Abstract
According to the United States census bureau 20% of Americans will be older than 65 years in 2030 and half of them will need an operation - equating to about 36 million older surgical patients. Older adults are prone to complications during gastrointestinal cancer treatment and therefore may need to undergo special pretreatment assessments that incorporate frailty and sarcopenia assessments. A focused, structured literature review on PubMed and Google Scholar was performed to identify primary research articles, review articles, as well as practice guidelines on frailty and sarcopenia among patients undergoing gastrointestinal surgery. The initial search identified 450 articles; after eliminating duplicates, reports that did not include surgical patients, case series, as well as case reports, 42 publications on the impact of frailty and/or sarcopenia on outcome of patients undergoing gastrointestinal surgery were included. Frailty is defined as a clinically recognizable state of increased vulnerability to physiologic stressors resulting from aging. Frailty is associated with a decline in physiologic reserve and function across multiple physiologic systems. Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength. Unlike cachexia, which is typically associated with weight loss due to chemotherapy or a general malignancy-related cachexia syndrome, sarcopenia relates to muscle mass rather than simply weight. As such, while weight reflects nutritional status, sarcopenia - the loss of muscle mass - is a more accurate and quantitative global marker of frailty. While chronologic age is an important element in assessing a patient’s peri-operative risk, physiologic age is a more important determinant of outcomes. Geriatric assessment tools are important components of the pre-operative work-up and can help identify patients who suffer from frailty. Such data are important, as frailty and sarcopenia have repeatedly been demonstrated among the strongest predictors of both short- and long-term outcome following complicated surgical procedures such as esophageal, gastric, colorectal, and hepato-pancreatico-biliary resections.
Keywords: Sarcopenia, Outcomes, Frailty, Morbidity, Mortality
Core tip: It is estimated that by the year 2030, 36 million Americans > 65 years will require surgery. Frailty as defined by a clinically recognizable state of increased vulnerability due to physiologic stressors resulting from aging has been associated with a decreased physiologic reserve and function across multiple physiological systems. Recently, a loss of muscle mass or sarcopenia has been proposed as an accurate and quantitative global marker of frailty. The current review demonstrates that frailty as defined by sarcopenia can be accurately used as a preoperative predictor of poor short- and long-term postoperative outcomes following complex gastrointestinal surgery.
INTRODUCTION
The life expectancy of the average person doubled over the course of the last century. In addition, between 1982 and 2003 the American population aged over 65 years doubled and the population older than 85 years quadrupled[1]. According to the United States census bureau 20% of Americans will be older than 65 years in 2030 and half of them will need an operation - equating to about 36 million older surgical patients[2]. The process of aging is associated with an increasing prevalence of frailty, comorbidities, a decline of functional reserve and a progressive restriction in personal and social resources. All of these factors can contribute to less favorable postoperative outcomes among older patients[3]. Older patients are at increased risk for complications which include delirium, urinary incontinence, pressure ulcers, depression, infection, functional decline and adverse drug affects[4-8]. Despite the fact that surgery is the most effective cancer therapy, complication rates, mortality, length of hospital stay and intensive care unit admissions increase with patient age, which can offset oncologic advantages[9-13].
Many cancer treatment guidelines have been formulated based on clinical data that may have under-represented older and more frail patients; therefore, more attention is needed to guide the management of this vulnerable population[14,15]. Several studies have noted potential differences in gastrointestinal surgical care between older and younger patients[16,17]. For example, commonly used predictor scores for postoperative complications like the American Society of Anesthesiology score have substantial limitations in older patients, as most are based on a single organ system, are subjective and none measures the patients’ physiologic reserve[18]. In fact, a recent review by McCleary et al[16] stressed that older adults are prone to complications during gastrointestinal cancer treatment and therefore need to undergo special pretreatment assessments incorporating frailty and sarcopenia assessments.
More recently, sarcopenia and frailty have increasingly been recognized as important factors that can be markers of decreased physiologic reserve. Several studies have highlighted the importance of frailty and sarcopenia to predict perioperative outcomes among patients undergoing surgery for gastrointestinal cancer[19-22]. Recent guidelines from the American College of Surgeons have highlighted the importance of assessing both frailty and sarcopenia prior to oncologic surgery in the elderly[23]. As such, there is increasing interest in screening patients for frailty and sarcopenia to better predict patients at highest risk of complications after surgery[24]. Given this, we sought to review the available literature on the association of frailty and sarcopenia with patient outcome, as well as the risk of perioperative morbidity and mortality after gastrointestinal surgeries.
SYSTEMATIC LITERATURE REVIEW
A focused, structured literature review was performed using PubMed and Google Scholar to identify primary research articles, review articles, as well as practice guidelines on frailty and sarcopenia among patients undergoing gastrointestinal surgery. Articles published between January 2000 to March 2015 were identified using the search terms “sarcopenia and gastrointestinal surgery”, “frailty and gastrointestinal surgery”, “sarcopenia and outcome and surgery”, as well as “frailty and outcome and surgery”. In addition, references of relevant articles were also reviewed to identify potentially eligible studies. As per the methodology specified under the PRISMA guidelines, only studies published in English were included, while conference abstracts that did not proceed to publication in peer-reviewed journals were excluded[25]. The initial search identified 343 articles; 53 duplicates were eliminated and 290 abstracts were reviewed for further assessment. Among these 25 editorials, 97 studies that did not include gastrointestinal patients, 99 articles that did not use standard frailty or sarcopenia assessments, 19 case series, as well as 5 case reports and 3 consensus statements were eliminated (Figure 1). In total 42 publications assessing the impact of frailty and/or sarcopenia on postoperative outcomes among patients undergoing gastrointestinal surgery were identified that met inclusion criteria. Among all studies that were included, 10 studies were performed prospectively (2 gastroesophageal surgery, 6 colorectal surgery, and 2 hepato-pancreatico-biliary surgery, Tables 1-3)[26-33]. Sixteen studies were conducted retrospectively on an unmatched cohort (2 gastroesophageal, 4 colorectal, and 10 hepato-pancreatico-biliary), 2 studies retrospectively analyzed prospectively collected data while two articles analyzed data from multiple centers in the United States[34-50]. Additionally, 15 narrative reviews were included in the study. The quality of each study was assessed using the Newcastle-Ottawa Scale based on case selection, comparability, and outcome reporting (Tables 1-3); the median quality score of the studies was 6.5 (range 4-9).
Figure 1.
Flow chart depicting the review process for the inclusion of publications.
Table 1.
Complies all studies evaluated in patients undergoing esophageal or gastric resection
| Ref. | Country | Quality score1 | Study design | Sample size | Age (yr) | Male sex (%) | Surgery type | Parameter used to define frailty | Postoperative complication rate | Follow-up (mo) | 30-d morbidity (%) | 30-d mortality (%) | 1-yr OS (%) | 5-yr OS (%) | Outcome parameter | Frailty/OS (OR) |
| Hodari et al[34] | United States | 5 | R | 2095 | NR | NR | Esophagectomy | Modified Canadian age index | 17.8 | NR | NR | NR | 96 | NR | Postoperative complications | OR = 31.84, P = 0.015 |
| Sheetz et al[35] | United States | 7 | R | 230 | 62 | 88 | Transhiatal esophagectomy | Lean psoas area (L4 level) | 57.8 | 12.8 | NR | NR | 11 | 0 | Overall survival | OR = 0.456; 95%CI: 0.197-1.054; P = 0.067 |
| Yip et al[26] | United Kingdom | 5 | P | 36 | 63 | 86 | Neoadjuvant chemotherapy and esophagectomy | Body composition | 26 | 30 | 26 | 0 | NR | NR | No multivariate outcome analysis | NR - significant increase in complications and decrease in survival |
| Awad et al[27] | United Kingdom | 7 | P | 47 | 63 | Esophagectomy gastrectomy | Body composition | NR | 24 | NR | 2.2 | 23.9 | 19 | No multivariate outcome analysis | NR - significant increase in complications with frailty | |
| Tegels et al[49] | The Netherlands | 5 | R/P | 70 | 59 | Gastrectomy | Groningen frailty index | 28 | 6 | NR | 9.1 | NR | NR | 30-d mortality | 3.96 (95%CI: 1.12-14.09, P = 0.03) |
According to the Newcastle-Ottawa Scale ranging from 1 to 9 stars. Age and OS are presented as median values unless indicated otherwise. NR: Not reported; OS: Overall survival; OR: Odds ratio; P: Prospective trial; P/R: Retrospective analysis on prospectively collected data; R: Retrospective trial.
Table 3.
Complies the characteristics of all trials which evaluated frailty in patients undergoing hepato-pancreatico-biliary resections
| Ref. | Country | Quality score1 | Study design | Sample size | Age (yr) | Male sex (%) | Surgery type | Parameter used to define frailty | Postoperative complication rate | Follow-up (mo) | 30-d morbidity (%) | 30-d mortality (%) | 1-yr OS (%) | 5-yr OS (%) | Outcome parameter | Frailty/outcome (OR) |
| Harimoto et al[40] | Japanese | 6 | R | 186 | 67 | 40 | Partial hepatectomy HCC | L3 muscle area | NR | 60 | NR | NR | NR | 71 | 5 yr survival | OR = 0.9; 95%CI: 0.84-093, P = 0.002 |
| van Vledder et al[41] | The Netherlands | 5 | R | 196 | 65 | 61 | Liver resection for CRLM | Skeletal muscle mass | NR | 29 | NR | NR | 94 | 43 | Overall survival | OR = 14.4; 95%CI: 18.76-31.2 |
| Valero et al[42] | United States | 7 | R | 96 | 62 | 61 | Liver resection liver transplantation | Total psoas area and total psoas volume | 29 | 26 | NR | NR | 82 | 47 | Complication rate | OR = 3.06; 95%CI: 1.07-8.52, P = 0.003 |
| Englesbe et al[43] | United States | 5 | R | 163 | 53 | NR | Liver transplantation | Total psoas area and psoas density | NR | 36 | NR | NR | NR | NR | Overall survival rate | OR = 0.27; 95%CI: 0.11-0.33, P = 0.001 |
| Waits et al[44] | United States | 8 | R | 348 | 51 | 62 | Liver transplantation | Total psoas area and psoas density and age - summarized in new parameter "monomorphometric age" | NR | 60 | NR | NR | 85 | 59 | 1 and 5 yr survival | OR = 1.04; 95%CI: 1.03-1.06, P = 0.001 |
| Masuda et al[76] | Japanese | 5 | R | 2014 | 48 | 50 | Living donor liver transplantation | Total psoas area | 18 | 60 | NR | NR | 75 | 89 | 1 and 5 yr survival | OR = 2,06; 95%CI: 1.1-4.2, P = 0.05 |
| Kaido et al[32] | Japanese | 6 | P | 124 | 54 | NR | Living donor liver transplantation | Skeletal muscle mass and bioimpedance analysis | NR | 60 | NR | NR | 80 | 73 | 1 and 5 yr survival | OR = 4.85; 95%CI: 2.092-11.79, P = 0.001 |
| Peng et al[46] | United States | 6 | R | 259 | 68 | 60 | Liver resection for CRLM | Total psoas area | 10 | 60 | NR | NR | 65 | 26 | Postoperative complications | OR = 3.1; 95%CI: 1.14-8.29, P = 0.02 |
| Amini et al[47] | United States | 7 | R | 763 | 67 | 57 | Pancreatic resection | Total psoas area and total psoas volume | 48 | 24 | 0.5 | 48 | 76 | 24 | Postoperative complications | OR = 1.79; 95%CI: 1.15-2.56, P = 0.002 |
| Dale et al[33] | United States | 9 | P | 76 | 67 | 55 | Pancreaticoduodenectomy | Fried’s criteria, Short Physical Performance Battery, Vulnerable Elderly Survey | 80 | 1 | 4 | 21 | NR | NR | Postoperative complications | OR = 4.06, P = 0.01 |
| Joglekar et al[48] | United States | 6 | R | 118 | 65 | 75 | Pancreatic resection | Total psoas index and psoas density | 78 | 3 | NR | 23 | NR | NR | Postoperative complications | OR = 2.78; 95%CI: 2.28-22, P = 0.02 |
| Peng et al[45] | United States | 6 | R | 557 | 66 | 53 | Pancreatic resection | Total psoas area | 47 | 36 | NR | NR | 62 | 3 a OS: 36 | 3 yr OS | OR = 1.68; 95%CI: 1.32-2.11; P = 0.001 |
According to the Newcastle-Ottawa Scale ranging from 1 to 9 stars. Age and OS are presented as median values unless indicated otherwise. NR: Not reported; OS: Overall survival; OR: Odds ratio; P: Prospective trial; P/R: Retrospective analysis on prospectively collected data; R: Retrospective trial.
Data pertaining to patient demographics (age and sex), assessment used, type of surgery and the number of patients were collected for each article and are displayed in Table 4. Additionally, data relating to short-term clinical outcomes such as 30-d morbidity and mortality, as well as long-term outcomes including median, 5-year overall and 1-year overall survival were recorded from each study. Sarcopenia and frailty, as well as other end points used for analyses were not homogenously defined throughout the studies. The different approaches to define sarcopenia and frailty along with relevant clinical and outcome parameters used along with the quality scale of the included studies (Tables 1-3). While a direct comparison between the studies was therefore not possible due to their heterogeneity, data were amassed from these studies to inform a comprehensive review.
Table 4.
Makary et al[22] did report on the surgical outcomes of a large cohort of older patients in which frailty was assessed using a frailty scale based on the fried frailty phenotype
| Characteristic | |
| Weakness | Weakness should be assessed by grip strength and measured directly with a hand held JAMAR dynamometer (Sammons, Preston Rolyan). Three serial tests of maximum grip strength with the dominant hand will be performed and a mean of the three values will be calculated and adjusted by body mass index and gender. Actual weakness will be defined in the lowest 20th percentile of a community dwelling adults of 65 yr and older |
| Shrinking | Shrinking should be defined through a self-report as unintentional weight loss above 10 pounds during the last year |
| Exhaustion | Exhaustion should be measured by responses following 2 statements from the modified 10 items Center for Epidemiological Studies - Depression scale: "I felt that everything I did was an effort and I could not get going" and "How often in the last week did you feel way?" and will be given the opportunity to reply with 0 = rarely or none of the time (< 1 d); 1 = some or a little time (1-2 d); 2 = a moderate amount of time (3-4 d); and 3 = most of the time. Patients answering either with 2 or 3 will be classified as exhausted |
| Low activity | Physical activities should be assessed using the Minnesota Leisure Time Activities Questionnaire which includes frequency and duration. The focus should be placed on activities in the past 2 wk prior to operation. Weekly tasks will be converted to equivalent kilocalories of expenditure, and individuals reporting a weekly kilocalorie expenditure in the lowest 20th percentile for their gender will be classified as having low activity |
| Slow walking speed | Walking speed should be measured combining 3 trials of walking 15 feet at a normal pace for the patient. Patients with a walking speed in the lowest 20th percentile, adjusted for gender and height, will be scored as having a slow walking speed |
FRAILTY AND SARCOPENIA IN OLDER ADULTS UNDERGOING SURGERY: GENERAL CONSIDERATIONS
Frailty is associated with a decline in physiologic reserve and function across multiple physiologic systems[51]. In the absence of a gold standard, frailty has been operationally defined by Fried et al[20] as meeting 3 out of 5 phenotypic criteria indicating compromised energetics: Low grip strength, low energy, slowed waking speed, low physical activity, and/or unintentional weight loss. While frailty has not been widely evaluated in surgical patients, Makary et al[22] did report on the surgical outcomes of a large cohort of older patients in which frailty was assessed using a frailty scale based on the Fried frailty phenotype (Table 4). The authors reported that preoperative frailty was associated with an increased risk of postoperative complications. Specifically, patients with moderate or severe frailty had roughly twice (moderate: OR = 2.06, 95%CI: 1.18-3.6; severe: OR = 2.54, 95%CI: 1.12-5.77) the odds of complications compared with non-frail patients. The authors also reported that frailty independently predicted length of stay with moderate or severe frailty having a 44%-53% and 65%-89%, respectively, longer hospital stays than non-frail patients. Of note, the power of frailty to predict worse outcomes was much higher than traditional peri-operative assessments alone (Figure 2). These data emphasize how frailty adds valuable information to standard preoperative risk assessments, yet highlight how defining frailty in the peri-operative period can be challenging.
Figure 2.
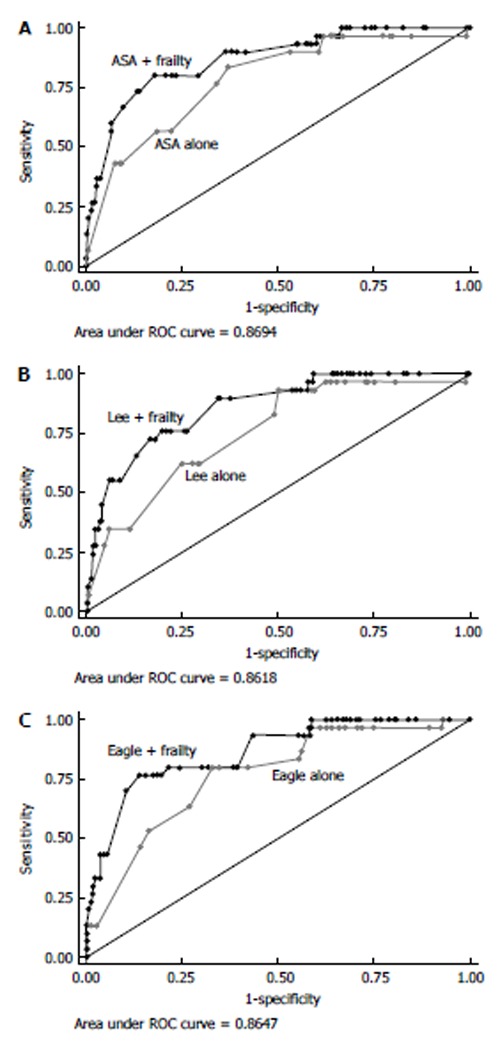
Power of frailty to predict worse outcomes was much higher than traditional peri-operative assessments. A: American Society of Anesthesiologists (ASA); B: Lee; C: Eagle risk indices. Each panel shows the area under the receiver operator characteristics (ROC) curve to demonstrate the ability of the specific risk index to predict surgical complications and discharge to an assisted or skilled nursing facility. Frailty was added to the risk index scoring to demonstrate the combined ability of these indices to predict discharge disposition. Used with permission Makary et al[22], 2010.
A full combined geriatric assessment (CGA) can take several hours and includes assessments such as activities of daily living, geriatric depression scores, and timed “up and go” tests[52]. Specifically, the risk of mortality among patients with frailty ranged from 1.1%-11.7%, with frail patients up to 12 times more likely to die compared with non-frail patients in a recent review on the use of CGA in gastrointestinal surgery[52]. Due to its time consuming nature, the National Cancer Institute and the National Institute of Aging recommends this scoring system only for patients with special needs who are deemed at high risk[7]. In addition to CGA, other parameters have been used to assess frailty and sarcopenia in older patients undergoing gastrointestinal surgery. For example, in a large cohort study of 76106 patients from the NSQIP database, Amrock et al[53] reported that preoperative impaired cognition, low albumin level, previous falls, low hematocrit levels and a high prevalence of comorbidities were associated with an increased 6 mo mortality and post discharge institutionalization among older patients undergoing major abdominal operations. While the authors concluded that preoperative data could help define frailty and predict the geriatric-specific surgical risk, the study failed to provide a clear definition for frailty in gastrointestinal surgical patients. Other studies have suggested that the Charlson index, timed “up and go” tests, Katz score or the Mini cog score, as well as serum albumin levels below 3.4 g/dL and the Braden score all may be associated with postoperative outcomes[28,54,55]. Each of these parameters have not been shown, however, to improve the risk prediction compared with the Fried Frailty Phenotype when used alone.
Sarcopenia has been proposed as another means to assess frailty. In fact, when Fried et al[20] first described the frailty phenotype and its association with mortality and morbidity, the potential link between frailty and sarcopenia was noted. Specifically, patients deemed to be frail who had a concomitant decrease in muscle mass were more likely to suffer from disabilities and a worsening in their mobility vs non-frail patients. Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength. Unlike cachexia, which is typically associated with weight loss due to chemotherapy or a general malignancy-related cachexia syndrome, sarcopenia relates to muscle mass rather than simply weight. As such, while weight reflects nutritional status, sarcopenia - the loss of muscle mass - is a more accurate and quantitative global marker of frailty[56]. Usually characterized as low muscle mass and low muscle function (strength/performance), sarcopenia is typically defined using an axial cross-sectional image of the psoas muscle at the level of L3[57,58]. Using this technique, sarcopenia is defined as the total psoas area (TPA), with sarcopenic patients having a smaller TPA[58]. More recent studies have suggested that assessment of the entire volume of the psoas muscle (TPV) may be a better means to define sarcopenia rather than a single axial image (Figure 3)[47,59]. In addition, other investigators have suggested the use of dual X-ray absorptiometry as an alternative means to screen for sarcopenia. This tool has not been widely adopted, however, as routine computed tomography is more commonly utilized in patients prior to surgery.
Figure 3.
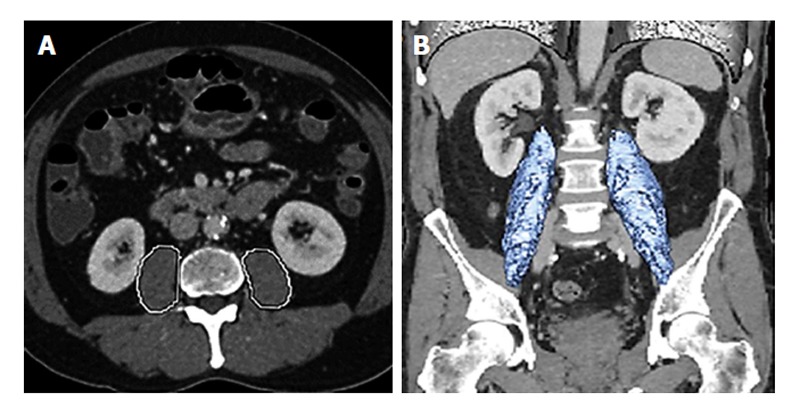
Define sarcopenia rather than a single axial image. A: Total psoas area is measured by circling both psoas muscles at the level of the patients computed tomography where both iliac crests are visible; B: Total psoas volume is measured at the full length of the psoas muscles and normalized for the patients body surface area. Used with permission Amini et al[47], 2015.
Both the European and the American Society of Ageing have recommended that sarcopenia be recognized as a geriatric syndrome[57]. In addition, several studies have noted an association between sarcopenia and increased risk of adverse outcomes following surgery[15,59]. For example, Brant et al[58] reported that patients with sarcopenia were particularly vulnerable in the setting of significant physiologic stressors like major surgery. The importance of sarcopenia in the prediction of outcome after gastrointestinal surgery has been particularly highlighted in several studies[43,47,59]. The hope is that identification of patients with frailty or sarcopenia who are at high risk of perioperative morbidity can guide patient-physician discussions prior to surgery, as well as identify appropriate patients for “pre-habilitation”[60].
GASTRO-ESOPHAGEAL MALIGNANCIES
Gastric and esophageal cancer represent a worldwide major health problem. While the incidence in Western countries is relatively low with 18170 and 22220 new esophageal and gastric malignancies, respectively, diagnosed in the United States in 2014, gastro-esophageal cancer is a leading indication for cancer resection in the Eastern hemisphere[61,62].
Frailty and gastro-esophageal malignancies
Because the incidence of esophageal and gastric cancer increases with age, there has been a particular interest in the management of these diseases in older patients. In fact, in 2014 Balducci et al[63] published guidelines on the treatment of older patients with gastro-esophageal malignancies. The authors noted that for individuals whose life expectancy without cancer exceed that with cancer, the estimated risk of chemotherapy complications may reveal those patients in need of additional care and those patients in whom the risk of treatment may exceed the potential benefits. Importantly, the authors noted that the individual’s general life expectancy should be defined using the CGA and an assessment of patient frailty.
Sarcopenia and gastro-esophageal malignancies
Specific publications on the impact of frailty and sarcopenia on postoperative outcomes following gastro-esophageal surgery are rather scarce (Table 1)[34,35,63]. In a small study, Pultrum et al[64] reported that esophagectomy was justified in older patients as advanced age alone had only a minor impact on a patients’ postoperative course. The authors noted, however, that frailty was much more strongly associated with both short- and long-term outcomes among patients undergoing esophageal surgery. In a separate study, Hodari et al[34] examined a much larger cohort of 2095 patients undergoing esophagectomy and reported that higher frailty scores were associated with increased postoperative morbidity and mortality. In this study, the frailty score was divided into 5 different categories and the incidence of peri-operative mortality incrementally increased with the frailty score, with mortality only 1.8% among patients with a frailty score 0 vs 23.1% among those patients with a frailty score 5 (P = 0.001). While the authors assessed several other parameters associated with postoperative outcomes, only age and frailty were significantly associated with risk of peri-operative morbidity and mortality. Examining a separate cohort of patients undergoing esophageal cancer, Sheetz et al[35] confirmed a strong association between frailty, sarcopenia and peri-operative risk of morbidity among patients undergoing esophagectomy. Using preoperative computed tomography scans in 230 subjects who had undergone transhiatal esophagectomy for malignancy, the authors assessed lean psoas area (LPA) and correlated it with overall and disease-free survival[35]. Analyses demonstrated that increasing LPA correlated with both overall and disease-free survival and the authors concluded that core muscle size appeared to be an independent predictor of outcome[35].
To date, the role of sarcopenia to predict peri-operative outcomes among patients undergoing esophagectomy has been evaluated in only a handful of studies[26,27]. Yip et al[26] studied 35 patients who received neoadjuvant chemotherapy followed by surgical resection for esophageal cancer. The authors noted that changes in computed tomography body composition were associated with outcomes. Specifically, fat mass, subcutaneous fat to muscle ratio and visceral to subcutaneous adipose tissue ratio were each associated with circumferential resection margin. While sarcopenia was more prevalent after neoadjuvant chemotherapy, changes in body composition were not associated with perioperative complication or survival. In a separate study, Awad et al[27] similarly noted marked changes in body composition following neoadjuvant chemotherapy for esophageal cancer. In this study, the authors reported on 47 patients treated with neoadjuvant chemotherapy for esophageal cancer. The proportion of patients with sarcopenia increased from 57% pre-therapy to 79% post-neoadjuvant therapy. Similar to the study by Yip et al[26], no association was demonstrated between sarcopenia and hospital stay, morbidity or mortality. Given the very small number of patients included in the studies by Yip et al[26] (n = 35) and Awad et al[27] (n = 47), the lack of association between sarcopenia and peri-operative outcomes may have been due to low sample size and a type II statistical error. Future larger studies are necessary to better delineate the impact of sarcopenia on peri-operative and long-term outcomes among patients with esophageal cancer undergoing surgical resection.
Similar to esophageal cancer, gastric cancer patients are at high risk for malnutrition and therefore older patients with gastric cancer may be at a particularly high risk of frailty. In fact, the prevalence of frailty and sarcopenia among patients with gastric cancer has been reported to be as high as 30% and 38%, respectively[49,65]. Despite the high incidence, data on the association of frailty, sarcopenia and outcomes of patients after gastric resection are limited. In a review on the topic of gastric cancer surgery, Tegels et al[49] described a strong association between frailty, sarcopenia and increased postoperative mortality after gastric resections. Specifically, the authors highlighted the need for better preoperative risk assessment using comorbidity index, assessment of nutritional status, and frailty assessment. In particular, Tegels et al[65] noted that assessment tools such as the Groningen Frailty Indicator (GFI), Edmonton frail scale, or the Hopkins frailty scale should be used to help identify patients for preoperative optimization using pre-habilitation. In a separate prospective study of 180 patients with gastric cancer, the same authors examined the association of frailty with morbidity and mortality after gastric cancer surgery. In this study, patients scheduled for gastric cancer surgery were preoperatively assessed with the GFI and the Short Nutritional Assessment Questionnaire (SNAQ). Of note, patients with a GFI ≥ 3 had a mortality of 23.3% vs 5.2% in the lower GFI group. Similarly, those patients who scored poorly on the SNAQ had a higher mortality (13.3%) vs those deemed to have better nutritional status (3.2%). The authors concluded that frailty and nutritional status were important factors in preoperative decision making among elderly patients being considered for gastric resection. While the impact of frailty and malnutrition on peri-operative outcomes has been examined, no study on the role sarcopenia to predict morbidity and mortality of patients undergoing gastric surgery has been reported to date.
COLORECTAL CANCER
In 2014, 132700 patients were diagnosed with colorectal cancer in the United States. More than half of patients with colorectal cancer are older than 65 years and approximately 70% are diagnosed at early stages, when surgical resection is feasible[66].
Frailty and colorectal cancer
Among older patients undergoing surgery for colorectal cancer, frailty and sarcopenia have been investigated as predictors of outcome in a small number (Table 2). In particular, pre-operative frailty has been associated with a decline in the patients’ activities of daily living and the instrumental activities of daily living after colon resection[67]. Other studies have noted that frailty can significantly impact peri-operative outcomes. For example, Obeid et al[36] reported on a large group of patients (n = 58448) with colorectal cancer derived from the NSQIP database. The authors noted that the proportion of patients who experienced a severe Clavien class IV-V complication following colorectal surgery increased from 5.8% to 56.3% when comparing non-frail vs frail patients (P = 0.0001). Frailty was also independently associated with a longer intensive care unit stay and increased peri-operative mortality. In a different study, Neuman et al[37] reported on 12979 patients from the SEER-Medicare database above the age of 80 who underwent a colorectal resection. Older age, male gender, frailty, and dementia were all associated with decreased survival at 1 year. Although only 4.4% of patients were considered frail, this factor had the strongest association with mortality with an odds ratio of 8.4. While the authors concluded that frailty was an important predictor of outcome, the study was limited due to the nature of the administrative data used in the analyses. In a different study that utilized institutional data, Robinson et al[68] reported on 201 subjects, many of whom underwent an elective colorectal surgery. Pre-operative frailty was associated with increased post-operative complications after colorectal surgery (frail 58% vs non-frail 21%); frail patients also had longer hospital stays and higher 30-d readmission rates. Furthermore, frailty has noted to be a good predictor of complications (AUC 0.702). Other authors have noted that an elderly modified Physiological and Operative Severity Score for the enumeration of Mortality and morbidity (E-POSSUM) is also a good tool for predicting mortality after major colorectal surgery in the elderly (AUC 0.86)[29,31].
Table 2.
Complies the characteristics of all studies evaluated in patients undergoing colorectal resections
| Ref. | Country | Quality score1 | Study design | Sample size | Age (yr) | Male sex (%) | Surgery type | Parameter used to define frailty | Postoperative complication rate | Follow-up (mo) | 30-d morbidity (%) | 30-d mortality (%) | 1-yr OS (%) | 5-yr OS (%) | Outcome parameter | Frailty/outcome (OR) |
| Rønning et al[67] | New Zealand | 6 | P | 84 | 82 | 41 | Colorectal surgery | Combined geriatric assessment | NR | 22 | NR | NR | NR | NR | No outcome analysis | NR - significant postoperative decrease of ADL |
| Obeid et al[36] | United States | 5 | R | 58448 | NR | 48 | Colectomy (33% malignant causes) | Canadian frailty index | 26 | NR | 15.9 | 4.6 | NR | NR | 30 d mortality and morbidity | OR = 14.4; 95%CI: 18.76-31.2 |
| Neuman et al[37] | United States | 6 | R | 12979 | 84 | 39 | Colectomy for colorectal cancer | Johns Hopkins adjusted case mix system | NR | 16 | NR | NR | 85.7 | NR | 1 yr survival | OR = 8.4; 95%CI: 6.4-11.1, P = 0.001 |
| Robinson et al[68] | United States | 4 | P | 60 | 75 | 97 | Colectomy for colorectal cancer | Individual frailty score | 10 | 6 | 10 | 2 | NR | NR | Hospital and health care costs | NR - significant association to costs and length of stay |
| Tran Ba Loc et al[29] | France | 7 | P | 1186 | 76 | NR | Major colorectal surgery | Elderly POSSUM score | 41 | 3 | NR | 2 | NR | NR | 30 d mortality | AUC 0.86 (0.81-0.92) |
| Tan et al[31] | China | 6 | P | 83 | 82 | NR | Colorectal resections | Fried frailty criteria | 22 | NR | 29 | 0 | NR | NR | 30 d morbidity | OR = 4.08; 95%CI: 1.43-11.64, P = 0.006 |
| Sabel et al[38] | United States | 5 | R | 302 | 68 | 52 | Colorectal resection | Psoas area; Psoas density | 58 | 34 | NR | NR | NR | NR | No outcome analysis | NR |
| Lieffers et al[39] | Canada | 5 | R | 234 | 63 | 135 | Colorectal resection | Skeletal muscle index | 6 | NR | NR | NR | NR | NR | Postoperative complications | OR = 4.6; 95%CI: 1.513.9, P = 0.007 |
| Reisinger et al[50] | The Netherlands | 5 | P/R | 340 | 69 | 50 | Colorectal resection | L3 muscle index | 21 | 24 | NR | 4.5 | NR | NR | Postoperative complications | OR = 43.3; 95%CI: 2.74-685.2, P = 0.007 |
| Huang et al[30] | China | 6 | P | 142 | 62 | 62 | Colorectal resection | L3 muscle index and gait speed and grip strength | 28 | NR | NR | NR | NR | NR | Postoperative complications | OR = 4.524, 95%CI: 1.584-12.921, P = 0.007 |
According to the Newcastle-Ottawa Scale ranging from 1 to 9 stars. Age and OS are presented as median values unless indicated otherwise. NR: Not reported; OS: Overall survival; OR: Odds ratio; P: Prospective trial; P/R: Retrospective analysis on prospectively collected data; R: Retrospective trial.
Sarcopenia and colorectal cancer
Similar to frailty, the effect of sarcopenia on post-surgical outcomes of patients with colorectal cancer has only been evaluated in a limited fashion. Robinson et al[68] prospectively examined 302 patients who underwent resection of colorectal cancer and noted that psoas density was a better predictor of postoperative complications compared with age, body mass index or preoperative patient comorbidities. The authors reviewed patient computed tomography scans to measure psoas area, density, subcutaneous fat, visceral fat and total body fat. Among the parameters studied, psoas density was found to be the best predictor of surgical complications among patients undergoing colectomy for colon cancer. In a separate prospective study by Lieffers et al[39] that included 234 older patients who underwent colon resection, sarcopenia was strongly associated with delayed recovery, postoperative infections (23.7% sarcopenic patients vs 12.5% non sarcopenic patients, P = 0.025), as well as an increased risk of discharge to a nursing facility (14.3% sarcopenic patients vs 5.6% non sarcopenic patients, P = 0.024)[39]. Similarly, Reisinger et al[50] reported a series of 331 older patients who underwent colorectal cancer surgery and demonstrated that a combination of age related parameters such as frailty, sarcopenia and malnutrition were strongly associated with adverse outcomes. Sarcopenia alone was predictive of 30 d in hospital mortality (8.8% sarcopenic vs 0.7% nonsarcopenic patients, P = 0.001). Most recently, Huang et al[30] defined sarcopenia through a combination of monomorphometric measurements and physical performance and used it to define low postoperative outcomes. By this, the authors showed, that including the muscles’ functional aspect (handgrip strength and 6-m usual gait speed) to the definition of sarcopenia results in a better prediction for postoperative complications as compared to measurement alone.
HEPATO-PANCREATO-BILIARY MALIGNANCIES
Surgery is commonly used to treat patients with a wide variety of hepato-pancreato-biliary (HBP) diseases. Many of these disease including liver, biliary, and pancreatic malignancies are more common in an aged population. In addition, HPB procedures tend to be complex operations that can be associated with substantial possible morbidity. As such, accurate preoperative assessment of aged patients being considered for HPB surgery is of particular importance.
In 1997, in one of the earlier studies to examine the impact of age on HPB surgery, Fong et al[69] reported on the outcome of 133 patients over the age of 65 years who underwent a hepatic resection. In this study, Fong et al[69] noted that age was an independent risk factor for increased risk of morbidity. Perhaps more importantly, however, the authors noted that major hepatic resection could be performed safely and with good functional outcomes among well-selected aged patients. Over the last several decades, multiple other investigators have similarly reported good outcomes in well-selected older patients undergoing hepatic resection[70,71]. For example, Reddy et al[71] reported on 856 patients who underwent a major hepatectomy (resection of 3 or more segments) and noted that increasing age was independently associated with postoperative mortality. In fact, each 1-year and 10-year increase in age resulted in an odds ratio of mortality after major hepatic resection of 1.036 and 1.426, respectively. In a separate study of 7764 patients who had colorectal liver metastasis, Adam et al[72] noted that age was associated with outcome, but major resection could be performed in elderly patients with acceptable morbidity. The authors found higher mortality and morbidity rates in older than in younger patients [3.8% and 32.3% in older, 1.6% and 28.7% in younger patients (both P < 0.001)] but did not further investigate frailty or sarcopenia in this cohort. Sixty-day postoperative mortality and morbidity were 3.8% and 32.3%, respectively, compared with 1.6% and 28.7% in younger patients. Of note, 5-year survival was relatively comparable even among very aged patients (70-75 years: 57.8% vs 75-80 years: 55.3% vs > 80 years: 54.1%), suggesting that surgery may have potential benefit even in very well selected aged patients.
Frailty and hepato pancreatico biliary malignancies
While age has been the topic of several investigations, the specific impact of frailty itself has been less well studied. Giovannini et al[73] suggested that a decrease in serum albumin may be a marker of frailty due to an altered albumin synthesis and the patient’s inability to compensate for albumin loss. Unlike frailty, while still limited, several papers have investigated the impact of sarcopenia on outcomes after liver surgery[40,43,74,75]. Several studies have noted an association between sarcopenia and both short- and long-term outcomes among patients undergoing hepatic surgery[40,41,43,74,75]. For example, Durand et al[74] studied whether muscle atrophy was of prognostic value among patients with cirrhosis undergoing surgery. The authors demonstrated that transversal psoas muscle thickness was significantly associated with mortality, independent of Model for End Stage Liver Disease (MELD) score. In a different study, Valero et al[42] examined whether sarcopenia impacted the risk of post-operative complications following resection or transplantation in patients with primary liver tumors. Among 96 patients, the presence of sarcopenia was an independent predictive factor of post-operative complications, but was not associated with long-term survival. In a study that examined only liver transplant recipients, Englesbe et al[43] noted that psoas area correlated poorly with MELD score and serum albumin. Central sarcopenia strongly correlated with mortality after liver transplantation, as 1-year survival was 49.7% among transplant recipients with the smallest psoas area vs 87.0% among transplant recipients with the largest psoas area. Kaido et al[32] reported a similar effect on a cohort of 124 living donor liver transplant patients in 2013. In this study the overall survival rate in patients with low skeletal muscle mass was significantly lower than in patients with normal/high skeletal muscle mass (P < 0.001). Other studies have similarly noted that morphometric age correlated with morbidity and mortality after liver transplantation with better discrimination than chronological age[44,76]. Sarcopenia has similarly been demonstrated to be an important prognostic factor for patients undergoing liver resection for colorectal liver metastasis. Peng et al[46] reported that sarcopenia was strongly associated with an increased risk of major complications, extended intensive care unit stay, and a longer overall hospital.
Sarcopenia and hepato pancreatico biliary malignancies
Similar to liver resection, frailty and sarcopenia have not been widely assessed in patients after pancreatic operations. Several studies have reported that age is a risk factor for increased morbidity and mortality[77-79]. For example, in one large study that investigated over three-thousand patients who underwent pancreatic resection in the state of Texas, Riall et al[77] reported that increased age was an independent risk factor for mortality after pancreatic resection. In fact, in-hospital mortality increased with each increasing age group from 2.4% among patients < 60 years to 11.4% among patients > 80 years. Likewise, postoperative length of stay increased with each increasing age group, going from 11 to 15 d. Of particular interest was the authors’ finding that the increase in mortality among older patients was most pronounced among those patients treated at a low vs high volume hospital. While these data and others suggest therefore that age may be associated with outcomes, multiple other studies have noted that pancreatic surgery can be performed safely in well selected older patients[78-80]. Dale et al[33] prospectively evaluated the additional value of geriatric assessment in a cohort of older patients undergoing a pancreaticoduodenectomy for pancreatic tumors. Among 76 older patients, significant unrecognized vulnerability was identified using the geriatric assessment. In turn, Fried’s exhaustion, a vulnerable elders survey score > 3, as well as a short physical performance battery score < 10 all correlated with an increased risk of severe complication after pancreaticoduodenectomy. As such, the authors concluded that geriatric assessment may help identify older patients at high risk for complication from pancreatic surgery.
Several series have similarly suggested that sarcopenia may be an important predictor of post-operative morbidity and mortality following pancreatic surgery[45-48]. For example, Joglekar et al[48] reported a relation between sarcopenia defined by the psoas muscle density and worse outcome after pancreatic resection. In a separate study, Peng et al[45] examined 557 patients undergoing resection of pancreatic adenocarcinoma and reported on the impact of sarcopenia on outcomes following surgery. Sarcopenia was associated with an increased three year mortality (HR = 1.63, P < 0.001) (Figure 4A). Of note, even after controlling for tumor-specific factors such as poor tumor differentiation, margin status, and lymph node metastasis, sarcopenia defined by TPA remained independently associated with risk of long-term death. More recently, rather than assessing sarcopenia using only two-dimensional imaging, the same group reported on the effect of three-dimensional psoas volume (TPV) on outcomes following pancreatic resection[47]. In this study, Amini et al[47] noted that more patients were identified as sarcopenic by TPA than TPV. Perhaps more importantly, while TPA-sarcopenia was not associated with a higher risk of postoperative complications (OR = 1.06), TPV-sarcopenia was as strong predictor of post-operative morbidity (OR = 1.79). On multivariate analysis, TPV - sarcopenia remained an independent risk factor of postoperative complications (OR = 1.69), as well as long-term survival (OR = 1.46) (both P < 0.05) (Figure 4B).
Figure 4.
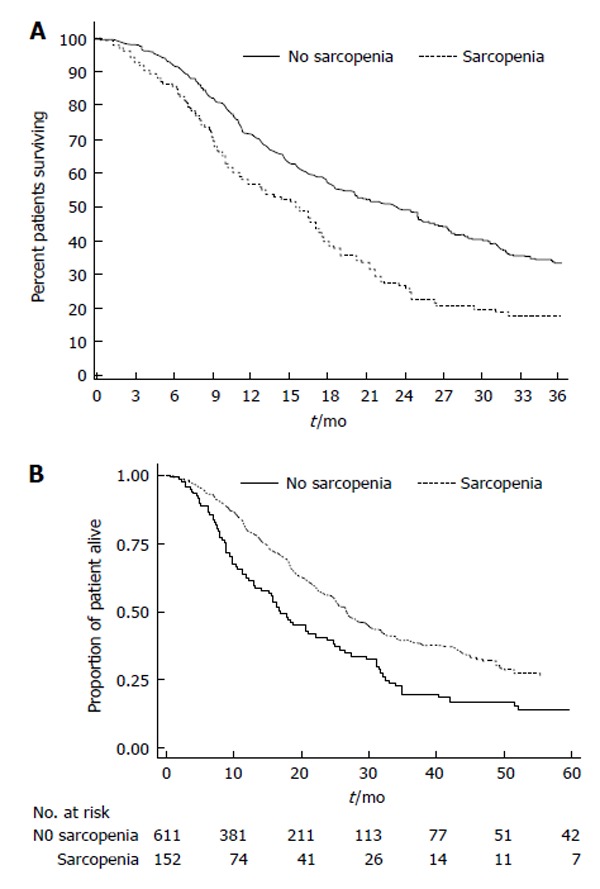
Sarcopenia was associated with an increased three year mortality. A: The presence of sarcopenia was also associated with the risk of death (no sarcopenia, 18.0 mo; 40.0% vs sarcopenia, 13.7 mo; 23.0% vs median, 3-yr survival, respectively; P = 0.01) in patients undergoing pancreatic surgery. Used with permission Peng et al[45], 2012; B: The overall survival according to total psoas volume stratified by sarcopenia patients vs no sarcopenia patients quartiles in patients undergoing pancreatic surgeries. Used with permission Amini et al[47], 2015.
CONCLUSION
As the population ages, an increasing number of older patients will require complex gastrointestinal surgical procedures. While chronologic age is an important element in assessing a patient’s peri-operative risk, physiologic age is a more important determinant of outcomes. Geriatric assessment tools are important components of the pre-operative work-up and can help identify patients who suffer from frailty. Such data are important, as frailty has repeatedly been demonstrated to be one of the strongest predictors of both short- and long-term outcome following complicated surgical procedures such as esophageal, gastric, colorectal, and HPB resections. Frailty can sometimes, however, be difficult to assess in an accurate and timely manner. As such, there has been an increasing interest in determining a patient’s “morphometric age”. Sarcopenia, or wasting of lean muscle mass, has been noted to be an emerging important metric of frailty that is associated with peri-operative outcomes. As demonstrated by the data herein reviewed, screening of patients being considered for gastrointestinal surgery should include an assessment of frailty and sarcopenia to target high risk patients for pre-habilitation. Future studies will need to continue to define the optimal combination of factors (e.g., clinical, performance, and morphometric) to predict optimally a patient’s peri-operative risk.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 24, 2015
First decision: September 17, 2015
Article in press: October 27, 2015
P- Reviewer: Bramhall S, Pera M S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.McCormack D, Mai X, Chen Y. Determinants of vitamin D supplement use in Canadians. Public Health Nutr. 2015:Epub ahead of print. doi: 10.1017/S1368980015001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;(260):1–161. [PubMed] [Google Scholar]
- 3.Audisio RA, van Leeuwen B. When reporting on older patients with cancer, frailty information is needed. Ann Surg Oncol. 2011;18:4–5. doi: 10.1245/s10434-010-1327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colloca G, Santoro M, Gambassi G. Age-related physiologic changes and perioperative management of elderly patients. Surg Oncol. 2010;19:124–130. doi: 10.1016/j.suronc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11:449–460. doi: 10.1097/00130404-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 7.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 9.Audisio RA, Bozzetti F, Gennari R, Jaklitsch MT, Koperna T, Longo WE, Wiggers T, Zbar AP. The surgical management of elderly cancer patients; recommendations of the SIOG surgical task force. Eur J Cancer. 2004;40:926–938. doi: 10.1016/j.ejca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Refaie WB, Parsons HM, Habermann EB, Kwaan M, Spencer MP, Henderson WG, Rothenberger DA. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg. 2011;253:947–952. doi: 10.1097/SLA.0b013e318216f56e. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Leung D, Trainor K, Borgen P, Norton L, Hudis C. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46:121–126. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 13.Zbar AP, Gravitz A, Audisio RA. Principles of surgical oncology in the elderly. Clin Geriatr Med. 2012;28:51–71. doi: 10.1016/j.cger.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny MM, Peterson BL, Kornblith AB, Muss HB, Wheeler J, Levine E, Bartlett N, Fleming G, Cohen HJ. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 16.McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol. 2014;32:2570–2580. doi: 10.1200/JCO.2014.55.1960. [DOI] [PubMed] [Google Scholar]
- 17.Adjuvant therapy for node-negative breast cancer. N Engl J Med. 1989;321:469–473. doi: 10.1056/NEJM198908173210711. [DOI] [PubMed] [Google Scholar]
- 18.Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen HO, Bakka A, Wyller TB. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg. 2007;246:215–221. doi: 10.1097/SLA.0b013e318070838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 21.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 22.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–466. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sørbye L, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, Maisey N, Ross P, Gaya A, Landau DB, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 27.Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, Catton JA, Lobo DN. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206:544–550. doi: 10.1016/j.amjsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran Ba Loc P, du Montcel ST, Duron JJ, Levard H, Suc B, Descottes B, Desrousseaux B, Hay JM. Elderly POSSUM, a dedicated score for prediction of mortality and morbidity after major colorectal surgery in older patients. Br J Surg. 2010;97:396–403. doi: 10.1002/bjs.6903. [DOI] [PubMed] [Google Scholar]
- 30.Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17:O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 31.Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, Tomiyama K, Yagi S, Mori A, Uemoto S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 33.Dale W, Hemmerich J, Kamm A, Posner MC, Matthews JB, Rothman R, Palakodeti A, Roggin KK. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg. 2014;259:960–965. doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodari A, Hammoud ZT, Borgi JF, Tsiouris A, Rubinfeld IS. Assessment of morbidity and mortality after esophagectomy using a modified frailty index. Ann Thorac Surg. 2013;96:1240–1245. doi: 10.1016/j.athoracsur.2013.05.051. [DOI] [PubMed] [Google Scholar]
- 35.Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, Orringer MB, Chang AC. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013;26:716–722. doi: 10.1111/dote.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obeid NM, Azuh O, Reddy S, Webb S, Reickert C, Velanovich V, Horst HM, Rubinfeld I. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72:878–883. doi: 10.1097/TA.0b013e31824d0f70. [DOI] [PubMed] [Google Scholar]
- 37.Neuman HB, Weiss JM, Leverson G, O’Connor ES, Greenblatt DY, Loconte NK, Greenberg CC, Smith MA. Predictors of short-term postoperative survival after elective colectomy in colon cancer patients ≥ 80 years of age. Ann Surg Oncol. 2013;20:1427–1435. doi: 10.1245/s10434-012-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabel MS, Terjimanian M, Conlon AS, Griffith KA, Morris AM, Mulholland MW, Englesbe MJ, Holcombe S, Wang SC. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 2013;108:169–175. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 39.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 41.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 42.Valero V, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, Wolfgang CL, Cameron AA, Philosophe B, Kamel IR, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272–281. doi: 10.1007/s11605-014-2680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waits SA, Kim EK, Terjimanian MN, Tishberg LM, Harbaugh CM, Sheetz KH, Sonnenday CJ, Sullivan J, Wang SC, Englesbe MJ. Morphometric age and mortality after liver transplant. JAMA Surg. 2014;149:335–340. doi: 10.1001/jamasurg.2013.4823. [DOI] [PubMed] [Google Scholar]
- 45.Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, Makary M, Hirose K, Edil B, Choti MA, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, Edil BH, Wolfgang CL, Schulick RD, Choti MA, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, et al. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tegels JJ, De Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH. Improving the outcomes in gastric cancer surgery. World J Gastroenterol. 2014;20:13692–13704. doi: 10.3748/wjg.v20.i38.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 51.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng MA, McMillan DT, Crowell K, Muss H, Nielsen ME, Smith AB. Geriatric assessment in surgical oncology: a systematic review. J Surg Res. 2015;193:265–272. doi: 10.1016/j.jss.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amrock LG, Neuman MD, Lin HM, Deiner S. Can routine preoperative data predict adverse outcomes in the elderly? Development and validation of a simple risk model incorporating a chart-derived frailty score. J Am Coll Surg. 2014;219:684–694. doi: 10.1016/j.jamcollsurg.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SW, Han HS, Jung HW, Kim KI, Hwang DW, Kang SB, Kim CH. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. doi: 10.1001/jamasurg.2014.241. [DOI] [PubMed] [Google Scholar]
- 55.Cohen RR, Lagoo-Deenadayalan SA, Heflin MT, Sloane R, Eisen I, Thacker JM, Whitson HE. Exploring predictors of complication in older surgical patients: a deficit accumulation index and the Braden Scale. J Am Geriatr Soc. 2012;60:1609–1615. doi: 10.1111/j.1532-5415.2012.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 57.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brant MG, Friedmann JN, Bohlken CG, Oliver AG, Wulff JE. Diastereoselective tandem reactions of substituted 3-sulfolenes with bis-vinyl ketones leading to highly functionalized bicyclic and tricyclic frameworks. Org Biomol Chem. 2015;13:4581–4588. doi: 10.1039/c5ob00387c. [DOI] [PubMed] [Google Scholar]
- 59.Hansen RD, Williamson DA, Finnegan TP, Lloyd BD, Grady JN, Diamond TH, Smith EU, Stavrinos TM, Thompson MW, Gwinn TH, et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007;86:952–958. doi: 10.1093/ajcn/86.4.952. [DOI] [PubMed] [Google Scholar]
- 60.Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156:521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 61.American Cancer Society. What are the key statistics about cancer of the esophagus? [Updated 2015 Mar 02] Available from: http://www.cancer.org/cancer/esophaguscancer/detailedguide/esophagus-cancer-key-statistics. [Google Scholar]
- 62.American Cancer Society. What are the key statistics about stomach cancer? [Updated 2015 Mar 06] Available from: http://www.cancer.org/cancer/stomachcancer/detailedguide/stomach-cancer-key-statistics. [Google Scholar]
- 63.Balducci L. Systemic treatment of gastric and esophageal adenocarcinoma in elderly patients. J Gastrointest Oncol. 2015;6:75–78. doi: 10.3978/j.issn.2078-6891.2014.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pultrum BB, Bosch DJ, Nijsten MW, Rodgers MG, Groen H, Slaets JP, Plukker JT. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–1580. doi: 10.1245/s10434-010-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tegels JJ, de Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH. Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg. 2014;18:439–445; discussion 445-446. doi: 10.1007/s11605-013-2443-7. [DOI] [PubMed] [Google Scholar]
- 66.Surveillance Research Program. Cancer of the Colon and Rectum - SEER Stat Fact Sheets. Available from: http://seer.cancer.gov/statfacts/html/colorect.html.
- 67.Rønning B, Wyller TB, Jordhøy MS, Nesbakken A, Bakka A, Seljeflot I, Kristjansson SR. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol. 2014;5:26–32. doi: 10.1016/j.jgo.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202:511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 70.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddy SK, Barbas AS, Turley RS, Gamblin TC, Geller DA, Marsh JW, Tsung A, Clary BM, Lagoo-Deenadayalan S. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg. 2011;212:787–795. doi: 10.1016/j.jamcollsurg.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 72.Adam R, Frilling A, Elias D, Laurent C, Ramos E, Capussotti L, Poston GJ, Wicherts DA, de Haas RJ. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97:366–376. doi: 10.1002/bjs.6889. [DOI] [PubMed] [Google Scholar]
- 73.Giovannini I, Chiarla C, Giuliante F, Vellone M, Ardito F, Nuzzo G. The relationship between albumin, other plasma proteins and variables, and age in the acute phase response after liver resection in man. Amino Acids. 2006;31:463–469. doi: 10.1007/s00726-005-0287-5. [DOI] [PubMed] [Google Scholar]
- 74.Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 75.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 76.Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, Uchiyama H, Ikeda T, Baba H, Maehara Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–407. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- 77.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kow AW, Sadayan NA, Ernest A, Wang B, Chan CY, Ho CK, Liau KH. Is pancreaticoduodenectomy justified in elderly patients? Surgeon. 2012;10:128–136. doi: 10.1016/j.surge.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Nishioka R, Shimizu A, Uchiyama K, Yamaue H. A pancreaticoduodenectomy is acceptable for periampullary tumors in the elderly, even in patients over 80 years of age. J Hepatobiliary Pancreat Surg. 2009;16:675–680. doi: 10.1007/s00534-009-0106-6. [DOI] [PubMed] [Google Scholar]
- 80.de la Fuente SG, Bennett KM, Pappas TN, Scarborough JE. Pre- and intraoperative variables affecting early outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB (Oxford) 2011;13:887–892. doi: 10.1111/j.1477-2574.2011.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


