two groups.
Saliba et al[104] (2013) found that TAC reduction with addition of EVR were associated with increased estimated GFR, demonstrating a significant benefit to renal function. Tsai et al[114] (2009) confirm that, for renal allograft recipients, CNI minimization with the introduction of SRL reduces acute rejection and improves renal function and survival.
CNI withdrawal and replacement with MMF is another promising approach to post-OLT immunosuppressive management in patients who have renal insufficiency. Orlando et al[115] (2007) report success with MMF monotherapy as a means of reducing the toxic effects of CNIs. Of 42 post-OLT individuals who were weaned off of CNI therapy and placed on subsequent MMF monotherapy, renal function improved in 89% and arterial hypertension decreased in 80% of cases. In a separate study examining post-OLT patients with severe side effects from CNI therapy, Dharancy et al[116] (2009) found that a switch to MMF monotherapy could lead to increased eGFR without significant increase in rejection. Several studies have concluded that MMF is less nephrotoxic, indicating that MMF could be used preferentially in patients with renal dysfunction[117,118].
Dyslipidemia
Incidence of dyslipidemia exceeds 70% and 40% for patients with and without pre-transplant MS, respectively[119]. Because MS affects a such great proportion of OLT patients[7], methods of preventing or reducing dyslipidemia could benefit a preponderance of patients.
In an evaluation of the metabolic impact of OLT, Luzi et al[120] (1996) reported that liver transplant recipients had abnormal FFA levels at 5 mo post-OLT. A follow-up at 26 mo found reduction in abnormal lipid and protein metabolism - in fact, plasma free fatty acids were reduced for transplant recipients with respect to the control group.
CNI therapy is associated with dyslipidemia post-OLT; but the level of dyslipidemia might be reduced upon use of mTOR inhibitors in combination with TAC[121]. Saliba et al[104] (2013) report that hyperlipidemia was more frequent in patients on EVR + reduced dose TAC compared to patients on only full dose TAC. In spite of an increase in dyslipidemia, mTOR inhibitors do seem to decrease arteriovascular plaque formation[122].
Orlando et al[115] (2007) found that CNI withdrawal and subsequent replacement with MMF improved dyslipidemia. Out of 41 patients, blood cholesterol decreased in 76% and blood TG decreased in 89%. Further supporting the use of MMF in patients who are at-risk for complications of atherosclerosis, Romero et al[123] (2000) also reported that MMF specifically reduces atherosclerosis in rabbits.
For patients with hyperlipidemia who are resistant to lifestyle changes, hydroxymethylglutaryl-CoA reductase inhibitors (statins) should be considered. Even with the potential for hepatotoxicity, the use of statins to counter immunosuppressive side effects can benefit patients[124]. Martin et al[125] (2007), in a retrospective review of 69 liver transplant patients, explain that there is a general tolerability and low incidence of adverse events in patients treated with lipid-lowering agents. Indeed, they report that there is no change in liver function tests.
Diabetes
Post-transplant diabetes mellitus (PTDM) is a well-recognized issue, and minimization of immunosuppression is currently the best treatment option for PTDM patients. The diabetogenic properties of immunosuppressive therapies seem to be intimately related to the signaling processes that are shared between pancreatic islets and leukocytes. Moreover, in contrast to the physiological proliferative signaling mechanisms used by white blood cells, renal calcineurin mediates glomerular hypertrophy and extracellular matrix accumulation[126].
Immunosuppressive regimens play a major role in new-onset diabetes, affecting patient and graft survival[127]. Rostambeigi et al[128] (2011) explain that beta cells exposed to TAC and CsA decreased insulin secretion and reduced mitochondrial density without affecting apoptosis rates, and posit that maybe there is a mitochondria-mediated dysfunction imposed by CNIs. Notably, the tacrolimus-exposed beta cells fared marginally better than their CsA counterparts. This is a reflection of the diabetogenic properties of TAC compared to CsA. On the other hand, some research finds no major metabolic differences between TAC and CsA post-OLT low-dose maintenance therapies[129,130].
HCV: FROM INVARIABLE TO INCONSEQUENTIAL
HCV recurrence after liver transplantation is nearly universal, immediate, and has an accelerated natural history[131]. However, the discovery of directly acting antiviral (DAA) protease inhibitors has dramatically reduced the impact of HCV. With SVR rates exceeding 90%[132], high safety[133], and a well-tolerated side-effects profile[134], Hepatitis C treatment will hopefully become a non-issue.
Still, there are OLT patients who experience HCV recurrence, and these patients deserve special consideration. Notably, in contrast to patients who do not have HCV, an episode of early ACR in post-OLT HCV patients is associated with a higher risk for mortality[135]. Despite the emphatic importance of treating ACR in this population, there has been a lack of consensus on the impact of using steroids - the front line of ACR treatment - in post-OLT HCV patients[136,137].
At the Cleveland Clinic Foundation, ACR treatment protocol first considers RAI of the HCV-positive post-OLT patient. For patients with RAI less than six, there is an increase in CNI dose and further monitoring for rejection before a bolus of steroids is administered. In contrast, patients who have HCV with a RAI greater than or equal to six are treated the same as patients who do not have HCV: 1 g of methylprednisone is administered daily for 3 d followed by steroid taper. Antibody therapy is used for steroid-resistant rejection.
Besides treatment of ACR, maintenance therapy for HCV-positive post-OLT patients can be nuanced. For example, use of the monoclonal antibody OKT3 is associated with early and severe post-OLT HCV recurrence, and must be approached with caution[138]. On the other hand, treatment with MMF and a 24-mo CNI taper appears to benefit liver function tests and presentation on histology for the hepatitis C patient[139].
With the introduction of DAAs, however, focus has shifted from accommodating immunosuppression to early HCV treatment for potential liver transplant recipients. Achieving SVR before the time of transplantation is ideal, and helps reduce risk of HCV recurrence post-OLT[140-142]. Still, the efficacy of recently-developed protease inhibitors must be evaluated specifically for post-OLT patients[143].
CONCLUSION
While the landscape of immunosuppressive medications remains steadfast, temperamental clinical weather demands that clinicians stay up to date on best practices. The good news is that HCV is nearly subdued as a post-transplant complication, increasing graft survival, perhaps even decreasing allocation of organs to retransplantation for HCV. The bad news is that MS is increasingly harming patients in ways that are exacerbated by immunosuppression - an issue in sore need of revolution like that in HCV treatment. Finally, given the confluence of MS and immunosuppressive side effects, treatment of early ACR could be excessive and must be reevaluated in light of today’s average patient.
Footnotes
Conflict-of-interest statement: All of the authors have no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 1, 2015
First decision: August 31, 2015
Article in press: January 7, 2016
P- Reviewer: Sanal MG S- Editor: Kong JX L- Editor: A E- Editor: Liu SQ
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, Everhart J, Detre KM. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 3.Banff Working Group on Liver Allograft Pathology. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl. 2012;18:1154–1170. doi: 10.1002/lt.23481. [DOI] [PubMed] [Google Scholar]
- 4.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruthi J, Medkiff KA, Esrason KT, Donovan JA, Yoshida EM, Erb SR, Steinbrecher UP, Fong TL. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811–815. doi: 10.1053/jlts.2001.27084. [DOI] [PubMed] [Google Scholar]
- 6.Madhwal S, Atreja A, Albeldawi M, Lopez R, Post A, Costa MA. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl. 2012;18:1140–1146. doi: 10.1002/lt.23508. [DOI] [PubMed] [Google Scholar]
- 7.Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 8.Alberti A, Piovesan S. The evolution of the therapeutic strategy in hepatitis C: features of sofosbuvir and indications. Dig Liver Dis. 2014;46 Suppl 5:S174–S178. doi: 10.1016/j.dld.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 10.Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, Harris NL, Junt T, Odermatt B, Clavien PA, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirkiran A, Kok A, Kwekkeboom J, Kusters JG, Metselaar HJ, Tilanus HW, van der Laan LJ. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12:277–284. doi: 10.1002/lt.20612. [DOI] [PubMed] [Google Scholar]
- 12.O’Leary JG, Demetris AJ, Friedman LS, Gebel HM, Halloran PF, Kirk AD, Knechtle SJ, McDiarmid SV, Shaked A, Terasaki PI, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14:779–787. doi: 10.1111/ajt.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541–1548. doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, Leverson GE, Bellingham JM, Fernandez LA, Foley DP, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindl MJ, Millar AM, Redhead DN, Fearon KC, Ross JA, Dejong CH, Garden OJ, Wigmore SJ. The adaptive response of the reticuloendothelial system to major liver resection in humans. Ann Surg. 2006;243:507–514. doi: 10.1097/01.sla.0000205826.62911.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 17.Wick MJ, Leithäuser F, Reimann J. The hepatic immune system. Crit Rev Immunol. 2002;22:47–103. [PubMed] [Google Scholar]
- 18.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 19.Starzl TE. The mystique of organ transplantation. J Am Coll Surg. 2005;201:160–170. doi: 10.1016/j.jamcollsurg.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos HC, Reyes J, Abu-Elmagd K, Zeevi A, Reinsmoen N, Tzakis A, Demetris AJ, Fung JJ, Flynn B, McMichael J. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong TL. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database. Transplantation. 2006;82:1298–1303. doi: 10.1097/01.tp.0000241104.58576.e6. [DOI] [PubMed] [Google Scholar]
- 22.Katznelson S, Cecka JM. The liver neither protects the kidney from rejection nor improves kidney graft survival after combined liver and kidney transplantation from the same donor. Transplantation. 1996;61:1403–1405. doi: 10.1097/00007890-199605150-00021. [DOI] [PubMed] [Google Scholar]
- 23.Castillo-Rama M, Castro MJ, Bernardo I, Meneu-Diaz JC, Elola-Olaso AM, Calleja-Antolin SM, Romo E, Morales P, Moreno E, Paz-Artal E. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 2008;14:554–562. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 24.Jakab SS, Navarro VJ, Colombe BW, Daskalakis C, Herrine SK, Rossi S. Human leukocyte antigen and adult living-donor liver transplantation outcomes: an analysis of the organ procurement and transplantation network database. Liver Transpl. 2007;13:1405–1413. doi: 10.1002/lt.21264. [DOI] [PubMed] [Google Scholar]
- 25.Neumann UP, Guckelberger O, Langrehr JM, Lang M, Schmitz V, Theruvath T, Schonemann C, Menzel S, Klupp J, Neuhaus P. Impact of human leukocyte antigen matching in liver transplantation. Transplantation. 2003;75:132–137. doi: 10.1097/00007890-200301150-00024. [DOI] [PubMed] [Google Scholar]
- 26.Knechtle SJ, Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin Liver Dis. 2009;29:91–101. doi: 10.1055/s-0029-1192058. [DOI] [PubMed] [Google Scholar]
- 27.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 28.Swiatecka-Urban A. Anti-interleukin-2 receptor antibodies for the prevention of rejection in pediatric renal transplant patients: current status. Paediatr Drugs. 2003;5:699–716. doi: 10.2165/00148581-200305100-00005. [DOI] [PubMed] [Google Scholar]
- 29.Waki K. UNOS Liver Registry: ten year survivals. Clin Transpl. 2006:29–39. [PubMed] [Google Scholar]
- 30.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 31.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 32.Basic-Jukic N, Kes P, Bubic-Filipi LJ, Puretic Z, Brunetta B, Pasini J. Does mycophenolate mofetil increase the incidence of cytomegalovirus disease compared with azathioprine after cadaveric kidney transplantation? Transplant Proc. 2005;37:850–851. doi: 10.1016/j.transproceed.2004.12.228. [DOI] [PubMed] [Google Scholar]
- 33.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 34.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 35.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araki M, Flechner SM, Ismail HR, Flechner LM, Zhou L, Derweesh IH, Goldfarb D, Modlin C, Novick AC, Faiman C. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation. 2006;81:335–341. doi: 10.1097/01.tp.0000195770.31960.18. [DOI] [PubMed] [Google Scholar]
- 38.Gómez R, Moreno E, Colina F, Loinaz C, Gonzalez-Pinto I, Lumbreras C, Perez-Cerdá F, Castellón C, García I. Steroid withdrawal is safe and beneficial in stable cyclosporine-treated liver transplant patients. J Hepatol. 1998;28:150–156. doi: 10.1016/s0168-8278(98)80214-6. [DOI] [PubMed] [Google Scholar]
- 39.Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, Eckhoff DE, Netto GJ, Katz E. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transpl. 2007;13:1521–1531. doi: 10.1002/lt.21182. [DOI] [PubMed] [Google Scholar]
- 40.Lerut JP, Pinheiro RS, Lai Q, Stouffs V, Orlando G, Juri JM, Ciccarelli O, Sempoux C, Roggen FM, De Reyck C, et al. Is minimal, [almost] steroid-free immunosuppression a safe approach in adult liver transplantation? Long-term outcome of a prospective, double blind, placebo-controlled, randomized, investigator-driven study. Ann Surg. 2014;260:886–891; discussion 891-892. doi: 10.1097/SLA.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 41.Tisone G, Angelico M, Palmieri G, Pisani F, Baiocchi L, Vennarecci G, Anselmo A, Orlando G, Negrini S, Casciani CU. Immunosuppression without prednisone after liver transplantion is safe and associated with normal early graft function: preliminary results of a randomized study. Transpl Int. 1998;11 Suppl 1:S267–S269. doi: 10.1007/s001470050475. [DOI] [PubMed] [Google Scholar]
- 42.Tisone G, Angelico M, Palmieri G, Pisani F, Anselmo A, Baiocchi L, Negrini S, Orlando G, Vennarecci G, Casciani CU. A pilot study on the safety and effectiveness of immunosuppression without prednisone after liver transplantation. Transplantation. 1999;67:1308–1313. doi: 10.1097/00007890-199905270-00003. [DOI] [PubMed] [Google Scholar]
- 43.Reding R. Steroid withdrawal in liver transplantation: benefits, risks, and unanswered questions. Transplantation. 2000;70:405–410. doi: 10.1097/00007890-200008150-00001. [DOI] [PubMed] [Google Scholar]
- 44.Kim JM, Joh JW, Kim SJ, Kwon CH, Song S, Shin M, Hong SH, Lee SK. Steroid withdrawal in adult liver transplantation: occurrence at a single center. Transplant Proc. 2010;42:4132–4136. doi: 10.1016/j.transproceed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid-free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transpl. 2001;7:693–697. doi: 10.1053/jlts.2001.26353. [DOI] [PubMed] [Google Scholar]
- 46.Bonnefoy-Berard N, Revillard JP. Mechanisms of immunosuppression induced by antithymocyte globulins and OKT3. J Heart Lung Transplant. 1996;15:435–442. [PubMed] [Google Scholar]
- 47.Bolt S, Routledge E, Lloyd I, Chatenoud L, Pope H, Gorman SD, Clark M, Waldmann H. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur J Immunol. 1993;23:403–411. doi: 10.1002/eji.1830230216. [DOI] [PubMed] [Google Scholar]
- 48.Shiheido H, Aoyama T, Takahashi H, Hanaoka K, Abe T, Nishida E, Chen C, Koga O, Hikida M, Shibagaki Y, et al. Novel CD3-specific antibody induces immunosuppression via impaired phosphorylation of LAT and PLCγ1 following T-cell stimulation. Eur J Immunol. 2014;44:1770–1780. doi: 10.1002/eji.201344146. [DOI] [PubMed] [Google Scholar]
- 49.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91:1057–1064. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]
- 50.Page EK, Dar WA, Knechtle SJ. Biologics in organ transplantation. Transpl Int. 2012;25:707–719. doi: 10.1111/j.1432-2277.2012.01456.x. [DOI] [PubMed] [Google Scholar]
- 51.Chatenoud L, Ferran C, Legendre C, Thouard I, Merite S, Reuter A, Gevaert Y, Kreis H, Franchimont P, Bach JF. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Frigault MJ, June CH. Predicting cytokine storms: it’s about density. Blood. 2011;118:6724–6726. doi: 10.1182/blood-2011-10-382598. [DOI] [PubMed] [Google Scholar]
- 53.Hanouneh IA, Zein NN, Lopez R, Yerian L, Fung J, Eghtesad B. IL-2 Receptor Antagonist (Basiliximab) Is Associated with Rapid Fibrosis Progression in Patients with Recurrent Hepatitis C after Liver Transplantation Using Serial Biopsy Specimens. Int J Organ Transplant Med. 2010;1:7–14. [PMC free article] [PubMed] [Google Scholar]
- 54.Perri R, Assi M, Talwalkar J, Heimbach J, Hogan W, Moore SB, Rosen CB. Graft vs. host disease after liver transplantation: a new approach is needed. Liver Transpl. 2007;13:1092–1099. doi: 10.1002/lt.21203. [DOI] [PubMed] [Google Scholar]
- 55.Bezabeh S, Flowers CM, Kortepeter C, Avigan M. Clinically significant liver injury in patients treated with natalizumab. Aliment Pharmacol Ther. 2010;31:1028–1035. doi: 10.1111/j.1365-2036.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- 56.Usuda M, Fujimori K, Koyamada N, Fukumori T, Sekiguchi S, Kawagishi N, Akamatsu Y, Enomoto Y, Satoh K, Satoh A, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005;79:12–16. doi: 10.1097/01.tp.0000149337.40911.e4. [DOI] [PubMed] [Google Scholar]
- 57.Yoshizawa A, Sakamoto S, Ogawa K, Kasahara M, Uryuhara K, Oike F, Ueda M, Takada Y, Egawa H, Tanaka K. New protocol of immunosuppression for liver transplantation across ABO barrier: the use of Rituximab, hepatic arterial infusion, and preservation of spleen. Transplant Proc. 2005;37:1718–1719. doi: 10.1016/j.transproceed.2005.03.148. [DOI] [PubMed] [Google Scholar]
- 58.Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, Wakabayashi G, Shimazu M, Kitajima M. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010;40:943–949. doi: 10.1111/j.1365-2362.2010.02339.x. [DOI] [PubMed] [Google Scholar]
- 59.Sanal MG. Future of liver transplantation: non-human primates for patient-specific organs from induced pluripotent stem cells. World J Gastroenterol. 2011;17:3684–3690. doi: 10.3748/wjg.v17.i32.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 61.Dickson RC, Lauwers GY, Rosen CB, Cantwell R, Nelson DR, Lau JY. The utility of noninvasive serologic markers in the management of early allograft rejection in liver transplantation recipients. Transplantation. 1999;68:247–253. doi: 10.1097/00007890-199907270-00015. [DOI] [PubMed] [Google Scholar]
- 62.Abraham SC, Furth EE. Receiver operating characteristic analysis of serum chemical parameters as tests of liver transplant rejection and correlation with histology. Transplantation. 1995;59:740–746. doi: 10.1097/00007890-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 63.Rodríguez-Perálvarez M, García-Caparrós C, Tsochatzis E, Germani G, Hogan B, Poyato-González A, O’Beirne J, Senzolo M, Guerrero-Misas M, Montero-Álvarez JL, et al. Lack of agreement for defining ‘clinical suspicion of rejection’ in liver transplantation: a model to select candidates for liver biopsy. Transpl Int. 2015;28:455–464. doi: 10.1111/tri.12514. [DOI] [PubMed] [Google Scholar]
- 64.Höroldt BS, Burattin M, Gunson BK, Bramhall SR, Nightingale P, Hübscher SG, Neuberger JM. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver Transpl. 2006;12:1144–1151. doi: 10.1002/lt.20779. [DOI] [PubMed] [Google Scholar]
- 65.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, Kulik LM, Pruett TL, Terrault NA. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aydogan C, Sevmis S, Aktas S, Karakayali H, Demirhan B, Haberal M. Steroid-resistant acute rejections after liver transplant. Exp Clin Transplant. 2010;8:172–177. [PubMed] [Google Scholar]
- 67.Fisher LR, Henley KS, Lucey MR. Acute cellular rejection after liver transplantation: variability, morbidity, and mortality. Liver Transpl Surg. 1995;1:10–15. doi: 10.1002/lt.500010104. [DOI] [PubMed] [Google Scholar]
- 68.Seiler CA, Renner EL, Czerniak A, Didonna D, Büchler MW, Reichen J. Early acute cellular rejection: no effect on late hepatic allograft function in man. Transpl Int. 1999;12:195–201. doi: 10.1007/s001470050210. [DOI] [PubMed] [Google Scholar]
- 69.Uemura T, Ikegami T, Sanchez EQ, Jennings LW, Narasimhan G, McKenna GJ, Randall HB, Chinnakotla S, Levy MF, Goldstein RM, et al. Late acute rejection after liver transplantation impacts patient survival. Clin Transplant. 2008;22:316–323. doi: 10.1111/j.1399-0012.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 70.Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, Gunson BK, Shah T, Neuberger J. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955–959. doi: 10.1097/TP.0b013e3182845f6c. [DOI] [PubMed] [Google Scholar]
- 71.Christina S, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, Zack Y, Florman S, Shneider BL, Shemesh E. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20:1168–1177. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartlett AS, Ramadas R, Furness S, Gane E, McCall JL. The natural history of acute histologic rejection without biochemical graft dysfunction in orthotopic liver transplantation: a systematic review. Liver Transpl. 2002;8:1147–1153. doi: 10.1053/jlts.2002.36240. [DOI] [PubMed] [Google Scholar]
- 73.Wishart DS. Metabolomics: the principles and potential applications to transplantation. Am J Transplant. 2005;5:2814–2820. doi: 10.1111/j.1600-6143.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 74.Hrydziuszko O, Silva MA, Perera MT, Richards DA, Murphy N, Mirza D, Viant MR. Application of metabolomics to investigate the process of human orthotopic liver transplantation: a proof-of-principle study. OMICS. 2010;14:143–150. doi: 10.1089/omi.2009.0139. [DOI] [PubMed] [Google Scholar]
- 75.Adolf J, Martin WG, Müller DF, Beckurts KT, Schneider-Eicke J, Wittekind C, Heidecke CD. [The effect of acute cellular rejection on liver function following orthoptic liver transplantation. Quantitative functional studies with the 14C-aminopyrine breath test] Dtsch Med Wochenschr. 1992;117:1823–1828. doi: 10.1055/s-2008-1062516. [DOI] [PubMed] [Google Scholar]
- 76.Volpin R, Angeli P, Galioto A, Fasolato S, Neri D, Barbazza F, Merenda R, Del Piccolo F, Strazzabosco M, Casagrande F, et al. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8:527–534. doi: 10.1053/jlts.2002.33456. [DOI] [PubMed] [Google Scholar]
- 77.Goddard S, Adams DH. Methylprednisolone therapy for acute rejection: too much of a good thing? Liver Transpl. 2002;8:535–536. doi: 10.1053/jlts.2002.33486. [DOI] [PubMed] [Google Scholar]
- 78.Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, Rolando N, Dhillon AP, Patch D, O’Beirne J, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013;58:262–270. doi: 10.1016/j.jhep.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 79.Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, Neff GW, Ranjan D, Toselli LT, Gane EJ, et al. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694–705. doi: 10.1111/j.1600-6143.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 80.Fung JJ, Jain A, Kwak EJ, Kusne S, Dvorchik I, Eghtesad B. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7:S109–S118. doi: 10.1053/jlts.2001.28645. [DOI] [PubMed] [Google Scholar]
- 81.Jiménez-Romero C, Justo-Alonso I, Cambra-Molero F, Calvo-Pulido J, García-Sesma Á, Abradelo-Usera M, Caso-Maestro O, Manrique-Municio A. Incidence, risk factors and outcome of de novo tumors in liver transplant recipients focusing on alcoholic cirrhosis. World J Hepatol. 2015;7:942–953. doi: 10.4254/wjh.v7.i7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baccarani U, Adani GL, Serraino D, Lorenzin D, Gambato M, Buda A, Zanus G, Vitale A, Piselli P, De Paoli A, et al. De novo tumors are a major cause of late mortality after orthotopic liver transplantation. Transplant Proc. 2009;41:1303–1305. doi: 10.1016/j.transproceed.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 83.Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, Winkler S, Houben P, Schultze D, Hatano E, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma - systematic review and meta-analysis. Cancer Med. 2013;2:862–871. doi: 10.1002/cam4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253–260. doi: 10.1097/MOT.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 86.Bonora BM, Marescotti M, Marcuzzo G, Avogaro A, Fadini GP. Synergistic interactions among metabolic syndrome components and homeostasis model assessment of insulin resistance in a middle-aged general population over time. Metab Syndr Relat Disord. 2015;13:171–178. doi: 10.1089/met.2014.0163. [DOI] [PubMed] [Google Scholar]
- 87.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 88.Björntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- 89.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 90.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stegall MD, Everson G, Schroter G, Bilir B, Karrer F, Kam I. Metabolic complications after liver transplantation. Diabetes, hypercholesterolemia, hypertension, and obesity. Transplantation. 1995;60:1057–1060. [PubMed] [Google Scholar]
- 92.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 93.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 94.Ducloux D, Kazory A, Simula-Faivre D, Chalopin JM. One-year post-transplant weight gain is a risk factor for graft loss. Am J Transplant. 2005;5:2922–2928. doi: 10.1111/j.1600-6143.2005.01104.x. [DOI] [PubMed] [Google Scholar]
- 95.Leonard J, Heimbach JK, Malinchoc M, Watt K, Charlton M. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008;8:667–672. doi: 10.1111/j.1600-6143.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 96.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 97.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 98.Sanal MG. The blind men ‘see’ the elephant-the many faces of fatty liver disease. World J Gastroenterol. 2008;14:831–844. doi: 10.3748/wjg.14.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ono M, Okamoto N, Saibara T. The latest idea in NAFLD/NASH pathogenesis. Clin J Gastroenterol. 2010;3:263–270. doi: 10.1007/s12328-010-0182-9. [DOI] [PubMed] [Google Scholar]
- 101.Purohit V, Russo D, Coates PM. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2004;34:3–8. doi: 10.1016/j.alcohol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Sanal MG. Adipose tissue transplantation may be a potential treatment for diabetes, atherosclerosis and nonalcoholic steatohepatitis. Med Hypotheses. 2009;72:247–249. doi: 10.1016/j.mehy.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Ersoy A, Baran B, Ersoy C, Kahvecioglu S, Akdag I. Calcineurin inhibitors and post-transplant weight gain. Nephrology (Carlton) 2008;13:433–439. doi: 10.1111/j.1440-1797.2008.00916.x. [DOI] [PubMed] [Google Scholar]
- 104.Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Jonas S, Sudan D, Fischer L, Duvoux C, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734–1745. doi: 10.1111/ajt.12280. [DOI] [PubMed] [Google Scholar]
- 105.Schlitt HJ, Barkmann A, Böker KH, Schmidt HH, Emmanouilidis N, Rosenau J, Bahr MJ, Tusch G, Manns MP, Nashan B, et al. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587–591. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 106.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 107.Hoorn EJ, Walsh SB, McCormick JA, Fürstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 109.Longenecker JC, Estrella MM, Segev DL, Atta MG. Patterns of Kidney Function Before and After Orthotopic Liver Transplant: Associations With Length of Hospital Stay, Progression to End-Stage Renal Disease, and Mortality. Transplantation. 2015;99:2556–2564. doi: 10.1097/TP.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 110.Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000;58:2111–2118. doi: 10.1111/j.1523-1755.2000.00384.x. [DOI] [PubMed] [Google Scholar]
- 111.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 112.Morales JM, Andres A, Rengel M, Rodicio JL. Influence of cyclosporin, tacrolimus and rapamycin on renal function and arterial hypertension after renal transplantation. Nephrol Dial Transplant. 2001;16 Suppl 1:121–124. doi: 10.1093/ndt/16.suppl_1.121. [DOI] [PubMed] [Google Scholar]
- 113.Masetti M, Montalti R, Rompianesi G, Codeluppi M, Gerring R, Romano A, Begliomini B, Di Benedetto F, Gerunda GE. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252–2262. doi: 10.1111/j.1600-6143.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 114.Tsai MK, Wu FL, Lai IR, Lee CY, Hu RH, Lee PH. Decreased acute rejection and improved renal allograft survival using sirolimus and low-dose calcineurin inhibitors without induction therapy. Int J Artif Organs. 2009;32:371–380. doi: 10.1177/039139880903200608. [DOI] [PubMed] [Google Scholar]
- 115.Orlando G, Baiocchi L, Cardillo A, Iaria G, De Liguori Carino N, De Luca L, Ielpo B, Tariciotti L, Angelico M, Tisone G. Switch to 1.5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007;13:46–54. doi: 10.1002/lt.20926. [DOI] [PubMed] [Google Scholar]
- 116.Dharancy S, Iannelli A, Hulin A, Declerck N, Schneck AS, Mathurin P, Boleslawski E, Gugenheim J, Pruvot FR. Mycophenolate mofetil monotherapy for severe side effects of calcineurin inhibitors following liver transplantation. Am J Transplant. 2009;9:610–613. doi: 10.1111/j.1600-6143.2008.02529.x. [DOI] [PubMed] [Google Scholar]
- 117.Wu YG, Lin H, Qi XM, Wu GZ, Qian H, Zhao M, Shen JJ, Lin ST. Prevention of early renal injury by mycophenolate mofetil and its mechanism in experimental diabetes. Int Immunopharmacol. 2006;6:445–453. doi: 10.1016/j.intimp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Rodríguez-Iturbe B, Quiroz Y, Shahkarami A, Li Z, Vaziri ND. Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int. 2005;68:1041–1047. doi: 10.1111/j.1523-1755.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 119.Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 120.Luzi L, Perseghin G, Regalia E, Sereni LP, Battezzati A, Baratti D, Bianchi E, Terruzzi I, Hilden H, Groop LC, et al. Metabolic effects of liver transplantation in cirrhotic patients. J Clin Invest. 1997;99:692–700. doi: 10.1172/JCI119213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trotter JF, Wachs ME, Trouillot TE, Bak T, Kugelmas M, Kam I, Everson G. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7:401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- 122.Martinet W, De Loof H, De Meyer GR. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis. 2014;233:601–607. doi: 10.1016/j.atherosclerosis.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 123.Romero F, Rodríguez-Iturbe B, Pons H, Parra G, Quiroz Y, Rincón J, González L. Mycophenolate mofetil treatment reduces cholesterol-induced atherosclerosis in the rabbit. Atherosclerosis. 2000;152:127–133. doi: 10.1016/s0021-9150(99)00458-x. [DOI] [PubMed] [Google Scholar]
- 124.Anfossi G, Massucco P, Bonomo K, Trovati M. Prescription of statins to dyslipidemic patients affected by liver diseases: a subtle balance between risks and benefits. Nutr Metab Cardiovasc Dis. 2004;14:215–224. doi: 10.1016/s0939-4753(04)80008-5. [DOI] [PubMed] [Google Scholar]
- 125.Martin JE, Cavanaugh TM, Trumbull L, Bass M, Weber F, Aranda-Michel J, Hanaway M, Rudich S. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transplant. 2008;22:113–119. doi: 10.1111/j.1399-0012.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 126.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003;284:F144–F154. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 127.Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, Vanholder R. Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am J Transplant. 2009;9:2140–2149. doi: 10.1111/j.1600-6143.2009.02752.x. [DOI] [PubMed] [Google Scholar]
- 128.Rostambeigi N, Lanza IR, Dzeja PP, Deeds MC, Irving BA, Reddi HV, Madde P, Zhang S, Asmann YW, Anderson JM, et al. Unique cellular and mitochondrial defects mediate FK506-induced islet β-cell dysfunction. Transplantation. 2011;91:615–623. doi: 10.1097/TP.0b013e3182094a33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fernandez LA, Lehmann R, Luzi L, Battezzati A, Angelico MC, Ricordi C, Tzakis A, Alejandro R. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation. 1999;68:1532–1541. doi: 10.1097/00007890-199911270-00017. [DOI] [PubMed] [Google Scholar]
- 130.Konrad T, Steinmüller T, Vicini P, Toffolo G, Grewerus D, Schüller A, Bechstein WO, Usadel KH, Cobelli C, Neuhaus P. Regulation of glucose tolerance in patients after liver transplantation: impact of cyclosporin versus tacrolimus therapy. Transplantation. 2000;69:2072–2078. doi: 10.1097/00007890-200005270-00017. [DOI] [PubMed] [Google Scholar]
- 131.Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9:S28–S34. doi: 10.1053/jlts.2003.50248. [DOI] [PubMed] [Google Scholar]
- 132.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 133.Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol. 2015;7:806–813. doi: 10.4254/wjh.v7.i5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fazel Y, Lam B, Golabi P, Younossi Z. Safety analysis of sofosbuvir and ledipasvir for treating hepatitis C. Expert Opin Drug Saf. 2015;14:1317–1326. doi: 10.1517/14740338.2015.1053868. [DOI] [PubMed] [Google Scholar]
- 135.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transpl Surg. 1999;5:S107–S114. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 136.Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, Nishida S, Moon J, Selvaggi G, Levi D, et al. Randomized trial of steroid-free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation. 2007;84:829–835. doi: 10.1097/01.tp.0000282914.20578.7b. [DOI] [PubMed] [Google Scholar]
- 137.Brillanti S, Vivarelli M, De Ruvo N, Aden AA, Camaggi V, D’Errico A, Furlini G, Bellusci R, Roda E, Cavallari A. Slowly tapering off steroids protects the graft against hepatitis C recurrence after liver transplantation. Liver Transpl. 2002;8:884–888. doi: 10.1053/jlts.2002.34640. [DOI] [PubMed] [Google Scholar]
- 138.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453–1457. [PubMed] [Google Scholar]
- 139.Bahra M, Neumann UI, Jacob D, Puhl G, Klupp J, Langrehr JM, Berg T, Neuhaus P. MMF and calcineurin taper in recurrent hepatitis C after liver transplantation: impact on histological course. Am J Transplant. 2005;5:406–411. doi: 10.1111/j.1600-6143.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- 140.Aghemo A, Donato MF. Sofosbuvir treatment in the pre and post liver transplantation phase: the sooner, the better. Gastroenterology. 2015;148:13–16. doi: 10.1053/j.gastro.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 141.Forns X, García-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, García-Valdecasas JC, Navasa M, Rimola A, Rodés J. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389–396. doi: 10.1016/s0168-8278(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 142.Everson GT, Terrault NA, Lok AS, Rodrigo del R, Brown RS, Saab S, Shiffman ML, Al-Osaimi AM, Kulik LM, Gillespie BW, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57:1752–1762. doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Price JC, Terrault NA. Treatment of hepatitis C in liver transplant patients: interferon out, direct antiviral combos in. Liver Transpl. 2015;21:423–434. doi: 10.1002/lt.24080. [DOI] [PubMed] [Google Scholar]
- 144.Siddiqui MS, Sterling RK. Posttransplant metabolic syndrome. Int J Hepatol. 2012;2012:891516. doi: 10.1155/2012/891516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rike AH, Mogilishetty G, Alloway RR, Succop P, Roy-Chaudhury P, Cardi M, Kaiser TE, Thomas M, Woodle ES. Cardiovascular risk, cardiovascular events, and metabolic syndrome in renal transplantation: comparison of early steroid withdrawal and chronic steroids. Clin Transplant. 2008;22:229–235. doi: 10.1111/j.1399-0012.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 146.Morrisett JD, Abdel-Fattah G, Kahan BD. Sirolimus changes lipid concentrations and lipoprotein metabolism in kidney transplant recipients. Transplant Proc. 2003;35:143S–150S. doi: 10.1016/s0041-1345(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 147.Manzia TM, De Liguori Carino N, Orlando G, Toti L, De Luca L, D’Andria D, Cardillo A, Anselmo A, Casciani CU, Tisone G. Use of mycophenolate mofetil in liver transplantation: a literature review. Transplant Proc. 2005;37:2616–2617. doi: 10.1016/j.transproceed.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 148.Peddi VR, Wiseman A, Chavin K, Slakey D. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando) 2013;27:97–107. doi: 10.1016/j.trre.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 149.Mathis AS, Egloff G, Ghin HL. Calcineurin inhibitor sparing strategies in renal transplantation, part one: Late sparing strategies. World J Transplant. 2014;4:57–80. doi: 10.5500/wjt.v4.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vodenik B, Rovira J, Campistol JM. Mammalian target of rapamycin and diabetes: what does the current evidence tell us? Transplant Proc. 2009;41:S31–S38. doi: 10.1016/j.transproceed.2009.06.159. [DOI] [PubMed] [Google Scholar]
- 151.Xiao X, Wang J, Chang X, Zhen J, Zhou G, Hu Z. Mycophenolate mofetil ameliorates diabetic nephropathy through epithelial mesenchymal transition in rats. Mol Med Rep. 2015;12:4043–4050. doi: 10.3892/mmr.2015.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pérez T, Segovia R, Castro L, Roblero JP, Estela R. Conversion to everolimus in liver transplant patients with renal dysfunction. Transplant Proc. 2011;43:2307–2310. doi: 10.1016/j.transproceed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 153.Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation. 2003;75:1213–1220. doi: 10.1097/01.TP.0000062837.99400.60. [DOI] [PubMed] [Google Scholar]
- 154.Knoll GA, MacDonald I, Khan A, Van Walraven C. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003;14:2381–2386. doi: 10.1097/01.asn.0000079616.71891.f5. [DOI] [PubMed] [Google Scholar]
- 155.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]


