Abstract
Aim
To develop and evaluate a method to detect circulation in the presence of organized rhythms (ORs) during resuscitation using signals acquired by defibrillation pads.
Methods
Segments containing electrocardiogram (ECG) and thoracic impedance (TI) signals free of artifacts were used. The ECG corresponded to ORs classified as pulseless electrical acitivity (PEA) or pulse-generating rhythm (PR). A first dataset containing 1091 segments was split into training and test sets to develop and validate the circulation detector. The method processed ECG and TI to obtain the impedance circulation component (ICC). Morphological features were extracted from ECG and ICC, and combined into a classifier to discriminate between PEA and PR. The performance of the method was evaluated in terms of sensitivity (PR) and specificity (PEA). A second dataset (86 segments from different patients) was used to assess two application of the method: confirmation of arrest by recognizing absence of circulation during ORs and detection of return of spontaneous circulation (ROSC) during resuscitation. In both cases, time to confirmation of arrest/ROSC was determined.
Results
The method showed a sensitivity/specificity of 92.1%/90.3% and 92.2%/91.9% for training and test sets respectively. The method confirmed cardiac arrest with a specificity of 93.3% with a median delay of 0 s after the first OR annotation. ROSC was detected with a sensitivity of 94.4% with a median delay of 57 s from ROSC onset.
Conclusion
The method showed good performance, and can be reliably used to distinguish perfusing from non-perfusing ORs.
Keywords: Circulation detection, Pulse, Thoracic Impedance, Pulseless Electrical Activity, Pulse-generating Rhythm, Defibrillator
1. INTRODUCTION
During resuscitation, recognition of cardiac arrest and detection of return of spontaneous circulation (ROSC) are challenging for both lay rescuers and healthcare personnel.1,2 The carotid pulse check was the protocol accepted to detect absence or presence of circulation until 1998.3 It was later proven both time-consuming and inaccurate,4–8 and is currently only recommended for experienced healthcare providers.9,10 Current resuscitation guidelines recommend checking for signs of life during cardiopulmonary resuscitation (CPR), such as purposeful movement, breathing or coughing.11,12 They also recommend using the capnogram, generally available only with intubation, as a decision support tool for ROSC detection. An abrupt increase in CO2 level to normal values (35 to 40mmHg) has been accepted as an indicator of ROSC.10,13–16 However, there is still a need for a real-time hemodynamic monitor in a resuscitation scenario.17
In the context of automated external defibrillators (AEDs), the capnogram is rarely available and the only signals recorded are often the electrocardiogram (ECG) and the thoracic impedance (TI) acquired by defibrillation pads. Both have proven valuable to detect pulse once a possible perfusing rhythm, an organized rhythm (OR), is present.18–22 The TI signal shows a very small fluctuation (usually less than 100m) with every effective heartbeat. Studies by Losert et al.18 and Risdal et al.19 concluded that a combination of features extracted from ECG and TI allows a proper discrimination between pulse-generating rhythms (PR) and pulseless electrical activity (PEA). Cromie et al. used a single feature computed from the spectrum of the processed TI as a clinical marker of circulatory collapse in animals20 and humans.21 In our previous work, we presented a reliable method to extract the impedance circulation component (ICC) using an adaptive processing scheme, and concluded that morphological features extracted from ICC showed great potential for PEA/PR discrimination.22
Here we propose a method to detect circulation based on the ECG and TI. The method is intended to be launched only when a possible perfusing rhythm has been detected, i.e when an OR is detected either by the shock advisory algorithm (SAA) of an AED, or by the healthcare professional using a monitor/defibrillator. For the rest of cardiac arrest rhythms, the procedure established by the resuscitation guidelines would be followed and the method would not be run. The method could be integrated into (1) AEDs to work together with the SAA and (2) monitor/defibrillators as a decision support tool which the healthcare professional could activate (e.g. by pressing a softkey). The circulation detector was designed to discriminate PR from PEA segments using a training set and validated with a test set. Finally, a case study was carried out using different complete episodes to assess two applications of the method: confirmation of cardiac arrest by recognizing absence of circulation during ORs and detection of circulation during resuscitation.
2. MATERIALS AND METHODS
2.1. Data materials
The dataset used in this study was a subset of a large out-of-hospital cardiac arrest (OHCA) registry containing 385 episodes maintained by the Tualatin Valley Fire & Rescue (Tigard, Oregon, USA). The episodes, one per patient, were collected using the Philips HeartStart MRx monitor/defibrillator between January 2010 and December 2014. First, the episodes were visually inspected to identify the segments of interest: the ECG signal to identify ORs with a duration of at least 5 s, and the TI signal to confirm that no artifacts due to chest compressions or ventilations were present. Second, those OR segments were classified as PEA or PR by three expert reviewers (a biomedical engineer, EAl; an emergency medicine physician, MD; and a cardiologist, LGT) using a majority criterion in the basis of available clinical information and the capnography signal. The clinical information derived from the prehospital record consisted of (1) the time of the first detected ROSC in field defined as a palpable pulse in any vessel for any length of time, (2) information about ROSC loss prior to arrival at emergency department, and (3) outcome (death in the field, expired in emergency department, expired post admission or discharged alive). The ROSC onset time was used to define the start of intervals with circulation which was confirmed by a sudden increase in EtCO2 levels to values above 35mmHg. The information about ROSC loss was used to define the stop of intervals with circulation. If ROSC was not lost, the stop time was defined as the end of the episode. However, if ROSC was lost the stop time was determined by either a sudden decrease in EtCO2 from values above 35mmHg to values below 20mmHg, or resume of CPR. The patient outcome was used to confirm the ROSC/noROSC annotations at the end of the episodes.
Each segment contained the ECG (resolution 1.03 μV per least significant bit with bandwidth 0–50Hz) and the TI (resolution 0.74mΩ per least significant bit with bandwidth 0–80Hz) signals. The TI was acquired by applying a sinusoidal excitation current (32 kHz, 3mA peak to peak) between the defibrillation pads.
A first dataset was composed of 1091 segments extracted from 158 episodes recorded until May 2014. The dataset was randomly split into training (60%) and test (40%) sets to develop and validate the circulation detector. The PR and PEA segments extracted from the same episode were all assigned to either training or test sets.
A second dataset, the case study dataset, was used to test the clinical applicability of the circulation detector. For this purpose 33 complete episodes gathered between June and December 2014 were used. In 15 episodes the patient never recovered ROSC and finally expired. The first three artefact-free OR segments from these episodes were used to assess the capacity of the circulation detector to confirm cardiac arrest by recognizing absence of circulation. In 18 episodes patients recovered ROSC and a clinical annotation of ROSC time was available. After ROSC, the first three segments from these episodes were used to evaluate the ability of the method to detect ROSC during resuscitation.
2.2. Circulation detector
Fig. 1 shows the overview of the detector. The ECG signal was processed to remove noise and detect the instants of QRS complexes. The TI signal was processed first, to remove noise and then, using an adaptive scheme based on a recursive least square (RLS) algorithm23,24 which used the instants of QRS complexes as reference to track and extract the ICC signal. Six waveform features were extracted from processed ECG, ICC and its first derivative that characterize PR and PEA and were then used to build the circulation detector based on a multivariate logistic regression classifier. The training set was used to develop and optimize the circulation detector. Appendix A gives a detailed technical description of the method.
Figure 1.
Overview of the circulation detector consisting of three different stages. First, the signal processing stage where the ECG signal and the TI signal are digitally processed to detect the instants of the QRS complexes, and to extract the ICC signal. Second, the feature extraction stage where features characterizing the waveform of the ECG, ICC, and its first derivative are extracted. Finally, the classifier distinguishes between PEA and PR based on the extracted features.
2.3. Evaluation and statistical analysis
The inter-rater agreement between the three expert reviewers during the annotation process of the gold standard was evaluated using the Fleiss’ Kappa coefficient (κ) and its 95% confidence interval. The performance of the circulation detector was assessed using the test set in terms of sensitivity (capacity to correctly detect PR), specificity (capacity to correctly detect PEA), and area under the curve (AUC). Results are presented as median (inter-quartile range, IQR) because their distributions did not pass the Anderson-Darling normality test. Consecutive diagnoses by the pulse detector within the same patient are correlated, so sensitivity and specificity and their 90% one-sided lower confidence limit were adjusted for clustering within patients using generalized estimating equations within a logistic regression model.25 The calculations were done using the Geepack library26 in the R statistical software.
2.4. Case study
Two applications of the circulation detector were assessed. First, the ability of the detector to confirm cardiac arrest was evaluated by computing the specificity of the circulation detector independently for the three segments, and determining the time to confirmation of arrest. Second, the capability of the detector to detect ROSC during resuscitation was evaluated by calculating the sensitivity of the circulation detector independently for the three segments, and determining the time to first detection of ROSC.
3. RESULTS
The PEA/PR annotation process resulted in an inter-rater agreement index of κ = 0.92 (0.91–0.93). Table 1 summarizes the number and median (IQR) duration of PEA and PR segments included in the datasets. Overall, 1177 segments from 191 patients with a duration of 8.3 (6.6 –11.7) s were analyzed. Specifically, 796 PR segments from 99 patients and duration of 7.9 (6.4 –11.0) s and 381 PEA segments from 92 patients and duration of 9.6 (7.0 –13.6) s.
Table 1.
Summary of segments grouped in training, test and case study datasets. The number of patients is indicated in parenthesis. Duration of the segments is expressed as median (IQR).
| Database | PEA
|
PR
|
||
|---|---|---|---|---|
| No. segments | Duration (s) | No. segments | Duration (s) | |
| Training | 205 (81) | 9.8 (7.4 – 14.3) | 449 (81) | 7.8 (6.5 – 10.9) |
| Test | 136 (77) | 9.3 (6.7 – 13.8) | 301 (77) | 8.2 (6.5 – 11.3) |
| Case study | 40 (15) | 8.9 (6.1 – 11.5) | 46 (18) | 7.4 (5.7 – 8.6) |
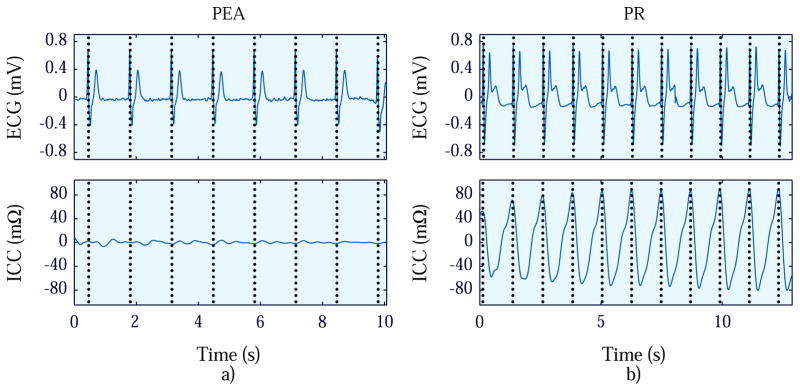
Panels a and b in Fig. 2 shows the ECG and ICC signals for a PEA and PR segment respectively. Both ECG signals are regular with similar mean RR interval, but the magnitude of the ICC is greater for PR than for PEA.
Figure 2.
Examples of PEA (panel a) and PR segments (panel b) where the ECG and ICC signals are depicted from top to bottom.
The circulation detector showed a sensitivity/specificity of 92.1% (90% one-sided lower confidence limit, 88.5%)/90.3% (86.2%) and AUC value of 0.95 for the training set. While, a sensitivity/specificity of 92.2% (87.7%)/91.9% (87.2%) and an AUC value of 0.97 for the test set.
In the case study, the specificities for the confirmation of cardiac arrest were 93.3%, 100%, and 100% respectively for the three PEA segments of the episodes. In these cases, the time instant when the circulation detector was launched was 0 (0–65) s, 122 (102–180) s, and 239 (195–416) s with respect to the first annotation of an OR. For the detection of ROSC during resuscitation, the sensitivities were 94.4%, 100%, and 100% respectively for the three PR segments of the episodes in the case study. The time instant when the circulation detector was launched was 57 (11 –38) s, 138 (46 –327) s, and 268 (136 –393) s with respect to the first clinical annotation of ROSC.
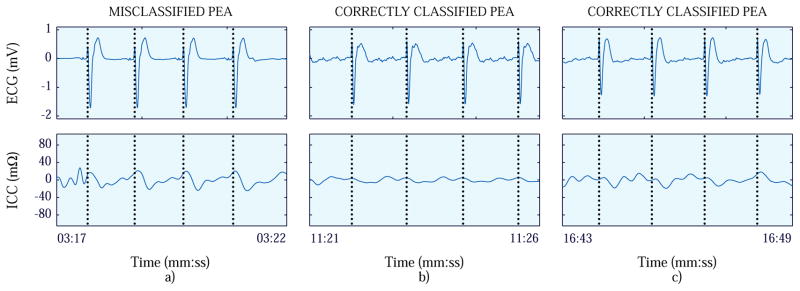
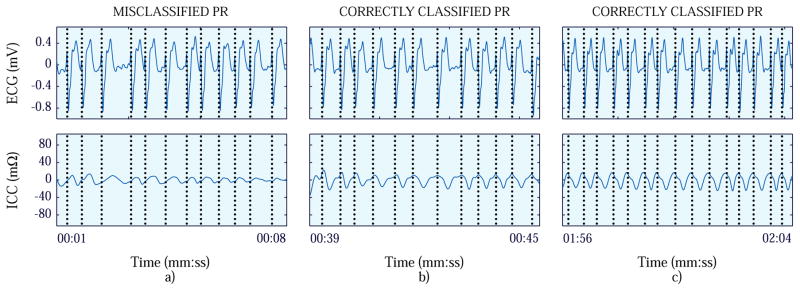
Fig. 3 and 4 show two case-study examples. Fig. 3 shows the case in which the first PEA segment was misclassified, and Fig. 4, the case in which the first PR segment was misclassified.
Figure 3.
First (panel a), second (panel b) and third (panel c) PEA segments of the patient of the case study where the first PEA segment was misclassified. The ECG signals are presented at the top, while the ICC signals are depicted at the bottom.
Figure 4.
First (panel a), second (panel b) and third (panel c) PR segments of the patient of the case study where the first PR segment was misclassified. The ECG signals are presented at the top, while the ICC signals are depicted at the bottom.
4. DISCUSSION
A method to detect the presence of circulation during resuscitation scenario has been presented. For every OR, the ECG and TI signals were analyzed, and the rhythm classified as PEA or PR. Through a case study two potential applications of the method were tested: confirmation of cardiac arrest and detection of ROSC.
4.1. The dataset and the gold standard
The circulation detector was developed, tested, and its applications assessed using a large dataset with 1177 PR/PEA segments extracted from OHCA episodes. The gold standard to annotate ORs as PR or PEA played a crucial role. It is accepted that additional information is needed to make that decision. In 2007 Losert et al.18 used the invasive measurement of the systolic blood pressure as gold standard in an in-hospital setting. Risdal et al.19 used as gold standard the manual rhythm annotations made by expert reviewers in an OHCA scenario. These annotations were made by visual inspection of the ECG and TI signals looking for fluctuations in the TI correlated with the heartbeats. In Ruiz et al.22 we based our study on the dataset used by Risdal et al.19 in which the gold standard PEA/PR annotations were made based on the ECG and TI signals and were therefore not independent of the methods used for PEA/PR discrimination. Furthermore, the criteria for segment selection were very restrictive since the data comprised only PR segments at the end of the episodes in patients admitted alive to hospital, and PEA segments on patients with no PR annotations along the episode. This study analyzes a comprehensive dataset that includes episodes with loss of ROSC and transient ROSC intervals. More importantly, the set of variables used to annotate ORs as PEA or PR are independent of the ECG and the impedance. These variables are, to the best of our knowledge, the most comprehensive set available in retrospective OHCA data, and included: ROSC onset time and ROSC loss information annotated in-situ by the rescuers, patient outcome data from the ROC registry, and the analysis of the capnogram.
4.2. The circulation detector
The circulation detector presented good performance, a sensitivity/specificity of 92.2% (87.7%)/91.9% (87.2%) for the test set, improving previously reported results.18,19,21 Combining ECG and ICC features improved the accuracy of the RLS based detector by over 7 points (see supplementary materials). The RLS filter we propose is a more efficient estimator of the ICC component than our previous LMS method,22 and permits a reliable PEA/PR discrimination using shorter ECG/TI segments of duration below 5 s with minimal computational burden, around 50ms (see supplementary materials). This would limit the duration of analysis segments to values comparable to those used for rhythm analysis in defibrillators27,28 as long as the segment stored in the defibrillator is immediately analyzed by the circulation detector after an OR is detected by the SAA. This would shorten pauses in chest compressions and may therefore contribute to improve survival since longer pauses compromise coronary and cerebral blood flow.29,30
4.3. Case study
The results obtained from the case study confirmed the expected good performance of the circulation detector and gave a time-perspective of its application as a support tool to confirm cardiac arrest and to detect ROSC during resuscitation.
When the ability to confirm cardiac arrest was evaluated, only the initial PEA segment of one patient was misclassified, as shown in panel a in Fig. 3. The magnitude of the ICC was significant which led the detector to make a classification error. Nevertheless, the following PEA segments (panels b and c in Fig. 3) were correctly detected. For the rhythm reassessments (2nd and 3rd segments), no PEA segment was misclassified in any episode. The reassessments were carried out at 122 s and 239 s respectively from the instant the OR was detected which reproduces the 2-min CPR cycle followed by a rhythm reassessment of the BLS protocol when an AED is available.
When the capability to detect ROSC during resuscitation was evaluated, only the initial PR segment of one patient was misclassified as shown in panel a in Fig. 4. The magnitude of the ICC was small, in contrast to the following PR segments (panels b and c in Fig. 4) which were correctly detected. No CPR was provided and half a minute passed between the first and second PR segments. Therefore, it could be that the mechanical activity of the heart was not sufficient to cause significant fluctuations in the TI during the first seconds of ROSC. For the rhythm reassessments (2nd and 3rd segments), no PR segment was misclassified in any episode. The reassessments were carried out at 138 s and 268 s respectively from the clinically annotated ROSC time. The presence of hyperventilation observed after ROSC delayed the launch of the detector.
4.4. Action protocol
An action protocol could be defined to integrate the circulation detector into BLS and ALS. In BLS, the detector could be integrated together with the SAA of an AED without varying the current protocol. The detector would be launched in a rhythm analysis interval, where no artifacts due to chest compressions or ventilations are present, and immediately after the detection of an OR by the SAA. In the current ALS protocol there are few spots without artifacts where the detector could be launched because normally several healthcare professionals are involved, and the rhythm assessment is carried out by visual inspection of the ECG while ventilating the patient. Therefore, we propose the detector to be integrated into a monitor/defibrillator as a decision support tool that could be manually activated by the healthcare professional when an OR is observed and presence of a pulse suspected.
4.5. Limitations
The results obtained are closely linked to specific features of signals acquired by the Philips MRx monitor/defibrillator. TI signals acquired by other equipment must first be validated for the purpose of detecting circulation.
5. Conclusions
In this study, a method to detect the presence of circulation has been proposed using the ECG and TI signals acquired via defibrillation pads. The method showed good performance when validated with the test set, and the case study revealed its applicability as a tool to confirm cardiac arrest and to detect ROSC during resuscitation. However, the method must be validated using signals acquired by AEDs, and it should be incorporated and assessed in a real time framework before integrating it into defibrillators.
Supplementary Material
Acknowledgments
Authors would like to thank Laura Zangróniz and Andoni Elola for their collaboration on this study. This work received financial support from the Ministerio de Economía y Competitividad of Spain through the project TEC2012-31928, and from the University of the Basque Country (UPV/EHU) through the unit UFI11/16 and the Convocatoria de Contratación de Doctores Recientes hasta su Integración en Programas de Formación Postdoctoral. Larisa G. Tereshchenko was partially supported by the NHLBI R01HL118277.
Appendix A. Appendix
Signal processing
The ECG signal, ecg[n], was band-pass filtered between 0.5–30Hz to remove baseline wander and high frequency noise. The instants of QRS complexes, nQRS, were automatically detected using the offline Matlab version of the QRS detector running in the Bexen cardio Reanibex 800 monitor/defibrillator. The TI signal, z[n], was band-pass filtered between 0.65–7Hz to suppress high frequency noise and low frequency components below the fundamental frequency of ICC. The resulting signal was denoted as zp[n].
Adaptive extraction of the ICC
The zp[n] signal was adaptively filtered to extract the ICC signal, , using the scheme illustrated in Fig. 5. The was modelled using 3 harmonics of slowly changing amplitude and phase22,31 as represented in the following equation:
| (A.1) |
where f[n] denotes the instantaneous frequency of the QRS complexes computed from the instants nQRS.
Figure 5.
Diagram of the adaptive scheme applying a RLS algorithm used to extract the from the filtered TI signal, zp[n].
Fig. 5 shows how the RLS algorithm recursively computes the amplitudes of the in-phase and quadrature components, âk[n] and b̂k[n], for each one of the three harmonics (k = 1, 2, and 3) to estimate the . The adaptive process of the RLS algorithm is defined by the forgetting factor, λ, a weighting factor to ensure that data in the close past are positively weighted over data in distant past so that the statistical variations of the data are tracked.24 The training set was used to optimize the value of λ (which is always close to, but less than one) resulting in an optimal value of 0.9997. The RLS algorithm has a shorter transient interval (quicker adaptation) than the LMS algorithm proposed in our previous work.22
Waveform feature selection
Up to 16 waveform features, v1 − v16,18,19,22 were considered in the optimization process to build the classifier. The training set was used to find the best feature combination that maximized the balanced accuracy, i.e. the average of the sensitivity and specificity. This ensures balanced detection results and avoids biases due to class imbalance in the training set. Features were selected using a greedy forward subset feature selection approach. The next 6 features were chosen in the following order:
-
Mean area of , v1: Area per sample under the absolute value of .
(A.2) where N represents the number of samples of the segment.
-
Mean RR interval, v2: Mean value of the RR intervals:
(A.3) where NQRS is the number of QRS complexes of the segment, and tRRi the time in seconds of ith RR interval.
-
Standard deviation of the amplitude of , v3: Standard deviation of the peak-to-trough amplitude of the fluctuations caused by each effective heartbeat in the :
(A.4) where ai denotes the peak-to-trough amplitude of the fluctuation in the ith RR interval, and μ the mean peak-to-trough amplitude of the fluctuations in the segment.
-
Mean amplitude of the QRS complexes, v4: The mean value of the peak-to-peak amplitude of the QRS complexes:
(A.5) where ppi represents the peak-to-peak amplitude of ith QRS complex, computed as the difference between maximum and minimum of the ecg[n] subsegment corresponding to the ith QRS complex.
- Mean area of , v5: Area per sample under the absolute value of .
(A.6) - Standard deviation of the amplitude of the QRS complexes, v6: The standard deviation of peak-to-peak amplitudes of the QRS complexes.
(A.7)
Classifier
The classifier was based on a multivariate logistic regression model using v1 –v6 features to assign a probability, PPR(z), to each segment as:
| (A.8) |
with z = θ0 + θ1 · v1 + θ2 · v2 + θ3 · v3 + θ4 · v4 + θ5 · v5 + θ6 · v6
The training set was used to compute the regression coefficients (θi). Segments with an assigned PPR > 0.5 (z > 0) were classified as PR, while the opposite (z ≤ 0) was classified as PEA. The imbalance between classes was compensated weighting each sample according to the proportion of samples in each class.
Footnotes
Ethical Approval
The CPR process files used in this study were collected as part of an effort to develop an airway check algorithm using the capnography signal. Since these raw data files have no identifying information, the Institutional Review Board at the Oregon Health & Science University determined that the proposed activity is not human subject research because the proposed activity does not meet the definition of human subject per 45 CFR 46.102(f).
Conflict of interest
Mohamud Daya is an unpaid consultant for Philips Healthcare.
References
- 1.Perkins GD, Stephenson B, Hulme J, Monsieurs KG. Birmingham assessment of breathing study (BABS) Resuscitation. 2005;64(1):109–113. doi: 10.1016/j.resuscitation.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Ruppert M, Reith MW, Widmann JH, et al. Checking for breathing: evaluation of the diagnostic capability of emergency medical services personnel, physicians, medical students, and medical laypersons. Annals of Emergency Medicine. 1999;34(6):720–729. doi: 10.1016/s0196-0644(99)70097-1. [DOI] [PubMed] [Google Scholar]
- 3.Bossaert L, Handley A, Marsden A, et al. European Resuscitation Council guidelines for the use of automated external defibrillators by EMS providers and first responders: A statement from the Early Defibrillation Task Force, with contributions from the Working Groups on Basic and Advanced Life Support, and approved by the Executive Committee of the European Resuscitation. Resuscitation. 1998;37(2):91–94. doi: 10.1016/s0300-9572(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 4.Eberle B, Dick WF, Schneider T, Wisser G, Doetsch S, Tzanova I. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33(2):107–116. doi: 10.1016/s0300-9572(96)01016-7. [DOI] [PubMed] [Google Scholar]
- 5.Ochoa FJ, Ramalle-Gómara E, Carpintero JM, García A, Saralegui I. Competence of health professionals to check the carotid pulse. Resuscitation. 1998;37(3):173–175. doi: 10.1016/s0300-9572(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 6.Bahr J, Klingler H, Panzer W, Rode H, Kettler D. Skills of lay people in checking the carotid pulse. Resuscitation. 1997;35(1):23–26. doi: 10.1016/s0300-9572(96)01092-1. [DOI] [PubMed] [Google Scholar]
- 7.Tibballs J, Russell P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation. 2009;80(1):61–64. doi: 10.1016/j.resuscitation.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Nyman J, Sihvonen M. Cardiopulmonary resuscitation skills in nurses and nursing students. Resuscitation. 2000;47(2):179–184. doi: 10.1016/s0300-9572(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 9.Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support Resuscitation. 2010;81(10):1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 11.Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S685–705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 12.Koster RW, Baubin MA, Bossaert LL, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81(10):1277–1292. doi: 10.1016/j.resuscitation.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pokorná M, Nec̆as E, Kratochvíl J, Skřipský R, Andrlík M, Franěk O. A sudden increase in partial pressure end-tidal carbon dioxide at the moment of return of spontaneous circulation. The Journal of Emergency Medicine. 2010;38(5):614–621. doi: 10.1016/j.jemermed.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 14.Bhende MS, Karasic DG, Karasic RB. End-tidal carbon dioxide changes during cardiopulmonary resuscitation after experimental asphyxial cardiac arrest. The American Journal of Emergency Medicine. 1996;14(4):349–350. doi: 10.1016/S0735-6757(96)90046-7. [DOI] [PubMed] [Google Scholar]
- 15.Sehra R, Underwood K, Checchia P. End tidal CO2 is a quantitative measure of cardiac arrest. Pacing and Clinical Electrophysiology: PACE. 2003;26(1):515–517. doi: 10.1046/j.1460-9592.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhende MS, Thompson AE. Evaluation of an end-tidal CO2 detector during pediatric cardiopulmonary resuscitation. Pediatrics. 1995;95(3):395–399. [PubMed] [Google Scholar]
- 17.Babbs CF. We still need a real-time hemodynamic monitor for CPR. Resuscitation. 2013;84(10):1297–1298. doi: 10.1016/j.resuscitation.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Losert H, Risdal M, Sterz F, et al. Thoracic-impedance changes measured via defibrillator pads can monitor signs of circulation. Resuscitation. 2007;73(2):221–228. doi: 10.1016/j.resuscitation.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Risdal M, Aase SO, Kramer-Johansen J, Eftestøl T. Automatic identification of return of spontaneous circulation during cardiopulmonary resuscitation. IEEE Transactions on Biomedical Engineering. 2008;55(1):60–68. doi: 10.1109/TBME.2007.910644. [DOI] [PubMed] [Google Scholar]
- 20.Cromie NA, Allen JD, Turner C, Anderson JM, Adgey AAJ. The impedance cardiogram recorded through two electrocardiogram/defibrillator pads as a determinant of cardiac arrest during experimental studies. Critical Care Medicine. 2008;36(5):1578–1584. doi: 10.1097/CCM.0b013e318170a03b. [DOI] [PubMed] [Google Scholar]
- 21.Cromie NA, Allen JD, Navarro C, Turner C, Anderson JM, Adgey AAJ. Assessment of the impedance cardiogram recorded by an automated external defibrillator during clinical cardiac arrest. Critical Care Medicine. 2010;38(2):510–517. doi: 10.1097/CCM.0b013e3181c02ca1. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz J, Alonso E, Aramendi E, et al. Reliable extraction of the circulation component in the thoracic impedance measured by defibrillation pads. Resuscitation. 2013;84(10):1345–1352. doi: 10.1016/j.resuscitation.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Widrow B, Stearns SD. Adaptive signal processing. Englewood Cliffs, NJ: Prentice-Hall; 1985. [Google Scholar]
- 24.Haykin SS. Adaptive filter theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 25.Sternberg MR, Hadgu A. A GEE approach to estimating sensitivity and specificity and coverage properties of the confidence intervals. Statistics in Medicine. 2001;20(9–10):1529–1539. doi: 10.1002/sim.688. [DOI] [PubMed] [Google Scholar]
- 26.Halekoh U, Højsgaard S, Yan J. The R package geepack for generalized estimating equations. Journal of Statistical Software. 2006;15(2):1–11. [Google Scholar]
- 27.Irusta U, Ruiz J, Aramendi E, Ruiz de Gauna S, Ayala U, Alonso E. A high-temporal resolution algorithm to discriminate shockable from nonshockable rhythms in adults and children. Resuscitation. 2012;83(9):1090–1097. doi: 10.1016/j.resuscitation.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Didon JP, Krasteva V, Ménétré S, Stoyanov T, Jekova I. Shock advisory system with minimal delay triggering after end of chest compressions: accuracy and gained hands-off time. Resuscitation. 2011;82(Suppl 2):S8–15. doi: 10.1016/S0300-9572(11)70145-9. [DOI] [PubMed] [Google Scholar]
- 29.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA: the Journal of the American Medical Association. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106(3):368–372. doi: 10.1161/01.cir.0000021429.22005.2e. [DOI] [PubMed] [Google Scholar]
- 31.Alonso E, Aramendi E, Ruiz J, Ayala U, González-Otero D. Suppression of the respiration artefact and extraction of the cardiac component in the thoracic impedance recorded through defibrillation pads. Computing in Cardiology (CinC) 2012;2012:677–680. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.