Abstract
Multiple robotic flexible endoscope platforms have been developed based on cross specialty collaboration between engineers and medical doctors. However, significant number of these platforms have been developed for the natural orifice transluminal endoscopic surgery paradigm. Increasing amount of evidence suggest the focus of development should be placed on advanced endolumenal procedures such as endoscopic submucosal dissection instead. A thorough literature analysis was performed to assess the current status of robotic flexible endoscopic platforms designed for advanced endolumenal procedures. Current efforts are mainly focused on robotic locomotion and robotic instrument control. In the future, advances in actuation and servoing technology, optical analysis, augmented reality and wireless power transmission technology will no doubt further advance the field of robotic endoscopy. Globally, health systems have become increasingly budget conscious; widespread acceptance of robotic endoscopy will depend on careful design to ensure its delivery of a cost effective service.
Keywords: Robotics/instrumentation, Endoscopes, Endoscopic submucosal dissection, Therapeutic endoscopy, Robotic surgery, Medical devices, Natural orifice endoscopic surgery/instrumentation
Core tip: The collaboration between clinicians, engineers and business entrepreneurs with advancements in visualization and actuation technologies have given rise to a new generation of advanced endoscopes with new capabilities. Current efforts have focused on the development of endoscopes with automated locomotion functions and improved instrument manipulation abilities. With further development these new endoscopes will enhance clinicians’ ability to perform advanced endolumenal procedures such as endoscopic submucosal dissection. It is vital that future robotic endoscope development will help deliver a cost effective health service.
INTRODUCTION
The introduction of advanced endoscopic therapies such as endoscopic submucosal dissection and the relative widespread acceptance of robotic laparoscopic surgery, namely the Da Vinci system, has fired up the imagination of engineers, medical doctors and business entrepreneurs to develop robotic systems for delivery of medical and healthcare services. Such cross-specialty collaboration fuelled by advances in computer aided design, micro-actuating technologies and rapid prototyping technologies has further enhanced the development of robotic flexible endoscopy[1].
The current design of flexible endoscopic system offers limited instrument freedom. In theory, there should be a significant scope for the development of a robotic system that would increase instrument dexterity and spatial awareness of the surgeon. With the development of advanced endoscopic resection techniques such as endoscopic submucosal dissection (ESD), there is an obvious demand for robotic enabling technology. ESD achieved a higher en-bloc resection rate for early gastrointestinal (GI) cancers compared to conventional endoscopic resection. This allowed better assessment of resection completeness and disease staging[2,3]. However, ESD is associated with higher risks of complication. Cohort studies on gastric ESD for early gastric cancer demonstrated a postoperative haemorrhage rate ranged from 3.6% to 15.6% and perforation rate of 1.2% to 5.3%, with a mean operative time range of 47 min to 60 min[4-10]. Low volume centres may have a higher perforation rate and longer operative time. The rate of complication and success of ESD is very much operator dependent. Under expert hands, excellent outcomes and low complication can be achieved even for oesophageal and colonic ESD[11]. Paradoxical endoscope movement, large tumour size and fibrosis have been cited as factors that exacerbate difficulty in performing colonic ESD[12]. Transitional techniques to improve endoscopic traction includes clip-with-line method[13], percutaneous traction method, sinker-assisted method, magnetic anchor[14], external forceps[15], internal traction, double scope method[16,17], have been reported. However, these adjuncts have not been widely adopted. A robotic endoscopic platform may act as an enabling technology to encourage widespread adoption of advanced endolumenal procedures. One of the developing areas of potential use of a robotic endolumenal platform will be gastric plication as a weight reduction surgery[18].
Mechanically actuated endoscopic platforms have been developed to improve tissue handling in the confined endolumenal environment[19]. These platforms rely on mechanically driven cables to actuate instruments arms. Some of the platforms have been tested in pre-clinical and clinical settings and have been shown to improve procedural performance efficiency. The R-scope (Olympus, Japan) is one of the earliest systems used to perform ESD[20-22]. However, the R-scope interface may be too difficult to use and therefore limits its uptake as an enabler technology[23]. The EndoSamurai (Olympus, Japan) has demonstrated obvious advantage in pin transfer and suturing using the two arms under the endoscope when compared to conventional dual channel endoscope[24]. The system has also been challenged to perform small bowel anastomosis in a bench top porcine model[25]. Bench top study of the Anubiscope (Karl Storz/IRCAD, Europe) have been shown to enhance the ability of novices to perform significantly faster ESD with lower perforation rate when compared to an experienced endoscopist using conventional double channel endoscope[26]. It has been used to perform cholecystectomy in human[27]. Access device based system such as the DDES (Boston Scientific, United States) has been used to perform complex task such as suturing and knot tying[28]. Other access device based system such as the incisionless operating platform (USGI, United States) has been used to perform mucosal resection and full thickness gastric wall resection on a bench top model, as well as cholecystectomy and fundoplication in animal and cadaveric models[29-31]. Clinical studies including transgastric cholecystectomy and obesity surgery revision have also been performed using the Incisionless Operating Platform[32,33]. The design of these mechanical systems generally required multiple endoscopists, which will incur significant increment in cost per procedure as well as the requirement of multiple operator collaboration.
Robotic surgery can potentially improve operational efficiency. Robotic surgery can be defined as the performance of surgery using an intelligent machine, which is capable of planning and executing surgical manoeuvres based on its ability to integrate various external information[34]. Current development is mainly focused on the development of electromechanical systems to execute surgical manoeuvres and autonomous locomotion. Numerous robotic systems have been developed for laparoscopic, endolumenal and transluminal paradigm[35]. The aim of this review is to assess the current status of development on robotic flexible endoscopy for diagnosis and treatment of gastrointestinal diseases.
PubMed search
PubMed search has been performed using search terms “Robotic” “Endoscopy” for articles published in the last five years from August 31, 2010 to August 31, 2015. Relevant articles pertaining to robotic flexible gastrointestinal endoscopy are identified from title and abstract. In addition, important references were identified through individual article references.
RESULTS
Currently, the application of robotic technology in endoscopy has been focused on autonomous locomotion and electromechanical instrument manipulation. A summary of the existing platforms can be seen in Table 1.
Table 1.
Summary of currently available robotic flexible endoscopic platforms
| Platforms |
Development status |
||
| Robotic driven locomotion | FDA | CE | Sale |
| Electromechanical control of a conventional endoscope | |||
| Robotic steering and automated lumen centralization (RS-ALC) (Netherlands) | - | - | - |
| Endoscopic operating robot (EOR) (Kyushu Institute of Technology, Japan) | - | - | - |
| Invendoscope (Invendo Medical Gmbh, Germany) | Y | Y | Y |
| Systems with elements of autonomous locomotion | |||
| Neoguide (Intuitive Surgical, United States) | Y | N | N |
| Aer-O-scope (GI View Ltd, Israel) | Y | Y | Y |
| Endotics (ERA Endoscopy SRL, Italy) | N | Y | Y |
| CUHK double -balloon endoscope (Chinese University of Hong Kong, China) | - | - | - |
| Robotic driven instrumentation | |||
| MASTER (EndoMASTER Pte, Singapore) | - | - | - |
| ISIS-Scope/STARS system (Karl Storz/IRCAD, Europe) | - | - | - |
| Endomina (Endo Tools Therapeutics, Belgium) | Y | - | Y |
| Scorpion shaped endoscopic robot (Kyushu University, Japan) | - | - | - |
| Viacath (Hansen Medical, United States) | Y | Y | Y |
| CUHK robotic gripper (Chinese University of Hong Kong, China) | - | - | - |
| Imperial College robotic flexible endoscope (Imperial College, United Kingdom) | - | - | - |
Currently, all systems are either in experimental stage of development or early commercialization. As such, no data is available to assess cost effectiveness of various systems.
ROBOTIC DRIVEN LOCOMOTION
Electromechanical control of a conventional endoscope
In this approach, the conventional endoscope control wheel is manipulated through electromechanical mechanism. The mechanism is in turn controlled through a joystick or touchpad control interface. This has been shown to be the preferred method of control by novices in endoscopy, however it is difficult to see its uptake by expert endoscopists[36]. Early efforts include the incorporation of hollow shaft motors directly replacing the conventional navigational wheels of an endoscope[37]. Other attempts included the use of automated horizon stabilization software. However, this has been shown to worsen endoscopist’s orientation and performance[38]. Notable systems using this approach include robotic steering and automated lumen centralization (RS-ALC), the endoscopic operating robot (EOR) and the Invendoscope.
RS-ALC (Netherlands)
The system consists of a remote drive unit which allows docking of the angulation wheels of a conventional endoscope (Figure 1). Open loop control is achieved through a joystick with operator visual feedback. Ex vivo phantom study showed that although it facilitated novices in reaching the caecum quicker, this effect did not persist for an experienced endoscopist; the median caecum intubation time with conventional endoscope was 129 s compared to 781 s with the RS-ALC system[39].
Figure 1.
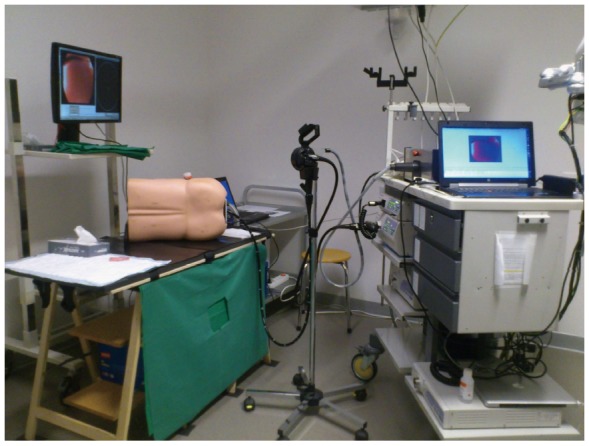
The RS-ALC system: A conventional endoscope is mounted onto electromechanical control wheels. Control of the system is through a joystick device. Courtesy of Dr Pullens, Meander MC, Netherlands.
EOR (Kyushu Institute of Technology, Japan)
The EOR is a master-slave robotic system mounted on a conventional endoscope (Figure 2). It is able to manipulate a conventional endoscope through actuator wheels that are controlled by two joysticks. The intention of the design is to replace the endoscopist thereby allowing a single operator to control a complex endoscopic multitasking platform such as the EndoSamurai. In the first version of EOR, the four axis of the endoscope is computer controlled by four separate motor actuated timing belt and pulleys. In addition to being able to manipulate the operation wheels, it is capable of rotating the scope 150 degrees. The system has been used on bench top models to perform colonoscopy and endoscopic submucosal dissection[40,41]. In the most updated version, torque sensors have been incorporated into the system to allow a degree of haptic feedback[42]. Clinical studies are awaited.
Figure 2.
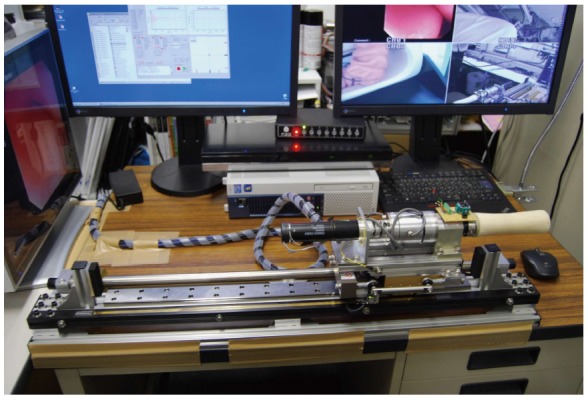
EOR version 3. The system consists of various actuators to execute forward/backward, rotational, up/down, left/right movement of a conventional endoscope. Courtesy of Professor Kume, Kyushu University, Japan.
Invendoscope (Invendo Medical Gmbh, Germany)
The device is a 10 mm endoscope driven by rotary actuators placed outside the patient. The scope and its channels are protected by an inverted sleeve. The bending section of the endoscope is controlled electrohydraulically (Figure 3). The system is controlled by a joy stick interface. During insertion, the inverted sleeve unrolls to protect the inserted section of the endoscope. A human clinical trial consisted of 34 patients was conducted to assess the functionality of different prototypes of the device. It demonstrated a caecal intubation rate of 82%. For those who failed to complete the examination, two patients developed severe pain resulting in procedure abandonment and in four patients, the invendoscope could not pass beyond the hepatic flexure or the transverse colon[43]. A recent clinical study recruited 61 patients to undergo colonoscopy with the CE marked InvendoSC20. It showed a caecal intubation rate of 98.4% with a median caecal intubation time of 15 min. Polypectomies were performed in 23 patients through the device’s 3.1 mm channel[44]. Further study on comparison between invendoscope and conventional colonoscopy is awaited.
Figure 3.
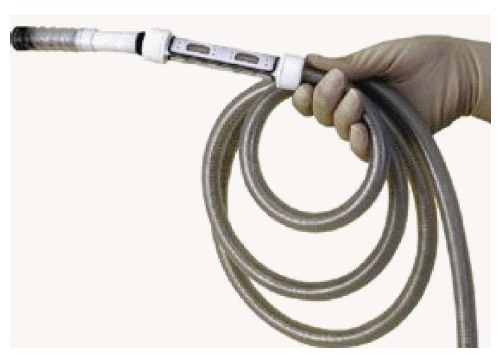
Invendoscope. The scope design is akin to a conventional endoscope. The scope is protected by a disposable inverted sheath which unfurls when the endoscope is pushed forward by the actuating wheels (Invendo Medical Ltd).
Systems with autonomous locomotion: In general, these systems utilize the inchworm locomotion concept akin to that used by double balloon enteroscopy[45], the snake-like tail-follow-nose concept or pneumatic propulsion. One of the earliest prototypes with autonomous locomotion was reported in 1999[46]. Notable systems using this approach include Neoguide, Aeroscope and Endotics. Early prototypes include the CUHK automated double balloon endoscope.
Neoguide (Intuitive Surgical, United States)
It is an endoscope system designed to traverse the natural shape of the colon and therefore overcomes the unintentional lateral forces generated during conventional colonoscopy[47] (Figure 4). It has a tip position sensor and an external position sensor to measure endoscope tip position and insertion depth. It has multiple independent segments which are electromechanically controlled to conform to the natural shape of the colon. However, it is advanced manually in the same manner as a conventional colonoscope. During active mode, the computer will adjust the proximal segments in a “tail follow nose” manner. Its tip diameter is approximately 14 mm and its proximal shaft is about 20 mm in diameter. Although it has been preliminarily tested in clinical trial, direct head to head comparison against conventional colonoscope is still awaited.
Figure 4.

Neoguide with its multiple bending segments enabling it to manoeuvre in a tail follow nose manner. Eickhoff et al[47], 2007.
Aeroscope (GI View Ltd, Israel)
The system consists of a camera vehicle with a contour conforming balloon. The vehicle is supplied by a 5.5 mm multi-lumen polyurethane cable for transmission of electricity, air, water, and suction (Figure 5). The vehicle is inserted into the rectum through an introducer. When the system is deployed the balloon around the camera is inflated to form an airtight seal with the colonic wall. Computer controlled positive pressure gradient is generated in the distal colon propelling the vehicle forward into the proximal colon, and vice versa during withdrawal. Colonic pressure is closely monitored to not exceed 54mbar[48]. An Omnivision camera which has 360 radial view and front viewing capability has been incorporated into the system[49]. A small single centre prospective study consisted of 56 subjects was conducted to assess Aeroscope performance with conventional colonoscopy performed immediately in tandem. This study showed that Aeroscope has a cecal intubation rate of 98.2% after initial learning curve but polyp detection rate is only 87.5% when compared with conventional colonoscopy[50].
Figure 5.
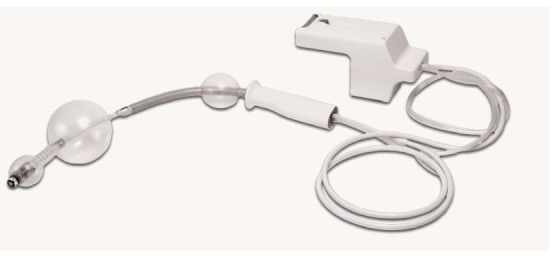
The Aeroscope relies on a balloon at the tip of the endoscope to form a seal with surrounding colonic wall. A computerized pump system generates a pressure gradient proximal and distal to the balloon. This pressure gradient propels the device. Courtesy of GIview Ltd.
Endotics (ERA Endoscopy SRL, Italy)
The Endotics system is composed of a disposable probe which has a steerable tip, flexible body and a special tank with electro-pneumatic connector. The tip has an integrated LED camera. It has an insufflation and suction channel. The probe is connected to the external workstation through a 7.5 mm supply cable. The probe has proximal and distal clampers to allow proper anchoring and performance of automated inch-worm locomotion (Figure 6). Initial study suggested a poor caecal intubation rate of only 27.5% with Endotics when compared to conventional colonoscopy which had an 82.5% caecal intubation rate. However, the group treated with Endotics had significantly lower patient discomfort[51]. A subsequent human study assessed 71 patients that underwent tandem examination with Endotics system and conventional endoscopy. The caecal intubation rate for Endotics was 81.6%, whereas conventional endoscopy achieved a caecal intubation rate of 94.3% (P = 0.03). Procedure time was significantly longer with Endotics system (45.1 ± 18.5 min vs 23.7 ± 7.2 min) (P < 0.0001). Furthermore, Endotics system demonstrated a significantly lower polyp detection rate. Although none of the patients required sedation during examination by Endotics, it appears that further refinement is necessary to improve the polyp detection[52].
Figure 6.

Endotics double balloon probe. Locomotion is executed using the inch-worm mechanism. Courtesy of Endotics SRL, Italy.
CUHK double balloon endoscope (Hong Kong, China)
This autonomous double balloon endoscope has a capsule camera at the tip. The body of the device consists of two balloon connected by an extension section. The most distal balloon wraps around the steering module. The balloons and extension section are actuated hydraulically[53] (Figure 7). Locomotion is achieved through standard inch-worm mechanism. Current development is still in the early prototype stage.
Figure 7.
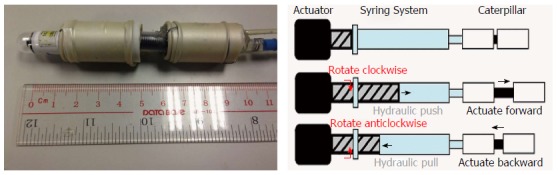
CUHK double balloon endoscope with its proximal and distal balloon connected by an extension section. A capsule endoscope is mounted at the tip of this endoscope prototype. Poon et al[53], 2015.
ROBOTIC DRIVEN INSTRUMENTATION
These tethered systems utilize traction cable actuation. Such actuation system has a significant level of hysteresis. Electromechanical control of these systems allow partial compensation and limit backlash and force reduction. Despite significant effort being made to overcome the challenge of hysteresis, this remains imperfect[54-62]. Notable system includes MASTER, ISIS-Scope, Viacath, Endomima and the Scorpion shaped endoscopic robot. Various early prototypes are also in development.
MASTER (EndoMASTER Pte, Singapore)
The first prototype of MASTER is a traction wire controlled robotic arm system that is mounted externally onto a conventional double channel endoscope. It is capable of delivering up to nine degrees of freedom of movement at the end effector[63] (Figure 8). Animal studies have shown its effectiveness in performing ESD, simulated gastric full thickness wedge resection and hepatic resection[63-67]. The MASTER system has been used to perform endoscopic submucosal dissection[64]. In a small clinical study consisted of 5 patients with lesions limited to gastric body or antrum, the median dissection time was 16 min (3-50 min). Although the MASTER system demonstrated its ability to perform endoscopic submucosal dissection[68], problems encountered included the lack of ability for instrument exchange and the requirement of passage of system through an overtube to protect the oesophagus. Large external actuator and bulky control units limited the manoeuvrability of the system. Currently, the second phase of development is driving on improvements in integrated control and streamlining performance. Haptic feedback and precision control is in development[69]. The value of such a robotic system will be especially useful for performing complex endoscopic surgical procedures in low volume centres and in localities where diagnosis of early GI cancers are relatively rare[70].
Figure 8.
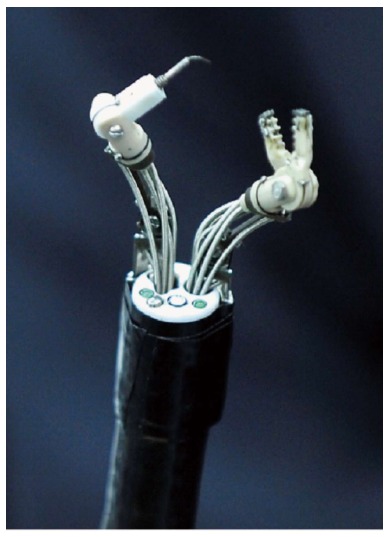
The MASTER system is a robotic arm system that can be mounted onto a conventional endoscope. Phee et al[68], 2012.
ISIS-Scope/STRAS system (Kark Storz/IRCAD, Europe)
The STRAS system is a robotized version of the Anubiscope[71] (Figure 9). The endoscope has a diameter of 18 mm. It has a 35 cm passive shaft and a 22 cm bending section with jaws at the front tip which opens to allow instrument triangulation. It has two 4.2 mm channels allowing passage of instruments capable of tip deflection on one axis, translation, rotation and end effector opening and closure. The endoscope element and the instrument are controlled electromechanically through externally actuated traction wires. Electromechanical control has been designed to improve instrument movement fluidity by cancelling out the friction sensation observed by operators using the purely mechanically designed Anubiscope. An open loop control architecture with special calibration and tracking procedures have been used in attempt to overcome hysteresis inherent in a traction cable system. Common work space is centred at 9 cm from the camera with a maximum aperture of 2.5 cm from the camera, but this may be considered too wide a working field for the gastrointestinal lumen. Visual feedback mechanisms are under development to allow a closed loop control system[72].
Figure 9.
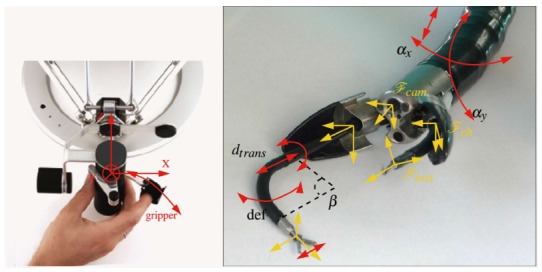
ISIS-Scope/STRAS system is a electromechanically controlled ANUBISCOPE. De Donno et al[72], 2013.
Endomina (Endo Tools Therapeutics, Belgium)
It is a universal triangulation platform which can be mounted on a conventional flexible endoscope similar to the aforementioned MASTER system (Figure 10). It has recently obtained CE mark certification in 2015. It has two instrument channels with 3 DOF of independent movement. These channels are able to guide two standard flexible instrument of up to 9 Fr in diameter. The system is actuated through electromechanically actuated traction cables. The control interface consists of two joysticks[73]. Currently, clinical human trials are ongoing.
Figure 10.
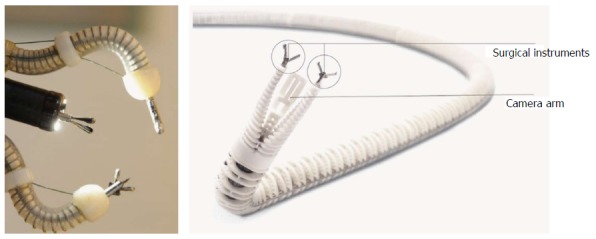
The Endomima system can be mounted onto a conventional endoscope. The arms allow passage of conventional flexible instruments. Each arm has up to 3 DOF of movement (Endotools Therapeutics).
Scorpion shaped endoscopic robot (Kyushu University, Japan)
The system consists of two external traction cable controlled robotic arms with an integrated camera[74] (Figure 11). Through the use of magnetic sensors, it is able to locate the tip of the endoscope. The scope tip position can be overlaid onto cross-sectional imaging data and fed back to the endoscopist through an integrated display. Each robotic arm is 40 mm in length and 6mm in width. Each arm is capable of up, down, left, right, and opening/closing of end effector and it can generate up to 3 N force. The system requires two operators; one operator controls the endoscope and one controls the robotic arms. Initial attempts were made to incorporating piezo pressure sensors to facilitate haptic feedback. However, adequate insulation proved to be very difficult. Therefore, haptic feedback was indirectly calculated through monitoring of wire traction. It is recognized by the authors that this is an imperfect method for generating haptic feedback because the various positions of the endoscope can add noise to the traction data. Despite this system appearing to be less bulky than the MASTER system, no pre-clinical or clinical data has been published for this system.
Figure 11.

Scorpion like endoscopic robot is an externally mountable robot arms system. It is actuated by external actuators through traction cables (right). Suzuki et al[74], 2010.
Viacath (Hansen Medical, United States)
This system consists of cable actuated robotic arms. It can be integrated with a conventional endoscope through the use of an overtube (Figure 12). It was reported that the instruments could only generate 0.5 N of lateral force, which may limit its ability to manipulate tissues within the GI tract. Its flex joint design allows infinite configurations of the flex section for the same cable displacement. Therefore, maximum force generation is based on bending stiffness of the flex section and the necessity for a small calibre instrument results in low lateral force generation[75]. There is as yet no clinical data published regarding its using in the gastrointestinal tract.
Figure 12.
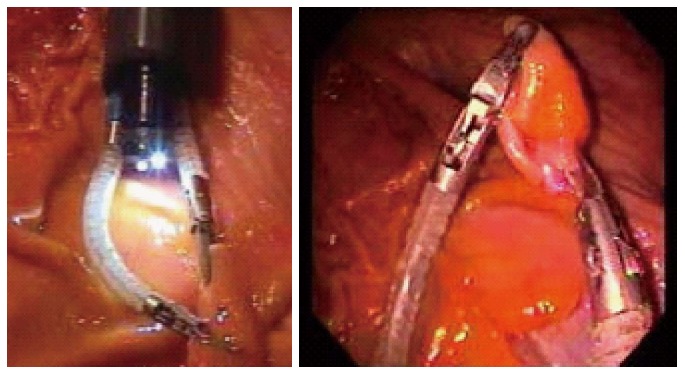
On the left, the endoscope and the viacath robotic arms are integrated using an overtube. Abbott et al[75], 2007.
OTHER PROTOTYPES
CUHK robotic gripper (Chinese University of Hong Kong, China)
CUHK 3 DOF robotic gripper designed to work through instrument channel of conventional endoscope is currently in development[76]. The same department has also suggested a bioinspired endoscopic robotic arm system using shape memory alloy traction wire actuation[77] (Figure 13). It is a roboticized flexible endoscopic instrument consisting of a 2 DOF bending section with an end effector. The bending section is controlled by 2 pairs of shape memory alloy wires guided by stainless steel tubes which reduces the level of hysteresis. The end effector also has 2 DOF[78]. The system is controlled by an external controller akin to other robotic systems. The system can be used in conjunction with other overtube system such as the USGI transport system[79]. Current evidence of its function is limited to bench top studies.
Figure 13.

CUHK robotic arm prototype. Poon et al[77], 2014.
Imperial College, London Prototype
Imperial College, London has also developed a robot prototype which has two instrument channels of 3 mm and 2.5 mm. Each instrument channel has 3 DOF of movement. Each DOF of movement is controlled by two NiTi tendon. The platform’s minimum overall diameter is 13 mm[80] (Figure 14).
Figure 14.
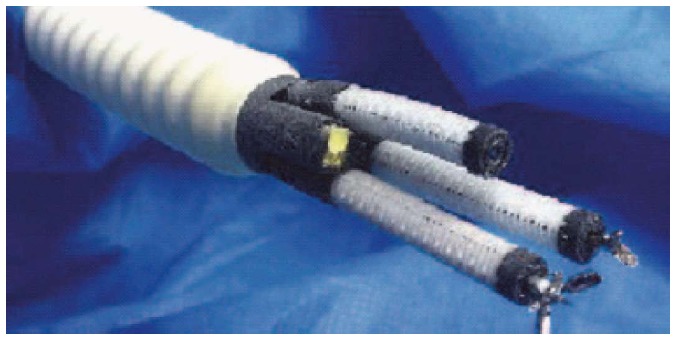
Imperial College robotic arm prototype. Seneci et al[80], 2014.
DISCUSSION
The application of robotics in gastrointestinal endoscopy had been focused on enhancing the manoeuvrability and the therapeutic capability of the endoscope. It is of note that one of the main driving forces for development of these advanced platforms, namely Natural Orifices Transluminal Endoscopic Surgery (NOTES), has waned significantly in recent years as evidence suggest that NOTES approach may actually result in increased morbidity[81,82]. The focus of future robotic flexible endoscope should target advanced endolumenal procedures. In the era of routine endoscopic screening, the demand for endolumenal resection of large polyps or early cancers will increase. Robotic endoscopy will enable many more clinicians across the globe to perform advanced endolumenal procedures such as ESD. As a result, many patients will avoid the complications and mortality associated with major resectional surgery. Therefore, the focus of this review has been on platforms that may have potential application in the endolumenal paradigm. As such, notable snake like platforms designed for transluminal procedures, such as the Carnegie mellon robotic system[83], the I-snake[84,85] have not been included in this review.
Robotic endoscopic platform: Mechanics
Further development and utilization of novel actuation technologies and feedback sensors will be vital for improvement in robotic instrument control. Current systems rely on traction cable actuation which makes accurate, efficient position feedback difficult[86]. This renders autonomous instrument control difficult. Development in new actuation technology will resolve the current difficulties. For example, a double screw drive mechanism has been described to ameliorate hysteresis. In this mechanism, flexible traction cables are placed in hollow tube rigid linkages[87]. The McKibbens fluidic actuators are another type of reinforced elastic actuation mechanism. These actuators consist of elastic tube structures reinforced with external braid of interwoven wire helixes. When the tube expands with fluid, it engages the external braid to twist and accommodate size change leading to actuation. These actuators are capable of generating contractile force of around 5 N[88]. Shape memory alloys and piezoelectric actuators are likely to play an increasing role in actuating robotic systems[89,90]. New programmable matters or phase change material, such as claytronics, can be incorporated into endoscope design[91,92]. Improvement in tactile and optical feedback mechanisms is also vital for autonomous instrument control. Novel microfabricated tactile sensors can be used to create haptic feedback[93]. Bragg grating sensors have been incorporated into flexible endoscopic instruments, such as an IT knife. The bending force can be inferred from the distortion of the sensors[94]. Alternatively, techniques such as visual servoing[87], where closed loop control is achieved through optical analysis of instrument positions can be considered. However, the lack of contrast of the gastrointestinal tract and the constant variation in illumination with current endoscopic visualization technology makes effective visual servoing difficult. Development of high resolution high frame rate imaging, 3D vision[95], image mosaicking[96] and advanced optical analytical and 3D modelling algorithms[97-101] may ameliorate this challenge. The use of artificial neural networks and different light conditions may also enhance visual servoing and improve the development of closed loop controlled automation[102]. Image based algorithm has been used to steer a conventional endoscope in a virtual simulation setting[103]. Improvement in optics may enhance the development of endoscopic micro-robotics to perform diagnosis and resection of early GI cancers automatically[104].
In order for robotic endoscopic systems to be adopted, it is pertinent that it delivers enhanced functions in addition to instrument dexterity and autonomous locomotion. A potential added function will be augmented reality. Optical and tactile augmented reality will help the surgeon to accurately excise target tissue through even more precise dissection[105]. It will be important for performing transluminal procedures such as endoscopic ultrasound guided drainage procedures[106,107]. Methods to simulate direct endolumenal palpation will in time become a reality. For example, palpation has been simulated through the use of an ultrasound probe which detects shear wave in tissue generated by an external exciter. A local frequency estimation method calculates the shear modulus of the tissue and provides an estimation of the elastic property of the tissue[108]. Other added features may include improvement in system interface which will reduce the barrier of entry for any endoscopic robotic system. Wearable gesture recognition interface have been attempted but have not yet found its utility[109,110]. Alternative control interface should be explored to improve control interface and instrument handling so as to enhance the acceptance of future robotic flexible platforms[111-114].
The ideal robotic platform should be cordless and small enough to be swallowed and retrieved without causing significant discomfort to the patient, while providing full therapeutic capability and added functions such as augmented reality.
Although great strides have been made since the introduction of the passive diagnostic capsule, it is likely that a robotic capsule capable of performing endoscopic interventions will require prolonged development. The development of autonomous locomotion through various mechanisms such as through paddling fins or spiral legs are at its infancy[115,116]. Magnetic steering of wireless capsule may allow better control of the capsule endoscope within the GI tract[117]. Localization systems is another challenging yet important issue to be refined[118]. Basic therapeutic procedure such as drug therapy delivery has also been attempted with capsule endoscopy[119]. Improvement in wireless power transmission will increase the capacity of miniature wireless robots to perform therapeutic procedures[120]. Until such a time when all these challenges have been overcome, the flexible robotic endoscope will likely be the mainstay of a clinically applicable platform for the foreseeable future.
Robotic endoscopic platform: Economics
Furthermore, not only does a robotic platform have to offer added functionality, it will also have to be cost effective. Despite the wide spread clinical application of the Da Vinci robotic assisted surgical system, majority of the studies did not demonstrate a significant improvement in robust clinical outcome parameters, patients’ safety or cost effectiveness[121]. The adoption of robotic surgery in the laparoscopic paradigm is largely driven by the advantages of the intuitive surgeons’ control of the robotic platform, as well as the market forces and competition between different health systems, rather than from genuine clinical benefits[122]. Globally, health systems are increasingly cost conscious[123]. It is likely that mainstream uptake of any robotic endolumenal platform will not take place until proper scrutiny into cost and benefit of such systems have taken place[124-128]. Therefore, a cost based paradigm for robotic design rather than a purely disease/procedure based design paradigm must be adopted. Currently, all robotic endoscopic systems presented in this review are either in experimental stage of development or in the process of being commercialization. As such, no data is available to assess cost effectiveness of various systems.
CONCLUSION
In short, the application of robotics in gastroenterological endoscopy is only at its infancy. Robotic flexible endoscopy is a rapidly emerging field of research. Although most of the systems currently in development will never reach widespread clinical application, it is likely that they will form the foundation for the next generation of robotic endoscope. The future robotic endoscope will not only be capable of delivering added instrument dexterity but also added functions such as augmented reality surgery. Importantly, it should be able to deliver effective therapy at an acceptable cost as well.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 9, 2015
First decision: November 27, 2015
Article in press: December 30, 2015
P- Reviewer: Amornyotin S, Nakayama Y S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.de Ruijter V, Halvax P, Dallemagne B, Swanström L, Marescaux J, Perretta S. The Business Engineering Surgical Technologies (BEST) teaching method: incubating talents for surgical innovation. Surg Endosc. 2015;29:48–54. doi: 10.1007/s00464-014-3652-1. [DOI] [PubMed] [Google Scholar]
- 2.Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng EK. Difficulties and outcomes in starting endoscopic submucosal dissection. Surg Endosc. 2010;24:1049–1054. doi: 10.1007/s00464-009-0724-8. [DOI] [PubMed] [Google Scholar]
- 3.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 4.Chiu PW. Novel endoscopic therapeutics for early gastric cancer. Clin Gastroenterol Hepatol. 2014;12:120–125. doi: 10.1016/j.cgh.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operative time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 6.Lee TH, Cho JY, Chang YW, Kim JO, Lee JS, Cho WY, Kim HG, Kim WJ, Park YS, Jin SY. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920–926. doi: 10.1016/j.gie.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136. doi: 10.1007/s10120-013-0241-2. [DOI] [PubMed] [Google Scholar]
- 9.Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 10.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H. Endoscopic submucosal dissection--current success and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:519–529. doi: 10.1038/nrgastro.2012.97. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Ito S, Kitagawa T, Kato M, Tominaga K, Suzuki T, Maetani I. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc. 2014;28:2959–2965. doi: 10.1007/s00464-014-3558-y. [DOI] [PubMed] [Google Scholar]
- 13.Oyama T, Kikuchi Y, Shimaya S, Tomori A, Hotta K, Miyata Y, Yamada S. Endoscopic mucosal resection using a hooking knife (hooking EMR) Stomach & Intestine. 2002;37:1155–1161. [Google Scholar]
- 14.Kobayashi T, Gotohda T, Tamakawa K, Ueda H, Kakizoe T. Magnetic anchor for more effective endoscopic mucosal resection. Jpn J Clin Oncol. 2004;34:118–123. doi: 10.1093/jjco/hyh025. [DOI] [PubMed] [Google Scholar]
- 15.Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N, et al. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007–1010. doi: 10.1055/s-2006-925264. [DOI] [PubMed] [Google Scholar]
- 16.Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264–269. doi: 10.1016/s0016-5107(88)71327-9. [DOI] [PubMed] [Google Scholar]
- 17.Imaeda H, Hosoe N, Kashiwagi K, Ohmori T, Yahagi N, Kanai T, Ogata H. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc. 2014;6:286–295. doi: 10.4253/wjge.v6.i7.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SM, Pryor AD. Future directions in bariatric surgery. Surg Clin North Am. 2011;91:1373–195, x. doi: 10.1016/j.suc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Yeung BP, Gourlay T. A technical review of flexible endoscopic multitasking platforms. Int J Surg. 2012;10:345–354. doi: 10.1016/j.ijsu.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Neuhaus H, Costamagna G, Devière J, Fockens P, Ponchon T, Rösch T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”) Endoscopy. 2006;38:1016–1023. doi: 10.1055/s-2006-944830. [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa J, Kaise M, Sumiyama K, Goda K, Arakawa H, Tajiri H. A novel double-channel therapeutic endoscope (“R-scope”) facilitates endoscopic submucosal dissection of superficial gastric neoplasms. Endoscopy. 2006;38:1011–1015. doi: 10.1055/s-2006-944779. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Gromski MA, Derevianko A, Jones DB, Pleskow DK, Sawhney M, Chuttani R, Matthes K. Efficacy of a prototype endoscope with two deflecting working channels for endoscopic submucosal dissection: a prospective, comparative, ex vivo study. Gastrointest Endosc. 2010;72:155–160. doi: 10.1016/j.gie.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spaun GO, Zheng B, Martinec DV, Cassera MA, Dunst CM, Swanström LL. Bimanual coordination in natural orifice transluminal endoscopic surgery: comparing the conventional dual-channel endoscope, the R-Scope, and a novel direct-drive system. Gastrointest Endosc. 2009;69:e39–e45. doi: 10.1016/j.gie.2008.12.239. [DOI] [PubMed] [Google Scholar]
- 24.Spaun GO, Zheng B, Swanström LL. A multitasking platform for natural orifice translumenal endoscopic surgery (NOTES): a benchtop comparison of a new device for flexible endoscopic surgery and a standard dual-channel endoscope. Surg Endosc. 2009;23:2720–2727. doi: 10.1007/s00464-009-0476-5. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs KH, Breithaupt W. Transgastric small bowel resection with the new multitasking platform EndoSAMURAI™ for natural orifice transluminal endoscopic surgery. Surg Endosc. 2012;26:2281–2287. doi: 10.1007/s00464-012-2173-z. [DOI] [PubMed] [Google Scholar]
- 26.Diana M, Chung H, Liu KH, Dallemagne B, Demartines N, Mutter D, Marescaux J. Endoluminal surgical triangulation: overcoming challenges of colonic endoscopic submucosal dissections using a novel flexible endoscopic surgical platform: feasibility study in a porcine model. Surg Endosc. 2013;27:4130–4135. doi: 10.1007/s00464-013-3049-6. [DOI] [PubMed] [Google Scholar]
- 27.Perretta S, Dallemagne B, Barry B, Marescaux J. The ANUBISCOPE® flexible platform ready for prime time: description of the first clinical case. Surg Endosc. 2013;27:2630. doi: 10.1007/s00464-013-2818-6. [DOI] [PubMed] [Google Scholar]
- 28.Thompson CC, Ryou M, Soper NJ, Hungess ES, Rothstein RI, Swanstrom LL. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery applications (with video) Gastrointest Endosc. 2009;70:121–125. doi: 10.1016/j.gie.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 29.DePaula AI, Kozarek RA, Birkett DH. A novel system for performing endolumenal antireflux surgery and other endosurgical procedures. M2286. Digestive Disease Week; 2006 May 19-25. Los Angeles, United States: Digestive Disease Week; 2006. [Google Scholar]
- 30.Pai RD, Fong DG, Bundga ME, Odze RD, Rattner DW, Thompson CC. Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model (with video) Gastrointest Endosc. 2006;64:428–434. doi: 10.1016/j.gie.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 31.Swain P, Rothe C, Bergstrom M, Park PO, Swanstrom L. Development and testing of a new platform for retroflexed flexible transgastric surgery: cholecystectomy, fundoplication, gastric restriction and diaphragmatic repair. Gastrointest Endosc. 2006;63:AB102. [Google Scholar]
- 32.Horgan S, Thompson K, Talamini M, Ferreres A, Jacobsen G, Spaun G, Cullen J, Swanstrom L. Clinical experience with a multifunctional, flexible surgery system for endolumenal, single-port, and NOTES procedures. Surg Endosc. 2011;25:586–592. doi: 10.1007/s00464-010-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horgan S, Jacobsen G, Weiss GD, Oldham JS, Denk PM, Borao F, Gorcey S, Watkins B, Mobley J, Thompson K, et al. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. 2010;6:290–295. doi: 10.1016/j.soard.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Royal College of Surgeons. Edinburgh: Short life working group on Robotic Surgery; 2014. [Google Scholar]
- 35.Vitiello V, Lee SL, Cundy TP, Yang GZ. Emerging robotic platforms for minimally invasive surgery. IEEE Rev Biomed Eng. 2013;6:111–126. doi: 10.1109/RBME.2012.2236311. [DOI] [PubMed] [Google Scholar]
- 36.Rozeboom E, Ruiter J, Franken M, Broeders I. Intuitive user interfaces increase efficiency in endoscope tip control. Surg Endosc. 2014;28:2600–2605. doi: 10.1007/s00464-014-3510-1. [DOI] [PubMed] [Google Scholar]
- 37.Ott L, Zanne P, Nageotte F, de Mathelin M. Physiological motion rejection in flexible endoscopy using visual servoing. IEEE International Conference on Robotics and Automation; 2008. pp. 2928–2933. [Google Scholar]
- 38.Sodergren MH, Warren A, Nehme J, Clark J, Gillen S, Feussner H, Teare J, Darzi A, Yang GZ. Endoscopic horizon stabilization in natural orifice translumenal endoscopic surgery: a randomized controlled trial. Surg Innov. 2014;21:74–79. doi: 10.1177/1553350613489187. [DOI] [PubMed] [Google Scholar]
- 39.Pullens HJ, van der Stap N, Rozeboom ED, Schwartz MP, van der Heijden F, van Oijen MG, Siersema PD, Broeders IA. Colonoscopy with robotic steering and automated lumen centralization: a feasibility study in a colon model. Endoscopy. 2015:Epub ahead of print. doi: 10.1055/s-0034-1392550. [DOI] [PubMed] [Google Scholar]
- 40.Kume K, Kuroki T, Sugihara T, Shinngai M. Development of a novel endoscopic manipulation system: The Endoscopic operation robot. World J Gastrointest Endosc. 2011;3:145–150. doi: 10.4253/wjge.v3.i7.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kume K, Kuroki T, Shingai M, Harada M. Endoscopic submucosal dissection using the endoscopic operation robot. Endoscopy. 2012;44 Suppl 2 UCTN:E399–E400. doi: 10.1055/s-0032-1310251. [DOI] [PubMed] [Google Scholar]
- 42.Kume K, Sakai N, Goto T. Development of a novel endoscopic manipulation system: the Endoscopic Operation Robot ver.3. Endoscopy. 2015;47:815–819. doi: 10.1055/s-0034-1391973. [DOI] [PubMed] [Google Scholar]
- 43.Rösch T, Adler A, Pohl H, Wettschureck E, Koch M, Wiedenmann B, Hoepffner N. A motor-driven single-use colonoscope controlled with a hand-held device: a feasibility study in volunteers. Gastrointest Endosc. 2008;67:1139–1146. doi: 10.1016/j.gie.2007.10.065. [DOI] [PubMed] [Google Scholar]
- 44.Groth S, Rex DK, Rösch T, Hoepffner N. High cecal intubation rates with a new computer-assisted colonoscope: a feasibility study. Am J Gastroenterol. 2011;106:1075–1080. doi: 10.1038/ajg.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 46.Dario P, Carrozza MC, Pietrabissa A. Development and in vitro testing of a miniature robotic system for computer-assisted colonoscopy. Comput Aided Surg. 1999;4:1–14. doi: 10.1002/(SICI)1097-0150(1999)4:1<1::AID-IGS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 47.Eickhoff A, van Dam J, Jakobs R, Kudis V, Hartmann D, Damian U, Weickert U, Schilling D, Riemann JF. Computer-assisted colonoscopy (the NeoGuide Endoscopy System): results of the first human clinical trial (“PACE study”) Am J Gastroenterol. 2007;102:261–266. doi: 10.1111/j.1572-0241.2006.01002.x. [DOI] [PubMed] [Google Scholar]
- 48.Pfeffer J, Grinshpon R, Rex D, Levin B, Rösch T, Arber N, Halpern Z. The Aer-O-Scope: proof of the concept of a pneumatic, skill-independent, self-propelling, self-navigating colonoscope in a pig model. Endoscopy. 2006;38:144–148. doi: 10.1055/s-2006-925089. [DOI] [PubMed] [Google Scholar]
- 49.Arber N, Grinshpon R, Pfeffer J, Maor L, Bar-Meir S, Rex D. Proof-of-concept study of the Aer-O-Scope omnidirectional colonoscopic viewing system in ex vivo and in vivo porcine models. Endoscopy. 2007;39:412–417. doi: 10.1055/s-2007-966452. [DOI] [PubMed] [Google Scholar]
- 50.Nathan G, Alaa M, Zamir H, Klaus M, Sharon G, Erwin S. Aer-O-Scope Colonoscope System Demonstrates Efficacy and Safety for Colorectal Cancer Screening in Humans. Gastrointestinal Endoscopy. 2015;81:AB386–AB386. [Google Scholar]
- 51.Cosentino F, Tumino E, Passoni GR, Morandi E, Capria A. Functional evaluation of the endotics system, a new disposable self-propelled robotic colonoscope: in vitro tests and clinical trial. Int J Artif Organs. 2009;32:517–527. doi: 10.1177/039139880903200806. [DOI] [PubMed] [Google Scholar]
- 52.Tumino E, Sacco R, Bertini M, Bertoni M, Parisi G, Capria A. Endotics system vs colonoscopy for the detection of polyps. World J Gastroenterol. 2010;16:5452–5456. doi: 10.3748/wjg.v16.i43.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poon CC, Leung B, Chan CK, Lau JY, Chiu PW. Design of wormlike automated robotic endoscope: dynamic interaction between endoscopic balloon and surrounding tissues. Surg Endosc. 2015:Epub ahead of print. doi: 10.1007/s00464-015-4224-8. [DOI] [PubMed] [Google Scholar]
- 54.Low SC, Phee SJ. Tendon Sheath Analysis for Estimation of Distal End Force and Elongation. IEEE/ASME International Conference on Advanced Intelligent Mechatronics; 2009. [Google Scholar]
- 55.Phee SJ, Darioa R, Menciassi A. Tendon sheath analysis for estimation of distal end force and elongation for sensorless distal end. IEEE/ASME Transactions on. 2010;28:1073–1082. [Google Scholar]
- 56.Phee SJ, Sun ZL. Elongation Modeling and Compensation for the Flexible Tendon–Sheath System. IEEE/ASME Transactions. 2014;19:1243–1250. [Google Scholar]
- 57.Do TN, Tjahjowidodo T, Lau MWS, Phee SJ. A investigation of friction-based tendon sheath model appropriate for control purposes. MSSP. 2014;42:97–114. [Google Scholar]
- 58.Do TN, Tjahjowidodo T, Lau MWS, Phee SJ. A new approach of friction model for tendon-sheath actuated surgical systems: Nonlinear modelling and parameter identification. MSSP. 2015;85:14–24. [Google Scholar]
- 59.Do TN, Tjahjowidodo T, Lau MWS, Phee SJ. Nonlinear friction modelling and compensation control of hysteresis phenomena for a pair of tendon-sheath actuated surgical robot. Mechanical Systems & Signal Processing. 2015;60-61:770–784. [Google Scholar]
- 60.Webster RJ III, Jones BA. Design and Kinematics Modeling of Constant Curvature Continuum Robots: A Review. Int J Rob Res. 2010;29:1661–1683. [Google Scholar]
- 61.Camarillo DB, Milne CF. Mechanics Modeling of Tendon-Driven Continuum Manipulators. IEEE/ASME Transactions. 2008;6:24. [Google Scholar]
- 62.Camarillo DB, Carlson CR. Configuration tracking for continuum manipulators with coupled tendon drive. IEEE/ASME Transactions. 2009;25:4. [Google Scholar]
- 63.Lomanto D, Wijerathne S, Ho LK, Phee LS. Flexible endoscopic robot. Minim Invasive Ther Allied Technol. 2015;24:37–44. doi: 10.3109/13645706.2014.996163. [DOI] [PubMed] [Google Scholar]
- 64.Ho KY, Phee SJ, Shabbir A, Low SC, Huynh VA, Kencana AP, Yang K, Lomanto D, So BY, Wong YY, et al. Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER) Gastrointest Endosc. 2010;72:593–599. doi: 10.1016/j.gie.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Phee SJ, Lomanto D, Goel R, Rebala P, Sun ZL, Trasti S, Reddy N, Wong JY, Ho KY. Endoscopic submucosal dissection of gastric lesions by using a master and slave transluminal endoscopic robot: an animal survival study. Endoscopy. 2012;44:690–694. doi: 10.1055/s-0032-1309404. [DOI] [PubMed] [Google Scholar]
- 66.Chiu PW, Phee SJ, Wang Z, Sun Z, Poon CC, Yamamoto T, Penny I, Wong JY, Lau JY, Ho KY. Feasibility of full-thickness gastric resection using master and slave transluminal endoscopic robot and closure by Overstitch: a preclinical study. Surg Endosc. 2014;28:319–324. doi: 10.1007/s00464-013-3149-3. [DOI] [PubMed] [Google Scholar]
- 67.Sun Z, Ang RY, Lim EW, Wang Z, Ho KY, Phee SJ. Enhancement of a master-slave robotic system for natural orifice transluminal endoscopic surgery. Ann Acad Med Singapore. 2011;40:223–230. [PubMed] [Google Scholar]
- 68.Phee SJ, Reddy N, Chiu PW, Rebala P, Rao GV, Wang Z, Sun Z, Wong JY, Ho KY. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol. 2012;10:1117–1121. doi: 10.1016/j.cgh.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z, Sun Z, Phee SJ. Haptic feedback and control of a flexible surgical endoscopic robot. Comput Methods Programs Biomed. 2013;112:260–271. doi: 10.1016/j.cmpb.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 70.Hawes RH, Eubanks S. Robotic endoscopy: a small case series, a giant step for endoscopy. Clin Gastroenterol Hepatol. 2012;10:1122–1123. doi: 10.1016/j.cgh.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 71.Dallemagne B, Marescaux J. The ANUBIS& amp; #x2122; project. Minim Invasive Ther Allied Technol. 2010;19:257–261. doi: 10.3109/13645706.2010.514741. [DOI] [PubMed] [Google Scholar]
- 72.De Donno A, Zorn L, Zanne P. Introducing STRAS: A new flexible robotic system for minimally invasive surgery. IEEE/ASME Transactions. 2013:1213–1220. [Google Scholar]
- 73.Cauche H, Hiernaux M, Chau A. Endomina: the endolumenal universal robotized triangulation system: description and preliminary results in isolated pig stomach. Gastrointest Endosc. 2013;77:AB204–AB205. [Google Scholar]
- 74.Suzuki N, Hattori A, Tanoue K. Scorpion shaped endoscopic surgical robot for NOTES and SPS with augmented reality functions. In: Medical Imaging and Augmented Reality. Springer; 2010. pp. 541–550. [Google Scholar]
- 75.Abbott DJ, Becke C, Rothstein RI. Design of an endolumenal NOTES robotic system. IEEE/ASME Transactions. 2007:410–416. [Google Scholar]
- 76.Xu W, Leung HK, Chiu PWY, Poon CCY. A feed forward friction compensation motion controller for a tendon-sheath driven flexible robotic gripper. IEEE ROBIO. 2013:2112–2117. [Google Scholar]
- 77.Poon CCY, Yang H, Lau KC, Xu WJ, Yam Y, Lau JYW, Chiu PWY. A bioinspired flexible robot with hybrid actuation mechanisms for endoscopic surgery. The Hamlyn Symposium on Medical Robot. 2014 [Google Scholar]
- 78.Lau KC, Hu Y, Poon CCY, Chiu PWY, Lau JYW, Yam Y. Design and Development of a Task specific surgical robot for endoscopic submucosal dissection. IEEE International Symposium on Optomechatronic technologies. Seattle: IEEE; 2014. [Google Scholar]
- 79.Swanstrom LL, Kozarek R, Pasricha PJ, Gross S, Birkett D, Park PO, Saadat V, Ewers R, Swain P. Development of a new access device for transgastric surgery. J Gastrointest Surg. 2005;9:1129–1136; discussion 1136-1137. doi: 10.1016/j.gassur.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Seneci CA, Shang J, Yang GZ. Design of a bimanual end-effector for an endoscopic surgical robot. The Hamlyn Symposium on Medical Robot; 2014. [Google Scholar]
- 81.Zorron R, Palanivelu C, Galvão Neto MP, Ramos A, Salinas G, Burghardt J, DeCarli L, Henrique Sousa L, Forgione A, Pugliese R, et al. International multicenter trial on clinical natural orifice surgery--NOTES IMTN study: preliminary results of 362 patients. Surg Innov. 2010;17:142–158. doi: 10.1177/1553350610370968. [DOI] [PubMed] [Google Scholar]
- 82.Lehmann KS, Ritz JP, Wibmer A, Gellert K, Zornig C, Burghardt J, Büsing M, Runkel N, Kohlhaw K, Albrecht R, et al. The German registry for natural orifice translumenal endoscopic surgery: report of the first 551 patients. Ann Surg. 2010;252:263–270. doi: 10.1097/SLA.0b013e3181e6240f. [DOI] [PubMed] [Google Scholar]
- 83.Thakkar S, Awad M, Gurram KC, Tully S, Wright C, Sanan S, Choset H. A novel, new robotic platform for natural orifice distal pancreatectomy. Surg Innov. 2015;22:274–282. doi: 10.1177/1553350614554232. [DOI] [PubMed] [Google Scholar]
- 84.Shang J, Noonan DP, Payne C, Clark J, Sodergren MH, Darzi A, Yang GZ. An articulated universal joint based flexible access robot for minimally invasive surgery. Robotics and Automation (ICRA). 2011 IEEE International Conference on; 2011 May 9-13; Shanghai, China. Los Angeles: IEEE; 2011. pp. 1147–1152. [Google Scholar]
- 85.Shang J, Payne CJ, Clark J, Noonan DP, Kwok KW, Darzi A, Yang GZ. Design of a multitasking robotic platform with flexible arms and articulated head for minimally invasive surgery. Intelligent Robots and Systems (IROS), 2012. IEEE/RSJ International Conference; 2012. pp. 1988–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azizian M, Khoshnam M, Najmaei N, Patel RV. Visual servoing in medical robotics: a survey. Part I: endoscopic and direct vision imaging - techniques and applications. Int J Med Robot. 2014;10:263–274. doi: 10.1002/rcs.1531. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi Y, Sekiguchi Y, Noguchi T, Takahashi Y, Liu Q, Oguri S, Toyoda K, Uemura M, Ieiri S, Tomikawa M, et al. Development of a robotic system with six-degrees-of-freedom robotic tool manipulators for single-port surgery. Int J Med Robot. 2015;11:235–246. doi: 10.1002/rcs.1600. [DOI] [PubMed] [Google Scholar]
- 88.Eastwood K, Looi T, Naguib HE, Drake JM. Fluidic actuators for minimally invasive neurosurgical instruments. The Hamlyn Symposium on Medical Robot; 2014. [Google Scholar]
- 89.Cerveri P, Zazzarini CC, Patete P, Baroni G. A micro-optical system for endoscopy based on mechanical compensation paradigm using miniature piezo-actuation. Med Eng Phys. 2014;36:684–693. doi: 10.1016/j.medengphy.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Webster RJ III, Romano JM, Cowan NJ. Mechanics of precurved-tube continuum robots. IEEE/ASME Transactions. 2009;25:7–78. [Google Scholar]
- 91.Smith K, Goldstein SC. Programmable matter: applications for gastrointestinal endoscopy and surgery. Gastroenterology. 2011;140:1884–1886. doi: 10.1053/j.gastro.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 92.Zhang GK, Wang SX, Lin JM, Sun YY, Xing Y. Prototype design of flexi-hand for single incision laparoscopic surgery. The Hamlyn Symposium. 2014 [Google Scholar]
- 93.Saccomandi P, Schena E, Oddo CM, Zollo L, Silvestri S, Guglielmelli E. Microfabricated tactile sensors for biomedical applications: a review. Biosensors (Basel) 2014;4:422–448. doi: 10.3390/bios4040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naito K, Ando T, Wang J, Kiyomatsu H, Kobayashi E, Fujishiro M, Sakuma I. Development of a force-sensing system for endoscopic submucosal dissection. The Hamlyn Symposium. 2014 [Google Scholar]
- 95.Hyun JJ, Chun HJ, Keum B, Seo YS, Kim YS, Jeen YT, Lee HS, Um SH, Kim CD, Ryu HS, et al. Feasibility of obtaining quantitative 3-dimensional information using conventional endoscope: a pilot study. Clin Endosc. 2012;45:182–188. doi: 10.5946/ce.2012.45.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loewke KE, Camarillo DB, Piyawattanametha W, Mandella MJ, Contag CH, Thrun S, Salisbury JK. In vivo micro-image mosaicing. IEEE Trans Biomed Eng. 2011;58:159–171. doi: 10.1109/TBME.2010.2085082. [DOI] [PubMed] [Google Scholar]
- 97.Puerto-Souza GA, Mariottini GL. A fast and accurate feature-matching algorithm for minimally-invasive endoscopic images. IEEE Trans Med Imaging. 2013;32:1201–1214. doi: 10.1109/TMI.2013.2239306. [DOI] [PubMed] [Google Scholar]
- 98.Reilink R, Stramigioli S, Misra S. 3D position estimation of flexible instruments: marker-less and marker-based methods. Int J Comput Assist Radiol Surg. 2013;8:407–417. doi: 10.1007/s11548-012-0795-1. [DOI] [PubMed] [Google Scholar]
- 99.Melo R, Barreto JP, Falcão G. A new solution for camera calibration and real-time image distortion correction in medical endoscopy-initial technical evaluation. IEEE Trans Biomed Eng. 2012;59:634–644. doi: 10.1109/TBME.2011.2177268. [DOI] [PubMed] [Google Scholar]
- 100.Totz J, Mountney P, Stoyanov D, Yang GZ. Dense surface reconstruction for enhanced navigation in MIS. Med Image Comput Comput Assist Interv. 2011;14:89–96. doi: 10.1007/978-3-642-23623-5_12. [DOI] [PubMed] [Google Scholar]
- 101.Chang PL, Stoyanov D, Davison AJ, Edwards PE. Real-time dense stereo reconstruction using convex optimisation with a cost-volume for image-guided robotic surgery. Med Image Comput Comput Assist Interv. 2013;16:42–49. doi: 10.1007/978-3-642-40811-3_6. [DOI] [PubMed] [Google Scholar]
- 102.Bell CS, Obstein KL, Valdastri P. Image partitioning and illumination in image-based pose detection for teleoperated flexible endoscopes. Artif Intell Med. 2013;59:185–196. doi: 10.1016/j.artmed.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Reilink R, Stramigioli S, Misra S. Image-based flexible endoscope steering. 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems. IEEE/RSJ; 2010. pp. 2339–2344. [Google Scholar]
- 104.Kim YT, Kim DE, Yang S, Yoon ES. Design of endoscopic micro-robotic end effectors: safety and performance evaluation based on physical intestinal tissue damage characteristics. Biomed Microdevices. 2014;16:397–413. doi: 10.1007/s10544-014-9843-7. [DOI] [PubMed] [Google Scholar]
- 105.Marescaux J, Diana M. Next step in minimally invasive surgery: hybrid image-guided surgery. J Pediatr Surg. 2015;50:30–36. doi: 10.1016/j.jpedsurg.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 106.Nakamoto M, Ukimura O, Faber K, Gill IS. Current progress on augmented reality visualization in endoscopic surgery. Curr Opin Urol. 2012;22:121–126. doi: 10.1097/MOU.0b013e3283501774. [DOI] [PubMed] [Google Scholar]
- 107.Mirota DJ, Ishii M, Hager GD. Vision-based navigation in image-guided interventions. Annu Rev Biomed Eng. 2011;13:297–319. doi: 10.1146/annurev-bioeng-071910-124757. [DOI] [PubMed] [Google Scholar]
- 108.Schneider C, Baghani A, Rohling R, Salcudean S. Remote ultrasound palpation for robotic interventions using absolute elastography. Med Image Comput Comput Assist Interv. 2012;15:42–49. doi: 10.1007/978-3-642-33415-3_6. [DOI] [PubMed] [Google Scholar]
- 109.A Novel User-Specific Wearable Controller for Surgical Robots. Proceedings of International Conference on Human-Computer Interaction; 2015 Aug 5-7. Los Angeles, USA: International Conference on Human-Computer Interaction; 2015. [Google Scholar]
- 110.Jung PG, Lim G, Kim S, Kong K. A Wearable gesture recognition device for detecting muscular activities based on air-pressure sensors. IEEE/ASME Transactions. 2015;11:485–494. [Google Scholar]
- 111.Simorov A, Otte RS, Kopietz CM, Oleynikov D. Review of surgical robotics user interface: what is the best way to control robotic surgery? Surg Endosc. 2012;26:2117–2125. doi: 10.1007/s00464-012-2182-y. [DOI] [PubMed] [Google Scholar]
- 112.Santos-Carreras L, Hagen M, Gassert R, Bleuler H. Survey on surgical instrument handle design: ergonomics and acceptance. Surg Innov. 2012;19:50–59. doi: 10.1177/1553350611413611. [DOI] [PubMed] [Google Scholar]
- 113.Reilink R, Stramigioli S, Kappers AM, Misra S. Evaluation of flexible endoscope steering using haptic guidance. Int J Med Robot. 2011;7:178–186. doi: 10.1002/rcs.386. [DOI] [PubMed] [Google Scholar]
- 114.Allemann P, Ott L, Asakuma M, Masson N, Perretta S, Dallemagne B, Coumaros D, De Mathelin M, Soler L, Marescaux J. Joystick interfaces are not suitable for robotized endoscope applied to NOTES. Surg Innov. 2009;16:111–116. doi: 10.1177/1553350609338181. [DOI] [PubMed] [Google Scholar]
- 115.Kim HM, Yang S, Kim J, Park S, Cho JH, Park JY, Kim TS, Yoon ES, Song SY, Bang S. Active locomotion of a paddling-based capsule endoscope in an in vitro and in vivo experiment (with videos) Gastrointest Endosc. 2010;72:381–387. doi: 10.1016/j.gie.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 116.Chen W, Yan G, Wang Z, Jiang P, Liu H. A wireless capsule robot with spiral legs for human intestine. Int J Med Robot. 2014;10:147–161. doi: 10.1002/rcs.1520. [DOI] [PubMed] [Google Scholar]
- 117.Carpi F, Kastelein N, Talcott M, Pappone C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans Biomed Eng. 2011;58:231–234. doi: 10.1109/TBME.2010.2087332. [DOI] [PubMed] [Google Scholar]
- 118.Than TD, Alici G, Zhou H, Li W. A review of localization systems for robotic endoscopic capsules. IEEE Trans Biomed Eng. 2012;59:2387–2399. doi: 10.1109/TBME.2012.2201715. [DOI] [PubMed] [Google Scholar]
- 119.Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y, Ye Y, Luo ZP, He F, Gong Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic Biol Med. 2014;68:234–246. doi: 10.1016/j.freeradbiomed.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Basar MR, Ahmad MY, Cho J, Ibrahim F. Application of wireless power transmission systems in wireless capsule endoscopy: an overview. Sensors (Basel) 2014;14:10929–10951. doi: 10.3390/s140610929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams SB, Prado K, Hu JC. Economics of robotic surgery: does it make sense and for whom? Urol Clin North Am. 2014;41:591–596. doi: 10.1016/j.ucl.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 122.Weissman JS, Zinner M. Comparative effectiveness research on robotic surgery. JAMA. 2013;309:721–722. doi: 10.1001/jama.2013.1107. [DOI] [PubMed] [Google Scholar]
- 123.Cormier L. Financial aspects, or how to use robot assistance without losing money. Perspectives from a public hospital. J Visc Surg. 2011;148:e19–e21. doi: 10.1016/j.jviscsurg.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Delaney CP, Senagore AJ, Ponsky L. Robot-assisted surgery and health care costs. N Engl J Med. 2010;363:2175; author reply 2176. doi: 10.1056/NEJMc1010658. [DOI] [PubMed] [Google Scholar]
- 125.Ibrahim AM, Makary MA. Robot-assisted surgery and health care costs. N Engl J Med. 2010;363:2175–216; author reply 2176. doi: 10.1056/NEJMc1010658. [DOI] [PubMed] [Google Scholar]
- 126.Shukla PJ, Scherr DS, Milsom JW. Robot-assisted surgery and health care costs. N Engl J Med. 2010;363:2174; author reply 2176. doi: 10.1056/NEJMc1010658. [DOI] [PubMed] [Google Scholar]
- 127.Awad MM, Fleshman JW. Robot-assisted surgery and health care costs. N Engl J Med. 2010;363:2174–2175; author reply 2176. doi: 10.1056/NEJMc1010658. [DOI] [PubMed] [Google Scholar]
- 128.Biehn Stewart S, Reed SD, Moul JW. Will the future of health care lead to the end of the robotic golden years? Eur Urol. 2014;65:325–327; discussion 327-328. doi: 10.1016/j.eururo.2012.10.019. [DOI] [PubMed] [Google Scholar]


