Abstract
The widespread popularity of new surgical technologies such as laparoscopy, thoracoscopy and robotics has led many surgeons to treat esophageal diseases with these methods. The expected benefits of minimally invasive surgery (MIS) mainly include reductions of postoperative complications, length of hospital stay, and pain and better cosmetic results. All of these benefits could potentially be of great interest when dealing with the esophagus due to the potentially severe complications that can occur after conventional surgery. Moreover, robotic platforms are expected to reduce many of the difficulties encountered during advanced laparoscopic and thoracoscopic procedures such as anastomotic reconstructions, accurate lymphadenectomies, and vascular sutures. Almost all esophageal diseases are approachable in a minimally invasive way, including diverticula, gastro-esophageal reflux disease, achalasia, perforations and cancer. Nevertheless, while the limits of MIS for benign esophageal diseases are mainly technical issues and costs, oncologic outcomes remain the cornerstone of any procedure to cure malignancies, for which the long-term results are critical. Furthermore, many of the minimally invasive esophageal operations should be compared to pharmacologic interventions and advanced pure endoscopic procedures; such a comparison requires a difficult literature analysis and leads to some confounding results of clinical trials. This review aims to examine the evidence for the use of MIS in both malignancies and more common benign disease of the esophagus, with a particular emphasis on future developments and ongoing areas of research.
Keywords: Esophageal disease, Esophageal cancer, Laparoscopic, Robotic, da Vinci, Heller, Reflux disease, Esophageal diverticula
Core tip: Minimally invasive surgery for esophageal diseases is very attractive for reducing potentially serious complications that can occur after conventional surgery. However, if the oncologic long-term results remain the cornerstone of any procedure to treat malignancies, determining the outcomes of surgery for benign diseases requires a deep analysis of published evidence and a comparison with alternative pharmaceutical or endoscopic treatments.
INTRODUCTION
For many years, esophageal surgery has been recognized as very challenging for surgeons and risky for patients[1-3]. However, subspecialized training of surgeons and a case-load centralization have been shown to reduce both perioperative mortality and the so-called “failure to rescue” rates after a life-threatening complication occurs[2,4].
This type of surgery is complicated by the deep location of the esophagus in the neck, the posterior mediastinum and the upper abdomen. Moreover, the esophagus crosses all of these sectors very close to major vascular structures, including the carotids, the jugular vein and the aorta, while the trachea and the pericardium have important connections. Furthermore, the absence of a formal serous layer leads to unsafe anastomosis with a great risk of leakage.
All of these issues, together with the older age and comorbidities of many patients affected by esophageal cancer, could explain the disappointing outcomes of patients who are candidates for surgery. In this scenario, the adoption of the concept of a minimally invasive (endoscopic, thoraco-laparoscopic and robotic) approach could represent an attractive and valuable option.
The introduction of the da Vinci® Robot system to surgical practice added other benefits in terms of feasibility of the most complex esophageal procedures, which were previously precluded by pure laparoscopy and thoracoscopy procedures.
The proven and unquestionable advantages of minimally invasive surgery (MIS) are mainly represented by a reduction in pulmonary complications, wound infections, postoperative pain, and length of postoperative stay compared to open surgery. A superior cosmetic result is an additional benefit, especially when dealing with benign diseases in younger patients. Another recent field of research has demonstrated the important role of MIS in decreasing the pro-inflammatory and immunologic responses to surgery, which is, hypothetically, related to improved immediate or even long-term oncologic results[5,6].
However, many of the minimally invasive surgical esophageal procedures failed to reach a consistent level of evidence-based efficacy to enable their routine application[5]. The evidence-based literature is limited for many reasons. First, there is an intrinsic and well-known difficulty in conducting clinical surgical research. Second, a relatively low incidence of esophageal diseases (i.e., cancer) compared to stomach and colo-rectal cancers limits the gain of sufficient experience in Western countries. Finally, the large spectrum of new technologies, including laparoscopy, thoracoscopy, robotics, hybrid procedures and endoscopy, contributes to unclear and confusing results in clinical trials[7].
We focused this review on minimally invasive surgical procedures, including laparoscopy, thoracoscopy and robotics, for the treatment of the more frequent esophageal diseases, with an emphasis on clinical outcomes rather than on the technical details of each approach. Pure endoscopy, although recognized as the standard of care in some esophageal impairments and as important in many others, does not represent the core focus of article and was treated marginally.
A search of the PubMed, EMBASE and Cochrane databases through March 2015 was conducted, including important cross-matched manual references. Randomized controlled clinical trials (RCTs) and meta-analyses were considered a priority. Data arising from English-written, multicenter, international studies and those with long-term follow-up and oncologic results were also of major interest. A few small studies on the feasibility of the newest procedure were also included.
REFLUX DISEASE AND HIATUS HERNIA
The largest number of medical consultations for esophageal diseases involve symptoms related to hiatus hernia and gastroesophageal reflux disease (GERD). Fortunately, most of the affected patients are managed properly by a medical regiment of proton pump inhibitors (PPIs) and drugs targeted to dyskinesia. However, a subgroup of patients requires further invasive approaches, including endoscopy and surgery, while a few with long-standing disease are at risk of developing cancer.
It is commonly accepted that laparoscopic fundoplication (LF) greatly improves GERD symptoms, and it is considered as the standard operation, although in some patients symptoms can recur, necessitating a return to PPI use[8]. Interestingly, the best surgical results are achieved in those patients with optimal responses to medical therapy, which reflects an ongoing health-policy and cost-efficacy problem[9-11]. Morbidly obese patients require peculiar integrated multidisciplinary surgical approaches and will not be considered further in this study.
A debate that has lasted for years still exists on the extent of the stomach wrap (total or partial). The most common approaches are the classical 360° posterior fundoplication [laparoscopic Nissen fundoplication (LNF)], the 270-degree posterior fundoplication [laparoscopic toupet fundoplication (LTF)], the 180-degree laparoscopic anterior fundoplication (180-degree LAF) and the 90-degree anterior laparoscopic anterior fundoplication (90-degree LAF or Dor fundoplication). All of these partial fundoplications have been adopted to avoid the post-operative negative symptoms associated with LNF (mainly gas bloating syndrome and dysphagia).
Neither of the two approaches (partial vs total plication) has been demonstrated to be sufficiently superior to justify abandoning the other completely. A recent, updated selective review[12] concluded that LTF is the therapy of choice for normal-weight GERD patients who qualify for surgery because no better pharmaceutical, endoluminal or surgical alternatives exist to date.
The technical option of performing a laparoscopic 180-degree LAF should be validated compared to the Toupet fundoplication, while the division of the short gastric vessels is not recommended, nor is the use of a boogie or a mesh in the vast majority of patients undergoing surgery[11]. Interestingly, anti-reflux surgery is considered to be a field for expert surgeons, although no consensus exists on the adequate learning curve[12].
Most of the benefits of LF for patients suffering from GERD still persist after long-term follow-up. A multicenter Scottish trial[13] included more than 350 patients randomized to medical management and surgery (or who expressed a preference for one arm over the other) who were followed for five years using structured questionnaires. The authors reported that 44% of those who underwent surgery and 82% of those who had initial medical management were still taking anti-reflux medications. Differences in the REFLUX scores significantly favored the surgery group (mean difference 8.5, 95%CI: 3.9-13.1, P < 0.001, at five years). Postoperative complications that required surgical intervention occurred in 3% of patients, while 4% had further reflux-related operations, most often revision of the wrap.
Few rigorous articles have been published on the robotic approach to GERD and most of those compared it to open or laparoscopic techniques. Globally, the updated surgical approach to GERD has led to a hard scientific comparison among medical therapies, the endoscopic approach and surgery using an open, laparoscopic or robotic route. Unfortunately, these types of studies are very difficult (if not utopian) to design and conduct[7].
One of the largest analyses was that published by Owen[14], which included more than 12000 patients from an American national database. The group was retrospectively divided into those who received open fundoplication (OF), LF, and robot-assisted fundoplication (RLF). Interestingly, RLF matched favorably with OF in terms of morbidity (5.6% vs 11%; P < 0.05), length of stay (LOS) (6.1 ± 7.2 d vs 3.0 ± 3.5 d; P < 0.05), intensive care unit (ICU) admissions (11.5% vs 23.1%; P < 0.05), and cost (United States $10644 ± 6041 vs United States $12766 ± 13982; P < 0.05), although LF remained superior to RLF when considering the 30-d re-admission rate (1.8% vs 3.6%; P < 0.05) and the cost (United States $7968 ± 6969 vs United States $10644 ± 6041; P < 0.05).
A meta-analysis[15] of 221 patients from six selected RCTs comparing LF and RLF found similar results, with RLF having a longer duration of surgery, higher costs and similar patient outcomes.
According to the current literature, it is very hard to consider robotic procedures as cost-effective (as compared to standard laparoscopy) when dealing with simple routine operations, such esophago-gastric junction and functional surgery[16,17].
Hiatus hernia has several epidemiologic, anatomic and pathophysiological correlations with GERD and its correction is often by LF. Moreover, some patients suffering from hiatus hernias experience gastric volvulus with life threatening complications or become highly symptomatic, which justifies a surgical repair. However, the early minimally invasive approaches could lead to an increased incidence of recurrence compared to traditional open surgery[18,19]. Currently, laparoscopic mesh crural reinforcement and Collis gastroplasty in selected cases have achieved excellent functional results, with a recurrence rate of less than 20%[20,21].
From a comprehensive point of view, laparoscopic surgery for GERD and hiatal hernia is considered as a standard of care in most hospitals worldwide. The high grade of effectiveness, together with the proven lower mortality and morbidity, are reasons for abandoning open surgery on a routine basis[22,23].
ESOPHAGEAL DIVERTICULA
Esophageal diverticula are rare pathologies. The exact incidence is unknown because patients are often asymptomatic, and diagnosis is mostly incidental. Confirmation is based on a barium esophagogram and a thorough endoscopy to exclude the presence of concomitant malignancies[24,25]. Many cases are acquired pulsion diverticula, caused by an impaired motility that results in higher intraluminal pressure and mucosa herniation through the muscular wall[25,26].
Zenker’s diverticulum (ZD) is the most common type in the esophagus (70%). It usually begins in the upper third, with an estimated prevalence of 0.01%-0.11%[26] and some age, geographic and gender-related differences[27].
The choice of treatment for ZD for many years has been an open surgical diverticulectomy with cricopharingeal myotomy, while an endoscopic myotomy with a rigid or flexible endoscope is a recent emerging option that can be achieved with multiple techniques[28-32]. Current literature is mostly based on retrospective studies with heterogeneous results, and the gold standard of treatment is not yet established[33]. However, the endoscopic staple-assisted esophago-diverticulostomy is often considered the first choice of treatment[34].
Endoscopic repair of ZD is safe and effective, allowing a shorter operative time, a reduction of hospital stay, and a quicker resumption of oral intake[35-37]. In the available literature, the endoscopic repair has a morbidity rate of up to 4% and a mortality rate lower than 1%. The mean recurrence rate is approximately 6% (0%-22%)[38].
The traditional surgical techniques consist of a stapled or manual diverticulectomy for larger diverticula associated with a myotomy; a myotomy alone for small diverticula (less than 1 cm); and a myotomy with suspension or inversion for moderate-sized diverticula (1-4 cm)[39,40]. Despite proven efficacy, open surgery is associated with a high rate of complications (ranging from 3% to 19% depending on the technique), such as pharyngeo-cutaneous fistula, mediastinitis, larynx muscles paralysis, recurrence and death (1.6%)[27,41-43].
The prevalence of epiphrenic diverticula (ED) is approximately 0.015%, and patients are usually elderly men. ED are usually localized in the terminal esophagus and tend to project into the right thoracic cavity, accounting for less than 20% of esophageal diverticula[44-46]. The remaining 10% of diverticula of the esophagus are located in the mediastinal space.
Because of the high morbidity and mortality rates, treatment of ED is recommended only for selected patients with severe symptoms and a high risk of ab-ingestis pneumonia, rather than being based on the dimension of the diverticular sac itself[44,46,47].
Surgical treatments of ED include diverticulectomy, myotomy and fundoplication (often partial) due to the higher recurrence rates of diverticulectomy alone[48]. The procedures could be achieved by a traditional thoracotomy, a thoracoscopy, or a laparoscopic and robotic-assisted transhiatal technique. The minimally invasive approach is generally preferred for its lower morbidity and mortality rates and a similar success rate (83%-100%)[49].
Fumagalli Romario et al[50] reported on 30 patients treated with a laparoscopic transhiatal diverticulectomy with only a suture leak (3%) and no recurrence after a median follow-up of 52 mo, while Zaninotto et al[45] reported on 17 laparoscopic diverticulectomies (associated with myotomy and anti-reflux procedures) and 7 that used a combined laparoscopic-thoracotomic approach. The latter study found 4 leakages (16.6%) and good functional outcomes in all patients.
Unfortunately, most of the studies published are single, monocentric case studies without robust statistical calculations.
ACHALASIA
Achalasia is the most common primary motility disorder of the esophagus and, after GERD, is the second most common functional disorder of the esophagus requiring operative treatment. Most people are diagnosed between the ages of 25 and 60. It initially presents with a difficulty in swallowing that progressively becomes chronic and is not resolved by conventional interventions[51].
A number of medical and endoscopic treatments, including dilatation and myotomy[52-55], are available for achalasia with promising results, but a surgical Heller myotomy (HM) with fundoplication has been recognized as having excellent long-term outcomes and is considered as the standard to which others options should be compared[56-58].
The goal of myotomy is to improve esophageal emptying by dividing the esophageal and gastric muscle fibers that contribute to the lower esophageal sphincter mechanism. The original operation was developed by Heller[59] in 1913 and consisted of anterior and posterior esophageal myotomies. Because this approach resulted in excessive gastroesophageal reflux, it later was modified to involve a single myotomy, which still is the mainstay of surgical treatment.
In the early 1990s, Shimi et al[60] and Pellegrini et al[61] were the first to describe the use of minimally invasive techniques for the treatment of achalasia. Laparoscopic HM (LHM) has been shown not only to be feasible but also to decrease hospital stay and costs[57]. The use of LHM spread rapidly, motivating a change in the treatment algorithm for esophageal achalasia[56]. The standard technique includes both myotomy and fundoplication, while the Dor partial anterior plasty has been shown to be superior to the Nissen total plication[62]. Most of the patients affected had consistent symptom relief within a few weeks of the operation, with clinical improvements maintained after several years[63].
Similar to many esophageal procedures, the surgical treatment of achalasia with robotic assistance has been studied[64]. The first study on a robotic HM (RHM) with a Toupet fundoplication was published by Melvin et al[65] in 2001. Since then, several larger studies on the use of a RHM have been published[66-68].
Interestingly, esophageal perforations represent a life-threatening complication but have rarely been studied[69,70]; the studies that do exist have included immediate repairs with good outcomes. In a meta-analysis of the efficacy of robotic abdominal surgery that included 3 studies relevant to RHM, the authors reported the risk of perforation to be lower with robotic assistance[71]. It should be noted, however, that the lower perforation rate of RHM may be subject to bias, as most authors compare their results with laparoscopic myotomy cases performed earlier in their learning curve.
Another retrospective multicenter trial suggested decreased esophageal mucosal perforations with the use of a robot (0% vs 16% with conventional laparoscopy; P < 0.05) with similar patient outcomes and equal operative times, after an appropriate learning curve[67]. Huffmann et al[72] reported a lower rate of esophageal perforations and better quality of life with RHM compared to LHM as well.
From a robust comparative perspective, Shaligram et al[73] analyzed 2683 patients suffering from achalasia who were treated by open Heller myotomy (OM), LHM, or RHM. No differences in mortality, morbidity, ICU admission, LOS, or 30-d re-admission were observed in the three groups. However, the overall hospital costs decreased in the LHM group (United States $7441 ± 7897 vs United States $9415 ± 5515; P = 0.0028). Interestingly, when comparing OM and RHM, the authors found significantly lower morbidity (9.08% vs 4.02%; P = 0.02), ICU admission rate (14.01% vs 3.36%, P = 0.0002), and LOS (4.42 ± 5.25 d vs 2.42 ± 2.69 d; P = 0.0001) in the RHM group. The authors concluded that the RHM group had also a slight improvement in perioperative outcomes compared to the LHM, at the price of increased costs.
Another large review[74] of LHM vs RHM, which including only 6 RCTs (of low quality), also reported comparable outcomes and increased costs for the robotic technique.
The Society of American Gastrointestinal and Endoscopic Surgeons’ guidelines[75] state that compared with laparoscopy, robotic assistance for the treatment of esophageal achalasia decreases the rate of intraoperative mucosal perforations, but no clear differences in postoperative morbidity, symptom relief, or long-term outcomes have been confirmed to date. Further studies are needed to better establish the role of RHM.
ESOPHAGEAL PERFORATION
Esophageal perforation (EP) is an uncommon situation, although its incidence has increased over the last 20 years. The most common cause is iatrogenic (60% of cases are caused by an endoscopic procedure)[76,77]. Otherwise, EP can occurs spontaneously after vomiting or in cases Boerhaave syndrome or a diseased esophagus (i.e., diverticula, Barrett’s esophagus, infective esophagitis, cancer)[78]. Other rare causes are blunt or penetrating trauma to the epigastrium and ingestion of foreign bodies or caustics. The mortality rate is as high as 60%[79-81] and is mainly secondary to the onset of a septic shock and the presence of comorbidities[82].
The ideal management of EP is not yet standardized, and no technique has shown a real superiority over the others. Nevertheless, the number of patients treated aggressively with surgery has been lower over the last several years[83], while many patients (approximately 25% of EP cases) are being managed non-operatively. Early total parenteral nutrition and antibiotic therapy, in those patients without signs of sepsis, can lead to a medical management success rate of more than 80%[78].
Endoscopic stenting, associated with or without a percutaneous or surgical thoracic drainage, has a success rate up to 90% in patients with EP due to benign perforations of less than 5 cm or an anastomotic leak with a minimal contamination if treated within 24 h of the perforation[84-86]. Endoscopic closure of the leak with clips or suture is also effective[87].
Nevertheless, the surgical approach to EP is still appropriate in case of severe acute sepsis, extended leaks or failure of endoscopic/percutaneous treatments. A feeding jejunostomy is often recommended[88]. Surgical drainage of the contaminated space, debridement with primary repair, esophageal diversion with delayed repair and esophagectomy with immediate or delayed repair have all been used for several years, with high morbidity and mortality rates[78,81,88,89].
Open surgery is widely consider the standard, even though some case studies have reported on the feasibility and safety of laparoscopic[90-93]/thoracoscopic[94] primary repair of EP associated with or without stent placement[95] in hemodynamically stable patients. Again, most of the published studies are monocentric case studies and anecdotal reports with short-term follow-up.
Pleural percutaneous drainage alone may achieve acceptable mortality rates in appropriately selected patients with cervical EP[96,97], although it is usually associated with thoracoscopy or laparoscopy for complete surgical debridement[98].
BENIGN AND MALIGNANT TUMORS
Both benign and malignant tumors arising in the esophageal tract are candidates for a minimally invasive approach, although the widespread adoption of minimally invasive techniques has been limited by many challenging technical issues. In addition, the oncologic outcomes remain the foundation of any procedure to cure malignancies, rather than the feasibility itself. Obviously, any laparoscopic or robotic procedure should follow the standards of oncologic surgery, including sufficient margins of resection and extended proper lymphadenectomy[99].
The need for a surgeon with advanced skills, the availability of instruments and the high case volume together have limited the use of MIS for esophageal neoplasms to few subspecialized centers.
Benign lesions are rare, representing only 20% of all esophageal neoplasms at autopsy, with more than 70% being leiomyoma[100]. Nevertheless, the anatomic location in the esophageal tract, together with the well-known challenges of esophageal reconstructions, lead to potential life-threatening complications after surgery. A minimally invasive surgical approach would be of crucial interest to limit the risks of perioperative deaths and the length of hospital stay.
Most studies have included a limited number of anecdotal experiences[101-103] with excellent results from a thoracoscopic or laparoscopic transhiatal enucleation for esophageal leiomyomas. However, the optimal approach should be tailored for each patient according to the location and size of the tumor[104]. For example, Palanivelu et al[105], in one of the largest single-center studies (18 cases), reported that leiomyomas are frequently located in the middle and lower third of the esophagus. The author suggested that the proximal ones should be best approached by a right thoracoscopy and the distal ones through an abdominal route. Nevertheless, a laparoscopic transhiatal operation is also feasible to manage benign lesions of the thoracic esophagus[106,107].
Many of the published studies include very few patients, and those comparing laparoscopic/thoracoscopic procedures with open traditional approaches have poor statistical relevance. However, most studies have reported superior results of MIS in terms of reductions of perioperative complications and length of hospital stay[108,109].
The robotic approach was also described as a procedure very suitable for managing benign esophageal masses that require careful dissection in deep, narrow spaces. Obviously, all these experiences were reported as case studies performed by skillful subspecialized surgeons[64,110-113].
The different interventions for esophageal benign diseases range from a simple enucleation achieved through a thoracic or an abdominal route to a formal Ivor-Lewis partial esophagectomy. Interestingly, Khalaileh et al[113] reported favorable results of robotic approaches compared to the corresponding open or traditional laparoscopic/thoracoscopic operations (overall complications of 0%, 10% and 13%, respectively). Unfortunately, that retrospective review included fewer than 100 patients in each group, with scarce homogeneity of characteristics and very different approaches.
Cancer of the esophagus is relatively rare in Europe, North America and other developed countries, although it represents a major concern in Eastern Asia, Eastern and Southern Africa, and, generally speaking, in less developed regions (Figure 1). In Eastern Asia, the incidence is almost double than in rest of the world (more than 10 per 100000 per year)[114], with some differences in the histopathological features (adenocarcinoma and squamous). The oncologic outcomes are still disappointing, with a 5-years survival rate of less than 40%[115]. New adjuvant regiments have been proven to significantly increase the survival after curative surgery, with few or no detrimental perioperative complications[115,116].
Figure 1.
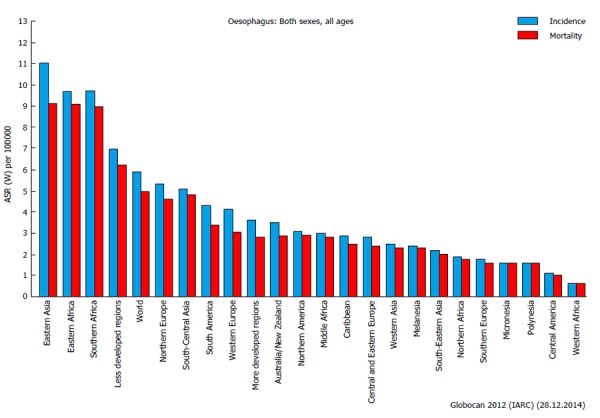
Incidence and mortality rates of esophageal cancer worldwide[114].
From a comprehensive point of view, the fundamental esophageal cancer cure is always resective surgery with regional lymphadenectomy and (neo) adjuvant chemo or radiochemotherapy. Conversely, many technical debates still exist regarding the opportunity of performing a partial or a total esophagectomy, with or without a transthoracic approach[117].
In brief, the three-field esophagectomy (McKeown procedure) has been the treatment of choice for esophageal cancer for many years and includes abdominal, thoracic and cervical incisions. The two-field partial esophagectomy with an esophagogastric intrathoracic anastomosis (Ivor-Lewis procedure) has gained popularity in recent years due to comparable oncologic results with the McKeown operation and minor complications. The transhiatal esophagectomy, which avoids the thoracotomy (Orringer procedure), probably offers inferior oncologic outcomes[118].
In the recent literature, many groups of esophageal surgeons have reported trends in reducing the use of the three-field McKeown total esophagectomy in favor of the two-field Ivor-Lewis partial esophagectomy (except for cases of cancer arising in the upper third of the esophagus). The significant reduction of perioperative complications, including leaks, recurrent laryngeal nerve injuries, alteration of swallowing and pharyngeal transit, is the major benefit of the limited approach[119,120].
Despite the different surgical techniques proposed, patients are expected to have a high incidence of complication of up to 60%. Most are pulmonary complications, with an increase in the postoperative stay, costs and mortality[1,3].
To improve such those disappointing figures, many minimally invasive approaches had been developed, replacing conventional operations with laparoscopy, thoracoscopy or hybrid routes (with open surgery combined), with excellent results[119,121,122].
The minimally invasive esophagectomy (MIE) is expected to reduce pulmonary impairment, intraoperative bleeding, wound infections and, consequently, length of hospital stay and mortality. Increases in the operative time and of the base costs are the principal concerns[123].
One recent multicenter (selected hospitals with specific credentials) prospective phase II trial[124] evaluated the feasibility of MIE in patients with high-grade dysplasia or esophageal cancer with a rigorous protocol. According to the authors’ results, surgery was completed in 95 of the 104 patients (91.3%), with a 30-d mortality rate of 2.1%. The major complications were anastomotic leak (8.6%), acute respiratory distress syndrome (5.7%), pneumonitis (3.8%), and atrial fibrillation (2.9%). The 3-year overall survival rate was 58.4% and a locoregional recurrence occurred in only 7 patients (6.7%).
However, the rapid worldwide use of MIS for esophageal cancer has not been followed by a rigorous scientific analysis of results, and the issue of cost-effectiveness is still unresolved[5,125]. Therefore, large-scale multicenter trials are still lacking, and few studies have had sufficient follow-up to judge the long-term oncologic results.
Aside from the intrinsic difficulty in conducing surgical clinical trials, the challenging learning curve and the numerous technical variables (including the patient’s position - prone vs supine or the transoral anvil introduction vs the transthoracic route during an Ivor-Lewis esophagectomy) have jeopardized the results[126].
One large retrospective cohort study also confirmed the superiority of MIE in terms of postoperative pulmonary complications (13% in the thoraco-laparoscopic MIE group, 38% in the thoracoscopic MIE group, and 39% in the open group)[122].
Nevertheless, to date, only one prospective, multicenter RCT that including 56 patients and compared open transthoracic oesophagectomy with the minimally invasive approach has been published[127]. The authors reported that 29% of patients in the open group had pulmonary infections in the first 2 wk compared to five (9%) in the minimally invasive group (P = 0.005), while 19 (34%) and 7 (12%) patients in the two groups had in-hospital pulmonary infections, respectively (P = 0.005)[127]. Another trial to evaluate the benefits of laparoscopic gastric mobilization during Ivor-Lewis intervention is still ongoing[128].
Conversely, Hanna et al[129], who selected thirty of the best published papers concerning MIE and open approaches for cancer (including only 1 RCT), found that in most studies a suboptimal lymphadenectomy was described (with the average number of nodes retrieved below 23 considered as the standard) and included a superficial description of the complications that occurred. However, the disease-free survival and the overall survival rates were similar to those achieved by open surgery[129].
In recent years, robotic-assisted MIE (RAMIE) has been introduced for the treatment of esophagogastric malignancies. The robotic platform would reduce the complexity of the laparoscopic-thoracoscopic maneuvers using endo-wrist arm technology (articulation of the instruments with 7 degrees of freedom). The deeper high-definition 3D vision, the motion scaling and the tremor filtration are other potential advantages of a robotic approach during esophageal dissection, allowing the execution of an extended lymphadenectomy and hand-sewn visceral anastomoses[130]. Another intriguing advantage of robotic surgery is the reduction of the learning curve (20 procedures in one study[131]), as compared to standard MIE, which increases the number of surgeons who can gain adequate and specific proficiency.
In the published literature, studies on all three types of esophageal resections (total esophagectomy, partial transthoracic and partial transhiatal resection) using a full robotic or a hybrid approach are available (Figure 2).
Figure 2.
da Vinci® docking during the thoracic step of a completely robotic esophagectomy at the Division of Oncologic Surgery and Robotics, Careggi Hospital.
For example, Boone et al[132] reported on 47 robotic three-field total esophagectomies with a pulmonary morbidity of 44% and a postoperative mortality of 6%, which were highly comparable with the results of historical open outcomes in terms of safety and short-terms results.
As in standard MIE, the robot-assisted Ivor-Lewis transthoracic esophageal resection has replaced the three-field approach in most cases[133-135]. The perioperative outcomes and the oncologic parameters reported were highly sufficient to judge the technique to be as safe as traditional MIE and the conventional open approach[133,136]. From a purely technical point of view, the transthoracic surgical step could be achieved throughout a standard supine or semi-lateral position, while recently some authors[137] have reported excellent results using the prone position (only a 6% rate of pulmonary complications).
Another peculiar issue of RAMIE is represented by the possibility of performing a hand-sewn intrathoracic esophagogastric anastomosis, which is virtually impossible or very time-consuming for even very skilled laparoscopists due to tremor and anti-ergonomic positions. However, only two papers[138,139] have specifically addressed the use of RAMIE with a hand-sewn intrathoracic anastomosis. The authors reported few leakages or cases of stenosis and no significant prolonging of the operative time.
Finally, even a transhiatal esophagectomy is feasible robotically, at the price of a higher complications rate reported in one of the very few anecdotal reports (35% of patients with temporary laryngeal nerve paresis and 25% of patients with self-limiting cervical leaks)[140].
In conclusion, although the first cases of RAMIE were described in the early 2000s[69,141], rigorous, well-designed, large comparative studies are still lacking, and none of the existing studies have demonstrated the tangible benefits of robotics over thoraco-laparoscopy or open surgery[133,142]. Interestingly, a monocentric trial specifically targeted to RAMIE was recently launched[143].
CONCLUSION
Most of the surgical operations for the treatment of benign and malignant esophageal diseases are suitable for a minimally invasive approach, with the goal of reducing the wide spectrum of perioperative complications.
Thoracoscopy, laparoscopy, hybrid procedures and robotic assistance have been shown to favorably impact pulmonary morbidity and length of hospital stay in many recent papers. However, most of these minimally invasive esophageal procedures were achieved in a limited number of subspecialized centers worldwide and were performed by surgeons with significant experience in esophageal surgery, advanced laparoscopy and robotics. Interestingly, the hypothesized learning curve for gaining sufficient confidence was more than 30 cases for major operations[144,145].
In addition, more of the published techniques, although very promising in terms of outcomes and results, are not yet completely validated. An authors’ comprehensive opinion of future developments in MIS for esophageal disease is reported in Table 1.
Table 1.
Recommended approaches to esophageal procedures
| Type of procedure | Open surgery | Laparoscopic surgery | Robotic | Level of evidence1 |
| Total esophagectomy (McKeown) | Standard | Accepted | Developing | LE 3 |
| Partial esophagectomy (Ivor-Lewis) | Standard | Accepted | Developing | LE 2 |
| Transhiatal esophagectomy (Orringer) | Standard | Accepted | Developing | LE 3 |
| Anti-reflux surgery | Abandoned | Standard | Developing | LE 1 |
| Heller myotomy | Abandoned | Standard | Developing | LE 1 |
| Local excision | Standard | Accepted | Developing | LE 4 |
| Others | Standard | Accepted | Developing | LE 4 |
Oxford Centre for Evidence-Based Medicine. Levels of Evidence Working Group. "The Oxford 2011 Levels of Evidence". http://www.cebm.net/index.aspx?o=5653
Centralization of the more challenging procedures and rigorous scientific approaches are needed before conventional open surgery can be abandoned completely.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 26, 2015
First decision: September 8, 2015
Article in press: December 8, 2015
P- Reviewer: Kumagai K, Zampieri N S- Editor: Kong JX L- Editor: A E- Editor: Liu SQ
References
- 1.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327:1192–1197. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopaldas RR, Bhamidipati CM, Dao TK, Markley JG. Impact of surgeon demographics and technique on outcomes after esophageal resections: a nationwide study. Ann Thorac Surg. 2013;95:1064–1069. doi: 10.1016/j.athoracsur.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Low DE, Bodnar A. Update on clinical impact, documentation, and management of complications associated with esophagectomy. Thorac Surg Clin. 2013;23:535–550. doi: 10.1016/j.thorsurg.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000-2011. J Gastrointest Surg. 2012;16:1055–1063. doi: 10.1007/s11605-011-1731-3. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb M, Brower S, Schwaitzberg SD. Minimally invasive surgery and cancer: controversies part 1. Surg Endosc. 2010;24:304–334. doi: 10.1007/s00464-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma B, Baxter N, Grantcharov T. Outcomes after laparoscopic techniques in major gastrointestinal surgery. Curr Opin Crit Care. 2010;16:371–376. doi: 10.1097/MCC.0b013e32833b0480. [DOI] [PubMed] [Google Scholar]
- 7.Garas G, Ibrahim A, Ashrafian H, Ahmed K, Patel V, Okabayashi K, Skapinakis P, Darzi A, Athanasiou T. Evidence-based surgery: barriers, solutions, and the role of evidence synthesis. World J Surg. 2012;36:1723–1731. doi: 10.1007/s00268-012-1597-x. [DOI] [PubMed] [Google Scholar]
- 8.Wileman SM, McCann S, Grant AM, Krukowski ZH, Bruce J. Medical versus surgical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev. 2010;(3):CD003243. doi: 10.1002/14651858.CD003243.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Grant AM, Boachie C, Cotton SC, Faria R, Bojke L, Epstein DM, Ramsay CR, Corbacho B, Sculpher M, Krukowski ZH, et al. Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro-oesophageal reflux disease: 5-year follow-up of multicentre randomised trial (the REFLUX trial) Health Technol Assess. 2013;17:1–167. doi: 10.3310/hta17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328; quiz 329. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 11.Lundell L. Borderline indications and selection of gastroesophageal reflux disease patients: ‘Is surgery better than medical therapy’? Dig Dis. 2014;32:152–155. doi: 10.1159/000357182. [DOI] [PubMed] [Google Scholar]
- 12.Schijven MP, Gisbertz SS, van Berge Henegouwen MI. Laparoscopic surgery for gastro-esophageal acid reflux disease. Best Pract Res Clin Gastroenterol. 2014;28:97–109. doi: 10.1016/j.bpg.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Grant AM, Cotton SC, Boachie C, Ramsay CR, Krukowski ZH, Heading RC, Campbell MK. Minimal access surgery compared with medical management for gastro-oesophageal reflux disease: five year follow-up of a randomised controlled trial (REFLUX) BMJ. 2013;346:f1908. doi: 10.1136/bmj.f1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen B, Simorov A, Siref A, Shostrom V, Oleynikov D. How does robotic anti-reflux surgery compare with traditional open and laparoscopic techniques: a cost and outcomes analysis. Surg Endosc. 2014;28:1686–1690. doi: 10.1007/s00464-013-3372-y. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zheng Q, Jin Z. Meta-analysis of robot-assisted versus conventional laparoscopic Nissen fundoplication for gastro-oesophageal reflux disease. ANZ J Surg. 2012;82:112–117. doi: 10.1111/j.1445-2197.2011.05964.x. [DOI] [PubMed] [Google Scholar]
- 16.Liberman D, Trinh QD, Jeldres C, Zorn KC. Is robotic surgery cost-effective: yes. Curr Opin Urol. 2012;22:61–65. doi: 10.1097/MOU.0b013e32834d543f. [DOI] [PubMed] [Google Scholar]
- 17.Salman M, Bell T, Martin J, Bhuva K, Grim R, Ahuja V. Use, cost, complications, and mortality of robotic versus nonrobotic general surgery procedures based on a nationwide database. Am Surg. 2013;79:553–560. [PubMed] [Google Scholar]
- 18.Dallemagne B, Kohnen L, Perretta S, Weerts J, Markiewicz S, Jehaes C. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg. 2011;253:291–296. doi: 10.1097/SLA.0b013e3181ff44c0. [DOI] [PubMed] [Google Scholar]
- 19.Zehetner J, Demeester SR, Ayazi S, Kilday P, Augustin F, Hagen JA, Lipham JC, Sohn HJ, Demeester TR. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg. 2011;212:813–820. doi: 10.1016/j.jamcollsurg.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 20.Zehetner J, DeMeester SR, Ayazi S, Costales JL, Augustin F, Oezcelik A, Lipham JC, Sohn HJ, Hagen JA, DeMeester TR. Long-term follow-up after anti-reflux surgery in patients with Barrett’s esophagus. J Gastrointest Surg. 2010;14:1483–1491. doi: 10.1007/s11605-010-1322-8. [DOI] [PubMed] [Google Scholar]
- 21.Petersen LF, McChesney SL, Daly SC, Millikan KW, Myers JA, Luu MB. Permanent mesh results in long-term symptom improvement and patient satisfaction without increasing adverse outcomes in hiatal hernia repair. Am J Surg. 2014;207:445–448; discussion 448. doi: 10.1016/j.amjsurg.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Molena D, Mungo B, Stem M, Feinberg RL, Lidor AO. Outcomes of operations for benign foregut disease in elderly patients: a National Surgical Quality Improvement Program database analysis. Surgery. 2014;156:352–360. doi: 10.1016/j.surg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Mungo B, Molena D, Stem M, Feinberg RL, Lidor AO. Thirty-day outcomes of paraesophageal hernia repair using the NSQIP database: should laparoscopy be the standard of care? J Am Coll Surg. 2014;219:229–236. doi: 10.1016/j.jamcollsurg.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 24.van Overbeek JJ. Pathogenesis and methods of treatment of Zenker’s diverticulum. Ann Otol Rhinol Laryngol. 2003;112:583–593. doi: 10.1177/000348940311200703. [DOI] [PubMed] [Google Scholar]
- 25.Herbella FA, Patti MG. Modern pathophysiology and treatment of esophageal diverticula. Langenbecks Arch Surg. 2012;397:29–35. doi: 10.1007/s00423-011-0843-2. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21:1–8. doi: 10.1111/j.1442-2050.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg GG, Kochman ML, Norton ID, Gostout CJ. Clinical Gastrointestinal Endoscopy, 2nd Ed. Saunders: Elsevier; 2012. [Google Scholar]
- 28.Rabenstein T, May A, Michel J, Manner H, Pech O, Gossner L, Ell C. Argon plasma coagulation for flexible endoscopic Zenker’s diverticulotomy. Endoscopy. 2007;39:141–145. doi: 10.1055/s-2007-966164. [DOI] [PubMed] [Google Scholar]
- 29.Tang SJ, Jazrawi SF, Chen E, Tang L, Myers LL. Flexible endoscopic clip-assisted Zenker’s diverticulotomy: the first case series (with videos) Laryngoscope. 2008;118:1199–1205. doi: 10.1097/MLG.0b013e31816e2eee. [DOI] [PubMed] [Google Scholar]
- 30.Repici A, Pagano N, Romeo F, Danese S, Arosio M, Rando G, Strangio G, Carlino A, Malesci A. Endoscopic flexible treatment of Zenker’s diverticulum: a modification of the needle-knife technique. Endoscopy. 2010;42:532–535. doi: 10.1055/s-0029-1244163. [DOI] [PubMed] [Google Scholar]
- 31.Hondo FY, Maluf-Filho F, Giordano-Nappi JH, Neves CZ, Cecconello I, Sakai P. Endoscopic treatment of Zenker’s diverticulum by harmonic scalpel. Gastrointest Endosc. 2011;74:666–671. doi: 10.1016/j.gie.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Verhaegen VJ, Feuth T, van den Hoogen FJ, Marres HA, Takes RP. Endoscopic carbon dioxide laser diverticulostomy versus endoscopic staple-assisted diverticulostomy to treat Zenker’s diverticulum. Head Neck. 2011;33:154–159. doi: 10.1002/hed.21413. [DOI] [PubMed] [Google Scholar]
- 33.Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital. 2013;33:219–229. [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiq MA, Sood S. Current management in pharyngeal pouch surgery by UK otorhinolaryngologists. Ann R Coll Surg Engl. 2004;86:247–252. doi: 10.1308/147870804524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richtsmeier WJ. Endoscopic management of Zenker diverticulum: the staple-assisted approach. Am J Med. 2003;115 Suppl 3A:175S–178S. doi: 10.1016/s0002-9343(03)00220-1. [DOI] [PubMed] [Google Scholar]
- 36.Luna RA, Collard JM. Transoral stapled diverticulotomy. Rev Col Bras Cir. 2009;36:268–270. [PubMed] [Google Scholar]
- 37.Bonavina L, Rottoli M, Bona D, Siboni S, Russo IS, Bernardi D. Transoral stapling for Zenker diverticulum: effect of the traction suture-assisted technique on long-term outcomes. Surg Endosc. 2012;26:2856–2861. doi: 10.1007/s00464-012-2261-0. [DOI] [PubMed] [Google Scholar]
- 38.Wasserzug O, Zikk D, Raziel A, Cavel O, Fleece D, Szold A. Endoscopically stapled diverticulostomy for Zenker’s diverticulum: results of a multidisciplinary team approach. Surg Endosc. 2010;24:637–641. doi: 10.1007/s00464-009-0651-8. [DOI] [PubMed] [Google Scholar]
- 39.Aly A, Devitt PG, Jamieson GG. Evolution of surgical treatment for pharyngeal pouch. Br J Surg. 2004;91:657–664. doi: 10.1002/bjs.4572. [DOI] [PubMed] [Google Scholar]
- 40.Simić A, Radovanović N, Stojakov D, Bjelović M, Kotarac M, Sabljak P, Skrobić O, Pesko P. Surgical experience of the national institution in the treatment of Zenker’s diverticula. Acta Chir Iugosl. 2009;56:25–33. doi: 10.2298/aci0901025s. [DOI] [PubMed] [Google Scholar]
- 41.Chang CY, Payyapilli RJ, Scher RL. Endoscopic staple diverticulostomy for Zenker’s diverticulum: review of literature and experience in 159 consecutive cases. Laryngoscope. 2003;113:957–965. doi: 10.1097/00005537-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Visosky AM, Parke RB, Donovan DT. Endoscopic management of Zenker’s diverticulum: factors predictive of success or failure. Ann Otol Rhinol Laryngol. 2008;117:531–537. doi: 10.1177/000348940811700712. [DOI] [PubMed] [Google Scholar]
- 43.Mantsopoulos K, Psychogios G, Künzel J, Zenk J, Iro H, Koch M. Evaluation of the different transcervical approaches for Zenker diverticulum. Otolaryngol Head Neck Surg. 2012;146:725–729. doi: 10.1177/0194599811435304. [DOI] [PubMed] [Google Scholar]
- 44.Del Genio A, Rossetti G, Maffetton V, Renzi A, Brusciano L, Limongelli P, Cuttitta D, Russo G, Del Genio G. Laparoscopic approach in the treatment of epiphrenic diverticula: long-term results. Surg Endosc. 2004;18:741–745. doi: 10.1007/s00464-003-9044-6. [DOI] [PubMed] [Google Scholar]
- 45.Zaninotto G, Parise P, Salvador R, Costantini M, Zanatta L, Rella A, Ancona E. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:218–222. doi: 10.1053/j.semtcvs.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Rossetti G, Fei L, del Genio G, Maffettone V, Brusciano L, Tolone S, Cimmino M, Moccia F, Terrone A, Romano G, et al. Epiphrenic diverticula mini-invasive surgery: a challenge for expert surgeons--personal experience and review of the literature. Scand J Surg. 2013;102:129–135. doi: 10.1177/1457496913482242. [DOI] [PubMed] [Google Scholar]
- 47.Zaninotto G, Portale G, Costantini M, Merigliano S, Guirroli E, Rizzetto C, Rampado S, Ancona E. Long-term outcome of operated and unoperated epiphrenic diverticula. J Gastrointest Surg. 2008;12:1485–1490. doi: 10.1007/s11605-008-0570-3. [DOI] [PubMed] [Google Scholar]
- 48.Rosati R, Fumagalli U, Bona S, Bonavina L, Peracchia A. Diverticulectomy, myotomy, and fundoplication through laparoscopy: a new option to treat epiphrenic esophageal diverticula? Ann Surg. 1998;227:174–178. doi: 10.1097/00000658-199802000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosati R, Fumagalli U, Elmore U, de Pascale S, Massaron S, Peracchia A. Long-term results of minimally invasive surgery for symptomatic epiphrenic diverticulum. Am J Surg. 2011;201:132–135. doi: 10.1016/j.amjsurg.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Fumagalli Romario U, Ceolin M, Porta M, Rosati R. Laparoscopic repair of epiphrenic diverticulum. Semin Thorac Cardiovasc Surg. 2012;24:213–217. doi: 10.1053/j.semtcvs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Vaezi MF, Richter JE. Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am J Gastroenterol. 1999;94:3406–3412. doi: 10.1111/j.1572-0241.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- 52.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 53.Meireles OR, Horgan S, Jacobsen GR, Katagiri T, Mathew A, Sedrak M, Sandler BJ, Dotai T, Savides TJ, Majid SF, et al. Transesophageal endoscopic myotomy (TEEM) for the treatment of achalasia: the United States human experience. Surg Endosc. 2013;27:1803–1809. doi: 10.1007/s00464-012-2666-9. [DOI] [PubMed] [Google Scholar]
- 54.Borges AA, Lemme EM, Abrahao LJ, Madureira D, Andrade MS, Soldan M, Helman L. Pneumatic dilation versus laparoscopic Heller myotomy for the treatment of achalasia: variables related to a good response. Dis Esophagus. 2014;27:18–23. doi: 10.1111/dote.12064. [DOI] [PubMed] [Google Scholar]
- 55.Leyden JE, Moss AC, MacMathuna P. Endoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasia. Cochrane Database Syst Rev. 2014;12:CD005046. doi: 10.1002/14651858.CD005046.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patti MG, Fisichella PM, Perretta S, Galvani C, Gorodner MV, Robinson T, Way LW. Impact of minimally invasive surgery on the treatment of esophageal achalasia: a decade of change. J Am Coll Surg. 2003;196:698–703; discussion 703-705. doi: 10.1016/S1072-7515(02)01837-9. [DOI] [PubMed] [Google Scholar]
- 57.Schuchert MJ, Luketich JD, Landreneau RJ, Kilic A, Gooding WE, Alvelo-Rivera M, Christie NA, Gilbert S, Pennathur A. Minimally-invasive esophagomyotomy in 200 consecutive patients: factors influencing postoperative outcomes. Ann Thorac Surg. 2008;85:1729–1734. doi: 10.1016/j.athoracsur.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Williams VA, Peters JH. Achalasia of the esophagus: a surgical disease. J Am Coll Surg. 2009;208:151–162. doi: 10.1016/j.jamcollsurg.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 59.Heller E. Extramucose cardioplastic beim chronischen cardiospasmus mit dilatation des oesophagus. Mitt Grengeb Med Chir. 1913;27:141–149. [Google Scholar]
- 60.Shimi S, Nathanson LK, Cuschieri A. Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edinb. 1991;36:152–154. [PubMed] [Google Scholar]
- 61.Pellegrini C, Wetter LA, Patti M, Leichter R, Mussan G, Mori T, Bernstein G, Way L. Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg. 1992;216:291–296; discussion 296-299. doi: 10.1097/00000658-199209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M. Randomized controlled trial of laparoscopic Heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results. Ann Surg. 2008;248:1023–1030. doi: 10.1097/SLA.0b013e318190a776. [DOI] [PubMed] [Google Scholar]
- 63.Costantini M, Zaninotto G, Guirroli E, Rizzetto C, Portale G, Ruol A, Nicoletti L, Ancona E. The laparoscopic Heller-Dor operation remains an effective treatment for esophageal achalasia at a minimum 6-year follow-up. Surg Endosc. 2005;19:345–351. doi: 10.1007/s00464-004-8941-7. [DOI] [PubMed] [Google Scholar]
- 64.Hanna JM, Onaitis MW. Robotic benign esophageal procedures. Thorac Surg Clin. 2014;24:223–229, vii. doi: 10.1016/j.thorsurg.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Melvin WS, Needleman BJ, Krause KR, Wolf RK, Michler RE, Ellison EC. Computer-assisted robotic heller myotomy: initial case report. J Laparoendosc Adv Surg Tech A. 2001;11:251–253. doi: 10.1089/109264201750539790. [DOI] [PubMed] [Google Scholar]
- 66.Melvin WS, Dundon JM, Talamini M, Horgan S. Computer-enhanced robotic telesurgery minimizes esophageal perforation during Heller myotomy. Surgery. 2005;138:553–558; discussion 558-559. doi: 10.1016/j.surg.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Horgan S, Galvani C, Gorodner MV, Omelanczuck P, Elli F, Moser F, Durand L, Caracoche M, Nefa J, Bustos S, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. J Gastrointest Surg. 2005;9:1020–1029; discussion 1029-1030. doi: 10.1016/j.gassur.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Galvani C, Gorodner MV, Moser F, Baptista M, Donahue P, Horgan S. Laparoscopic Heller myotomy for achalasia facilitated by robotic assistance. Surg Endosc. 2006;20:1105–1112. doi: 10.1007/s00464-005-0272-9. [DOI] [PubMed] [Google Scholar]
- 69.Ruurda JP, Gooszen HG, Broeders IA. Early experience in robot-assisted laparoscopic Heller myotomy. Scand J Gastroenterol Suppl. 2004;(241):4–8. doi: 10.1080/00855920410010924. [DOI] [PubMed] [Google Scholar]
- 70.Undre S, Moorthy K, Munz Y, Aggarwal R, Hance J, Rockall T, Darzi A. Robot-assisted laparoscopic Heller cardiomyotomy: preliminary UK results. Dig Surg. 2004;21:396–400. doi: 10.1159/000082316. [DOI] [PubMed] [Google Scholar]
- 71.Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, Plana MN. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg. 2010;252:254–262. doi: 10.1097/SLA.0b013e3181e6239e. [DOI] [PubMed] [Google Scholar]
- 72.Huffmanm LC, Pandalai PK, Boulton BJ, James L, Starnes SL, Reed MF, Howington JA, Nussbaum MS. Robotic Heller myotomy: a safe operation with higher postoperative quality-of-life indices. Surgery. 2007;142:613–618; discussion 618-620. doi: 10.1016/j.surg.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Shaligram A, Unnirevi J, Simorov A, Kothari VM, Oleynikov D. How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc. 2012;26:1047–1050. doi: 10.1007/s00464-011-1994-5. [DOI] [PubMed] [Google Scholar]
- 74.Falkenback D, Lehane CW, Lord RV. Robot-assisted oesophageal and gastric surgery for benign disease: antireflux operations and Heller’s myotomy. ANZ J Surg. 2015;85:113–120. doi: 10.1111/ans.12731. [DOI] [PubMed] [Google Scholar]
- 75.Stefanidis D, Richardson W, Farrell TM, Kohn GP, Augenstein V, Fanelli RD. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc. 2012;26:296–311. doi: 10.1007/s00464-011-2017-2. [DOI] [PubMed] [Google Scholar]
- 76.Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77:1475–1483. doi: 10.1016/j.athoracsur.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 77.Hasimoto CN, Cataneo C, Eldib R, Thomazi R, Pereira RS, Minossi JG, Cataneo AJ. Efficacy of surgical versus conservative treatment in esophageal perforation: a systematic review of case series studies. Acta Cir Bras. 2013;28:266–271. doi: 10.1590/s0102-86502013000400006. [DOI] [PubMed] [Google Scholar]
- 78.Chirica M, Champault A, Dray X, Sulpice L, Munoz-Bongrand N, Sarfati E, Cattan P. Esophageal perforations. J Visc Surg. 2010;147:e117–e128. doi: 10.1016/j.jviscsurg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Reeder LB, DeFilippi VJ, Ferguson MK. Current results of therapy for esophageal perforation. Am J Surg. 1995;169:615–617. doi: 10.1016/s0002-9610(99)80232-3. [DOI] [PubMed] [Google Scholar]
- 80.Muir AD, White J, McGuigan JA, McManus KG, Graham AN. Treatment and outcomes of oesophageal perforation in a tertiary referral centre. Eur J Cardiothorac Surg. 2003;23:799–804; discussion 804. doi: 10.1016/s1010-7940(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 81.Vallböhmer D, Hölscher AH, Hölscher M, Bludau M, Gutschow C, Stippel D, Bollschweiler E, Schröder W. Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus. 2010;23:185–190. doi: 10.1111/j.1442-2050.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 82.Bhatia P, Fortin D, Inculet RI, Malthaner RA. Current concepts in the management of esophageal perforations: a twenty-seven year Canadian experience. Ann Thorac Surg. 2011;92:209–215. doi: 10.1016/j.athoracsur.2011.03.131. [DOI] [PubMed] [Google Scholar]
- 83.Sepesi B, Raymond DP, Peters JH. Esophageal perforation: surgical, endoscopic and medical management strategies. Curr Opin Gastroenterol. 2010;26:379–383. doi: 10.1097/MOG.0b013e32833ae2d7. [DOI] [PubMed] [Google Scholar]
- 84.Dai Y, Chopra SS, Kneif S, Hünerbein M. Management of esophageal anastomotic leaks, perforations, and fistulae with self-expanding plastic stents. J Thorac Cardiovasc Surg. 2011;141:1213–1217. doi: 10.1016/j.jtcvs.2010.07.096. [DOI] [PubMed] [Google Scholar]
- 85.Freeman RK, Ascioti AJ, Giannini T, Mahidhara RJ. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg. 2012;94:959–964; discussion 964-965. doi: 10.1016/j.athoracsur.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 86.Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Esquival R, Raju GS. Endoscopic closure of acute esophageal perforations. Curr Gastroenterol Rep. 2013;15:321. doi: 10.1007/s11894-013-0321-9. [DOI] [PubMed] [Google Scholar]
- 88.Nirula R. Esophageal perforation. Surg Clin North Am. 2014;94:35–41. doi: 10.1016/j.suc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 89.de Aquino JL, de Camargo JG, Cecchino GN, Pereira DA, Bento CA, Leandro-Merhi VA. Evaluation of urgent esophagectomy in esophageal perforation. Arq Bras Cir Dig. 2014;27:247–250. doi: 10.1590/S0102-67202014000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landen S, El Nakadi I. Minimally invasive approach to Boerhaave’s syndrome: a pilot study of three cases. Surg Endosc. 2002;16:1354–1357. doi: 10.1007/s00464-001-9185-4. [DOI] [PubMed] [Google Scholar]
- 91.Ashrafi AS, Awais O, Alvelo-Rivera M. Minimally invasive management of Boerhaave’s syndrome. Ann Thorac Surg. 2007;83:317–319. doi: 10.1016/j.athoracsur.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 92.Toelen C, Hendrickx L, Van Hee R. Laparoscopic treatment of Boerhaave’s syndrome: a case report and review of the literature. Acta Chir Belg. 2007;107:402–404. doi: 10.1080/00015458.2007.11680082. [DOI] [PubMed] [Google Scholar]
- 93.Kimberley KL, Ganesh R, Anton CK. Laparoscopic repair of esophageal perforation due to Boerhaave syndrome. Surg Laparosc Endosc Percutan Tech. 2011;21:e203–e205. doi: 10.1097/SLE.0b013e3182245771. [DOI] [PubMed] [Google Scholar]
- 94.Cho JS, Kim YD, Kim JW, I HS, Kim MS. Thoracoscopic primary esophageal repair in patients with Boerhaave’s syndrome. Ann Thorac Surg. 2011;91:1552–1555. doi: 10.1016/j.athoracsur.2011.01.082. [DOI] [PubMed] [Google Scholar]
- 95.Ben-David K, Behrns K, Hochwald S, Rossidis G, Caban A, Crippen C, Caranasos T, Hughes S, Draganov P, Forsmark C, et al. Esophageal perforation management using a multidisciplinary minimally invasive treatment algorithm. J Am Coll Surg. 2014;218:768–774. doi: 10.1016/j.jamcollsurg.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 96.Martinez L, Rivas S, Hernández F, Avila LF, Lassaletta L, Murcia J, Olivares P, Queizán A, Fernandez A, López-Santamaría M, et al. Aggressive conservative treatment of esophageal perforations in children. J Pediatr Surg. 2003;38:685–689. doi: 10.1016/jpsu.2003.50183. [DOI] [PubMed] [Google Scholar]
- 97.Vogel SB, Rout WR, Martin TD, Abbitt PL. Esophageal perforation in adults: aggressive, conservative treatment lowers morbidity and mortality. Ann Surg. 2005;241:1016–1021; discussion 1021-1023. doi: 10.1097/01.sla.0000164183.91898.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biancari F, D’Andrea V, Paone R, Di Marco C, Savino G, Koivukangas V, Saarnio J, Lucenteforte E. Current treatment and outcome of esophageal perforations in adults: systematic review and meta-analysis of 75 studies. World J Surg. 2013;37:1051–1059. doi: 10.1007/s00268-013-1951-7. [DOI] [PubMed] [Google Scholar]
- 99.Bencini L, Bernini M, Farsi M. Laparoscopic approach to gastrointestinal malignancies: toward the future with caution. World J Gastroenterol. 2014;20:1777–1789. doi: 10.3748/wjg.v20.i7.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsai SJ, Lin CC, Chang CW, Hung CY, Shieh TY, Wang HY, Shih SC, Chen MJ. Benign esophageal lesions: endoscopic and pathologic features. World J Gastroenterol. 2015;21:1091–1098. doi: 10.3748/wjg.v21.i4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Obuchi T, Sasaki A, Nitta H, Koeda K, Ikeda K, Wakabayashi G. Minimally invasive surgical enucleation for esophageal leiomyoma: report of seven cases. Dis Esophagus. 2010;23:E1–E4. doi: 10.1111/j.1442-2050.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 102.Dapri G, Himpens J, Ntounda R, Alard S, Dereeper E, Cadière GB. Enucleation of a leiomyoma of the mid-esophagus through a right thoracoscopy with the patient in prone position. Surg Endosc. 2010;24:215–218. doi: 10.1007/s00464-009-0514-3. [DOI] [PubMed] [Google Scholar]
- 103.Tsalis K, Antoniou N, Kalfadis S, Dimoulas A, Dagdilelis AK, Lazaridis C. Laparoscopic enucleation of a giant submucosal esophageal lipoma. Case report and literature review. Am J Case Rep. 2013;14:179–183. doi: 10.12659/AJCR.883928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaninotto G, Portale G, Costantini M, Rizzetto C, Salvador R, Rampado S, Pennelli G, Ancona E. Minimally invasive enucleation of esophageal leiomyoma. Surg Endosc. 2006;20:1904–1908. doi: 10.1007/s00464-005-0838-6. [DOI] [PubMed] [Google Scholar]
- 105.Palanivelu C, Rangarajan M, Madankumar MV, John SJ, Senthilkumar R. Minimally invasive therapy for benign tumors of the distal third of the esophagus--a single institute’s experience. J Laparoendosc Adv Surg Tech A. 2008;18:20–26. doi: 10.1089/lap.2007.0052. [DOI] [PubMed] [Google Scholar]
- 106.Dulucq JL, Wintringer P, Mahajna A. Totally laparoscopic trans-hiatal gastroesophagectomy for benign diseases of the esophago-gastric junction. World J Gastroenterol. 2007;13:285–288. doi: 10.3748/wjg.v13.i2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palanivelu C, Rangarajan M, John SJ, Parthasarathi R, Senthilkumar R. Laparoscopic transhiatal approach for benign supra-diaphragmatic lesions of the esophagus: a replacement for thoracoscopy? Dis Esophagus. 2008;21:176–180. doi: 10.1111/j.1442-2050.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 108.Kent M, d’Amato T, Nordman C, Schuchert M, Landreneau R, Alvelo-Rivera M, Luketich J. Minimally invasive resection of benign esophageal tumors. J Thorac Cardiovasc Surg. 2007;134:176–181. doi: 10.1016/j.jtcvs.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 109.Vallböhmer D, Hölscher AH. Laparoscopic excision of leiomyomas in the esophageal and gastric wall. Surg Technol Int. 2007;16:82–88. [PubMed] [Google Scholar]
- 110.DeUgarte DA, Teitelbaum D, Hirschl RB, Geiger JD. Robotic extirpation of complex massive esophageal leiomyoma. J Laparoendosc Adv Surg Tech A. 2008;18:286–289. doi: 10.1089/lap.2007.0067. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen NT, Reavis KM, El-Badawi K, Hinojosa MW, Smith BR. Minimally invasive surgical enucleation or esophagogastrectomy for benign tumor of the esophagus. Surg Innov. 2008;15:120–125. doi: 10.1177/1553350608317353. [DOI] [PubMed] [Google Scholar]
- 112.Gullo R, Herbella FA, Patti MG. Laparoscopic excision of esophageal leiomyoma. Updates Surg. 2012;64:315–318. doi: 10.1007/s13304-011-0108-1. [DOI] [PubMed] [Google Scholar]
- 113.Khalaileh A, Savetsky I, Adileh M, Elazary R, Abu-Gazala M, Abu Gazala S, Schlager A, Rivkind A, Mintz Y. Robotic-assisted enucleation of a large lower esophageal leiomyoma and review of literature. Int J Med Robot. 2013;9:253–257. doi: 10.1002/rcs.1484. [DOI] [PubMed] [Google Scholar]
- 114.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray , et al. GLOBOCAN 2012 v1. Dec 28]. Lyon, France. 0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. [Accessed; 2014. p. International Agency for Research on Cancer, 2013. Available from: http://globocan.iarc.fr. [Google Scholar]
- 115.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 116.Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, Ye W, Lundell L, Nilsson M. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 117.Pennathur A, Zhang J, Chen H, Luketich JD. The “best operation” for esophageal cancer? Ann Thorac Surg. 2010;89:S2163–S2167. doi: 10.1016/j.athoracsur.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kutup A, Nentwich MF, Bollschweiler E, Bogoevski D, Izbicki JR, Hölscher AH. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg. 2014;260:1016–1022. doi: 10.1097/SLA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 119.Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, Christie NA, Weksler B, Landreneau RJ, Abbas G, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 121.Butler N, Collins S, Memon B, Memon MA. Minimally invasive oesophagectomy: current status and future direction. Surg Endosc. 2011;25:2071–2083. doi: 10.1007/s00464-010-1511-2. [DOI] [PubMed] [Google Scholar]
- 122.Kinjo Y, Kurita N, Nakamura F, Okabe H, Tanaka E, Kataoka Y, Itami A, Sakai Y, Fukuhara S. Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc. 2012;26:381–390. doi: 10.1007/s00464-011-1883-y. [DOI] [PubMed] [Google Scholar]
- 123.Schumer E, Perry K, Melvin WS. Minimally invasive esophagectomy for esophageal cancer: evolution and review. Surg Laparosc Endosc Percutan Tech. 2012;22:383–386. doi: 10.1097/SLE.0b013e31826295a4. [DOI] [PubMed] [Google Scholar]
- 124.Luketich JD, Pennathur A, Franchetti Y, Catalano PJ, Swanson S, Sugarbaker DJ, De Hoyos A, Maddaus MA, Nguyen NT, Benson AB, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg. 2015;261:702–707. doi: 10.1097/SLA.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Uttley L, Campbell F, Rhodes M, Cantrell A, Stegenga H, Lloyd-Jones M. Minimally invasive oesophagectomy versus open surgery: is there an advantage? Surg Endosc. 2013;27:724–731. doi: 10.1007/s00464-012-2546-3. [DOI] [PubMed] [Google Scholar]
- 126.Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, Turrado Rodriguez VT, van der Peet DL, Cuesta MA. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc. 2012;26:1795–1802. doi: 10.1007/s00464-012-2149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 128.Briez N, Piessen G, Bonnetain F, Brigand C, Carrere N, Collet D, Doddoli C, Flamein R, Mabrut JY, Meunier B, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer. 2011;11:310. doi: 10.1186/1471-2407-11-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanna GB, Arya S, Markar SR. Variation in the standard of minimally invasive esophagectomy for cancer--systematic review. Semin Thorac Cardiovasc Surg. 2012;24:176–187. doi: 10.1053/j.semtcvs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Lehenbauer D, Kernstine KH. Robotic esophagectomy: modified McKeown approach. Thorac Surg Clin. 2014;24:203–209, vii. doi: 10.1016/j.thorsurg.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 131.Hernandez JM, Dimou F, Weber J, Almhanna K, Hoffe S, Shridhar R, Karl R, Meredith K. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg. 2013;17:1346–1351. doi: 10.1007/s11605-013-2225-2. [DOI] [PubMed] [Google Scholar]
- 132.Boone J, Schipper ME, Moojen WA, Borel Rinkes IH, Cromheecke GJ, van Hillegersberg R. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg. 2009;96:878–886. doi: 10.1002/bjs.6647. [DOI] [PubMed] [Google Scholar]
- 133.de la Fuente SG, Weber J, Hoffe SE, Shridhar R, Karl R, Meredith KL. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc. 2013;27:3339–3347. doi: 10.1007/s00464-013-2915-6. [DOI] [PubMed] [Google Scholar]
- 134.Sarkaria IS, Rizk NP, Finley DJ, Bains MS, Adusumilli PS, Huang J, Rusch VW. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg. 2013;43:e107–e115. doi: 10.1093/ejcts/ezt013. [DOI] [PubMed] [Google Scholar]
- 135.Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin. 2014;24:211–222, vii. doi: 10.1016/j.thorsurg.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 136.Weksler B, Sharma P, Moudgill N, Chojnacki KA, Rosato EL. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus. 2012;25:403–409. doi: 10.1111/j.1442-2050.2011.01246.x. [DOI] [PubMed] [Google Scholar]
- 137.Puntambekar SP, Rayate N, Joshi S, Agarwal G. Robotic transthoracic esophagectomy in the prone position: experience with 32 patients with esophageal cancer. J Thorac Cardiovasc Surg. 2011;142:1283–1284. doi: 10.1016/j.jtcvs.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 138.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg. 2013;145:90–96. doi: 10.1016/j.jtcvs.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 139.Trugeda S, Fernández-Díaz MJ, Rodríguez-Sanjuán JC, Palazuelos CM, Fernández-Escalante C, Gómez-Fleitas M. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot. 2014;10:397–403. doi: 10.1002/rcs.1587. [DOI] [PubMed] [Google Scholar]
- 140.Dunn DH, Johnson EM, Morphew JA, Dilworth HP, Krueger JL, Banerji N. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus. 2013;26:159–166. doi: 10.1111/j.1442-2050.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 141.Espat NJ, Jacobsen G, Horgan S, Donahue P. Minimally invasive treatment of esophageal cancer: laparoscopic staging to robotic esophagectomy. Cancer J. 2005;11:10–17. doi: 10.1097/00130404-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 142.Clark J, Sodergren MH, Purkayastha S, Mayer EK, James D, Athanasiou T, Yang GZ, Darzi A. The role of robotic assisted laparoscopy for oesophagogastric oncological resection; an appraisal of the literature. Dis Esophagus. 2011;24:240–250. doi: 10.1111/j.1442-2050.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 143.van der Sluis PC, Ruurda JP, van der Horst S, Verhage RJ, Besselink MG, Prins MJ, Haverkamp L, Schippers C, Rinkes IH, Joore HC, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial) Trials. 2012;13:230. doi: 10.1186/1745-6215-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo W, Zou YB, Ma Z, Niu HJ, Jiang YG, Zhao YP, Gong TQ, Wang RW. One surgeon’s learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc. 2013;27:1346–1352. doi: 10.1007/s00464-012-2614-8. [DOI] [PubMed] [Google Scholar]
- 145.Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg. 2014;218:1130–1140. doi: 10.1016/j.jamcollsurg.2014.02.014. [DOI] [PubMed] [Google Scholar]


