Abstract
Background
Racial and ethnic disparities have been identified in the provision of neuraxial labor analgesia. These disparities may exist in other key aspects of obstetric anesthesia care. We sought to determine if racial/ethnic disparities exist in mode of anesthesia for cesarean delivery (CD).
Methods
Women who underwent CD between 1999 and 2002 at 19 different obstetric centers in the United States were identified from the Maternal-Fetal Medicine Units Network Cesarean Registry. Race/ethnicity was categorized as: Caucasian, African-American, Hispanic, Non-Hispanic Others (NHOs). Mode of anesthesia was classified as neuraxial anesthesia (spinal, epidural or combined spinal-epidural anesthesia) or general anesthesia. To account for obstetric and nonobstetric covariates that may have influenced mode of anesthesia, multiple logistic regression analyses were performed using sequential sets of covariates.
Results
The study cohort comprised 50,974 women who underwent CD. Rates of general anesthesia among racial/ethnic groups were: 5.2% for Caucasians, 11.3% for African Americans, 5.8% for Hispanics and 6.6% for NHOs. After adjustment for obstetric and nonobstetric covariates, African Americans had the highest odds of receiving general anesthesia compared to Caucasians (adjusted odds ratio (aOR) = 1.7; 95% CI: 1.5 – 1.8; P<0.001). The odds of receiving general anesthesia were also higher among Hispanics (aOR = 1.1; 95% CI: 1.0 – 1.3; P=0.02) and NHOs (aOR = 1.2; 95% CI: 1.0 – 1.4; P=0.03) compared to Caucasians, respectively. In our sensitivity analysis, we reconstructed the models after excluding women who underwent neuraxial anesthesia prior to general anesthesia. The adjusted odds of receiving general anesthesia were similar to those in the main analysis: African-Americans (aOR=1.7; 95% CI=1.5 – 1.9; P<0.001; Hispanics (aOR=1.2; 95% CI=1.1 – 1.4; P=0.006); and NHOs (aOR=1.2; 95% CI=1.0 – 1.5; P=0.05).
Conclusion
Based on data from the Cesarean Registry, African-American women had the highest odds of undergoing general anesthesia for CD compared to Caucasian women. It is uncertain whether this disparity exists in current obstetric practice.
Introduction
Neuraxial anesthesia is the preferred anesthetic modality for cesarean delivery (CD).1–3 Widespread adoption of neuraxial anesthetic techniques into contemporary obstetric anesthetic practice has resulted in major improvements for maternal safety. Maternal mortality is lower among women who receive neuraxial anesthesia (3.8 deaths per million) compared to general anesthesia (6.5 deaths per million) in the United States.4 Furthermore, rates of anesthetic-related maternal morbidity have decreased as the rate of neuraxial anesthesia for CD has increased.5,6 Complications from general anesthesia, such as aspiration and airway management disasters, can be avoided by using a neuraxial technique. 4 Other maternal-fetal benefits of neuraxial anesthesia include lower rates of surgical site infection and postpartum hemorrhage,7,8 superior quality post-CD analgesia,9 improved ambulation and an earlier return of bowel function.10,11 Neuraxial anesthesia is also associated with less neonatal morbidity and post-neonatal developmental delay compared with general anesthesia.12–16
Despite this strong evidence in favor of neuraxial anesthesia, the whether mode of anesthesia (general vs. neuraxial) for CD differs according to race/ethnicity. In a previous study of deliveries occurring in New York State, the odds of general anesthesia were 1.5 fold higher for African-Americans compared to Caucasians,17 however risk estimates for women in other racial/ethnic groups were not described. With national rates of CD for African-Americans and Hispanic women currently at record highs (35.8% and 32.2% respectively),18 identifying and addressing anesthesia-related disparities may improve maternal outcomes and the overall quality of obstetric anesthesia care.
The primary aim of this secondary analysis of data from an observational study was to investigate whether racial/ethnic disparities exist for mode of anesthesia (general vs. neuraxial) among women undergoing CD, and to examine whether these associations are influenced by demographic and maternal factors, obstetric morbidities and indications for CD.
Methods
Our study received permission to waive consent from the Stanford University IRB as the Cesarean Registry contains de-identified data. The study cohort was identified using a dataset (the Cesarean Registry) sourced from a previous multicenter study by the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network.19 Details of this study were previously reported.19 Between 1999 and 2000, data were collected in women who underwent delivery by primary CD, repeat CD or vaginal delivery after CD and who delivered infants ≥20 weeks’ gestation or ≥500 g at 19 academic centers in the United States. For the final two years of the study (between 2001 and 2002), only women undergoing repeat CD or vaginal birth after CD who delivered infants ≥20 weeks’ gestation or ≥500 g were enrolled. Data regarding patient and hospital were de-identified by the MFMU. All data, including data on patients’ predominant race and ethnicity, were abstracted from medical records by trained research nurses and submitted to a biostatistical coordinating center. The center housed a centralized data management system and regular audits were performed of the entire database and specific subsets to assess data quality.
For our study, we identified women who had undergone CD, hence excluding successful vaginal births after CD. In the Cesarean Registry there were six classifications for the predominant patients’ race/ethnicity: African-American ; Caucasian; Hispanic; Asian; Native American or Alaskan; and Unknown. The cohort comprised relatively limited numbers of Asians (n=884) and Native American or Alaskans (n=98). Within these groups, low numbers of Asians (n=46) and Native Americans or Alaskans (n=8) underwent general anesthesia. Due to concern about the adequacy of patient numbers in these subgroups for our primary and sensitivity analyses, we reclassified race/ethnicity categories into the following groups: African-American, Caucasian, Hispanic, and Non-Hispanic Others (hereafter referred to as Others). Based on previously published data20 and our clinical experience, emergency CD is one of the most common reasons for considering general anesthesia. Using criteria for emergency CD from a prior publication using the Cesarean Registry data,21 we identified conditions that may warrant urgent or emergency CD (hereafter referred to as emergency CD), which included: umbilical cord prolapse, non-reassuring fetal tracing, placental abruption, placenta previa with hemorrhage. For our primary outcome, we classified mode of anesthesia for CD into two types: neuraxial anesthesia and general anesthesia. Women who received spinal, epidural or spinal with epidural anesthesia were classified as receiving neuraxial anesthesia. For women who had codes for both neuraxial and general anesthesia, we classified women as receiving general anesthesia. Rates of general anesthesia and neuraxial anesthesia in our study cohort, calculated as percentages, were determined by race/ethnicity.
Statistical Analysis
The relationships between race/ethnicity and mode of anesthesia were investigated using univariate and multivariate analyses. Proportions were compared using the chi-square test. For the univariate and multivariate analyses, we performed logistic regression analyses to assess the associations between race/ethnicity with mode of anesthesia for CD. To assess the influence of other factors on the associations between race/ethnicity and mode anesthesia, we created a series of models by sequentially adding groups of predictors to each model. This approach has been previously used in other studies investigating race/ethnicity disparities in obstetric outcomes.22,23 Independent variables included in each model are described as follows: Model 1 = only race/ethnicity; Model 2 = covariates in Model 1 + maternal age, insurance class,; Model 3 = covariates in Model 2 + chronic hypertension, gestational age at delivery, singleton/multiple pregnancy, number of prior cesarean deliveries, pregnancy-associated hypertensive disease, labor or attempted induction of labor; Model 4 = covariates in Model 3 + emergency indications for CD. With each series of covariates, we performed a likelihood ratio test to compare each “full” model with the model with fewer variables (“reduced model”) that immediately preceded it. We calculated the Akaike Information Criteria for each model which provides an indication of model goodness-of-fit. We tested for multicollinearity between independent variables by calculating the variance inflation factors. Collinearity was determined to be insignificant as variance inflation scores ranged from 1.03 to 1.85 with a mean variance inflation score=1.22. Model discrimination was determined by calculating the c-statistic for the final model for each logistic regression sequence.
In order to determine whether the point estimates were influenced by women who received neuraxial block prior to general anesthesia, we performed sensitivity analyses for the following cohorts: women who did not receive a neuraxial block prior to general anesthesia; women who underwent primary CD; women who underwent repeat CD; and women who underwent CD without prior labor or induction. We also performed additional sensitivity analyses to investigate potential interactions between race/ethnicity and maternal age, body mass index (BMI) and the presence/absence of an indication for emergency CD. We included the main effect and a cross-product term in the full model (Model 4) and compared nested models with and without each cross-product term using a likelihood ratio test. Data analyses were performed using STATA version 12 (Statacorp, College Station, TX).
Results
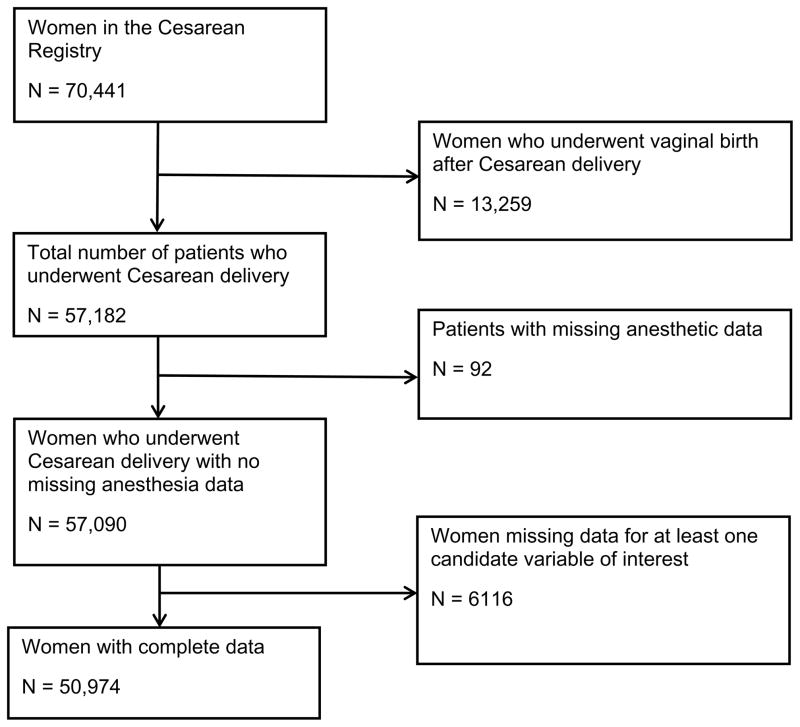
In the Cesarean Registry, 57,182 women underwent CD. We excluded 92 women who had missing anesthetic data and 6,116 women with missing data for at least one of the covariates. A flow diagram of patients included in the final cohort is presented in Figure 1. Our final study cohort comprised 50,974 women; 3,629 (7.1%) women underwent general anesthesia and 47,343 (92.9%) women underwent neuraxial anesthesia. The major indications for CD by racial/ethnic group are presented in the Appendix.
Figure 1. Flow Diagram.
The Cesarean Registry of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network comprises data collected between 1999 and 2002. Data were collected in women who underwent delivery by primary CD, repeat CD or vaginal delivery after CD and who delivered infants ≥20 weeks’ gestation or ≥500 g at 19 academic centers in the United States from 1999–2000. For the final two years of the study (between 2001 and 2002), only women undergoing repeat CD or vaginal birth after CD who delivered infants ≥20 weeks’ gestation or ≥500 g were enrolled.
Within the final cohort, 21,113 (41.4%) were Caucasians, 14,338 (28.1%) were African-Americans, 12,990 (25.5%) were Hispanics and 2,533 (5%) were Others. The unadjusted rate of general anesthesia was highest for African-Americans (11.3%) compared to other ethnicities and races: Caucasians = 5.2%, Hispanics = 5.8%, and Others = 6.6%. Baseline and obstetric characteristics of the study cohort are presented in Table 1. We observed statistically significant differences in all demographic, obstetric and perioperative characteristics among racial and ethnic groups. Among the women who received general anesthesia, 1,187 women received a neuraxial block (epidural and/or spinal anesthesia) prior to general anesthesia and 2,442 women received no neuraxial block prior to general anesthesia.
Table 1.
Maternal Demographic and Obstetric Characteristics
| Race / Ethnicity | |||||
|---|---|---|---|---|---|
|
| |||||
| Caucasian N=21,113 |
African-American N=14,338 |
Hispanic N=12,990 |
Other N=2,533 |
P value | |
|
| |||||
| Age (yr): | <0.001 | ||||
| <20 | 867 (4.1%) | 2,021 (14.1%) | 897 (6.9%) | 85 (3.3%) | |
| 20–34 | 15,212 (72.1%) | 10,324 (72%) | 10,380 (79.9%) | 1,773 (70%) | |
| >34 | 5,034 (23.8%) | 1,993 (13.9%) | 1,713 (13.2%) | 675 (26.7%) | |
|
| |||||
| Insurance Class: | <0.001 | ||||
| Government-Assisted | 4,301 (20.4%) | 9,098 (63.5%) | 7,228 (55.6%) | 895 (35.3%) | |
| Private Insurance | 16,103 (76.3%) | 4,151 (28.9%) | 1,311 (10.1%) | 1,335 (52.7%) | |
| Self-Pay or Other | 709 (3.3%) | 1,089 (7.6%) | 4,451 (34.3%) | 303 (12%) | |
|
| |||||
| BMI at delivery (kg/m2) | <0.001 | ||||
| ≤24.9 | 2,199 (10.4%) | 1,124 (7.8%) | 899 (6.9%) | 304 (12%) | |
| 25–29.9 | 6,882 (32.6%) | 3,029 (21.1%) | 3,881 (29.9%) | 964 (38.1%) | |
| 30–34.9 | 6,034 (28.6%) | 3,822 (26.7%) | 4,617 (35.5%) | 715 (28.2%) | |
| 35–39.9 | 3,282 (15.5%) | 2,859 (19.9%) | 2,288 (17.6%) | 331 (13.1%) | |
| ≥40 | 2,716 (12.9%) | 3,504 (24.5%) | 1,305 (10.1%) | 219 (8.6%) | |
|
| |||||
| Chronic Hypertension | 547 (2.6%) | 665 (4.6%) | 146 (1.1%) | 94 (3.7%) | <0.001 |
|
| |||||
| Gestational Age at delivery (weeks): | <0.001 | ||||
| <37 | 4,738 (22.4%) | 3,140 (21.9%) | 1,586 (12.2%) | 466 (18.4%) | |
| 37–41 | 15,279 (72.4%) | 9,858 (68.8%) | 10,037 (77.3%) | 1,897 (74.9%) | |
| >41 | 1,096 (5.2%) | 1,340 (9.3%) | 1,367 (10.5%) | 170 (6.7%) | |
|
| |||||
| Type of pregnancy: | <0.001 | ||||
| Multiple Pregnancy | 1,175 (5.6%) | 614 (4.3%) | 314 (2.4%) | 93 (3.7%) | |
| Singleton Pregnancy | 19,938 (94.4%) | 13,724 (95.7%) | 12,676 (97.6%) | 2,440 (96.3%) | |
|
| |||||
| Number of prior CD: | <0.001 | ||||
| None | 9,031 (42.8%) | 6,576 (45.9%) | 4,061 (31.3%) | 1,082 (42.7%) | |
| 1 prior CD | 8,773 (41.5%) | 5,385 (37.5%) | 6,018 (46.3%) | 1,069 (42.2%) | |
| 2 or more prior CD | 3,309 (15.7%) | 2,377 (16.6%) | 2,911 (22.4%) | 382 (15.1%) | |
|
| |||||
| Pregnancy associated hypertension: | <0.001 | ||||
| None | 18,565 (87.9%) | 12,078 (84.3%) | 11,539 (88.8%) | 2,245 (88.6%) | |
| Gestational hypertension | 788 (3.7%) | 496 (3.5%) | 372 (2.9%) | 83 (3.3%) | |
| Preeclampsia | 1,519 (7.2%) | 1,637 (11.4%) | 1,027 (7.9%) | 187 (7.4%) | |
| Eclampsia or HELLP syndrome | 241 (1.1%) | 127 (0.9%) | 52 (0.4%) | 18 (0.7%) | |
|
| |||||
| Labor or attempted induction | 8,863 (42%) | 8,107 (56.5%) | 5,603 (43.1%) | 1,251 (49.4%) | <0.001 |
|
| |||||
| Emergency CD* | 2,709 (12.8%) | 3,212 (22.4%) | 1,275 (9.8%) | 378 (14.9%) | <0.001 |
Data presented as n (%).
BMI, body mass index; CD, cesarean delivery; HELLP, hemolysis, elevated liver enzymes, low platelet count
Emergency Cesarean delivery is defined by at least one of the following conditions or events: placental abruption, cord prolapse, placenta previa with antenatal bleeding, or a non-reassuring fetal tracing.
Using Caucasians as the reference group, the unadjusted odds of general anesthesia was increased for African-Americans (odds ratio (OR) = 2.3), Hispanics (OR=1.1) and Others (OR=1.3) (Model 1; Table 2). With sequential addition of each series of covariates to each model, the odds for African-American race was moderately reduced (adjusted odds ratio (aOR) = 1.7 [Model 4]) after accounting for mediating factors, whereas, the odds were only marginally altered for Hispanics (aOR = 1.1 [Model 4]) and Others (aOR = 1.2 [Model 4]). For African-Americans, most of the decrease in the odds for general anesthesia occurred with adjustment of demographic factors [Model 2]. The likelihood ratio test and AIC improved with sequential addition of covariates to each model indicating improved goodness-of-fit. The c-statistic for the final model was 0.80, which suggests moderate model discrimination. We also compared the full model (Model 4) with models that included a cross-product term between race/ethnicity and maternal age, BMI, and emergency CD respectively. We found no evidence of a significant improvement in model fit by including a cross-product term between race/ethnicity*maternal age (χ2 = 5.3; P=0.5) or race/ethnicity*BMI (χ2 = 7.6; P=0.8) in the full models. In contrast, we did observe evidence of improved model fit after adding a cross-product term between race/ethnicity*emergency CD (χ2 = 95.3; P=<0.001). We examined whether the racial disparity for mode of anesthesia persisted when the results were stratified by the presence or absence of an indication for emergency CD. Among women with an emergency indication, only African-Americans (aOR=1.5; 95% CI=1.3–1.7) and Hispanics (aOR=1.6; 95% CI=1.3–1.9) were at increased odds of receiving general anesthesia in the full model. For women without an emergency indication, only African-Americans (aOR=1.8; 95% CI=1.6–2.0) and Others (aOR=1.3; 95% CI=1.0–1.7) were at significantly increased odds of receiving general anesthesia.
Table 2.
Associations between Race/Ethnicity and Mode of Anesthesia (General Compared to Neuraxial) for Cesarean Delivery
| Model 1a | Model 2b | Model 3c | Model 4d | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Caucasian | Reference | Reference | Reference | Reference | ||||
| African-American | 2.3 (2.2–2.5) | <0.001 | 1.7 (1.6–1.9) | <0.001 | 1.9 (1.7–2.1) | <0.001 | 1.7 (1.5–1.8) | <0.001 |
| Hispanic | 1.1 (1.0–1.2) | 0.01 | 0.8 (0.7–0.9) | <0.001 | 1.1 (1.0–1.2) | 0.14 | 1.1 (1.0–1.3) | 0.02 |
| Other | 1.3 (1.1–1.5) | 0.003 | 1.1 (0.9–1.3) | 0.2 | 1.2 (1.0–1.4) | 0.03 | 1.2 (1.0–1.4) | 0.03 |
| Log likelihood | −12,837 | −12,714 | −11,731 | −10,925.3 | ||||
| LRT | 246.3 | <0.001 | 1965.2 | <0.001 | 1,613.4 | <0.001 | ||
| AIC | 25,683.5 | 25,445.1 | 23,507.9 | 21,896.6 | ||||
AIC = Akaike Information Criteria; CI = Confidence Intervals; LRT = Likelihood Ratio Test; OR = Odds Ratio
Model 1: only race/ethnicity.
Model 2: adjusted for maternal age and insurance class.
Model 3: adjusted for maternal age, insurance class, body mass index at time of delivery, chronic hypertension, gestational age at delivery, singleton/multiple pregnancy, number of prior cesarean deliveries, pregnancy-associated hypertensive disease, labor or attempted induction.
Model 4: adjusted for maternal age, insurance class, body mass index at time of delivery, chronic hypertension, gestational age at delivery, singleton/multiple pregnancy, number of prior cesarean deliveries, pregnancy-associated hypertensive disease, labor or attempted induction, and emergency indication for cesarean delivery.
In our sensitivity analysis, we reconstructed the models after excluding women who underwent neuraxial anesthesia prior to general anesthesia. The odds ratios calculated from the logistic regression analyses are presented in Table 3. The point estimates for mode of anesthesia according to race/ethnicity were similar to those observed in our primary analysis. In the final model, all non-Caucasian ethnicities and races had significantly increased odds of receiving general anesthesia compared to Caucasians, with African-Americans having the highest adjusted odds of general anesthesia. For African-Americans, with sequential addition of each series of covariates, the odds of general anesthesia remained high (aOR = 2.2 [Model 1] to 1.7 [Model 4]). In contrast, the adjusted odds remained relatively unchanged for rHispanics and Others with addition of each series of covariates. The c-statistic from the final model in our sensitivity analysis was 0.84 which indicated good model discrimination.
Table 3.
Associations between Race/Ethnicity and Mode of Anesthesia in Women with no Prior Neuraxial Block who Received General Anesthesia Compared to Neuraxial Anesthesia
| Model 1a | Model 2b | Model 3c | Model 4d | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Caucasian | Reference | Reference | Reference | Reference | ||||
| African-American | 2.2 (2.0–2.4) | <0.001 | 1.6 (1.4–1.8) | <0.001 | 1.9 (1.7–2.2) | <0.001 | 1.7 (1.5–1.9) | <0.001 |
| Hispanic | 1.1 (1.0–1.2) | 0.06 | 0.7 (0.6–0.8) | <0.001 | 1.1 (1.0–1.3) | 0.05 | 1.2 (1.1–1.4) | 0.006 |
| Other | 1.3 (1.0–1.6) | 0.01 | 1.1 (0.9–1.3) | 0.6 | 1.2 (1.0–1.5) | 0.04 | 1.2 (1.0–1.5) | 0.05 |
| Log likelihood | −9,595 | −9,743 | −8,418.4 | −7,606.1 | ||||
| LRT | 189 | <0.001 | 2,164.5 | <0.001 | 1,624.6 | <0.001 | ||
| AIC | 19,198.4 | 19,017.4 | 16,880.9 | 15,258.3 | ||||
AIC = Akaike Information Criteria; CI = Confidence Interval; LRT = Likelihood Ratio Test; OR = Odds Ratio
Model 1: only race and ethnicity.
Model 2: adjusted for maternal age and insurance class
Model 3: adjusted for maternal age, insurance class, body mass index at time of delivery, chronic hypertension, gestational age at delivery, singleton/multiple pregnancy, number of prior cesarean deliveries, pregnancy-associated hypertensive disease, labor or attempted induction.
Model 4: adjusted for maternal age, insurance class, body mass index at time of delivery, chronic hypertension, gestational age at delivery, singleton/multiple pregnancy, number of prior cesarean deliveries, pregnancy-associated hypertensive disease, labor or attempted induction, and emergency indication for cesarean delivery.
We performed additional sensitivity analyses to separately examine the estimates in the following subpopulations: primary CD, repeat CD, and women who underwent CD without prior labor or induction. Among women who underwent primary CD, only African American (aOR = 1.6; 95% CI = 1.4–1.8) and Hispanic (aOR = 1.5; 95% CI = 1.3–1.7) women were at significantly increased odds of general anesthesia in the full model. Among women who underwent repeat CD, only African Americans (aOR = 1.8; 95% CI = 1.5–2.1) had significantly greater odds for general anesthesia in the full model. Among women who did not experience labor or induction of labor before CD, the association for general anesthesia was increased for African American women (aOR = 1.9; 95% CI = 1.6–2.2) and Others (aOR = 1.4; 95% CI = 1.1–1.9) in the full model.
Discussion
Using clinical data from over 50,000 women who delivered by CD at 19 obstetric centers in the United States, our results suggest that there were racial/ethnic disparities in the use of general vs. neuraxial anesthesia for women undergoing CD. After adjustment, African American women had a 1.7 fold increased odds of receiving general anesthesia compared to Caucasian women. Due to the inherent nature of our observational study design, the potential etiologies for this disparity are unclear. Furthermore, we analyzed data from a cohort undergoing CD between 1999 and 2002, therefore our findings may not be applicable in current obstetric anesthesia practice.
The findings of our main analysis and sensitivity analyses indicate that African-African women were at increased odds of receiving general anesthesia for CD compared to Caucasian women. Although demographic and obstetric factors mediated the likelihood of receiving general anesthesia, African-American women were at increased odds of receiving general anesthesia in all logistic models. In our sensitivity analyses, we investigated whether this disparity was present in specific cesarean subpopulations: primary CD, repeat CD or CD without prior labor or induction of labor, and in a population that excluded women who received neuraxial anesthesia before general anesthesia. Within each cesarean subpopulation, African-American women had increased odds of receiving general anesthesia compared to Caucasian women. In contrast, the odds of general anesthesia, although significant, were only modestly increased among Hispanics (aOR = 1.1) and Others (aOR=1.2) in our main analyses. It is possible that the mediating effects of other unmeasured factors may have further attenuated the observed associations for Hispanics and Others. Our findings are in keeping with those of Obst et al. who observed evidence of racial/ethnic disparities for mode of anesthesia using a database of deliveries in New York State in 1992.17 In their study, African-American women were more likely than Caucasian women to undergo general anesthesia for CD (aOR=1.53).17 However, the authors did not account for demographic and clinical factors in their analyses, and these findings predate our findings.
Our findings may have important public health and clinical relevance. Between 1998 and 2005, the rate of maternal mortality among African-Americans (37.5 per 100,000 live births) was approximately 4-fold higher compared to the rate among Caucasians (13.4 deaths per 100,000 live births).24 African-American women have also been shown to be high risk for inpatient maternal mortality and events linked to perinatal morbidity, such as CD for fetal distress.25–27 Although the pregnancy-related mortality ratio from anesthesia complications has decreased from 4.3 per million live births between 1979–1981 to 1.0 per million live births between 2000–2002,4 anesthesia-related maternal death is more common among African-Americans (46.4%) compared to women from other ethnic and racial groups (Caucasians = 42.9%; Others = 10.7%).4 Future population-wide studies are needed to determine national rates of general anesthesia for CD and to investigate associations between general anesthesia for urgent or emergent CD and anesthesia-related maternal morbidity.
Due to our observational study design, we are only able to determine associations and not causality. Therefore, the underlying reasons why African-American women were at increased odds of general anesthesia are unclear and likely complex. In 2003, the Institute of Medicine published a detailed report examining racial and ethnic disparities in US healthcare.28 In their report, healthcare disparities are described as ‘rooted in historic and contemporary inequities’ and include variations in healthcare financing and in the institutional and organizational characteristics of health-care systems; clinical interaction between care providers and patients; and influences of the attitudes, beliefs and perceptions of care providers and patients. Although we can only speculate about possible etiologic factors for the disparities in our study, possible patient-level and healthcare-related factors include cultural barriers between minority patients and their providers, mistrust, misunderstanding, limited interaction with healthcare systems, limited health literacy, and a lack of knowledge about healthcare services and anesthesia options related to labor and delivery.28–32 Limited data suggest that minority patients are more likely that Caucasian patients to refuse treatment, however studies reporting these differences are small and patient refusal is unlikely to fully explain all healthcare disparities.28 Provider-level biases may also be important etiologic factors. Three suggested mechanisms may explain perceived provider discriminatory behavior: bias (or prejudice) against minorities; clinical uncertainty during patient-provider interactions; and provider beliefs or stereotypes about the behavior or health of patients belonging to minority groups.28,33 In the setting of CD, it is possible that medical decisions regarding mode of anesthesia may reflect subjective variability and physician preference. Furthermore, there is evidence that time pressure may increase the likelihood of applying stereotypes to decision making,33 such as a situation in which mode of anesthesia is chosen for a patient requiring urgent CD.
Our study has a number of important limitations. We could not account for key hospital-level factors in our analyses because hospital identifiers were not included in the Cesarean Registry. Furthermore, we could not determine whether rates of general anesthesia varied within or between institutions in our analysis. Hypothetically, if complete data were available, a hierarchical model would be preferred for nested data structures,34 specifically, patients being nested according to the anesthesia care provider, who is in turn nested by hospital, with the hospital nested by type or geographical location. Furthermore, due to the nonlinearity of logistic regression, odds ratios are highly sensitive to the statistical model that represents an independent variable and the logit function for an outcome of interest. This statistical issue has been highlighted previously in an Anesthesia & Analgesia statistical grand round by Dexter et al.35 Although we lacked hospital-specific data on rates of anesthesia, the overall rate of general anesthesia in our cohort (7.9%) was within the range reported from other high-volume obstetric centers with >1,500 births per year in 2001 (3% for elective CD; 15% for emergency CD).3 Another limitation is the age of our dataset. As the data were collected between 1999 and 2002, we cannot state that our findings are applicable to current obstetric anesthesia practice. However, there is a surprising lack of population-wide data on rates of neuraxial and general anesthesia for CD, therefore it is uncertain if, and to what degree, rates of general and neuraxial anesthesia have changed since 2002.
Missing data were also a concern. Approximately 10% of our original study cohort was excluded from our analysis due to missing data. Despite our study cohort comprising >50,000 women, the exclusion of patients with missing data may have introduced bias. Within the study cohort, low numbers of Asians (n=8) and Native Americans or Alaskans (n=8) who underwent general anesthesia; therefore, risk estimates for these subgroups could not be computed. We collapsed women who were Asian, Native American or Alaskans, or other race into one group, therefore we appreciate that the heterogeneity of women within this group limits inference of the risk estimate for Others. The accuracy of race/ethnicity documentation in the medical records could not be determined. Underreporting and variability can occur in the documentation of race/ethnicity data in medical records, data registries and other administrative datasets.36,37 Therefore misclassification bias is a potential concern when these data sources are used for secondary analyses.
Unmeasured anesthetic factors may also have biased our risk estimates. Epidural “top-up” is recommended for women with pre-existing labor epidural analgesia for providing surgical anesthesia for intrapartum CD.38 However, disparities in rates of labor epidural analgesia use 30 may have resulted in different rates of epidural top-up for CD among racial/ethnic groups. Unfortunately, we were unable to determine if epidural catheters were originally sited for labor analgesia or CD anesthesia. In addition, we were not able to deduce whether anesthetic-related complications, such as failed epidural top-up, failed spinal anesthesia or unanticipated perioperative breakthrough pain, were primary indications for general anesthesia. Despite these limitations, the risk estimates for general anesthesia among women who did not receive any neuraxial block prior to general anesthesia were similar to those observed in our primary analysis. These results suggest that the anesthesia-related complications did not influence the risk estimates across racial/ethnic groups. For women classified as having an emergent CD, the clinical determinants that influence the degree of urgency could not be ascertained, especially for women with a non-reassuring fetal trace. In light of controversies related to interpretation of intrapartum fetal heart tracings,39 we appreciate that a non-reassuring heart rate may cover a broad spectrum of fetal trace abnormalities. Nonetheless, in a prior examination of Cesarean Registry, the most common indication of emergency CD was a non-reassuring fetal trace, with 62% of women with a non-reassuring trace incurring a decision-to-delivery period of less than 30 minutes,21 implying a degree of urgency to deliver. In our study, African-Americans had the highest rates of non-assuring fetal trace as the main indication for CD compare to other races/ethnicities. This is consistent with prior research suggesting that African-Americans are at higher odds of CD for a non-reassuring trace compared to Caucasians.25 Further work is needed to determine whether these disparities are due to biological factors that may influence fetal tolerance of labor or whether provider biases may influence management of labor and interpretation of fetal traces and the subsequent decision to perform urgent operative delivery under general anesthesia.
Using clinical data from the MFMU Cesarean Registry, our findings suggest that racial/ethnic disparities may have existed for African American women regarding mode of anesthesia for CD at the turn of the millennium. Population-wide prospective studies are needed to validate these findings in current obstetric anesthesia practice, and to identify etiologic factors to explain why rates of general anesthesia may vary according to patients’ race/ethnicity.
Acknowledgments
Funding: This study was supported and funded internally by the Department of Anesthesia and the Department of Obstetrics and Gynecology, Stanford University School of Medicine. Dr. Butwick is also supported by an award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1K23HD070972).
We acknowledge the assistance of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Maternal Fetal Medicine Units Network, and the Protocol Subcommittee in making the database available.
Appendix A. Major Indications for Cesarean Delivery according to Race / Ethnicity
| Caucasian (n=21,113) | African-American (n=14,338) | Hispanic (n=12,990) | Others (n=2,533) | |
|---|---|---|---|---|
| Cephalo-pelvic disproportion | 1,344 (6.37%) | 501 (3.49%) | 422 (3.25%) | 146 (5.76%) |
| Failure to progress | 2,373 (11.24%) | 2,389 (16.66%) | 2,023 (15.57%) | 385 (15.2%) |
| Cord Prolapse | 154 (0.73%) | 139 (0.97%) | 69 (0.53%) | 17 (0.67%) |
| Non-reassuring trace | 2,153 (10.2%) | 2,818 (19.65%) | 1,088 (8.38%) | 310 (12.24%) |
| Malpresentation – no prior ECV | 2,546 (12.06%) | 1,224 (8.54%) | 993 (7.64%) | 242 (9.55%) |
| Malpresentation – prior unsuccessful ECV | 384 (1.82%) | 127 (0.89%) | 124 (0.95%) | 32 (1.26%) |
| Multiple gestation | 400 (1.89%) | 132 (0.92%) | 93 (0.72%) | 35 (1.38%) |
| Suspected macrosomia | 342 (1.62%) | 184 (1.28%) | 144 (1.11%) | 43 (1.7%) |
| Placental abruption | 244 (1.16%) | 149 (1.04%) | 54 (0.42%) | 18 (0.71%) |
| Previa with hemorrhage | 158 (0.75%) | 106 (0.74%) | 64 (0.49%) | 33 (1.3%) |
| Previa without hemorrhage | 153 (0.72%) | 96 (0.67%) | 57 (0.44%) | 17 (0.67%) |
| Preclampsia or hypertension | 672 (3.18%) | 512 (3.57%) | 339 (2.61%) | 83 (3.28%) |
| Prior myomectomy | 95 (0.45%) | 108 (0.75%) | 36 (0.28%) | 30 (1.18%) |
| Herpes | 148 (0.7%) | 183 (1.28%) | 37 (0.28%) | 11 (0.43%) |
| Prior classical, vertical, T-shape or J-shaped incision | 288 (1.36%) | 368 (2.57%) | 175 (1.35%) | 20 (0.79%) |
| Prior cesarean – unknown prior hysterotomy incision | 59 (0.28%) | 121 (0.84%) | 1,277 (9.83%) | 58 (2.29%) |
| Elective repeat | 8,538 (40.44%) | 4,447 (31.02%) | 5,458 (42.02%) | 929 (36.68%) |
| Other | 515 (2.44%) | 265 (1.85%) | 118 (0.91%) | 44 (1.74%) |
| Non-reassuring antepartum fetal testing | 340 (1.61%) | 219 (1.53%) | 317 (2.44%) | 44 (1.74%) |
| Failed induction | 207 (0.98%) | 250 (1.74%) | 102 (0.79%) | 36 (1.42%) |
ECV = external cephalic version
Data presented as n (%)
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
This report was previously presented, in part, at the Annual Meeting of the Society of Maternal-Fetal Medicine in New Orleans, LA (02/03/14-02/08/14) and at the 46th Annual Meeting of the Society for Obstetric Anesthesia and Perinatology in Toronto, Canada (05/14/14-05/18/14).
The contents of this report represent the views of the authors and do not represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network or the National Institutes of Health.
DISCLOSURES:
Name: Alexander J Butwick, MBBS, FRCA, MS
Contribution: This author helped design the study, conduct the study, analyse the data, and write the manuscript
Attestation: Alexander Butwick has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Yair J Blumenfeld, MD
Contribution: This author helped design the study, conduct the study, analyse the data, and write the manuscript
Attestation: Yair Blumenfeld has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Kathleen Brookfield, MD, PhD, MPH
Contribution: This author helped design the study, conduct the study, analyse the data, and write the manuscript
Attestation: Kathleen Brookfield has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Lorene M Nelson, PhD, MS
Contribution: This author helped design the study, conduct the study, analyse the data, and write the manuscript
Attestation: Lorene M Nelson has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Carolyn F Weiniger, MBCHB
Contribution: This author helped design the study, conduct the study, analyse the data, and write the manuscript
Attestation: Carolyn F Weiniger has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Contributor Information
Alexander J Butwick, Department of Anesthesia, Stanford University School of Medicine, Stanford, California.
Yair J Blumenfeld, Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California.
Kathleen F. Brookfield, Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California.
Lorene M Nelson, Department of Health Research Policy, Stanford University School of Medicine, Stanford, California.
Carolyn F Weiniger, Department of Anesthesia, Stanford University School of Medicine and Department of Anesthesiology and Critical Care Medicine, Stanford, California; Hadassah Hebrew University Medical Center, Jerusalem, Israel.
References
- 1.Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–63. doi: 10.1097/01.anes.0000264744.63275.10. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins JG, Khan MM. Anaesthesia for Caesarean section: a survey in a UK region from 1992 to 2002. Anaesthesia. 2003;58:1114–8. doi: 10.1046/j.1365-2044.2003.03446.x. [DOI] [PubMed] [Google Scholar]
- 3.Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–53. doi: 10.1097/00000542-200509000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins JL, Chang J, Palmer SK, Gibbs CP, Callaghan WM. Anesthesia-related maternal mortality in the United States: 1979–2002. Obstet Gynecol. 2011;117:69–74. doi: 10.1097/AOG.0b013e31820093a9. [DOI] [PubMed] [Google Scholar]
- 5.Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008*. Crit Care Med. 2013;41:1844–52. doi: 10.1097/CCM.0b013e31828a3e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuklina EV, Meikle SF, Jamieson DJ, Whiteman MK, Barfield WD, Hillis SD, Posner SF. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113:293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai PS, Hsu CS, Fan YC, Huang CJ. General anaesthesia is associated with increased risk of surgical site infection after Caesarean delivery compared with neuraxial anaesthesia: a population-based study. Br J Anaesth. 2011;107:757–61. doi: 10.1093/bja/aer262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Wang IT, Chen YH, Lin HC. Anesthetic management as a risk factor for postpartum hemorrhage after cesarean deliveries. Am J Obstet Gynecol. 2011;205:462.e1–7. doi: 10.1016/j.ajog.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet MP, Mignon A, Mazoit JX, Ozier Y, Marret E. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. 2010;14:894.e1–9. doi: 10.1016/j.ejpain.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Rosaeg OP, Lui AC, Cicutti NJ, Bragg PR, Crossan ML, Krepski B. Peri-operative multimodal pain therapy for caesarean section: analgesia and fitness for discharge. Can J Anaesth. 1997;44:803–9. doi: 10.1007/BF03013154. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SE, Woods WA. The role of epidural morphine in the postcesarean patient: efficacy and effects on bonding. Anesthesiology. 1983;58:500–4. doi: 10.1097/00000542-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BM, Aulakh JM, Barker JP, Reginald PW, Goroszeniuk T, Trojanowski A. Anaesthetic morbidity following caesarean section under epidural or general anaesthesia. Lancet. 1984;1:328–30. doi: 10.1016/s0140-6736(84)90371-4. [DOI] [PubMed] [Google Scholar]
- 13.Beckmann M, Calderbank S. Mode of anaesthetic for category 1 caesarean sections and neonatal outcomes. Aust N Z J Obstet Gynaecol. 2012;52:316–20. doi: 10.1111/j.1479-828X.2012.01457.x. [DOI] [PubMed] [Google Scholar]
- 14.Algert CS, Bowen JR, Giles WB, Knoblanche GE, Lain SJ, Roberts CL. Regional block versus general anaesthesia for caesarean section and neonatal outcomes: a population-based study. BMC Med. 2009;7:20. doi: 10.1186/1741-7015-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lie B, Juul J. Effect of epidural vs. general anesthesia on breastfeeding. Acta Obstet Gynecol Scand. 1988;67:207–9. doi: 10.3109/00016348809004203. [DOI] [PubMed] [Google Scholar]
- 17.Obst TE, Nauenberg E, Buck GM. Maternal health insurance coverage as a determinant of obstetrical anesthesia care. J Health Care Poor Underserved. 2001;12:177–91. doi: 10.1353/hpu.2010.0780. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton BE, Martin JA, Ventura SJ. National vital statistics reports. 3. Vol. 62. Hyattsville, MD: National Center for Health Statistics; 2013. [Accessed November 6, 2014.]. Births: Preliminary data for 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_09.pdf. [PubMed] [Google Scholar]
- 19.Landon MB, Hauth JC, Leveno KJ, Spong CY, Leindecker S, Varner MW, Moawad AH, Caritis SN, Harper M, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai B, Langer O, Thorp JM, Ramin SM, Mercer BM, Gabbe SG. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–9. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 20.Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: a retrospective analysis and 10-year update. Int J Obstet Anesth. 2011;20:10–6. doi: 10.1016/j.ijoa.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Bloom SL, Leveno KJ, Spong CY, Gilbert S, Hauth JC, Landon MB, Varner MW, Moawad AH, Caritis SN, Harper M, Wapner RJ, Sorokin Y, Miodovnik M, O’Sullivan MJ, Sibai BM, Langer O, Gabbe SG. Decision-to-incision times and maternal and infant outcomes. Obstet Gynecol. 2006;108:6–11. doi: 10.1097/01.AOG.0000224693.07785.14. [DOI] [PubMed] [Google Scholar]
- 22.Bryant A, Mhyre JM, Leffert LR, Hoban RA, Yakoob MY, Bateman BT. The association of maternal race and ethnicity and the risk of postpartum hemorrhage. Anesth Analg. 2012;115:1127–36. doi: 10.1213/ANE.0b013e3182691e62. [DOI] [PubMed] [Google Scholar]
- 23.Bryant AS, Washington S, Kuppermann M, Cheng YW, Caughey AB. Quality and equality in obstetric care: racial and ethnic differences in caesarean section delivery rates. Paediatr Perinat Epidemiol. 2009;23:454–62. doi: 10.1111/j.1365-3016.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 24.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 25.Washington S, Caughey AB, Cheng YW, Bryant AS. Racial and ethnic differences in indication for primary cesarean delivery at term: experience at one U.S. Institution Birth. 2012;39:128–34. doi: 10.1111/j.1523-536X.2012.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmonds JK, Yehezkel R, Liao X, Moore Simas TA. Racial and ethnic differences in primary, unscheduled cesarean deliveries among low-risk primiparous women at an academic medical center: a retrospective cohort study. BMC Pregnancy Childbirth. 2013;13:168. doi: 10.1186/1471-2393-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell EA, Zeitlin J, Hebert P, Balbierz A, Egorova N. Paradoxical trends and racial differences in obstetric quality and neonatal and maternal mortality. Obstet Gynecol. 2013;121:1201–8. doi: 10.1097/AOG.0b013e3182932238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smedley BD, Stith AY, Nelson AR, editors. Institute of Medicine. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, D.C: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 29.Toledo P, Caballero JA. Racial and ethnic disparities in obstetrics and obstetric anesthesia in the United States. Curr Anesthesiol Rep. 2013;3:292–9. [Google Scholar]
- 30.Glance LG, Wissler R, Glantz C, Osler TM, Mukamel DB, Dick AW. Racial differences in the use of epidural analgesia for labor. Anesthesiology. 2007;106:19–25. doi: 10.1097/00000542-200701000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–52. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 32.Henderson J, Gao H, Redshaw M. Experiencing maternity care: the care received and perceptions of women from different ethnic groups. BMC Pregnancy Childbirth. 2013;13:196. doi: 10.1186/1471-2393-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med. 2000;50:813–28. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 34.Glaser D, Hastings RH. An introduction to multilevel modeling for anesthesiologists. Anesth Analg. 2011;113:877–87. doi: 10.1213/ANE.0b013e3182198a01. [DOI] [PubMed] [Google Scholar]
- 35.Dexter F, Dexter EU, Ledolter J. Statistical grand rounds: Importance of appropriately modeling procedure and duration in logistic regression studies of perioperative morbidity and mortality. Anesth Analg. 2011;113:1197–201. doi: 10.1213/ANE.0b013e318229d450. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease C and Prevention. Use of Race and Ethnicity in Public Health Surveillance. MMWR Recomm Rep; Summary of the DC/ATSDR workshop; Atlanta, Georgia. March 1–2, 1993; 1993. pp. 1–16. [Google Scholar]
- 37.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93:1685–8. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahl V, Spreng UJ. Anaesthesia for urgent (grade 1) caesarean section. Curr Opin Anaesthesiol. 2009;22:352–6. doi: 10.1097/aco.0b013e3283294c37. [DOI] [PubMed] [Google Scholar]
- 39.Santo S, Ayres-de-Campos D. Human factors affecting the interpretation of fetal heart rate tracings: an update. Curr Opin Obstet Gynecol. 2012;24:84–8. doi: 10.1097/GCO.0b013e3283505b3c. [DOI] [PubMed] [Google Scholar]