Abstract
AIM: To prospectively evaluate the effectiveness and safety of continuous wound infiltration (CWI) for pain management after open gastrectomy.
METHODS: Seventy-five adult patients with American Society of Anesthesiologists (ASA) Physical Status Classification System (ASA) grade 1-3 undergoing open gastrectomy were randomized to three groups. Group 1 patients received CWI with 0.3% ropivacaine (group CWI). Group 2 patients received 0.5 mg/mL morphine intravenously by a patient-controlled analgesia pump (PCIA) (group PCIA). Group 3 patients received epidural analgesia (EA) with 0.12% ropivacaine and 20 µg/mL morphine with an infusion at 6-8 mL/h for 48 h (group EA). A standard general anesthetic technique was used for all three groups. Rescue analgesia (2 mg bolus of morphine, intravenous) was given when the visual analogue scale (VAS) score was ≥ 4. The outcomes measured over 48 h after the operation were VAS scores both at rest and during mobilization, total morphine consumption, relative side effects, and basic vital signs. Further results including time to extubation, recovery of bowel function, surgical wound healing, mean length of hospitalization after surgery, and the patient’s satisfaction were also recorded.
RESULTS: All three groups had similar VAS scores during the first 48 h after surgery. Group CWI and group EA, compared with group PCIA, had lower morphine consumption (P < 0.001), less postoperative nausea and vomiting (1.20 ± 0.41 vs 1.96 ± 0.67, 1.32 ± 0.56 vs 1.96 ± 0.67, respectively, P < 0.001), earlier extubation (16.56 ± 5.24 min vs 19.76 ± 5.75 min, P < 0.05, 15.48 ± 4.59 min vs 19.76 ± 5.75 min, P < 0.01), and earlier recovery of bowel function (2.96 ± 1.17 d vs 3.60 ± 1.04 d, 2.80 ± 1.38 d vs 3.60 ± 1.04 d, respectively, P < 0.05). The mean length of hospitalization after surgery was reduced in groups CWI (8.20 ± 2.58 d vs 10.08 ± 3.15 d, P < 0.05) and EA (7.96 ± 2.30 d vs 10.08 ± 3.15 d, P < 0.01) compared with group PCIA. All three groups had similar patient satisfaction and wound healing, but group PCIA was prone to higher sedation scores when compared with groups CWI and EA, especially during the first 12 h after surgery. Group EA had a lower mean arterial pressure within the first postoperative 12 h compared with the other two groups.
CONCLUSION: CWI with ropivacaine yields a satisfactory analgesic effect within the first 48 h after open gastrectomy, with lower morphine consumption and accelerated recovery.
Keywords: Postoperative pain, Gastrectomy, Wound infiltration, Epidural analgesia, Patient-controlled analgesia, Incision infection, Ropivacaine
Core tip: This prospective study compared the analgesic effectiveness and safety of continuous wound infiltration (CWI) with ropivacaine after open gastrectomy with epidural analgesia and patient-controlled intravenous analgesia. CWI could provide similar analgesia compared with epidural analgesia and patient-controlled intravenous analgesia within the first 48 h after surgery, but with lower morphine consumption, fewer side effects, and an accelerated early recovery. These results suggest that CWI with local anesthetics could be a suitable option for postoperative pain management after major abdominal surgery.
INTRODUCTION
The intense pain in the postoperative period of abdominal laparotomy may have a major impact on patients. Inadequate pain control causes suffering and distress, and might lead to some postoperative complications, prolong hospitalization, and trigger chronic pain syndromes[1]. Postoperative pain is likely to impair respiratory effort by restricting thoracic and abdominal breathing, reducing tidal volume and vital capacity, and may also cause respiratory and cardiovascular depression and cognitive, gastrointestinal and neuroendocrine dysfunction[2,3]. These changes will probably negatively interfere with the postoperative recovery course.
Multimodal analgesia techniques, including pharmacological and non-pharmacological techniques, can reduce the opioid consumption and prevent common postoperative side effects[4,5]. Therefore, multimodal analgesia seems to be the best mean of managing pain after major surgery. Intravenous analgesia or epidural analgesia with a patient-controlled analgesia (PCA) pump is often adopted as the main element for pain management after open gastrectomy[6]. Patient-controlled intravenous analgesia (PCIA) is frequently used after major abdominal surgery, although there is evidence that PCIA can retard postoperative recovery since the analgesia is often accompanied by side effects, such as postoperative nausea and vomiting (PONV), sedation, and dizziness[7,8]. Epidural analgesia (EA) with local anesthetics provides better analgesia than PCIA, especially for mobilization pain. However, it is limited in daily practice by contraindications, technical failure, and side effects[9-13]. For medical staff, effectiveness, tolerance, convenience and potential benefit for recovery are the critical factors of analgesia techniques. EA has been identified as the more expensive postoperative analgesic strategy, in terms of pain-free days, as compared with PCIA[14].
As a useful supplement component of multimodal postoperative analgesia, local anesthetic wound infiltration is widely applied to different surgeries. Continuous wound infiltration (CWI) is an analgesic technique to administer local anesthetics directly into the surgical wound at a constant speed, through a multi-holed catheter that is placed by the surgeon at the end of the surgery. Its analgesic efficacy was studied after major abdominal surgery[15], but there is little evidence for open gastrectomy in particular. This technique has a high rate of success, good tolerance and is easy to implement compared with PCIA or EA, and may provide better analgesia with a reduction in length of hospitalization[14,16]. CWI has recently gained popularity as an alternative method of providing postoperative pain management. This technique remains relatively untested in China, and there are few studies that have examined whether it can provide satisfactory analgesia after open abdominal surgery without PCIA. This study aimed to evaluate the effectiveness of CWI with ropivacaine as the main component of a multimodal analgesic strategy, as an alternative to PCIA or EA following open gastrectomy.
MATERIALS AND METHODS
After gaining approval by the institutional ethics board, a prospective, randomized and double-blinded study of the patients scheduled for open gastrectomy was undertaken from January 2012 to March 2014 at Sir Run Run Shaw Hospital, which is affiliated with Zhejiang University, School of Medicine. Written informed consent was obtained from each patient. The patients were divided randomly into the following three groups according to a computer-generated randomization code: CWI with 0.3% ropivacaine (group CWI), PCIA with morphine (group PCIA), and epidural analgesia (group EA). The inclusion criteria were defined as adult patients (aged 18-75 years) who would undergo open gastrectomy with grades 1-3 according to the American Society of Anesthesiologists Physical Status Classification System (ASA). Exclusion criteria included a history of allergy to local anesthetics, contraindication of EA, inability to use a PCA device, chronic hepatic disease, obesity (body mass index > 30 kg/m2). Patients with chronic pain, opioids addiction, and psychiatric disorders - which would prevent postoperative assessments - were also excluded. Effectiveness and safety of the three analgesic methods were evaluated by the same acute pain service (APS) team who were blinded to the entire anesthesia procedure and analgesia approach.
All the patients were induced with propofol (2 mg/kg), sufentanil (1 μg/kg), and cisatracurium (0.15 mg/kg). After tracheal intubation, mechanical ventilation with a mixture of 50% O2 and 50% air was initiated, and maintenance was obtained with combined intravenous-inhalational anesthesia to keep an appropriate anesthetic state (bispectral index 40-60). Remifentanil was also administrated to maintain anesthesia with a dose of 0.2 μg/kg per min. Both intravenous and halogenated agents were stopped 20 min before awakening. The surgical incision was designed from the xiphoid process to above or below the umbilicus. All the patients received 10 mL of 0.75% ropivacaine infiltrated into the tissues around the incision, including the peritoneum, muscles and subcutaneous tissue, before the skin was sutured. Residual neuromuscular block was reversed if necessary.
For the patients in group CWI, two multiholed catheters were inserted by the surgeons through a separate puncture of the layer of deep fascia adjacent to the surgical incision before closure. These multiholed catheters could deliver local anesthetics at a constant rate (2.5 mL/h for each) through the side holes in the front portion (8 cm). The introducer needle was placed 2-3 cm from both ends of the incision and the multiholed catheters were inserted into the incision along the full length of the wound before the needle was removed and secured by suturing to the skin. The catheters were then connected to an elastomeric pump (CWI device, TJPS120-1-250-5; Surgiland, Beijing, China) filled with 250 mL of 0.3% ropivacaine. The CWI device delivered the solution with a 5 mL/h constant flow (2.5 mL/h per catheter) within the first 48 h after surgery.
The patients in group PCIA used electronic controlled analgesia pumps (GemStar®; Abbott Hospira, Chicago, IL, United States) containing 0.5 mg/mL morphine with a size of 200 mL that delivered a bolus of 2 mg with a 5 min lockout time. Patients over 70-years-old, or weighing less than 40 kg, took a half dose of the bolus. When the skin was sutured, the PCA pump was connected to the venous catheter.
Group EA underwent mid-thoracic epidural catheter insertion at approximately T 7-8, and a test dose of 4 mL of 2% lidocaine was given before anesthesia induction. At the end of surgery, the epidural catheter was connected to a PCA pump for 48 h with an infusion of 0.1% ropivacaine and 20 μg/mL morphine at 6-8 mL/h. A bolus infusion of 3-4 mL/h was also set for breakthrough pain with a lock time of 15 min.
All the patients were transferred to the post-anesthesia care unit (PACU) as soon as the surgery was finished. The anesthesiologists and nurses of the PACU were then in charge of pain assessment and recording of the VAS score. In the PACU, a supplementary dose of 2 mg morphine was administered if the VAS score was over 6 in group CWI. The time to extubation was also recorded. While in the wards, the same APS team was responsible for the management of postoperative pain for a 48 h period. Postoperative pain at rest was measured using VAS for the first 48 h after surgery (0: no pain to 10: very severe pain), and mobilization pain was defined as the pain experienced when coughing using the same scale. Interventions were undertaken, such as reprogramming of the PCA pump or administration of supplementary morphine, if rescue analgesia was needed. When contraindications were eliminated, 40 mg of parecoxib was administered routinely every 12 h within the first 48 h after surgery. Other variables were recorded, including but not limited to consumption of morphine, recovery of bowel function, PONV, sedation score, and wound healing score (1: no effusion, 2: effusion, 3: infection). Time to bowel recovery was defined as the first anal exhaust. PONV was recorded as a three-point rating scale (1: no PONV, 2: nausea without vomiting, 3: nausea with vomiting). The level of sedation was measured using the Ramsay sedation score as follows: (1) anxious and irritable or dysphoric or both; (2) co-operational, oriented and quiet; (3) responsive to command; (4) asleep, quickly responsive to light tap or loud auditory stimulus; (5) asleep, slowly responsive to light tap or loud auditory stimulus; and (6) asleep, no response to light tap or loud auditory stimulus. Finally, patient satisfaction (using a four-point rating scale of 1: poor, 2: fair, 3: good, 4: excellent) and length of hospitalization were recorded and compared between the three groups.
Statistical analysis
Statistical analysis was performed using SPSS (version 17.0; SPSS Inc., Chicago, IL, United States). All data were examined for normal distribution. The length of hospitalization was analyzed using the Mann-Whitney U test, and the other variables were then compared and analyzed using one-way ANOVA analysis (Bonferroni test) and χ2 test. P < 0.05 was considered to be statistically significant. The statistical methods of this study were reviewed by Prof. Yi Shen from the Teaching-Researching Office of Epidemiology and Health Statistics, School of Public Health, Zhejiang University.
RESULTS
Overall, 75 cases were recruited to this study (25 per group) and all patients successfully completed the study. Demographic characteristics had no significant difference in terms of age, sex, body mass index, ASA status, and length of wound between the three groups. No statistically significant difference was observed between the three groups with regards to operating time, blood loss, length of wound, and surgical approach. Patients’ characteristics and intraoperative data are presented in Table 1.
Table 1.
Demographic characteristics of the patients studied (mean ± SD)
| Parameter | Group PCIA | Group EA | Group CWI | P value |
| Age (yr) | 63.56 ± 10.21 | 62.40 ± 9.69 | 61.96 ± 12.72 | 0.831 |
| BMI (kg/m2 ) | 23.37 ± 3.87 | 22.90 ± 3.46 | 22.60 ± 2.27 | 0.701 |
| Sex | ||||
| F/M, n/n | 8/17 | 11/14 | 9/16 | 0.671 |
| %/% | 32/68 | 44/56 | 36/64 | |
| ASA status | ||||
| I/II/III, n/n/n | 6/17/2 | 6/14/5 | 4/18/3 | 0.657 |
| %/%/% | 24/68/8 | 24/56/20 | 16/72/12 | |
| Surgical approach | ||||
| Subtotal/total, n/n | 16/9 | 19/6 | 18/7 | 0.637 |
| %/% | 64/36 | 76/24 | 72/28 | |
| Ulcer/tumor, n/n | 4/21 | 2/23 | 1/24 | 0.032 |
| %/% | 16/84 | 8/92 | 4/96 | |
| Blood loss (mL) | 161.60 ± 58.50 | 167.60 ± 48.93 | 157.20 ± 60.66 | 0.807 |
| Time of operation (min) | 251.20 ± 63.02 | 268.40 ± 66.67 | 263.20 ± 52.10 | 0.594 |
| Length of wound (cm) | 16.48 ± 3.49 | 16.68 ± 3.53 | 15.48 ± 3.23 | 0.418 |
BMI: Body mass index; ASA: American Society of Anesthesiologists.
The postoperative VAS scores both at rest and during mobilization were similar between the three groups for the first 48 h after surgery (Figure 1), but morphine consumption was significantly lower in groups CWI and EA than in group PCIA (Figure 2A). The mean total morphine consumption was 12.84 ± 4.07 mg in group CWI, 11.52 ± 4.62 mg in group EA, and 42.32 ± 7.25 mg in group PCIA over the first 48 h after surgery, which had significant difference (P < 0.001). The patients in groups CWI and EA had earlier extubation and earlier bowel recovery than the patients in group PCIA. With reference to side effects, higher scores for PONV and higher sedation scores were observed in group PCIA compared with the other two groups, especially within the first 12 h after surgery (Table 2 and Figure 2B). The length of hospitalization was reduced in groups CWI and EA compared with group PCIA (P < 0.05 and P < 0.01, respectively; Table 2), but no difference in wound healing scores or the satisfactory score of postoperative pain management was observed between the three groups (P > 0.05; Table 2 and Figure 2C). Group EA had a lower mean arterial pressure (MAP) than the other two groups, especially within the first 12 h after surgery, while no difference was observed in heart rate between the three groups (Figure 2D and E). No severe complications or deaths occurred in any of the groups.
Figure 1.
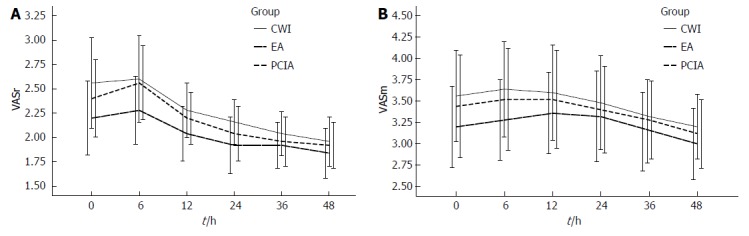
Visual analogue scale score at rest and during mobilization within the first 48 h after surgery. A: VASr: Visual analogue scale (VAS) score at rest; B: VASm: VAS score on mobilization.
Figure 2.
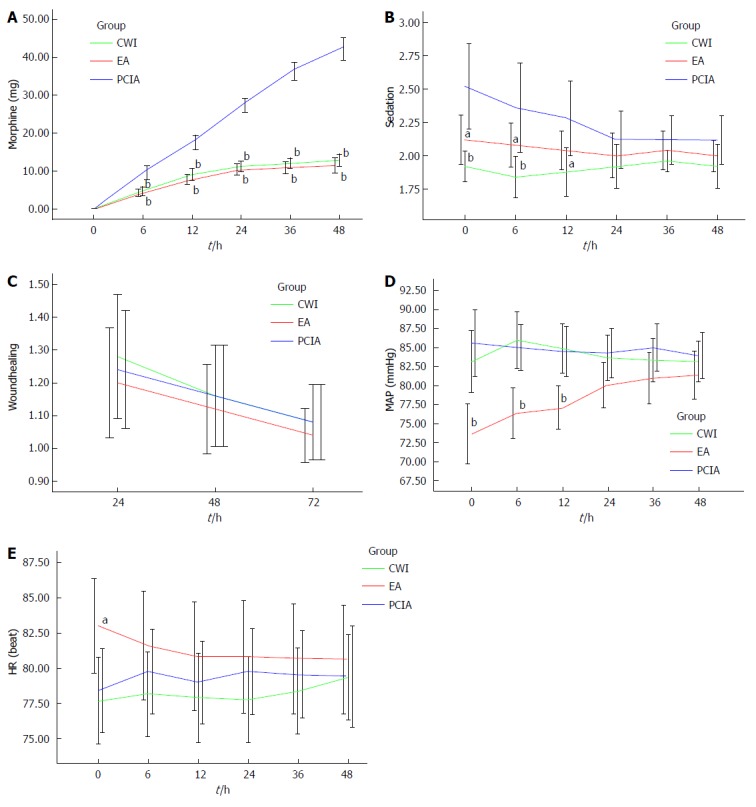
Characteristic after surgery. A: Cumulative morphine consumption within the first 48 h after surgery. bP < 0.01 vs group patient-controlled intravenous analgesia (PCIA); B: Ramsay sedation score within the first 48 h after surgery. aP < 0.05, bP < 0.01 vs group PCIA; C: Wound healing score within 72 h after surgery; D: Mean arterial pressure within the first 48 h after surgery. bP < 0.01 vs group PCIA; E: Heart rate within the first 48 h after surgery. aP < 0.05 vs group PCIA.
Table 2.
Early recovery, postoperative nausea and vomiting, patient’s satisfaction, and hospitalization length of the three groups (mean ± SD)
| Group PCIA | Group EA | Group CWI | P value | |
| Extubation (min) | 19.76 ± 5.75 | 15.48 ± 4.59b | 16.56 ± 5.24a | 0.014 |
| Bowel recovery (d) | 3.60 ± 1.04 | 2.80 ± 1.38a | 2.96 ± 1.17a | 0.048 |
| PONV | 1.96 ± 0.67 | 1.32 ± 0.56d | 1.20 ± 0.41d | < 0.001 |
| Satisfaction | 2.88 ± 0.78 | 3.04 ± 0.84 | 3.24 ± 0.72 | 0.272 |
| Hospitalization (d) | 10.08 ± 3.15 | 7.96 ± 2.30b | 8.20 ± 2.58a | 0.013 |
P < 0.05,
P < 0.01,
P < 0.001 vs group patient-controlled intravenous analgesia (PCIA). PONV: Postoperative nausea and vomiting; EA: Epidural analgesia; CWI: Continuous wound infiltration.
DISCUSSION
Inadequate postoperative analgesia can cause a multitude of problems. Three types of pain may occur after surgery: constant incisional pain, incision-associated pain, and pain caused by elevated tension in the wound[17,18].
PCIA and EA are widely used as a basic component of postoperative pain management, although they are associated with adverse drug effects and risks of epidural puncture. A means of reducing opioid consumption, avoiding the risks and complications of epidural puncture, and at the same time providing effective postoperative analgesia would be the ideal choice.
Multimodal analgesia is based on the treatment of various components of postoperative pain. The combined use of different analgesic techniques leads to further decrease in pain and doses of analgesic drugs, and the lower doses contribute to decrease or avoidance of adverse drug effects[19]. There is evidence in the literature that if the wound is infused with local anesthetics, spinal dorsal horn neuron sensitization may be reduced due to the block of parietal afferents, thereby providing analgesia over the duration of wound infiltration[20]. Data from Hopf’s[21] study indicated that postoperative pain aggravated the inflammatory response and reduced infusion and oxygenation of the wound site. Therefore, wound infiltration with local anesthetics may be a better choice for postoperative analgesia.
We observed that a single-shot infiltration with 0.75% ropivacaine into the incision below the xiphoid process appeared to be an effective means of early postoperative analgesia after laparoscopic cholecystectomy. This technique is convenient but does not provide long-term benefits in terms of pain control. This time-limited effect of a single shot administration of local anesthetics has been resolved by a CWI technique through multiholed catheters. Systematic studies confirmed the benefits and the safety of this technique when applied in several major pain surgeries[22-24], but in most cases it was combined with other analgesic methods[25,26] and there was no uniform standard of concentration since different local anesthetics were adopted in these studies. Our study is the first to evaluate the effectiveness of CWI as the principal component of multimodal postoperative analgesia. We chose ropivacaine, a pure levorotatory stereoisomer, considering its higher safety in cardiotoxicity. As the only means of postoperative analgesia in our study, we chose 0.3% ropivacaine to ensure the effectiveness since continuous preperitoneal infusion of 0.2% ropivacaine could provide effective analgesia combined with morphine PCA[27].
In our study, all patients in the three groups underwent similar surgical procedures with broadly comparable surgical wounds from the xiphoid process down to the umbilicus or below the umbilicus, suggesting similar pain suffering. VAS scores at rest and during mobilization of group CWI were similar to those of the other two groups within the first 48 h after surgery, suggesting that continuous infiltration with local anesthetics was as effective as the other two techniques. The similar consumption of morphine in groups CWI and EA that was obviously lower than that of group PCIA also demonstrated the good analgesic effectiveness of CWI. The analgesic effectiveness of CWI was affected by several factors, however. First, the effectiveness of surgical wound infiltration with local anesthetics depends in part on the level of tissue where the infiltration takes place. Inappropriate placement of the catheters may impair the efficacy of this wound infiltration technique after open abdominal surgeries. Second, the surgical wound should be covered by the multiholed catheters completely. If the size of surgical incision was beyond the length of the catheters, the patients might need other types of analgesic techniques or to be changed to another model of CWI device with a longer catheter. Third, the analgesic effect also depended on appropriate concentration of local anesthetics and sufficient infiltrating area. Other studies illustrated the need for deep placement with proper concentration and volume of local anesthetics[26,27].
The risk of increased surgical wound infection and systemic toxicity of local anesthetics were the main problems causing surgeons to hesitate to adopt the CWI technique. However, the results of our study highlight that CWI was not only an effective but also a safe postoperative analgesic technique. None of the 25 patients in group CWI developed a surgical wound infection, consistent with Lluis’[28] study, or local anesthetic intoxication. Even if larger amounts of ropivacaine were used, the total plasma concentration of ropivacaine had been reported to remain far below the known toxic threshold[29]. Therefore, we did not measure the plasma ropivacaine concentration in our study. Only one patient in group PCIA suffered from skin itching, but the symptom was not severe. No urinary retention was observed in the three groups. In our study, group CWI had a lower PONV score and a lower Ramsay sedation score within the first 12 h after surgery compared with group PCIA, suggesting that CWI may be safer than intravenous PCA, perhaps due to the lower consumption of morphine. The similar wound healing scores within the first 48 h of the three groups showed that CWI did not hinder the recovery of the surgical wound, although the elastic pump infiltrated the surgical wound with local anesthetics continuously. EA also showed good effectiveness with almost parallel VAS scores compared with intravenous PCA in our study, and with less side effects related to morphine. However, the obvious lower MAP in group EA in our study suggested that a higher incidence of hypotension accompanied with EA should not be ignored, in addition to the risks of epidural puncture. Epidural-associated hypotension is a frequently encountered problem that may result in dizziness and an excessive volume of fluid, and which can slow down the early postoperative recovery. Thus, as a postoperative analgesic technique, CWI showed its superiority in safety compared with epidural and intravenous PCA. However, our study did not include elderly patients (aged > 75 years) or those with severe systemic disease (ASA status > 3) who may be more prone to severe sedation or respiratory depression. We cannot generalize our findings to these patients, although it could be argued that CWI with local anesthetics might benefit these patients even more.
In some studies, the endpoints evaluated the impact of a treatment on postoperative pain, including the pain score directly and morphine consumption indirectly. Patients’ satisfaction and hospitalization or the cost-effectiveness should also be included in the indicators of assessing the postoperative analgesic treatment. The elastic pump infiltrating the surgical wound continuously with local anesthetics at a constant rate alleviated postoperative pain at rest effectively and encouraged early mobilization. It is well recognized that early mobilization after surgery favors early recovery of bowel function and reduces risk of pulmonary complications[30]. In our study, the patients in group CWI were extubated earlier and bowel recovery suggested a faster recovery in open gastrectomy patients with a CWI. The shorter hospitalization in group CWI compared to group PCIA also suggested a faster postoperative recovery. Our study showed higher satisfaction scores in patients of group CWI compared to the other two groups, presumably related to the earlier recovery with reduced morphine consumption, experience of less side effects, and need for fewer interventions.
With a lower morphine consumption and lower risk of hypotension related to EA, the workload of the APS team was reduced greatly and they could focus on assessing the effectiveness of CWI, providing additional analgesics when necessary and even taking charge of more patients. In groups PCIA and EA, patients were informed about PCA before surgery, and were able to demonstrate their ability to use the device. It can take a lot of time for the APS team and nurses in wards to educate patients of different educational levels regarding how to use a PCA pump. In group CWI, avoiding the PCA pump reduced the occurrence of mistakes caused by patients’ misuse or mechanical faults of the pumps. All the patients in our study underwent the same standard surgical procedure and anesthesia scheme with nonsteroidal anti-inflammatory drugs included in the multimodal postoperative analgesic plan and additional morphine as the only means to alleviate breakthrough pain. This unified protocol of rescue management for breakthrough pain eased the burden of the APS team and made it easy to compare the effectiveness of different analgesic techniques between the three groups. So, it seems that CWI with local anesthetics is an effective, safer and more economic postoperative analgesic technique.
We could not make any firm conclusions that CWI of local anesthetics shortened the length of hospital stay or reduced abdominal complications in our study, as it was influenced by many other factors. For example, hospital stay exceeded 60 d in three cases due to unexpected cerebral infarction and pulmonary embolus. Furthermore, the incidence of abdominal complications was influenced by various factors, such as nutritional status, time of drain removal and healing of the anastomotic sites[31-33]. High quality, multicenter, large-sample randomized controlled trials are expected to eliminate such effects.
In conclusion, CWI with local anesthetics after open gastrectomy provided comparable postoperative analgesia to intravenous PCA with morphine and EA, with lower morphine consumption and fewer adverse reactions, favoring earlier recovery after surgery. This technique appears to be a safe and effective means of providing postoperative analgesia after open abdominal surgery.
ACKNOWLEDGMENTS
The authors thank the workmates who participated in the anesthesia of the patients in this study, Dr. Su-Ming Tian and Ms. Min-Jun Liu from the Acute Pain Service team who traced all the cases for evaluation of analgesic effectiveness and performing rescue analgesia, as well as the nurses in PACU who assisted in evaluation of analgesia and recording of the VAS scores when the patients stayed in the PACU. The authors are also grateful for the statistics guidance given by Prof. Yi Shen from the Teaching-Researching Office of Epidemiology and Health Statistics, School of Public Health, Zhejiang University.
COMMENTS
Background
Multimodal analgesic techniques are the best means of addressing pain after major surgery. Patient-controlled intravenous analgesia (PCIA) and epidural analgesia (EA) are two routine methods with clinical application after major abdominal surgery, although each has side effects and limitations. Continuous wound infiltration (CWI) has shown its superiority in avoiding risks of invasive manipulation or opioids. The current study was designed to evaluate the effectiveness and safety of CWI compared with PCIA and EA after open gastrectomy.
Research frontiers
Pain has been considered to be the 5th vital sign of patients in many Western countries in recent years, and pain management is a very important element of “fast-track anesthesia” for surgical patients. Analgesia with a lower opioid consumption is a research hotspot for anesthesiologists, such as wound infiltration, peripheral nerve blocks, paravertebral blocks, and transversus abdominal plane blocks.
Innovations and breakthroughs
This study suggests that CWI with ropivacaine as the only main component of a multimodal analgesic regimen can provide satisfactory and safe analgesia after open gastrectomy. CWI could be an alternative to PCIA or EA after major abdominal surgeries.
Applications
This study provides additional evidence supporting CWI with local anesthetics as a safe and effective analgesia after open gastrectomy.
Terminology
Visual analogue scale/score (VAS) is a method using a 10 cm ruler to evaluate the degree of pain, with 0 as no pain and 10 as very severe pain. VAS score over 4 is moderate or severe pain that needs intervention. Multimodal analgesia is an approach to treat various components of postoperative pain due to different physiological mechanisms. The rationale for this strategy is achievement of sufficient analgesia due to additive or synergistic effects between different analgesics, stages and passes, with concomitant reduction of side effects, due to resulting lower doses of analgesics and differences in side effect profiles.
Peer-review
The authors have performed a good study and the manuscript is well written.
Footnotes
Supported by Foundation of Health Department of Zhejiang Province, China, No. 2011RCA207; and Foundation of Education Department of Zhejiang Province, China, No. Y201431914.
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board and Ethics Committee of Sir Run Run Shaw Hospital, Hangzhou, China.
Clinical trial registration statement: We applied for waiver of the clinical trial registration.
Informed consent statement: All study participants, or their legal guardian, provided written informed consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this study.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 19, 2015
First decision: September 29, 2015
Article in press: November 19, 2015
P- Reviewer: Murata A S- Editor: Qi Y L- Editor: Filipodia E- Editor: Ma S
References
- 1.Dajczman E, Gordon A, Kreisman H, Wolkove N. Long-term postthoracotomy pain. Chest. 1991;99:270–274. doi: 10.1378/chest.99.2.270. [DOI] [PubMed] [Google Scholar]
- 2.Wightman JA. A prospective survey of the incidence of postoperative pulmonary complications. Br J Surg. 1968;55:85–91. doi: 10.1002/bjs.1800550202. [DOI] [PubMed] [Google Scholar]
- 3.Latimer RG, Dickman M, Day WC, Gunn ML, Schmidt CD. Ventilatory patterns and pulmonary complications after upper abdominal surgery determined by preoperative and postoperative computerized spirometry and blood gas analysis. Am J Surg. 1971;122:622–632. doi: 10.1016/0002-9610(71)90290-x. [DOI] [PubMed] [Google Scholar]
- 4.Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin. 2012;30:91–100. doi: 10.1016/j.anclin.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Elvir-Lazo OL, White PF. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28:217–224. doi: 10.1016/j.anclin.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Wang C, Xu C, Cai Q. Influence of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer. 2013;16:193–200. doi: 10.1007/s10120-012-0168-z. [DOI] [PubMed] [Google Scholar]
- 7.Hankin CS, Schein J, Clark JA, Panchal S. Adverse events involving intravenous patient-controlled analgesia. Am J Health Syst Pharm. 2007;64:1492–1499. doi: 10.2146/ajhp060220. [DOI] [PubMed] [Google Scholar]
- 8.Choi JB, Shim YH, Lee YW, Lee JS, Choi JR, Chang CH. Incidence and risk factors of postoperative nausea and vomiting in patients with fentanyl-based intravenous patient-controlled analgesia and single antiemetic prophylaxis. Yonsei Med J. 2014;55:1430–1435. doi: 10.3349/ymj.2014.55.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient-controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457. doi: 10.1097/00000542-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Bruce DL, Gerken MV, Lyon GD. Postcholecystectomy pain relief by intrapleural bupivacaine in patients with cystic fibrosis. Anesth Analg. 1987;66:1187–1189. [PubMed] [Google Scholar]
- 11.Mohta M, Ophrii LE, Agarwal D, Bhatt S, Sethi AK, Chilkoti G. Vocal cord palsy: an unusual complication of paravertebral block. Anaesth Intensive Care. 2011;39:969–971. [PubMed] [Google Scholar]
- 12.Lucas SD, Higdon T, Boezaart AP. Unintended epidural placement of a thoracic paravertebral catheter in a patient with severe chest trauma. Pain Med. 2011;12:1284–1289. doi: 10.1111/j.1526-4637.2011.01180.x. [DOI] [PubMed] [Google Scholar]
- 13.Faheem M, Sarwar N. Sliding of the skin over subcutaneous tissue is another important factor in epidural catheter migration. Can J Anaesth. 2002;49:634. doi: 10.1007/BF03017395. [DOI] [PubMed] [Google Scholar]
- 14.Tilleul P, Aissou M, Bocquet F, Thiriat N, le Grelle O, Burke MJ, Hutton J, Beaussier M. Cost-effectiveness analysis comparing epidural, patient-controlled intravenous morphine, and continuous wound infiltration for postoperative pain management after open abdominal surgery. Br J Anaesth. 2012;108:998–1005. doi: 10.1093/bja/aes091. [DOI] [PubMed] [Google Scholar]
- 15.Abadir AR, Nicolas F, Gharabawy R, Shah T, Michael R. Efficacy of postoperative continuous wound infiltration with local anesthetic after major abdominal surgery. Proc West Pharmacol Soc. 2009;52:35–38. [PubMed] [Google Scholar]
- 16.Xin Y, Hong Y, Yong LZ. Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Clin J Pain. 2014;30:571–576. doi: 10.1097/AJP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tverskoy M, Cozacov C, Ayache M, Bradley EL, Kissin I. Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. Anesth Analg. 1990;70:29–35. doi: 10.1213/00000539-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett WJ, Baldini G. Optimal analgesia during major open and laparoscopic abdominal surgery. Anesthesiol Clin. 2015;33:65–78. doi: 10.1016/j.anclin.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Gritsenko K, Khelemsky Y, Kaye AD, Vadivelu N, Urman RD. Multimodal therapy in perioperative analgesia. Best Pract Res Clin Anaesthesiol. 2014;28:59–79. doi: 10.1016/j.bpa.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. doi: 10.1016/j.atc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Hopf HW, Hunt TK, West JM, Blomquist P, Goodson WH, Jensen JA, Jonsson K, Paty PB, Rabkin JM, Upton RA, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997–1004; discussion 1005. doi: 10.1001/archsurg.1997.01430330063010. [DOI] [PubMed] [Google Scholar]
- 22.Baig MK, Zmora O, Derdemezi J, Weiss EG, Nogueras JJ, Wexner SD. Use of the ON-Q pain management system is associated with decreased postoperative analgesic requirement: double blind randomized placebo pilot study. J Am Coll Surg. 2006;202:297–305. doi: 10.1016/j.jamcollsurg.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Wheatley GH, Rosenbaum DH, Paul MC, Dine AP, Wait MA, Meyer DM, Jessen ME, Ring WS, DiMaio JM. Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg. 2005;130:464–468. doi: 10.1016/j.jtcvs.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Kelemen SE, Autieri MV. Expression of granulocyte colony-stimulating factor is induced in injured rat carotid arteries and mediates vascular smooth muscle cell migration. Am J Physiol Cell Physiol. 2005;288:C81–C88. doi: 10.1152/ajpcell.00322.2004. [DOI] [PubMed] [Google Scholar]
- 25.Revie EJ, McKeown DW, Wilson JA, Garden OJ, Wigmore SJ. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 2012;14:611–618. doi: 10.1111/j.1477-2574.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forastiere E, Sofra M, Giannarelli D, Fabrizi L, Simone G. Effectiveness of continuous wound infusion of 0.5% ropivacaine by On-Q pain relief system for postoperative pain management after open nephrectomy. Br J Anaesth. 2008;101:841–847. doi: 10.1093/bja/aen309. [DOI] [PubMed] [Google Scholar]
- 27.Beaussier M, El’Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit JX, Azizi L, Gervaz P, Rohr S, Biermann C, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double-blind, placebo-controlled study. Anesthesiology. 2007;107:461–468. doi: 10.1097/01.anes.0000278903.91986.19. [DOI] [PubMed] [Google Scholar]
- 28.Lluis F, Romero Simó M, Márquez Peiró JF, Selva Otaolaurruchi J, Zarco A. Safety of a multiperforated catheter implanted in the surgical wound for the continuous infusion of local anaesthetics in post-operative analgesia. Cir Esp. 2011;89:613–617. doi: 10.1016/j.ciresp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Fredman B, Shapiro A, Zohar E, Feldman E, Shorer S, Rawal N, Jedeikin R. The analgesic efficacy of patient-controlled ropivacaine instillation after Cesarean delivery. Anesth Analg. 2000;91:1436–1440. doi: 10.1097/00000539-200012000-00025. [DOI] [PubMed] [Google Scholar]
- 30.Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. doi: 10.1007/s10350-004-6672-4. [DOI] [PubMed] [Google Scholar]
- 31.ten Broek RP, Strik C, Issa Y, Bleichrodt RP, van Goor H. Adhesiolysis-related morbidity in abdominal surgery. Ann Surg. 2013;258:98–106. doi: 10.1097/SLA.0b013e31826f4969. [DOI] [PubMed] [Google Scholar]
- 32.Hoem D, Viste A. Improving survival following surgery for pancreatic ductal adenocarcinoma--a ten-year experience. Eur J Surg Oncol. 2012;38:245–251. doi: 10.1016/j.ejso.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, Talamini G, Pederzoli P. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010;252:207–214. doi: 10.1097/SLA.0b013e3181e61e88. [DOI] [PubMed] [Google Scholar]


