Abstract
Objective
To investigate fertilization and embryo quality of dysmorphic mature oocytes with specific morphological abnormalities obtained from intracytoplasmic sperm injection (ICSI).
Methods
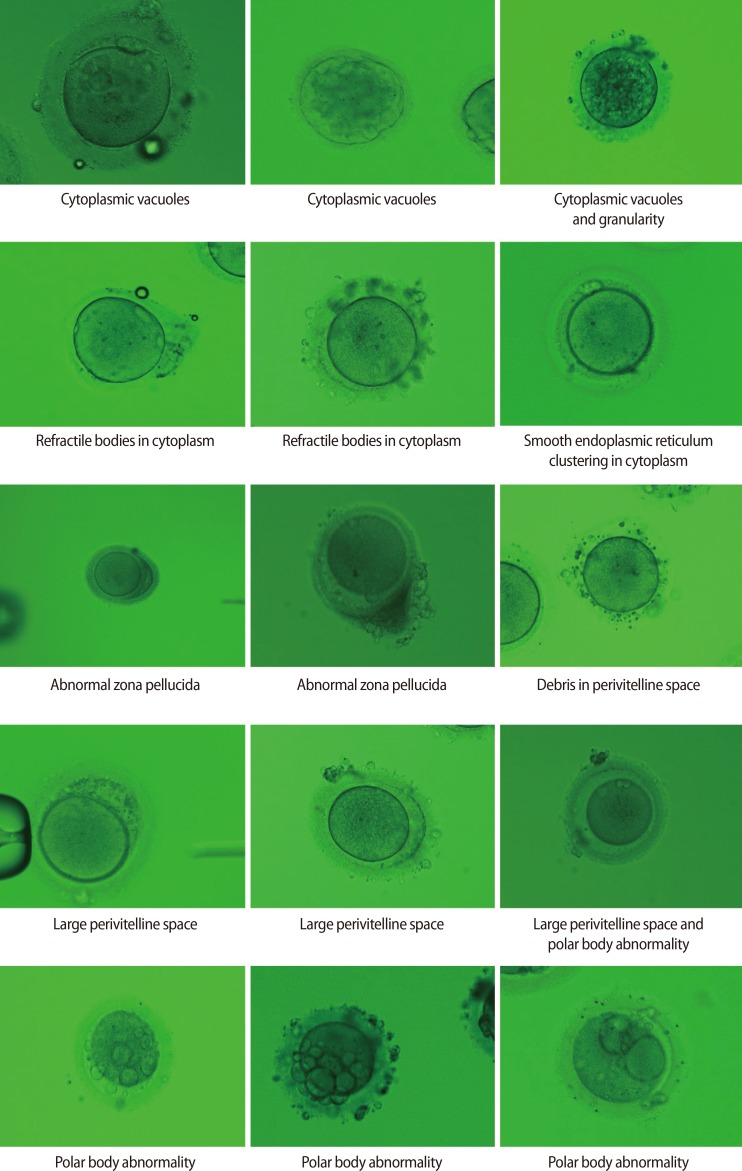
The fertilization rate (FR) and embryo quality were compared among 58 dysmorphic and 42 normal form oocytes (control 1) obtained from 35 consecutive ICSI cycles, each of which yielded at least one dysmorphic mature oocyte, performed over a period of 5 years. The FR and embryo quality of 441 normal form oocytes from another 119 ICSI cycles that did not involve dysmorphic oocytes served as control 2. Dysmorphic oocytes were classified as having a dark cytoplasm, cytoplasmic granularity, cytoplasmic vacuoles, refractile bodies in the cytoplasm, smooth endoplasmic reticulum in the cytoplasm, an oval shape, an abnormal zona pellucida, a large perivitelline space, debris in the perivitelline space, or an abnormal polar body (PB).
Results
The overall FR was significantly lower in dysmorphic oocytes than in normal form oocytes in both the control 1 and control 2 groups. However, embryo quality in the dysmorphic oocyte group and the normal form oocyte groups at day 3 was similar. The FR and embryo quality were similar in the oocyte groups with a single abnormality and multiple abnormalities. Specific abnormalities related with a higher percentage of top-quality embryos were dark cytoplasm (66.7%), abnormal PB (50%), and cytoplasmic vacuoles (25%).
Conclusion
The fertilization potential of dysmorphic oocytes in our study was lower, but their subsequent embryonic development and embryo quality was relatively good. We were able to define several specific abnormalities related with good or poor embryo quality.
Keywords: Dysmorphism, Embryo, Intracytoplasmic sperm injections, Oocytes
Introduction
Good-quality mature human oocytes are thought to have a clear, moderately granular cytoplasm; a small perivitelline space (PVS); a clear, colorless zona pellucida (ZP); and contain a single unfragmented polar body (PB) [1]. However, some oocytes exhibit variations in shape, color, granularity and homogeneity of the cytoplasm, cytoplasmic incorporations, size of the PVS, color of the ZP, and/or regularity of PB [2]. It has been reported that a majority (60% to 70%) of retrieved oocytes exhibit one or more of these abnormal morphologic characteristics [1,3,4,5,6].
Dysmorphic oocytes are commonly classified as having abnormal cytoplasm (dark cytoplasm or cytoplasmic granularity), cytoplasmic inclusions (vacuoles, refractile bodies, or smooth endoplasmic reticulum [SER] clustering), an abnormal oocyte shape (e.g., an oval), an abnormal ZP, an abnormal PVS (e.g., a large PVS or debris in the PVS), and an abnormal PB [7]. Currently there are several reports that describe whether a specific oocyte abnormality has a good prognosis in terms of successful fertilization and formation of good quality embryos, but conflicting results exist regarding the influence of a given oocyte morphological abnormality on the fertilization rate (FR) and embryo quality.
Poor FR has been reported in oocytes with cytoplasmic inclusions [8,9,10], vacuoles [6,10], refractile bodies [1,6], dark ZP [11], a large PVS [6,12,13], and abnormal PB [6,7]. Nonetheless, a relatively good FR was also reported in oocytes exhibiting cytoplasmic inclusions [14], refractile bodies [10], a dark ZP [10,15], a large PVS [16], debris in the PVS [15,17], and an abnormal PB [15,18,19].
Thus, the fate of certain types of dysmorphic oocytes remains unclear. Moreover, the FR and embryo quality in dysmorphic oocytes possessing two or more abnormalities are largely unknown. Here we retrospectively assessed the influence of specific morphological oocyte abnormalities on the success rate of intracytoplasmic sperm injection (ICSI) in terms of FR and embryo quality. We also assessed the FR and embryo quality in oocytes demonstrating multiple abnormalities.
Methods
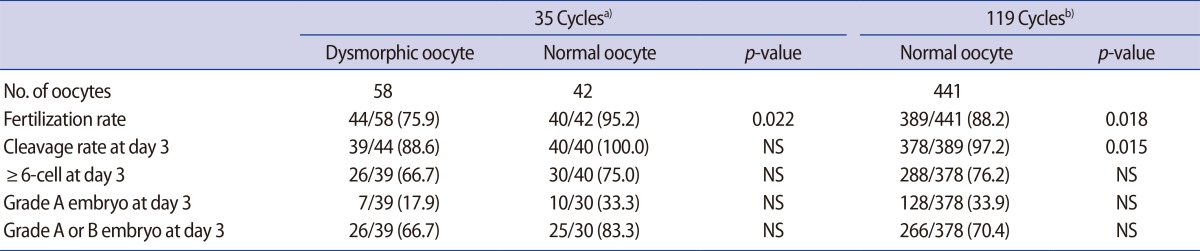
Data from 35 consecutive ICSI cycles, conducted during a period of 5 years (2010 to 2014) and which each yielded at least one dysmorphic mature oocyte were selected for analysis. The FR and embryonic development of dysmorphic oocytes and normal oocytes (control 1) from these cycles were assessed. The FR and embryonic development of normal oocytes from another 119 ICSI cycles during the same period, in which at least one normal oocyte and no dysmorphic oocytes were obtained (control 2), were also assessed.
The mean age of women in the 35 ICSI cycles was 36.1±3.9 years (range, 29 to 45 years). Male factor infertility diagnosis was present in 14 cycles and non-male factor infertility in 21 cycles; non-male factors included tubal factor infertility (n=6), decreased ovarian reserve or advanced maternal age (≥41 years old; n=6), unexplained (n=5), endometriosis (n=2), ovulatory dysfunction (n=1), and uterine factors (n=1). The 14 obligatory ICSI cycles involving male factor infertility included azoospermia (n=1), oligoasthenozoospermia (n=1), asthenozoospermia (n=2), oligoasthenoteratozoospermia (n=4), and teratozoospermia (n=6). Testicular sperm were used for ICSI in the azoospermic case; ejaculated sperm were used in the remaining 34 ICSI cycles.
Stimulation was performed in a standard manner, using a luteal long protocol of gonadotropin-releasing hormone (GnRH) agonist (Decapeptyl, Ferring, Malmo, Sweden) or GnRH antagonist (Cetrotide, Serono, Geneva, Switzerland) with daily administration of recombinant follicle-stimulating hormone (FSH) (GONAL-f, Serono). Oocyte retrieval was performed by transvaginal aspiration under ultrasound guidance 35 to 36 hours after the injection of recombinant human chorionic gonadotropin (Ovidrel, Serono).
The retrieved oocytes were exposed briefly to 80 IU/mL hyaluronidase (Sydney IVF Hyaluronidase, Cook Medical, Spencer, IA, USA), and mechanically separated from their surrounding cumulus cells by aspiration through a glass pipette. Next, all oocytes were examined under standard light microscope at a magnification of ×400, and those with a first PB present were selected for micromanipulation. At this time, all oocytes were assessed by an embryologist, and abnormal oocytes were classified as having a dark cytoplasm, cytoplasmic granularity, cytoplasmic vacuoles, refractile bodies in the cytoplasm, SER in the cytoplasm, an oval shape, an abnormal ZP, a large PVS, debris in the PVS, or an abnormal PB (Figure 1).
Figure 1. Various abnormalities of dysmorphic oocytes. Observed by light microscopy (×400).
Oocytes were incubated in the insemination medium (Sydney IVF fertilization medium, Cook Medical) at 37℃ in an atmosphere of 5% CO2 in air prior to and following injection. Sperm injection was performed, and oocytes with two pronuclei and two PBs at 14 to 18 hours post-ICSI were considered normally fertilized. Further embryonic development was assessed daily, and the final embryo quality was assessed at day 3 using a morphological grading system: grade A, equal-sized blastomeres without fragments or apparent morphologic abnormalities; grade B, equal-sized blastomeres and <20% of fragments without apparent morphologic abnormalities; grade C, irregularity of blastomeres and 20% to 50% of fragments without apparent morphologic abnormalities; grade D, irregularity of blastomeres and >50% fragments with apparent morphologic abnormalities [20].
Data were analyzed with MedCalc Software ver. 6.10 (Medcalc Software, Ostend, Belgium). Differences in FR at day 1, cleavage rate at day 3, and the proportion of embryos at the 6-cell stage or beyond at day 3 and the percentage of top-quality (grade A) and good-quality (grade A or grade B) embryos at day 3 were compared between groups of oocytes using the chi-square test. Differences were considered statistically significant when the p-value was <0.05.
Results
During the study period, 58 dysmorphic mature oocytes were obtained from 35 ICSI cycles, from each of which at least one dysmorphic mature oocyte was obtained. Forty-two normal form oocytes were retrieved simultaneously from the same cohort (control 1). From another 119 ICSI cycles in which at least one normal mature oocyte and no dysmorphic oocytes were retrieved, 441 normal form oocytes were obtained (control 2). The frequency of dysmorphic oocytes was 58% in the first group of 35 ICSI cycles, and 10.7% (58/541) in the whole study population.
Overall, the FR was significantly lower in the dysmorphic oocytes than in the normal form oocytes from the control 1 and 2 groups (Table 1). Development up to the day 3 cleavage stage was significantly lower in the dysmorphic oocytes than in the normal form oocytes in the control 2 group. However, the percentage of ≥6-cell embryos and the percentage of embryos of top or good quality at day 3 were similar between the dysmorphic oocyte group and the normal form oocytes in either the control 1 or control 2 groups.
Table 1. Overall fertilization rate and embryonic development in dysmorphic and normal form oocyte.
Values are presented as number (%).
NS, not significant.
a)Yielded at least one dysmorphic mature oocyte; b)Yielded at least one normal oocyte and no dysmorphic oocytes.
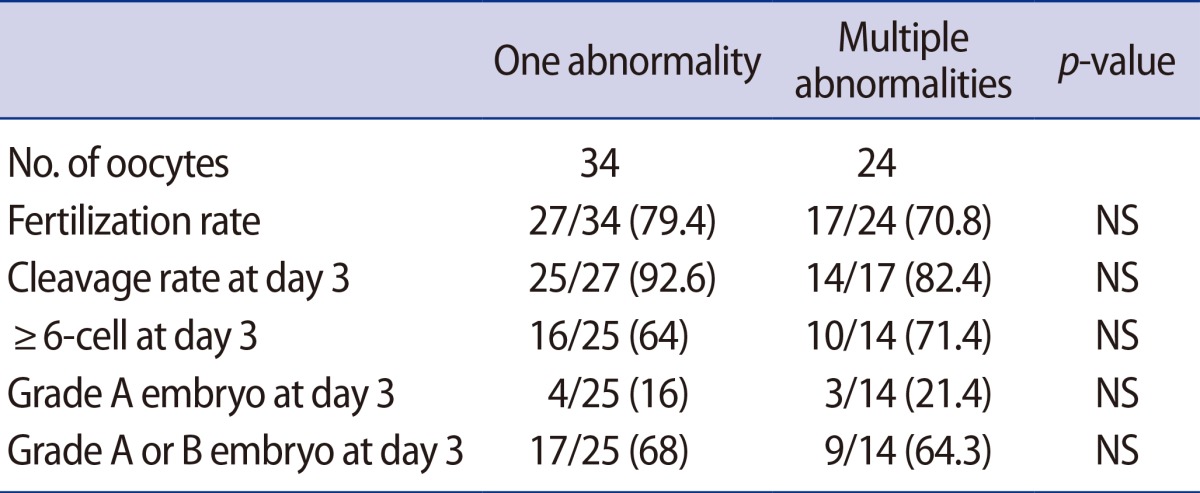
Among 58 dysmorphic oocytes, 34 oocytes (58.6%) exhibited a single abnormality and 24 oocytes (41.4%) exhibited two or more abnormalities. The FR, embryonic development, and the percentage of top- or good-quality embryos were similar between these two groups (Table 2).
Table 2. Overall fertilization rate and embryonic development in dysmorphic oocytes with single or multiple abnormalities.
Values are presented as number (%).
NS, not significant.
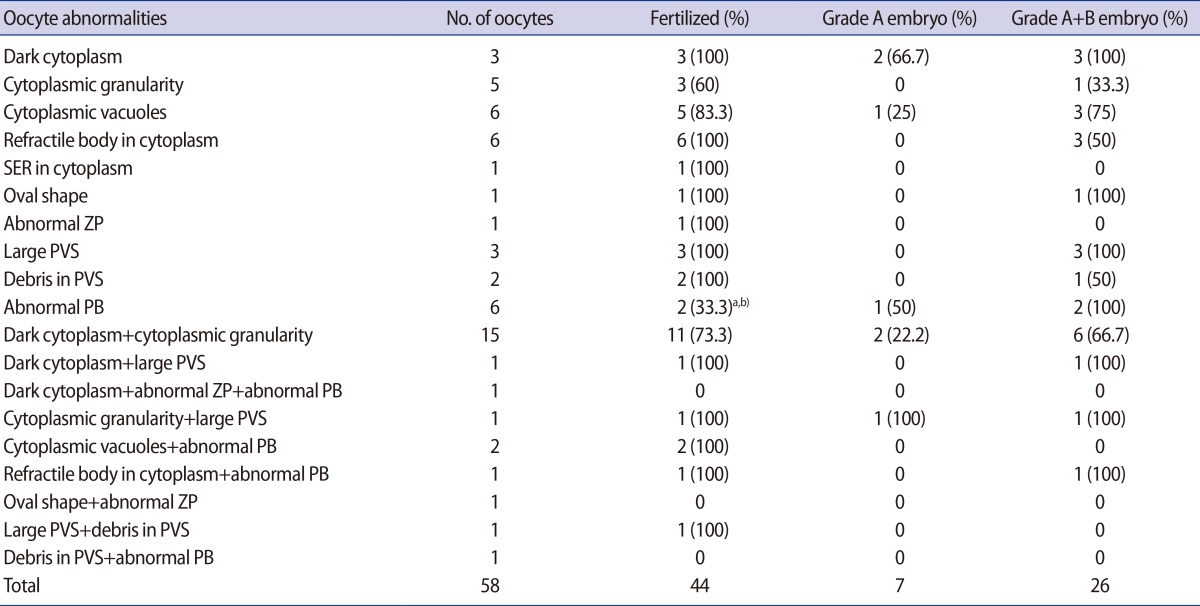
The FR and embryo quality in dysmorphic oocytes with single or multiple abnormalities are presented in Table 3. The lowest FR (33.3%) was evident in the oocytes with an abnormal PB and this was significantly lower than the FR of normal form oocytes in the control 1 and 2 groups. No fertilization occurred in oocytes with three of the combinations of multiple abnormalities, but each had only one oocyte.
Table 3. Specific fertilization rate and embryonic development in dysmorphic oocytes with single or multiple abnormalities.
SER, smooth endoplasmic reticulum; ZP, zona pellucida; PVS, perivitelline space; PB, polar body.
a)p<0.05 when compared with the corresponding data in normal form oocytes from 35 cycles; b)p<0.05 when compared with the corresponding data in normal form oocytes from 119 cycles.
The percentage of top- or good-quality embryos varied from 0% to 100% and all values were similar to that in the normal form oocyte groups. The majority of oocytes with single or multiple abnormalities yielded at least one good-quality embryo. However, no good-quality embryo could be obtained from oocytes with two kinds of single abnormalities and five of the combinations of multiple abnormalities. In terms of the percentage of top-quality embryos, oocytes with a dark cytoplasm (66.7%), oocytes with an abnormal PB (50%), and oocytes with cytoplasmic vacuoles (25%) had a good prognosis. Although a single oocyte with both cytoplasmic granularity and a large PVS formed a top-quality embryo, five oocytes with cytoplasmic granularity alone and three oocytes with a large PVS alone did not form a top-quality embryo.
Discussion
In the present study, dysmorphic oocytes had a significantly lower FR and cleavage rate, but a similar rate of forming top- or good-quality embryos, compared with normal form oocytes. While this is in contrast to an earlier report by De Sutter et al. [10], a relatively lower FR in dysmorphic oocytes has also been reported previously (66.7% to 71.2%) [15,21].
There have been few studies examining the prognosis of specific oocyte abnormalities in terms of successful fertilization and/or formation of good-quality embryos. Two kinds of dysmorphic oocytes in our study, specifically those with a dark cytoplasm or an abnormal PB, had a relatively good prognosis, as they had a 50% or greater chance of forming top-quality embryos. All three oocytes with a dark cytoplasm were fertilized and yielded good-quality embryos. Although only two oocytes were fertilized among six oocytes with an abnormal PB, both of them developed into good-quality embryos.
It has also been reported that oocytes with a dark cytoplasm exhibit a FR and embryo quality similar to normal oocytes with a clear cytoplasm [15,22,23,24]. Taken together, the darkness of the cytoplasm appears to represent a milder form of dysmorphism. In contrast, cytoplasmic granularity appears to represent a severe form of dysmorphism. In our study, five oocytes with cytoplasmic granularity displayed an acceptable FR, but the resulting embryo quality was poor. Cytoplasmic granularity can be homogeneous or heterogeneous or concentrated in the center [14]. Although we did not define the specific type of cytoplasmic granularity, oocytes of the heterogeneous type have been reported to show a better FR than those with the concentrated type [23]. It has also been reported that those with the concentrated type of granulation often fail to become fertilized and have reduced developmental potential [1,8,9].
With regards to the FR of oocytes with an abnormal PB, conflicting results have been published [6,7,15,18,19]. We observed a poor FR in the oocytes with an abnormal PB, but all fertilized oocytes developed into good-quality embryos. Since an abnormal PB represents abnormal cytokinesis, oocytes with an abnormal PB may have a high potential for a cytoplasmic defect, leading to unsuccessful fertilization. However, if they are successfully fertilized, a pre-existent cytoplasmic defect appears not to hinder subsequent embryonic development.
We observed a relatively good FR and embryo quality in oocytes with cytoplasmic vacuoles, but this is in contrast with the previous understanding. In general, oocyte vacuoles are a sign of severe degeneration [2]. Cytoplasmic vacuoles vary in size, as well as in number, and can be observed in 5% to 12% of oocytes [4,25]. Previous studies suggest that larger vacuoles may physically displace the metaphase II spindle from its usual polar position [15,26]. This is in line with a recent report describing a significant correlation between vacuole diameter and the presence of two pronuclei at the zygote stage, in which no fertilizations were observed above a vacuole size of 14 µm [27]. Larger or multiple vacuoles would result in a more detrimental impact on the oocytes than smaller ones, but this association could not be quantified because the vacuole parameters were not measured in this study.
The refractile body is a structure approximately 10 µm in diameter first described by Veeck [8]. Its evolution and its relationship to oocyte maturity and viability are not yet fully understood. Both mature and immature oocytes can develop refractile bodies, and there is a strong tendency toward their recurrence in repetitive treatment cycles in the same patient [8].
Extracytoplasmic defects include abnormal morphology of the ZP, the PVS, and first PB. Morphological abnormalities of the ZP include a thick, indented, or dark ZP, and can emerge differently in different IVF cycles with the same woman [16,28,29]. This type of oocyte presents with a total or partial absence of the PVS, an absence of resistance to ZP and oolemma penetration during ICSI, and low ooplasm viscosity during aspiration [16].
Although several studies have described FR and embryo quality in oocytes with a specific abnormality, few reports have examined oocytes with multiple abnormalities [14]. Because approximately 40% of dysmorphic oocytes exhibited two or more abnormalities in the present study, its occurrence is not rare. We here report a similar FR and similar embryo quality between oocytes that exhibited a single abnormality and those with two or more abnormalities. Interestingly, cytoplasmic granularity frequently occurred in combination with dark cytoplasm; 15 oocytes with both dark and granular cytoplasm had an acceptable FR, cleavage, and embryo quality. Thus oocytes displaying both dark cytoplasm and cytoplasmic granularity appear to have a good prognosis, although oocytes with cytoplasmic granularity alone have a poor prognosis. Since dysmorphic oocytes commonly exhibit multiple abnormalities, our data could help to assess the prognosis in oocytes with certain combinations of multiple abnormalities.
Pregnancy rates from oocytes with specific single abnormalities are difficult to assess because a typical embryo transfer includes mixed embryos generated from normal and dysmorphic oocytes. Nonetheless, a poor pregnancy rate has been reported in oocytes with cytoplasmic granularity [14], cytoplasmic inclusions [8,9,10,14], an indented ZP [16], oval shape [30,31], a dark ZP [11], and an abnormal ZP [16], compared with those without such anomalies.
In the present study, the frequency of dysmorphic oocytes was 10.7% in the population examined (154 cycles). However, this frequency was 58% in the 35 ICSI cycles in which at least one dysmorphic mature oocyte was obtained. Such a high rate has also been reported in the literature [10,32].
The origin of morphological abnormalities of oocytes is largely unknown but is likely multifactorial. Intrinsic factors such as age and genetic defects, or extrinsic factors such as ovarian stimulation protocolor handling procedures immediately following aspiration have been proposed [12]. Additionally, ovarian stimulation may result in the maturation of abnormal oocytes that would otherwise have become atretic in the absence of stimulation. Oocyte quality may also be directly affected by the supraphysiological level of estrogen and progesterone, which are intimately involved in the initiation of cytoplasmic maturation and the final stage of nuclear maturation of oocytes.
Human oocytes recovered from stimulated IVF cycles have an approximately 40% incidence of numerical chromosomal abnormalities. In addition, oocytes with abnormal cytoplasmic morphologies were found to have a high frequency of aneuploidy [33]. A high degree of morphological and nuclear anomalies has been shown in oocytes after stimulation with pure FSH following pituitary desensitization [34]. Debris in the PVS has been associated with high levels of gonadotropin during stimulation [35]. Mature oocytes exhibiting severe cytoplasmic disorganization have a lower intracytoplasmic pH and adenosine triphosphate content, as well as an increased incidence of aneuploidy and chromosomal scattering [36].
One limitation of our study was that we did not investigate specific conditions or stimulation protocols that could produce dysmorphic oocytes; instead, we focused on the individual developmental competence of oocytes with single or multiple abnormalities. Larger studies are needed to investigate patient-related factors and the benefits of ICSI in patients with dysmorphic oocytes.
Dysmorphic oocytes showed a significantly lower FR than normal-form oocytes, but subsequent embryonic development and embryo quality was relatively good. We here demonstrated that the FR and embryo quality are similar between oocyte groups with a single abnormality and multiple abnormalities. We also defined several specific abnormalities of dysmorphic oocytes related with top-quality embryos such as dark cytoplasm, abnormal PB, or cytoplasmic vacuoles. Because our cases were rather small, further large-scaled studies will be needed.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259–274. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16:324–346. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen AL, Lindenberg S. Morphology of in-vitro matured oocytes: impact on fertility potential and embryo quality. Hum Reprod. 2001;16:1714–1718. doi: 10.1093/humrep/16.8.1714. [DOI] [PubMed] [Google Scholar]
- 4.Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod Biomed Online. 2006;12:507–512. doi: 10.1016/s1472-6483(10)62006-8. [DOI] [PubMed] [Google Scholar]
- 5.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–615. doi: 10.1016/s1472-6483(10)61187-x. [DOI] [PubMed] [Google Scholar]
- 6.Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–1700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams & Wilkins; 1991. [Google Scholar]
- 9.Bedford JM, Kim HH. Sperm/egg binding patterns and oocyte cytology in retrospective analysis of fertilization failure in vitro. Hum Reprod. 1993;8:453–463. doi: 10.1093/oxfordjournals.humrep.a138071. [DOI] [PubMed] [Google Scholar]
- 10.De Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 11.Shi W, Xu B, Wu LM, Jin RT, Luan HB, Luo LH, et al. Oocytes with a dark zona pellucida demonstrate lower fertilization, implantation and clinical pregnancy rates in IVF/ICSI cycles. PLoS One. 2014;9:e89409. doi: 10.1371/journal.pone.0089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Cassia S Figueira R, de Almeida Ferreira Braga DP, Semiao-Francisco L, Madaschi C, Iaconelli A, Jr, Borges E., Jr Metaphase II human oocyte morphology: contributing factors and effects on fertilization potential and embryo developmental ability in ICSI cycles. Fertil Steril. 2010;94:1115–1117. doi: 10.1016/j.fertnstert.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Setti AS, Figueira RC, Braga DP, Colturato SS, Iaconelli A, Jr, Borges E., Jr Relationship between oocyte abnormal morphology and intracytoplasmic sperm injection outcomes: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;159:364–370. doi: 10.1016/j.ejogrb.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 15.Ten J, Mendiola J, Vioque J, de Juan J, Bernabeu R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod Biomed Online. 2007;14:40–48. doi: 10.1016/s1472-6483(10)60762-6. [DOI] [PubMed] [Google Scholar]
- 16.Sousa M, Teixeira da Silva J, Silva J, Cunha M, Viana P, Oliveira E, et al. Embryological, clinical and ultrastructural study of human oocytes presenting indented zona pellucida. Zygote. 2015;23:145–157. doi: 10.1017/S0967199413000403. [DOI] [PubMed] [Google Scholar]
- 17.Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J. Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod. 1998;13:3425–3430. doi: 10.1093/humrep/13.12.3425. [DOI] [PubMed] [Google Scholar]
- 18.Verlinsky Y, Lerner S, Illkevitch N, Kuznetsov V, Kuznetsov I, Cieslak J, et al. Is there any predictive value of first polar body morphology for embryo genotype or developmental potential? Reprod Biomed Online. 2003;7:336–341. doi: 10.1016/s1472-6483(10)61874-3. [DOI] [PubMed] [Google Scholar]
- 19.De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 20.Bolton VN, Hawes SM, Taylor CT, Parsons JH. Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastocyst. J In Vitro Fert Embryo Transf. 1989;6:30–35. doi: 10.1007/BF01134578. [DOI] [PubMed] [Google Scholar]
- 21.Kahraman S, Yakin K, Donmez E, Samli H, Bahce M, Cengiz G, et al. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15:2390–2393. doi: 10.1093/humrep/15.11.2390. [DOI] [PubMed] [Google Scholar]
- 22.Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Brown oocytes: implications for assisted reproductive technology. Fertil Steril. 2006;86:1522–1525. doi: 10.1016/j.fertnstert.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 23.Wilding M, Di Matteo L, D'Andretti S, Montanaro N, Capobianco C, Dale B. An oocyte score for use in assisted reproduction. J Assist Reprod Genet. 2007;24:350–358. doi: 10.1007/s10815-007-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaban B, Ata B, Isiklar A, Yakin K, Urman B. Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos. Hum Reprod. 2008;23:1778–1785. doi: 10.1093/humrep/den127. [DOI] [PubMed] [Google Scholar]
- 25.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3:283–288. doi: 10.1017/s0967199400002707. [DOI] [PubMed] [Google Scholar]
- 26.Nayudu PL, Lopata A, Jones GM, Gook DA, Bourne HM, Sheather SJ, et al. An analysis of human oocytes and follicles from stimulated cycles: oocyte morphology and associated follicular fluid characteristics. Hum Reprod. 1989;4:558–567. doi: 10.1093/oxfordjournals.humrep.a136944. [DOI] [PubMed] [Google Scholar]
- 27.Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fertil Steril. 2005;83:1635–1640. doi: 10.1016/j.fertnstert.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Stalf T, Mehnert C, Eichenlaub-Ritter U, Tinneberg HR. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum Reprod. 2005;20:1596–1606. doi: 10.1093/humrep/deh811. [DOI] [PubMed] [Google Scholar]
- 29.Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update. 2008;14:431–446. doi: 10.1093/humupd/dmn025. [DOI] [PubMed] [Google Scholar]
- 30.Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online. 2006;13:661–667. doi: 10.1016/s1472-6483(10)60656-6. [DOI] [PubMed] [Google Scholar]
- 31.Ebner T, Shebl O, Moser M, Sommergruber M, Tews G. Developmental fate of ovoid oocytes. Hum Reprod. 2008;23:62–66. doi: 10.1093/humrep/dem280. [DOI] [PubMed] [Google Scholar]
- 32.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3433. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 33.Van Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod. 1992;7:379–390. doi: 10.1093/oxfordjournals.humrep.a137655. [DOI] [PubMed] [Google Scholar]
- 34.Wojcik C, Guerin JF, Pinatel MC, Bied V, Boulieu D, Czyba JC. Morphological and cytogenetic observations of unfertilized human oocytes and abnormal embryos obtained after ovarian stimulation with pure follicle stimulating hormone following pituitary desensitization. Hum Reprod. 1995;10:2617–2622. [PubMed] [Google Scholar]
- 35.Halvaei I, Ali Khalili M, Razi MH, Nottola SA. The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J Assist Reprod Genet. 2012;29:803–810. doi: 10.1007/s10815-012-9799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]