Abstract
Inflammasomes monitor the cytosol for microbial contamination or perturbation, and are thus predicted to provide potent defense against infection. However, the compendium of data from murine infection models suggests that inflammasomes merely delay the course of disease, allowing the host time to mount an adaptive response. Interpretations of such results are confounded by inflammasome evasion strategies of vertebrate-adapted pathogens. Conversely, environmental opportunistic pathogens have not evolved in the context of inflammasomes, and are therefore less likely to evade them. Indeed, opportunistic pathogens do not normally cause disease in wild type animals. Accordantly, the extreme virulence of two opportunistic bacterial pathogens, Burkholderia thailandensis and Chromobacterium violaceum, is fully counteracted by inflammasomes in murine models. This leads us to propose a new hypothesis: perhaps animals maintain inflammasomes over evolutionary time not to defend against vertebrate-adapted pathogens, but instead to counteract infection by a plethora of undiscovered opportunistic pathogens residing in the environment.
Introduction
Sensors in the innate immune system survey either the extracellular/vacuolar space or the cytosol. Toll-like receptors (TLRs) and C-type lectin receptors survey the extracellular/vacuolar compartment. In contrast, RIG-I-like receptors (RLRs), Nod-like receptors (NLRs), AIM2-like receptors (ALRs), and certain TRIM family proteins survey the cytosolic space. Amongst these cytosolic sensors, the canonical inflammasomes activate caspase-1. The exception to this paradigm of upstream sensors activating downstream signaling molecules is the so called “noncanonical inflammasome” pathway, in which murine caspase-11 (and its human orthologs caspase-4 and -5) have the cytosolic sensor and effector functions built into the same protein (1–2).
Caspase-1 activation leads to processing and secretion of the inflammatory cytokines IL-1β and IL-18, and also to a form of programmed lytic cell death called pyroptosis. Inflammasomes detect a wide range of cytosolic contaminants. AIM2 detects cytosolic DNA(3). NLRP3 responds to diverse stimuli that seem to trigger catastrophic cellular events(3). In a relatively unique mechanism, NLRC4 is activated by an upstream helper NLR in the NAIP family that is the direct sensor for one of three bacterial proteins: T3SS rod, T3SS needle, or flagellin. These components aberrantly enter the host cytosol through the T3SS, presumably while the bacteria is injecting effector proteins to alter host cell function(s) (4). NLRP1b in the mouse responds to the anthrax lethal toxin by serving as a “lure” for this pathogen protease (5). Finally, Pyrin (also called TRIM20) senses perturbation of Rho GTPases by bacterial toxins (6). In contrast to these canonical inflammasomes, caspase-11/4/5 detect cytosolic LPS by direct interaction between LPS and their CARD domains (2). These noncanonical inflammasomes trigger pyroptosis, but do not process IL-1β directly.
Inflammasomes are evolutionarily maintained in vertebrates (7), although the repertoire may be expanded or contracted in various species. Inflammasomes are proposed to aid in defense against a variety of infections. However, inflammasome activation can also drive septic shock, leading to death (8, 9). This would exert a selective pressure to lose inflammasomes. With this in mind, does the current published evidence support a positive selective pressure that explains the maintenance of inflammasomes over evolutionary time?
In vivo data suggests that inflammasomes delay, but do not eradicate infection
In considering the positive selective pressure to maintain inflammasomes in defense against infection, many infectious studies have been performed using knockout mice, typically on the C57BL/6 background. Although inflammasome-deficient mice have increased susceptibility to many pathogens, the effect of inflammasomes on experimental survival studies is typically incremental. Salmonella enterica serovar Typhimurium (S. typhimurium) was one of the best and earliest examples. S. typhimurium replicates somewhat faster in Casp1−/−Casp11−/− mice, resulting in significantly increased burdens at any given time point post infection; for example at day 5 post infection, Casp1−/−Casp11−/− mice have 10 fold higher burdens than wild type (WT) mice (10). This translates into the WT mice surviving two days longer than the Casp1−/−Casp11−/− mice (11), however it is important to remember that the WT mice still succumb, and thus the ultimate outcome of the infection remained unchanged. Our interpretation of these results is that inflammasomes alone cannot eradicate S. typhimurium, but merely slow its kinetics, which could be beneficial in that the animal may survive long enough to develop an adaptive immune response. In further support of this hypothesis, mice infected with Listeria monocytogenes that survive to days 7–8 post infection are able to mount a cytotoxic T lymphocyte (CTL) response that will combat and clear the infection. It is well established that this CTL response will eliminate L. monocytogenes (12). Therefore, slowing the course of infection could be the positive selective pressure that maintains inflammasomes over evolutionary time.
Conversely, some infectious studies would, at first glance, suggest that inflammasomes have a critical role in defense. For example, we published that B. pseudomallei is lethal in Casp1−/−Casp11−/− mice, while WT mice survive the infection (13). On its surface, such a result seems to have incredible power, leading to the interpretation that inflammasomes fully prevent lethal infection. However, interpretation of this result is confounded by the fact that we chose the dose specifically because it was not lethal in WT mice; in other words, we defined the dose as being sublethal. When a deeper analysis of the dose response is performed, the results lead to a more nuanced interpretation. In this regard, Fabio Re’s group examined three doses of B. pseudomallei infection. They demonstrated that as few as 25 CFU resulted in 100% lethality in Casp1−/−Casp11−/− mice, whereas all the WT mice survive, consistent with our results. However, when Re’s group increased the dose, they found that 100 CFU caused an intermediate lethality in WT mice, and 200 CFU was sufficient to cause 100% lethality in WT mice (14). Therefore, inflammasome detection of B. pseudomallei resulted in approximately an 8-fold shift in the lethal infectious dose. While this is not an insignificant degree of protection, the general conclusion could be that both WT and Casp1−/−Casp11−/− mice succumb to extremely low dose B. pseudomallei challenge. Thus, our interpretation is that although experiments performed at doses just under the lethal dose can appear fully penetrant, they may actually reflect a more subtle phenotype.
We next considered whether this interpretation is consistent with other published data in infectious models. In contrast to Re’s work (14), most studies only use one infectious dose. Although this limits our ability to precisely evaluate the shift in lethal dose, we can estimate the minimum change supported by the data (rubric is described in Table 1 legend). As we wished to contrast various infectious models, we realized the difficulty of comparing pathogen burdens, pathology, or cytokine responses at various time points. On the other hand, the lethal challenge provides the same readout (survival) for an array of pathogens, routes, and doses; the survival assay also integrates temporal effects. We therefore attempted to identify and collate all lethal challenge studies that have been performed in inflammasome knockout mice in order to illustrate the relative importance of inflammasomes in defense against numerous pathogens (Table 1). Unless otherwise indicated (Table 1, Notes column), all KO mice in Table 1 are Casp1−/−Casp11−/− mice. It should be noted that while many survival studies have been performed with bacteria, fewer have been published with viral, fungal, and parasitic infection (further discussed below) (15–18). After collation of published survival studies (Table 1), it was striking that most changes in lethal dose were estimated to be in the 1–5 fold range.
Table I.
Inflammasome survival studies reveal incremental inflammasome protection.
| Pathogen | Vert. Adapted | Dose | Route | Time to death | % Survival | Δ lethal dose* | Notes | Ref | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | KO | WT | KO | ||||||||
| BACTERIA | |||||||||||
|
| |||||||||||
| Bacillus anthracis | Y | 105 | sc | ∞ | 80 h | 100% | 0% | >5 | † | (42) | |
| Bacillus anthracis Ames | Y | 4×102 | ip | 3 d | 3 d | 50% | 25% | >1 | ‡ | (43) | |
| Bacillus anthracis Sterne | Y | 2.5×107 | ip | ∞ | 4 d | 100% | 0% | >5 | ‡ | (43) | |
| Burkholderia cepacia | N | 106 | ip | ∞ | ∞ | 100% | 100% | 1 | (33) | ||
| Burkholderia pseudomallei | Y | 200 | in | 4 d | n.d. | 0% | n.d. |
|
(14) | ||
| 100 | in | 4 d | 4 d | 65% | 0% | >8 | (14) | ||||
| 25 | in | ∞ | 4 d | 100% | 0% | (14) | |||||
| 100 | in | ∞ | 2–3 d | 100% | 0% | >5 | (44) | ||||
| 100 | in | ∞ | 3.5 d | 100% | 0% | >5 | (13) | ||||
| Burkholderia thailandensis | N | 2×107 | ip | ∞ | 1 d | 100% | 0% |
|
(29) | ||
| 106 | ip | ∞ | 2 d | 100% | 0% | (29) | |||||
| 105 | ip | ∞ | 2 d | 100% | 0% | >1,000,000 | (29) | ||||
| 104 | ip | ∞ | 2 d | 100% | 0% | (29) | |||||
| 1000 | ip | ∞ | 3 d | 100% | 0% | (29) | |||||
| 100 | ip | ∞ | 3 d | 100% | 0% | (29) | |||||
| Chromobacterium violaceum | N | 106 | ip | ∞ | n.d. | 100% | n.d. |
|
(33) | ||
| 104 | ip | ∞ | 3 d | 100% | 0% | >50,000 | (33) | ||||
| 100 | Ip | ∞ | 4 d | 100% | 0% | (33) | |||||
| Francisella tularensis subsp. novicida | Y | 1.5×105 | sc | 4 d | 3 d | 65% | 0% | >2 | # | (45) | |
| 1.5×105 | sc | 6 d | 4 d | 25% | 0% | >1 | (46) | ||||
| 5×103 | sc | 3 d | 2.5 d | 75% | 0% | >2 | (47) | ||||
| Francisella philomiragia | N | 106 | ip | ∞ | ∞ | 100% | 100% | 1 | (33) | ||
| Klebsiella pneumoniae | N | 7.4×104 | it | 50 h | 45 h | 15% | 0% | >1 | # | (35) | |
| 1000 | in | 5 d | 5 d | 75% | 40% | >1 | (48) | ||||
| 104 | in | 4 d | 6 d | 50% | 15% | >1 | (48) | ||||
| Listeria monocytogenes | Y | 106 | iv | 5 d | 3–4 d | 35–65% | 0% | >2 | (49) | ||
| Mycobacterium tuberculosis | Y | 250–350 | in | 200 d | 148 d | 0% | 0% | 1 | # | (50) | |
| 250–350 | in | 170 d | 170 d | 0% | 0% | 1 | (50) | ||||
| 50–100 | in | 200 d | 110 d | 90% | 0% | >2 | (51) | ||||
| 106 | it | ∞ | 6 d | 0% | 0% | >5 | # | (52) | |||
| Pseudomonas aeruginosa | N | 2×107 | in | 36 h | 40 h | 20% | 65% | >1 | # | (53) | |
| 7×105 | it | 3 d | ∞ | 10% | 0% | 1 | # | (54) | |||
| Salmonella enterica serovar Typhimurium | Y | 100 | ip | 5 d | 5 d | 0% | 0% | 1 | (55) | ||
| 106 | oral | 9 d | 6 d | 0% | 0% | 1 | (10) | ||||
| 108 | oral | 8 d | 5.5 d | 0% | 0% | 1 | (11) | ||||
| Shigella flexneri | Y | 2×108 | in | 20 h | 45 h | 75% | 20% | >2 | (56) | ||
| Staphylococcus aureus | Y | 1×104 | ic | 20 h | 18 h | 60% | 25% | >1 | (57) | ||
| Streptococcus agalactiae (Group B) | Y | 105 | ip | ∞ | 24 h | 100% | 40% | >2 | (58) | ||
| Streptococcus pneumoniae | Y | 105 | In | 3 d | 2.5 d | 87% | 55% | >1 | (59) | ||
| Vibrio vulnificus | N | 1.5×104 | ip | ∞ | 24 h | 100% | 60% | >1 | (36) | ||
| Yersinia pestis | Y | 1×104 | in | 72 h | 72 h | 0% | 0% | 1 | (60) | ||
| Yersinia pseudotuberculosis | Y | 1000 | ip | 6 d | 4 d | 0% | 0% | 1 | (23) | ||
| 1×109 | oral | 7 d | 6 d | 0% | 0% | 1 | (61) | ||||
|
| |||||||||||
| VIRUSES | |||||||||||
|
| |||||||||||
| Encephalomyocarditis virus | Y | 2× LD50 | ip | 5 d | 5 d | 10% | 15% | >1 | (62) | ||
| Influenza A virus | Y | 6×104 | in | 8 d | 7 d | 65% | 40% | >1 | (63) | ||
| 8×103 | in | 11 d | 10 d | 65% | 35% | >1 | (64) | ||||
| 10 | in | ∞ | 11 d | 100% | 0% | >5 | (65) | ||||
| Vesicular stomatitis virus | Y | 2×105 | in | 7 d | 7 d | 40% | 20% | >1 | (62) | ||
| West Nile virus | Y | 100 | sc | 9 d | 9 d | 80% | 50% | >1 | (66, 67) | ||
|
| |||||||||||
| FUNGI | |||||||||||
|
| |||||||||||
| Aspergillus fumigatus | N | 1×105 | ip | 6 d | 4 d | 70% | 0% | >2 | (68) | ||
| Candida albicans | Y | 105 | iv | 9 d | 5 d | 40% | 0% | >1 | # | (69) | |
| 2×105 | iv | 18 d | 17 d | 83% | 50% | >1 | (70) | ||||
| 5×106 | oral | N/A | 3 d | 100% | 60% | >1 | # | (71) | |||
| n.s. | oral | ∞ | 5 d | 97% | 60% | >1 | (72) | ||||
| Paracoccidioides brasiliensis | N | 2×106 | iv | 90 d | 75 d | 50% | 0% | >2 | (73) | ||
|
| |||||||||||
| PARASITES | |||||||||||
|
| |||||||||||
| Plasmodium berghei | Y | 10 | iv | 9 d | 10 d | 40% | 75% | >1 | # | (74) | |
| Plasmodium berghei iRBCs | Y | 104 | iv | 6.5 d | 6.5 d | 0% | 0% | 1 | (75) | ||
| Plasmodium berghei sporozites | Y | 104 | iv | 6.5 d | 6.5 d | 0% | 0% | 1 | (75) | ||
| Plasmodium chabaudi adami | Y | 5×104 | ip | 11 d | 12 d | 0% | 0% | 1 | # | (76) | |
| Plasmodium falciparum | Y | 106 | ip | 6 d | 6 d | 0% | 0% | 1 | (77) | ||
| Toxoplasma gondii | Y | 104 | ip | 10 d | 9 d | 75% | 10% | >2 | (78) | ||
| Trypanosoma cruzi | Y | 103 | ip | 20 d | 20 d | 70% | 10% | >2 | (79) | ||
| 103 | sc | 22 d | 28 d | 80% | 90% | >1 | (80) | ||||
Change in 100% lethality between WT and KO mice. Values based on ref 3 because difference was 8 fold between WT 100% lethality and lowest dose listed. However, since there was no dose where Casp1−/−Casp11−/− mice did not die, the difference was listed as >8 fold. Thus, a difference in survival percentages of <50% was estimated to be >1 fold increase in the infectious dose, >50% was >2 fold, and >100% was >5 fold.
Mice encoding NLRP1b that could detect anthrax lethal toxin, leading to caspase-1 activation. KO mice have this sensitive NLRP1b but lack caspase-1 and -11.
KO are transgenic mice expressing a 129S1/SvImJ(129S1)-derived lethal toxin-sensitive allele of Nlrp1b on a B6 background. WT mice are normal B6.
Aim2−/−, Nlrp3−/−, or Nlrc4−/− mice.
Therefore, the combined results of all infection survival studies in inflammasome-deficient mice supports our interpretation that inflammasomes delay the course of infection, but do not have a dominant role in defense. Conversely, what if inflammasomes could provide rapid and potent defense against infection? This would exert a strong selective pressure for pathogens to evolve inflammasome evasion strategies, minimizing the utility of inflammasomes. The difference between these two interpretations has a profound influence on how we think about the importance of inflammasomes.
Vertebrate adapted pathogens evade inflammasomes
Indeed, many pathogens evade inflammasome detection. For example, S. typhimurium induces rapid and profound caspase-1 activation in vitro. However, S. typhimurium encodes two T3SSs (SPI-1 and SPI-2), of which only SPI-1 is detected by NLRC4. Therefore, upon host cell entry and during the systemic phase of infection in vivo, S. typhimurium suppresses SPI-1 expression in favor of the “silent” SPI-2 system. Further, S. typhimurium represses flagellin expression (19). The significance of these strategies in vivo cannot be understated, since S. typhimurium engineered to express flagellin or SPI1 rod protein during systemic infection are completely attenuated in WT mice but fully virulent in inflammasome-deficient mice (4, 20). L. monocytogenes also represses flagellin, and is attenuated when engineered to persistently express it (21, 22). By virtue of being a Gram-positive bacterium, L. monocytogenes also naturally evades caspase-11, simply because it lacks LPS. On the other hand, cytosol-invasive Francisella species have evolved to actively modify their LPS structure to evade caspase-11 detection (9). Another mechanism to evade cytosolic LPS detection by caspase-11 is by replicating in the vacuole, as is the case with numerous vacuolar pathogens, including S. typhimurium (13).
In contrast, Yersinia species encode the effector YopM, which is capable of inhibiting caspase-1 by directly binding to the active site. This is critically important in vivo, as Y. pseudotuberculosis yopM mutants are attenuated in WT mice, but retain full virulence in Casp1−/−Casp11−/− mice (23). Similarly, Shigella flexneri encode the OspC3 T3SS effector that inhibits human caspase-4 (24), thus preventing detection of its LPS as the bacterium invades the cytosol. Direct inhibition of inflammasome components is not limited to bacteria. Indeed, poxviruses encode crmA, a serpin that can inhibit several caspases (both inflammatory and apoptotic) and was identified as a caspase-1 inhibitor before the discovery of pyroptosis (25). Further, Kaposi’s sarcoma-associated herpesvirus (KSHV) encodes an NLR analog (the tegument protein Orf63) that inhibits NLRP1 and NLRP3 activation of caspase-1 (26).
Another possible strategy for pathogens to cope with inflammasomes is to avoid the consequences downstream of inflammasome detection. B. pseudomallei may be an example of this strategy by resisting the neutrophil killing (27) that occurs after the bacteria are ejected into the extracellular space by pyroptosis (4). Staphylococcus aureus may be another example, as they are highly resistant to killing by neutrophils and have numerous toxins that inhibit neutrophil chemotaxis (28).
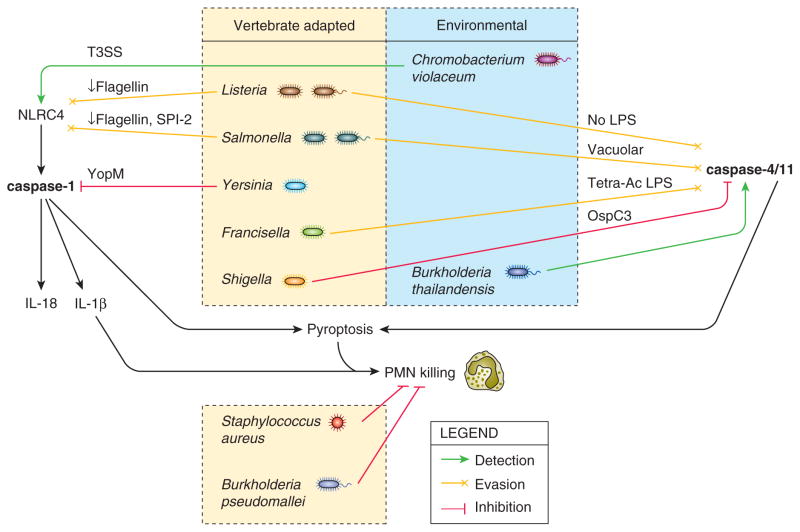
These pathogens are striking examples that demonstrate the importance of evading inflammasome detection (Figure 1). They show that inflammasomes could provide extremely potent defense against infection in vivo, but fail to do so because many pathogens minimize detection.
Figure 1. Inflammasomes readily detect opportunistic microbes while vertebrate-adapted pathogens evade.
Numerous pathogens evade inflammasome detection by repression or modification of ligands (yellow arrows) or by direct inhibition with specific virulence factors (red arrows). Concomitantly, the beneficial effect of inflammasomes against such pathogens is typically blunted in vivo (see Table 1). In contrast, two environmental bacteria (Chromobacterium violaceum and Burkholderia thailandensis) are potently detected by inflammasomes (green arrows), and inflammasome defense in vivo is accordingly robust. Thus, we hypothesize that inflammasomes defend against environmental pathogens with specific virulence traits and may have limited utility against vertebrate-adapted bacterial pathogens, which have evolved to evade and/or inhibit them.
Inflammasomes defend against opportunistic pathogens
Vertebrate-adapted pathogens have evolved in the context of selective pressure exerted by the host immune system, and have accordingly potent virulence traits. In contrast, for opportunistic pathogens where humans are dead-end accidental hosts, there is no selective pressure to evade the human immune response. However, such microbes could also encode an impressive array of virulence factors, which can enable extreme pathogenicity. For example, B. thailandensis encodes cytosol-invasive T3SS, but almost never causes infection in people (29). As such, it has been used as an experimental surrogate for its close relative B. pseudomallei (a BSL3 pathogen); in this capacity, our laboratory has studied B. thailandensis infection in vivo in mice.
Shockingly, during systemic infection with B. thailandensis there is a 1,000,000-fold change between Casp1−/−Casp11−/− and WT mice (Table 1). The resistance conferred by inflammasomes is extremely efficient; WT mice fully sterilize even high dose B. thailandensis infection (2×107 CFU) within just one day (29). Clearly, inflammasomes can provide potent protection when presented with this specific pathogen. The strength of this phenotype was extremely surprising, rivaled only by the effect of inflammasomes upon pathogens that were engineered to remove inflammasome evasion strategies. This made us re-evaluate the nature of B. thailandensis; we had simplistically considered it to be a surrogate for B. pseudomallei. However, it is important to keep in mind that B. thailandensis occupies a specific environmental niche as a soil microbe, where its T3SS is presumably used to invade the cytosol of an unknown eukaryotic host (30).
Is B. thailandensis an isolated unique case? With these thoughts in mind, we began to screen for other environmental opportunistic pathogens that primarily cause disease in immunocompromised individuals, hypothesizing that healthy individuals would competently clear them via the activity of inflammasomes.
In this endeavor, we discovered another ubiquitous environmental opportunistic pathogen against which inflammasomes play a similarly striking role, conferring a greater than 50,000 fold shift in the lethal dose (Table 1). Chromobacterium violaceum is a Gram-negative bacterium that lives in the sediment of fresh-water rivers and lakes. Although C. violaceum encodes two T3SSs that are similar to Salmonella SPI-1 and SPI-2 and can be detected by NLRC4 (31), it virtually never causes disease in immunocompetent people (32).
A confounding factor in the identification of these examples is that there may be redundant host defense pathways that independently confer sterilizing innate immunity. Indeed, it is quite surprising that defense against C. violaceum and B. thailandensis is mono-allellic. However, our studies with C. violaceum did reveal that pyroptosis is partially redundant with natural killer (NK) cell cytotoxicity, although both are dependent on caspase-1 activation (33). Further, if an opportunistic pathogen does not kill the host within one week, the adaptive immune response could compensate for the loss of inflammasomes.
Specific virulence traits are required for inflammasome detection and defense
As evidenced by the vertebrate-adapted column in Table 1, several opportunistic pathogens have been examined in Casp1−/−Casp11−/− mice, yet none have demonstrated such large changes in the lethal dose as seen with C. violaceum and B. thailandensis. C. violaceum encodes two T3SSs (34), and concomitantly, defense is conferred by NLRC4 in vivo (33). B. thailandensis invades the host cytosol, and its LPS is detected by caspase-11 (13, 29), which confers defense in vivo. In both cases, it is the specific virulence traits that are detected by the inflammasomes. The evasion strategies employed by vertebrate-adapted pathogens are likely not present in these environmental bacteria, as they did not evolve in the presence of potential inflammasome detection. For example, both Klebsiella pneumoniae and Vibrio vulnificus are opportunistic pathogens that typically cause disease in immunocompromised patients (35, 36), which fits our model that healthy individuals would be protected by inflammasomes. However, neither has a strong in vivo phenotype in inflammasome-deficient mice (Table 1). Additionally, neither encode a T3SS nor are cytosol-invasive, as is the case for B. thailandensis and C. violaceum. Thus, they may lack inflammasome agonists.
Hypothesis
The predominance of evidence suggests that vertebrate-adapted pathogens have evolved to minimize the effectiveness of inflammasomes; in vivo phenotypes are typically incremental, and evasion strategies are prevalent. Why, then, are inflammasomes maintained in the human genome? The extreme virulence of B. thailandensis and C. violaceum in inflammasome-deficient mice leads us to propose a new hypothesis regarding the importance of inflammasomes in the immune system. We hypothesize that inflammasomes defend against ubiquitous environmental microbes with specific virulence traits. Further, we propose that inflammasome-directed defense is so efficient that the infection never progresses to clinically apparent symptoms. It is interesting to note that inflammasome-deficient patients have not been identified. We speculate that a survey of apparently immunocompetent people with susceptibility to specific opportunistic pathogens, such as C. violaceum, could identify patients with inflammasome mutations. For example, in a case series of 106 C. violaceum infections, only 15 percent were directly attributed to an immunocompromising comorbidity (such as chronic granulomatous disease) (32).
Caveats to the hypothesis
There are several caveats that temper interpretation of the extreme virulence of B. thailandensis and C. violaceum in Casp1−/−Casp11−/− mice. One consideration is that Casp1−/−Casp11−/− animals are inbred and typically maintained on a C57BL/6 background that naturally lacks NRAMP1 (37). Mice lacking NRAMP1 have increased susceptibility to numerous intracellular pathogens, including S. typhimurium, L. monocytogenes, and Mycobacterium tuberculosis. This caveat has not gone unnoticed. In 2006, Lara-Tejero et al investigated the roles of inflammasomes in Nramp1-sufficient mice during S. typhimurium infection. However, regardless of the presence of a functional Nramp1 gene, Casp1−/−Casp11−/− mice still showed an incremental susceptibility to S. typhimurium infection in comparison to their WT counterparts (10).
Further, 129/SvEv mice are Nramp1-sufficient, but carry a spontaneous mutation in caspase-11 (38). Aachoui et al infected 129/SvEv (Casp11−/−Nramp1+/+) mice and found that they have extreme susceptibility to B. thailandensis that is comparable to C57BL/6 Casp11−/− (Nramp1−/−) mice, whereas BALB/c (Casp11+/+Nramp1−/−) remained resistant (29). Thus, the potency of inflammasome defense against this environmental bacterium was not significantly altered by the presence or absence of Nramp1.
In addition to the influence of Nramp1 on different inbred mouse strains, there is also the fact that mice are not men; while this may seem to be an obvious caveat, it is one worth expanding upon. There are species barriers that can influence the virulence of pathogens. There are several examples of bacteria (Shigella spp, Salmonella typhi) that fail to establish mouse infections despite infectivity in humans. However, there are far more examples of viruses that have human-specific strains that do not infect mice: human cytomegalovirus, HIV, HBV, HCV, Epstein-Barr virus, measles, mumps, and many more (39). Therefore, there could be more stringent non-inflammasome barriers restricting interspecies dissemination of viruses in comparison to bacteria. Therefore, our hypothesis that inflammasomes defend against environmental microbes might not hold true for viruses.
Certain inflammasomes, but not all, are conserved between mice and humans. NLRC4, NLRP3, caspase-1, and AIM2 are highly conserved over evolutionary time, from sharks to humans (Maltez and Miao BLAST searches). Yet, despite the high conservation of the ALR family member AIM2, there are 12 additional ALRs in mice but only 3 more in humans, suggesting active evolution of this gene family (40). Because AIM2/ALRs are often attributed to recognizing viral infections, the diversity within this family over evolutionary time could argue against the hypothesis that they defend against specific environmental pathogens. Similarly, mice encode three NLRP1 genes that are highly polymorphic between inbred mouse strains, and there is only one human NLRP1 (5). This again suggests active evolution of the NLRP1 locus. Thus, our hypothesis might only hold true for certain highly conserved inflammasomes, and not for the more divergent or polymorphic inflammasomes.
Conclusion
If vertebrate-adapted pathogens minimize the effectiveness of inflammasomes, why should we continue to study them? First, even sub-optimal inflammasome responses may have clinically beneficial effects during infection by vertebrate-adapted pathogens. Second, inflammasomes seem to be important drivers of the pathology of severe sepsis and septic shock (8, 9, 41). There are no immunologically directed therapies for sepsis; supportive treatments are useful, but nevertheless 28% of patients with sepsis die. Study of the downstream effect(s) of aberrant inflammasome activation could aid in the development of new directed treatments.
If vertebrate-adapted pathogens minimize the effectiveness of inflammasomes, how should we best study them? Opportunistic pathogens have the potential to lead us to novel insights and to reveal therapeutic approaches. Our work with C. violaceum demonstrates the reality of this potential – we discovered a novel link between inflammasome activation and in vivo perforin-mediated defense that was then applicable to L. monocytogenes infection as a cytokine therapy (33). With the rise in antibiotic-resistant microbes, there is a pressing need for new immunotherapeutic approaches to treat infection.
Acknowledgments
This work was supported by the following NIH grants: AI119073, AI097518, AI097518-02S
We would like to apologize if we missed any publications in our table. We attempted to be as thorough as possible, and only cited studies with live infections and both WT and inflammasome-deficient mice.
References
- 1.Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013;17:873–882. doi: 10.1016/j.cmet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innateimmune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 3.Wen H, Miao EA, Ting JPY. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao EA, I, Leaf A, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of Bacteria by Inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong Y-N, Peng X, Xi JJ, Chen S, Wang F, Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014:1–17. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 7.Sakamaki K, Satou Y. Caspases: evolutionary aspects of their functions in vertebrates. Journal of Fish Biology. 2009;74:727–753. doi: 10.1111/j.1095-8649.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science. 2013 doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 9.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galán JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raupach B, Peuschel SK, Monack DM, Zychlinsky A. Caspase-1-Mediated Activation of Interleukin-1 (IL-1 ) and IL-18 Contributes to Innate Immune Defenses against Salmonella enterica Serovar Typhimurium Infection. Infect Immun. 2006;74:4922–4926. doi: 10.1128/IAI.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aachoui Y, I, Leaf A, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceballos-Olvera I, Sahoo M, Miller MA, Barrio LD, Re F. Inflammasome-dependent Pyroptosis and IL-18 Protect against Burkholderia pseudomallei Lung Infection while IL-1β Is Deleterious. PLoS Pathog. 2011;7:e1002452. doi: 10.1371/journal.ppat.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen IY, Ichinohe T. Response of host inflammasomes toviral infection. Trends in Microbiology. 2015;23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Lupfer C, Malik A, Kanneganti TD. ScienceDirectInflammasome control of viral infection. Current Opinion in Virology. 2015;12:38–46. doi: 10.1016/j.coviro.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamboni DS, Lima-Junior DS. Inflammasomes in host response to protozoan parasites. Immunol Rev. 2015;265:156–171. doi: 10.1111/imr.12291. [DOI] [PubMed] [Google Scholar]
- 18.Tavares AH, Bürgel PH, Bocca AL. Turning Up the Heat: Inflammasome Activation by Fungal Pathogens. PLoS Pathog. 2015;11:e1004948. doi: 10.1371/journal.ppat.1004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 20.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proceedings of the National Academy of Sciences. 2011;108:12419–12424. doi: 10.1073/pnas.1019041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren SE, Duong H, Mao DP, Armstrong A, Rajan J, Miao EA, Aderem A. Generation of a Listeriavaccine strain by enhanced caspase-1 activation. Eur J Immunol. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaRock CN, Cookson BT. The Yersinia Virulence Effector YopM Binds Caspase-1 to Arrest Inflammasome Assembly and Processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Ogawa M, Sanada T, Mimuro H, Kim M, Ashida H, Akakura R, Yoshida M, Kawalec M, Reichhart JM, Mizushima T, Sasakawa C. The Shigella OspC3 Effector Inhibits Caspase-4, Antagonizes Inflammatory Cell Death, and Promotes Epithelial Infection. Cell Host Microbe. 2013;13:570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Turner S, Kenshole B, Ruby J. Viral modulation of the host response via crmA/SPI-2 expression. Immunol Cell Biol. 1999;77:236–241. doi: 10.1046/j.1440-1711.1999.00820.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa SI, Reed JC, Ting JPY, Damania B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riyapa D, Buddhisa S, Korbsrisate S, Cuccui J, Wren BW, Stevens MP, Ato M, Lertmemongkolchai G. Neutrophil Extracellular Traps Exhibit Antibacterial Activity against Burkholderia pseudomallei and Are Influenced by Bacterial and Host Factors. Infect Immun. 2012;80:3921–3929. doi: 10.1128/IAI.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaan AN, Surewaard BGJ, Nijland R, van Strijp JAG. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War*. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 29.Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JPY, Coers J, Aderem A, Buxbaum JD, Miao EA. Canonical Inflammasomes Drive IFN-γ to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 32.Yang CH, Li YH. Chromobacterium violaceum infection: A clinical review of an important but neglected infection. Journal of the Chinese Medical Association. 2011;74:435–441. doi: 10.1016/j.jcma.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Liana EF, Holland SM, Whitmire JK, Miao EA. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity. doi: 10.1016/j.immuni.2015.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Brito CFA, Carvalho CB, Santos F, Gazzinelli RT, Oliveira SC, Azevedo V, Teixeira SMR. Chromobacterium violaceum genome: molecular mechanisms associated with pathogenicity. Genet Mol Res. 2004;3:148–161. [PubMed] [Google Scholar]
- 35.Willingham SB, I, Allen C, Bergstralh DT, Brickey WJ, Huang MTH, Taxman DJ, Duncan JA, Ting JPY. NLRP3 (NALP3, Cryopyrin) Facilitates In Vivo Caspase-1 Activation, Necrosis, and HMGB1 Release via Inflammasome-Dependent and -Independent Pathways. The Journal of Immunology. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Núñez G, Suzuki T. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. The Journal of Immunology. 2010;184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 37.Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological Variation Between Inbred Laboratory Mouse Strains: Points to Consider in Phenotyping Genetically Immunomodified Mice. Veterinary Pathology. 2012;49:32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- 38.Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 39.Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Research. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunette RL, Young JM, Whitley DG, Brodsky IE, Malik HS, Stetson DB. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. Journal of Experimental Medicine. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, Liu S, Sastalla I, Leppla SH. Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. Cutting Edge: Resistance to Bacillus anthracis Infection Mediated by a Lethal Toxin Sensitive Allele of Nalp1b/Nlrp1b. The Journal of Immunology. 2009;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breitbach K, Sun GW, Kohler J, Eske K, Wongprompitak P, Tan G, Liu Y, Gan YH, Steinmetz I. Caspase-1 Mediates Resistance in Murine Melioidosis. Infect Immun. 2009;77:1589–1595. doi: 10.1128/IAI.01257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, Mcdermott E, Eisenlohr L, Landel CP, Alnemri ES. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariathasan S. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. Journal of Experimental Medicine. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meunier E, Wallet P, Dreier RF, Costanzo SEP, Anton L, Rühl S, Dussurgey SEB, Dick MS, Kistner A, Rigard MEL, Degrandi D, Pfeffer K, Yamamoto M, Henry T, Broz P. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015:1–11. doi: 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 Inflammasome-Mediated Production of IL-1 Modulates Mucosal Immunity in the Lung against Gram-Negative Bacterial Infection. The Journal of Immunology. 2012;188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji NM, Tsutsui H, Seki E, Kuida K, Okamura H, Nakanishi K, Flavell RA. Roles of caspase-1 in Listeria infection in mice. International Immunology. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 50.McElvania-TeKippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, Braunstein M, Ting JPY. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS ONE. 2010;5:e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Cutting Edge: Caspase-1 Independent IL-1 Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling In Vivo. The Journal of Immunology. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. Critical role of AIM2 in Mycobacterium tuberculosis infection. International Immunology. 2012;24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 53.Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, Grandjean T, Balloy V, Ryffel B, Dessein R, Chignard M, Uyttenhove C, Guery B, Gosset P, Chamaillard M, Kipnis E. Pseudomonas aeruginosaType-3 Secretion System Dampens Host Defense by Exploiting the NLRC4-coupled Inflammasome. Am J Respir Crit Care Med. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- 54.Tolle L, Yu F-S, Kovach MA, Ballinger MN, Newstead MW, Zeng X, Núñez G, Standiford TJ. Redundant and Cooperative Interactions between TLR5 and NLRC4 in Protective Lung Mucosal Immunity against Pseudomonas aeruginosa. J Innate Immun. 2014;7:177–186. doi: 10.1159/000367790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monack DM, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer’s patches in a murine typhoid model. J Exp Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 57.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem. 2014;129:704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, Midiri A, Galbo R, Trieu-Cuot P, Papasergi S, Teti G, Henneke P, Mancuso G, Golenbock DT, Beninati C. Activation of the NLRP3 inflammasome by group B streptococci. The Journal of Immunology. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 60.Sivaraman V, Pechous RD, Stasulli NM, Miao EA, Goldman WE. Yersinia pestis activates both IL-1β and IL-1 receptor antagonist to modulate lung inflammation during pneumonic plague. PLoS Pathog. 2015;11:e1004688. doi: 10.1371/journal.ppat.1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng Y, Lilo S, Mena P, Bliska JB. YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense. PLoS ONE. 2012;7:e36019. doi: 10.1371/journal.pone.0036019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajan JV, Rodriguez D, Miao EA, Aderem A. The NLRP3 Inflammasome Detects Encephalomyocarditis Virus and Vesicular Stomatitis Virus Infection. Journal of Virology. 2011;85:4167–4172. doi: 10.1128/JVI.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JPY. The NLRP3 Inflammasome MediatesIn Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti T-D. The Intracellular Sensor NLRP3 MediatesKey Innate and Healing Responses to Influenza A Virus via the Regulation of Caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. Journal of Experimental Medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M. IL-1β Signaling Promotes CNS-Intrinsic Immune Control of West Nile Virus Infection. PLoS Pathog. 2012;8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M, Verma S. Inflammasome Adaptor Protein Apoptosis-Associated Speck-Like Protein Containing CARD (ASC) Is Critical for the Immune Response and Survival in West Nile Virus Encephalitis. Journal of Virology. 2013;87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karki R, Man SM, Malireddi RKS, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 70.van de Veerdonk FL, Joosten LAB, Shaw PJ, Smeekens SP, Malireddi RKS, van der Meer JWM, Kullberg BJ, Netea MG, Kanneganti TD. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An Essential Role for the NLRP3 Inflammasome in Host Defense against the HumanFungal Pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ketelut-Carneiro N, Silva GK, Rocha FA, Milanezi CM, Cavalcanti-Neto FF, Zamboni DS, Silva JS. IL-18 Triggered by the Nlrp3 Inflammasome Induces Host Innate Resistance in a Pulmonary Model of Fungal Infection. The Journal of Immunology. 2015 doi: 10.4049/jimmunol.1402321. [DOI] [PubMed] [Google Scholar]
- 74.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial Hemozoin Is a Nalp3 Inflammasome Activating Danger Signal. PLoS ONE. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kordes M, Matuschewski K, Hafalla JCR. Caspase-1 Activation of Interleukin-1 (IL-1 ) and IL-18 Is Dispensable for Induction of Experimental Cerebral Malaria. Infect Immun. 2011;79:3633–3641. doi: 10.1128/IAI.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tiemi Shio M, Eisenbarth SC, Savaria M, Vinet AF, Bellemare M-J, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial Hemozoin Activates the NLRP3 Inflammasome through Lyn and Syk Kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reimer T, Shaw MH, Franchi L, Coban C, Ishii KJ, Akira S, Horii T, Rodriguez A, Núñez G. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol. 2010;40:764–769. doi: 10.1002/eji.200939996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M, Saeij JPJ, Grigg ME. Dual Role for Inflammasome Sensors NLRP1 and NLRP3 in Murine Resistance to Toxoplasma gondii. mBio. 2013;5:e01117–13–e01117–13. doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva GK, Costa RS, Silveira TN, Caetano BC, Horta CV, Gutierrez FRS, Guedes PMDM, Andrade WA, De Niz M, Gazzinelli RT, Zamboni DS, Silva JS. Apoptosis-Associated Speck-like Protein Containing a Caspase Recruitment Domain Inflammasomes Mediate IL-1 Response and Host Resistance to Trypanosoma cruzi Infection. The Journal of Immunology. 2013;191:3373–3383. doi: 10.4049/jimmunol.1203293. [DOI] [PubMed] [Google Scholar]
- 80.GonÃalves VM, Matteucci KC, Buzzo CL, Miollo BH, Ferrante D, Torrecilhas AC, Rodrigues MM, Alvarez JM, Bortoluci KR. NLRP3 Controls Trypanosoma cruzi Infection through a Caspase-1-Dependent IL-1R-Independent NO Production. PLoS Negl Trop Dis. 2013;7:e2469. doi: 10.1371/journal.pntd.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]