Abstract
Amines with remote stereocenters (stereocenters that are three or more bonds away from the C–N bond) are important structural elements in many pharmaceutical agents and natural products. However, previously reported methods to prepare these compounds in an enantioselective manner are indirect and require multistep synthesis. Here we report a copper hydride-catalysed, enantioselective synthesis of γ- or δ-chiral amines from readily available allylic alcohols, esters, and ethers using a reductive relay hydroamination strategy (a net reductive process in which an amino group is installed at a site remote from the original C–C double bond). The protocol was suitable for substrates containing a wide range of functional groups and provided remote chiral amine products with high levels of regio- and enantioselectivity. Sequential amination of substrates containing several carbon-carbon double bonds could be achieved, demonstrating the high chemoselectivity of this process.
Graphical Abstract
Single operation transformations that enantioselectively install a stereogenic center while introducing a distal functional group are synthetically valuable but rare processes. Now, a copper-catalysed reductive relay hydroamination process that simultaneously creates a remote chiral center is described. The resulting γ- and δ-chiral amines are important structural elements in many pharmaceutical agents and natural products.
Aliphatic amines are featured prominently in therapeutic agents and clinically useful natural products and are often crucial for their biological activity1. Consequently, synthetic organic chemists have long pursued general, efficient, and selective methods for the introduction of this functional group. Moreover, because the biological activities of stereoisomers may differ, methods for the synthesis of amines in high stereochemical purity are particularly valuable2. Although approaches to chiral amines have been developed using a variety of strategies1, these generally only allow control over the stereocenters α or β to the newly introduced amine. The concomitant construction of well-defined stereocenters at sites remote from a newly introduced functional group remains a long-standing challenge for synthetic organic chemists3–5. Despite the presence of amines containing remote stereocenters in a considerable number of biologically active molecules (see Fig. 1b), there are no reported direct asymmetric transformations that allow for the preparation of this structural motif. Known approaches to install this subunit require time-consuming multistep sequences, severely slowing, for example, high throughput production of analogues for screening in medicinal chemistry.
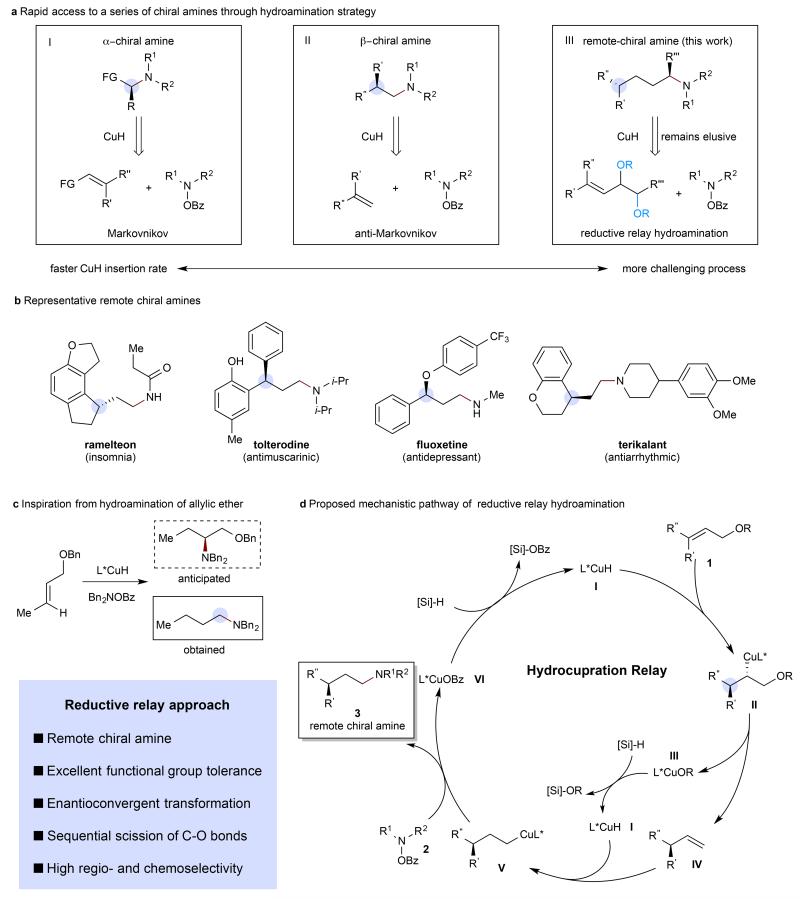
Figure 1. Design of a CuH-catalysed relay hydroamination reaction.
a, Rapid access to a series of chiral amines through a hydroamination strategy. b, Representative γ-chiral amines. c, Inspiration from hydroamination of allylic ether. d, Proposed mechanistic pathway of reductive relay hydroamination. Me, methyl; Bz, benzoyl; Bn, benzyl; FG, functional group.
In this work, we describe a CuH-catalysed6–8 reductive relay9-12 hydroamination13–15 strategy for the enantioselective synthesis of chiral amines bearing stereogenic centers γ- and δ- to the amino group (γ- and δ-chiral amines). Previously, we have reported16–19 (as have Hirano and Miura20,21) the CuH-catalysed syntheses of α-chiral amines by the Markovnikov hydroamination of functionalized olefins (Fig. 1a, box I) and β-chiral amines by the anti-Markovnikov hydroamination of 1,1-disubstituted aliphatic alkenes (Fig. 1a, box II)22. The idea for our approach to the synthesis of remote-chiral amines stemmed from the observation that the reaction of allylic ethers, under our previously reported hydroamination conditions16–19, gave the corresponding terminal amine product instead of the anticipated 1,2-amino alcohol (Fig. 1c). We reasoned that this product was formed via initial insertion to produce II followed by β-alkoxide elimination and subsequent anti-Markovnikov hydroamination of the intermediate terminal olefin. Based on this, we hypothesized that a trisubstituted allylic ether might likewise deliver the terminal amine product while concurrently generating a chiral center distal from the amine.
A more complete depiction of the presumptive mechanism for this reductive relay hydroamination is shown in Fig. 1d. Copper(I) hydride I reacts with allylic ether (or ester) 1 to generate alkylcopper intermediate II, which readily undergoes β-alkoxide elimination to afford transient enantioenriched terminal alkene IV and ligated copper(I) alkoxide III in a net allylic substitution process. Alkene IV then undergoes anti-Markovnikov hydrocupration to form terminal alkylcopper species V. Subsequent interception of V by the hydroxylamine O-carboxylate aminating reagent 2 furnishes the desired γ-chiral amine 3 and ligated copper(I) benzoate VI. Copper(I) alkoxide III and copper(I) benzoate VI could both undergo transmetalation with a stoichiometric hydrosilane reagent to regenerate copper(I) hydride I. Although the Cu(I)-catalysed enantioselective allylic substitution reaction is a well-precedented and versatile tool for the enantioselective introduction of carbon10,23,24, boron25–28, and silicon29 nucleophiles, the proposed enantioselective delivery of a hydride is an unprecedented process. Under our previously developed hydroamination conditions16–19, unactivated (e.g. nonstyrenyl) trisubstituted olefins were generally unreactive. We ascribe this to the disfavored nature of the insertion reaction of the highly substituted alkene. We believe that the combination of the β-alkoxide elimination step followed by formation of a Si-O bond renders the overall process thermodynamically favorable and allows the desired process to take place.
Herein we report the development, substrate scope, and applications of the reductive relay hydroamination reaction. The protocol developed was found to be a flexible and general process for the preparation of a variety of γ-chiral amines. Notably, this approach was applicable to easily obtainable achiral allylic esters with both aliphatic and aromatic substituents. Moreover, chiral amines containing minimally differentiated alkyl substituents at the γ-position could be prepared with excellent enantioselectivity. In addition, the delivery of hydride and elimination of alkoxide could proceed iteratively in the case of allylic epoxides and acetonides to afford the corresponding δ-chiral amines. Several applications of the reductive relay hydroamination reaction are also described.
Results and discussion
Reaction development and optimization
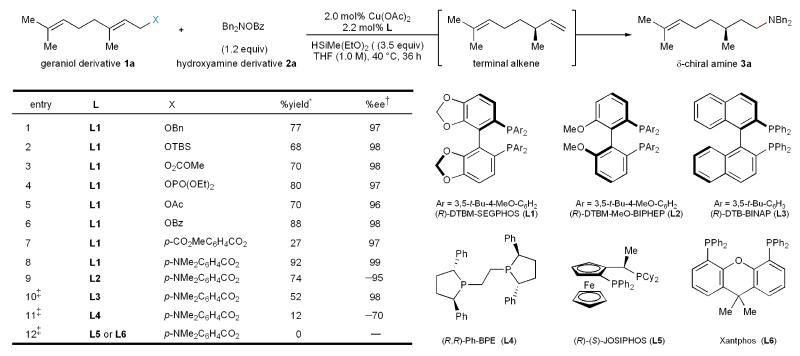
We initiated our study by investigating the reactivity of substrates derived from geraniol under our previously reported hydroamination conditions (Table 1). A variety of leaving groups, including alkoxy, silyloxy, carbonate, phosphate, and carboxylates, provided the desired product with high levels of enantioselectivity (entries 1–6). The stable and readily-prepared allylic benzoate was found to provide the desired product in high yield. Hence, we subsequently investigated substituted benzoates as leaving groups. Incorporation of an electron-withdrawing group on the benzoate to increase the nucleofugality of the leaving group was found instead to significantly reduce the yield (entry 7). In contrast, the use of electron-donating substituents on the benzoate, such as a 4-dimethylamino group (entry 8), produced near quantitative yield of the γ-chiral amine (S)-3a. An evaluation of ligands revealed DTBM-SEGPHOS to give superior results compared to all others tested (entry 8 vs. entries 9–12).
Table 1. Optimization of reductive relay hydroamination.
Conditions: 1a (0.20 mmol), 2a (0.24 mmol), HSiMe(OEt)2 (0.70 mmol), Cu(OAc)2 (2.0 mol %), ligand (2.2 mol %), THF (1.0 M), 40 °C, 36 h.
Yield refers to isolated yield of purified product and is an average of two runs (0.20 mmol scale).
The e.e. was determined by HPLC analysis using chiral stationary phases.
10 mol% Cu(OAc)2, 11 mol% ligand was used. Me, methyl; Bz, benzoyl; Ph, phenyl; Bn, benzyl; TBS, tert-butyldimethylsilyl; Ac, acetyl. (See supplementary information for experimental details.)
Substrate scope
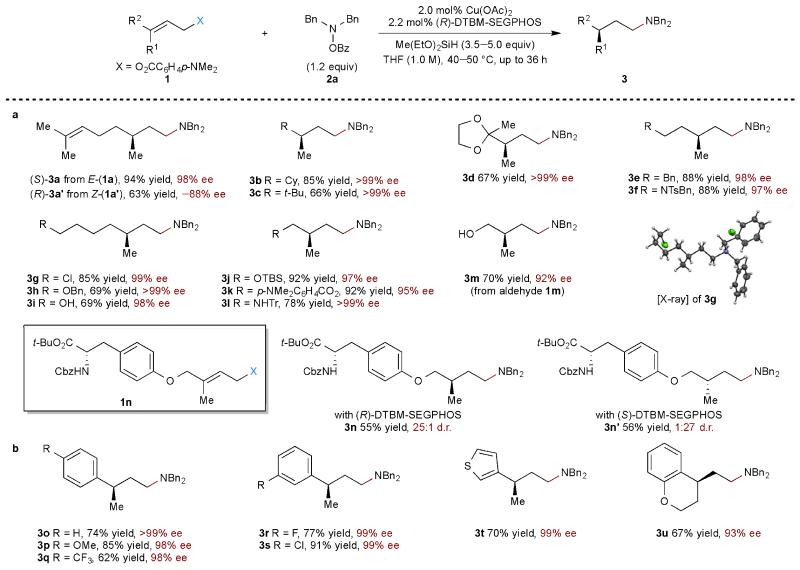
Under the optimized conditions, the scope of allylic benzoates that could be transformed was investigated (Table 2). A variety of substrates were converted into the corresponding chiral amines with high enantioselectivity and in moderate to excellent yields. A variety of 3,3-dialkyl substituted allylic 4-(dimethylamino)benzoates were first investigated (Table 2a). When the isomeric nerol-derived 4-(dimethylamino)benzoate containing a (Z)-configured allylic double bond was exposed to the optimized conditions, the opposite enantiomer (R)-3a’ was obtained with slightly lower yield and enantioselectivity (3a vs. 3a’). Bulky groups at the 3-position of the allylic system were tolerated and provided chiral amine products in moderate to good yields and exceptionally high enantioselectivity (3b–d). A variety of functional groups were readily accommodated, including a ketal (3d), an aryl group (3e), a sulfonamide (3f), an alkyl chloride (3g), ethers (3h, 3j), an ester (3k), a free alcohol (3i) and an unprotected secondary amine (3l). When an aldehyde-containing substrate was employed, not surprisingly, reduction to the alcohol was observed to produce the amino alcohol product (3m)30,31. Protected amino acid-containing substrate 1n could be transformed to γ-chiral amine (3n, 3n’) without carbonyl reduction or epimerization, reflecting the mildness of the reaction conditions.
Table 2. Substrate scope of allylic esters.
a, Substrates bearing 3,3-dialkyl substituted allylic ester. b, Substrates bearing 3,3-alkyl,aryl substituted allylic ester. Under each product are given yield in percent, and either enantiomeric excess (e.e.) or diastereomer ratio (d.r.). Yield refers to isolated yield of purified product (1 mmol scale, average of two runs). The e.e. was determined by HPLC analysis using chiral stationary phases. Me, methyl; Ph, phenyl; Bn, benzyl; Ts, tosyl; Tr, triphenylmethyl; Cbz, carboxybenzyl; TBS, tert-butyldimethylsilyl. (See supplementary information for experimental details.)
Additionally, 3-aryl-substituted allylic benzoates could also undergo reductive relay hydroamination (Table 2b). Substrates bearing electron-rich (3p, 3u) and electron-poor (3q−s) aryl substituents were tolerated. A 3-thienyl substituted substrate (3t), as well as a chromane-derived bicyclic substrate (3u), were also compatible with these conditions. Likewise, a silyl-substituted allylic benzoate (Table 3a, 1v) was converted into the γ-chiral silylamine (3v). In addition to 3,3-disubstituted allylic benzoates, this protocol was also applicable to racemic 1-aryl-3-alkyl-substituted allylic benzoate (Table 3a, 1w), which reacted in an enantioconvergent manner to provide the α-branched chiral amine product (3w).
Table 3. Extension of scope to other substrate classes and reaction on large scale.
a. Extension of reductive relay hydroamination to the synthesis of a chiral γ-silylamine, enantioconvergent transformation of an allylic ester, and synthesis of δ-chiral amines. b. Reductive relay hydroamination of allylic alcohols. c. A 10 mmol-scale reductive relay hydroamination with 0.5% catalyst loading. Yield, e.e., and d.r. are as defined in Table 2 legend. (See supplementary information for experimental details.)
We reasoned that substrates containing an appropriate duo of vicinal allylic and homoallylic substituents would undergo sequential insertion/elimination sequences before undergoing hydroamination. Such a cascade process would lead to the formation of δ-chiral amines. To test this idea, allylic epoxide (Table 3a, 1x) and allylic acetonide (Table 3a, 1y) substrates were subjected to our previously developed reaction conditions. As predicted, racemic epoxide 1x reacted in an enantioconvergent manner to provide the δ-chiral amine 3x with high enantioselectivity. Likewise, enantioenriched acetonide 1y also underwent the cascade hydroamination process to afford either enantiomer (3y, 3y’) of the δ-chiral amine product under catalyst control. The successful extension of the reductive relay hydroamination protocol to this complex cascade serves to illustrate the flexibility and generality of this strategy.
In some cases, the free allylic alcohols could be used directly in the current catalytic system (Table 3b). By adding an extra equivalent of silane, the alcohol is first converted into silyl ether. Subsequently, in situ insertion, β-siloxy elimination and hydroamination provides the desired product. The use of the free alcohol did not affect the enantioselectivity of the process, although the yields obtained in this extended cascade were somewhat diminished relative to the use of the corresponding (4-dimethylamino)benzoate esters (Table 2a, 3a, 3e, 3g).
To demonstrate the scalability of this process, we conducted the reaction on a 10-mmol scale. Under slightly modified reaction conditions, a catalyst loading of 0.5 mol% proved sufficient for a reaction on this scale (Table 3c). As first observed by Lipshutz32, the inclusion of triphenylphosphine as an additive led to improved stability of the active catalyst, allowing higher catalyst turnover numbers to be attained without any significant effect on the enantioselectivity.
The utility of this catalytic system was further demonstrated through the use of a number of hydroxylamine esters as aminating reagents (Table 4). Despite the presence of a stereocenters adjacent to the nitrogen atom of the electrophilic aminating reagent, the hydroamination reaction proceeded in a completely catalyst-controlled manner (4a, 4a’) to form a minimally differentiated stereocenter (methyl vs ethyl) with excellent selectivity. In addition, acyclic (4b), cyclic (4d), and sterically hindered (4c) hydroxylamine esters could be utilized, as could one containing a carbamate protecting group (4d). Furthermore, substrates containing heterocycles, such as pyridine (4e), pyrimidine (4f), benzothiadiazole (4g), and piperazine (4f, 4g) were handled without event. Finally, duloxetine, a drug used to treat depression and generalized anxiety disorder, could be functionalized with complete control of diastereoselectivity (4h, 4h’), demonstrating the potential utility of this protocol in the late-stage functionalization of complex molecules.
Table 4. Scope of hydroxylamine electrophiles.
Yield, e.e., and d.r. are as defined in Table 2 legend. (See supplementary information for experimental details.)
Applications of chemoselectivity
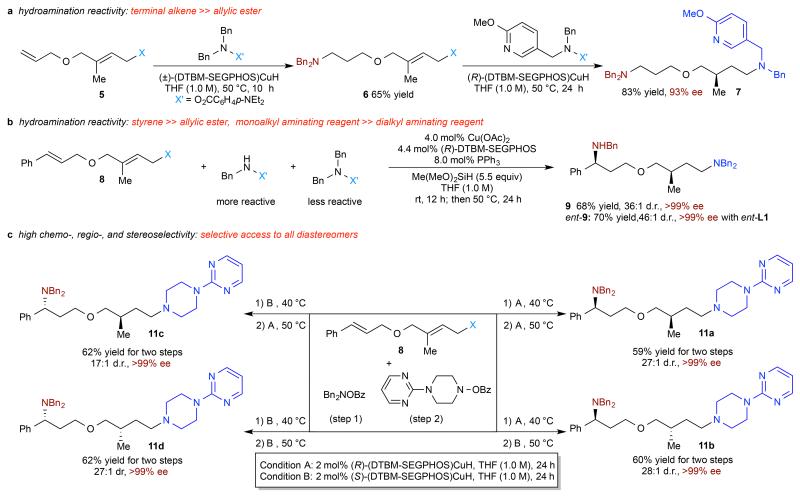
We investigated the reactivity of substrates containing multiple C–C double bonds and found that the hydroamination protocols described here and previously16,22 exhibited excellent chemoselectivity when different types of double bonds were present. In general, styrenyl and terminal double bonds both react in preference to trisubstituted allylic esters, and this difference in reactivity was exploited for sequential chemoselective hydroamination. For example, sequential hydroamination of diolefin 5 containing a trisubstituted allylic ester and terminal olefin with two different aminating reagents resulted in the formation of diamine 7 with excellent chemo- and enantioselectivity (Fig. 2a). Furthermore, by simultaneously taking advantage of the difference in reactivity between mono- and dialkyl aminating reagents19, as well as between styrenyl double bonds and trisubstituted allylic esters, a three component coupling of two aminating reagents with diolefin 8 could be achieved with good chemo-, diastereo-, and enantioselectivity (Fig. 2b). Importantly, this strategy can also be employed to form all four stereoisomers of a diamine product in uniformly high stereoselectivity depending on the enantiomer of chiral ligand used in each step (17:1 to 28:1 dr, >99% ee) (Fig. 2c, 11a-d).
Figure 2. Synthetic applications of CuH-catalysed reductive relay hydroamination.
a, Sequential hydroamination of an allylic ester substrate bearing a terminal olefin. b, Three-component hydroamination to install secondary and tertiary amines sequentially. c, High chemo-, regio-, and stereoselective hydroamination of an allylic ester substrate bearing a styrenyl olefin, stereodivergent access to all four diastereomers. Yield and e.e. are as defined in Table 2 legend. (See supplementary information for experimental details.)
Conclusions
In summary, we have developed a CuH-catalysed reductive relay process to access γ- and δ-chiral amines. This method allows for the installation of a stereocenter and a distal amino group in a single operation under mild conditions. Excellent enantio-, regio-, and chemoselectivity were observed for a broad range of substrates with high functional group tolerance. Furthermore, this system was found to be applicable to the late-stage modification of a pharmaceutical agent and was suitable for large-scale synthesis. Lastly, we also demonstrated that the CuH-catalysed protocol could be applied to the chemoselective sequential amination of substrates containing more than one olefin. The expansion of this relay strategy to other areas including drug and natural product synthesis is currently underway and will be reported in due course.
Methods
General procedure for reductive relay hydroamination
To an oven-dried 4 mL screw-cap vial equipped with a magnetic stir bar was added Cu(OAc)2 (2.0–5.0 mol%) and (R)-DTBM-SEGPHOS (2.2–5.5 mol%). The tube was sealed with a teflon-lined screw cap, evacuated, and backfilled with argon (this process was repeated a total of three times) by piercing with a needle attached to a Schlenk line. Anhydrous THF (1.0 mL) was added by syringe, and the mixture was stirred for 10 min at room temperature. At this time diethoxymethylsilane (560–720 μL, 3.5–4.5 mmol, 3.5–4.5 equiv) was added by syringe and stirring was continued for another 5 min. Into a separate oven-dried medium-sized screw-cap test tube was added γ-disubstituted allylic benzoate (1.0 mmol, 1.0 equiv) and O-benzoyl-N,N-dibenzylhydroxylamine (381 mg, 1.2 mmol, 1.2 equiv). The tube was sealed with a teflon-lined screw cap, evacuated, and backfilled with argon (this process was repeated a total of three times). The catalyst solution was then transferred via syringe to the reaction tube containing the substrates, and the reaction mixture was stirred at 40–50 °C for up to 36 h. After the reaction was complete, the reaction mixture was allowed to cool to room temperature and was directly filtered through a short pad of silica gel (using ethyl acetate in hexanes) to give the crude product. Dodecane (100 μL) was added as an internal standard for GC analysis. 1,1,2,2-Tetrachloroethane (84 mg, 0.50 mmol) was added as internal standard for 1H NMR analysis of the crude material. The product was purified by chromatography on silica gel or by acid-base extraction as indicated for each substrate. The enantiomeric excesses (% ee) were determined by HPLC analysis using chiral stationary phases as described in the Supplementary Information.
The X-ray crystallographic coordinate for 3g is deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number CCDC 1413400. These data can be obtained free of charge (http://www.ccdc.cam.ac.uk/data_request/cif).
Supplementary Material
Acknowledgements
This paper is dedicated to Professor Doctor Paul Knochel on the occasion of his 60th birthday. Research reported in this publication was supported by the National Institutes of Health under award number GM58160. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Peter Müller (MIT) for X-ray analysis of 3g and Dr. Yi-Ming Wang, Dr. Michael T. Pirnot, and Dr. Christine Nguyen for their advice on the preparation of this manuscript.
Footnotes
Author contributions
S.Z. and S.L.B designed the project. S.Z., N.N., and S.L.B. co-wrote the manuscript, analyzed the data, discussed the results and commented on the manuscript. S.Z. and N.N. performed the experiments.
Supplementary information is available in the online version of the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Nugent TC. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley-VCH; Weinheim, Germany: 2010. [Google Scholar]
- 2.Nguyen LA, He H, Pham-Huy C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- 3.Werner EW, Mei T-S, Burckle AJ, Sigman MS. Enantioselective heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei T-S, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocenters. Nature. 2014;508:340–344. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science. 2013;339:1593–1596. doi: 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch C, Krause N, Lipshutz BH. CuH-catalyzed reactions. Chem. Rev. 2008;108:2916–2927. doi: 10.1021/cr0684321. [DOI] [PubMed] [Google Scholar]
- 7.Lipshutz BH. Rediscovering organocopper chemistry through copper hydride. It’s all about the ligand. Synlett. 2009:509–524. [Google Scholar]
- 8.Alexakis A, Krause N, Woodward S. Copper-catalyzed asymmetric synthesis. Wiley; Weinheim, Germany: 2014. [Google Scholar]
- 9.Ketcham JM, Shin I, Montgomery TP, Krische MJ. Catalytic enantioselective C−H functionalization of alcohols by redox-triggered carbonyl addition: Borrowing hydrogen, returning carbon. Angew. Chem. Int. Ed. 2014;53:9142–9150. doi: 10.1002/anie.201403873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui J, et al. Practical olefin hydroamination with nitroarenes. Science. 2015;348:886–891. doi: 10.1126/science.aab0245. [DOI] [PubMed] [Google Scholar]
- 11.Sahli Z, Sundararaju B, Achard M, Bruneau C. Ruthenium-catalyzed reductive amination of allylic alcohols. Org. Lett. 2011;13:3964–3967. doi: 10.1021/ol201485e. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Achard M, Bruneau C, Sortais J-B, Darcel C. Iron-catalysed tandem isomerisation/hydrosilylation reaction of allylic alcohols with amines. RSC Adv. 2014;4:25892–25897. [Google Scholar]
- 13.Müller TE, Hultzch KC, Yus M, Foubelo F, Tada M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]
- 14.Hesp KD. Copper-catalyzed regio- and enantioselective hydroamination of alkenes with hydroxylamines. Angew. Chem. Int. Ed. 2014;53:2034–2036. doi: 10.1002/anie.201309262. [DOI] [PubMed] [Google Scholar]
- 15.Huang L, Arndt M, Gooßen K, Heydt H, Gooßen LJ. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]
- 16.Zhu S, Niljianskul N, Buchwald SL. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 2013;135:15746–15749. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niljianskul N, Zhu S, Buchwald SL. Enantioselective synthesis of α-aminosilanes by copper-catalyzed hydroamination of vinylsilanes. Angew. Chem. Int. Ed. 2015;54:1638–1641. doi: 10.1002/anie.201410326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S-L, Buchwald SL. Copper-catalysed Selective hydroamination reactions of alkynes. Nature Chem. 2015;7:38–44. doi: 10.1038/nchem.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu D, Buchwald SL. The design of modified amine transfer reagents allows the synthesis of α-chiral secondary amines via CuH-catalyzed hydroamination. J. Am. Chem. Soc. 2015;137:9716–9721. doi: 10.1021/jacs.5b05446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miki Y, Hirano K, Satoh T, Miura M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Ed. 2013;52:10830–10834. doi: 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]
- 21.Miki Y, Hirano K, Satoh T, Miura M. Org. Lett. Vol. 16. Copper-catalyzed enantioselective formal hydroamination of oxa- and azabicyclic alkenes with hydrosilanes and hydroxylamines; 2014. pp. 1498–1501. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S, Buchwald SL. Enantioselective CuH-catalyzed anti-Markovnikov hydroamination of 1,1-disubstituted alkenes. J. Am. Chem. Soc. 2014;136:15913–15916. doi: 10.1021/ja509786v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yorimitsu H, Oshima K. Recent progress in asymmetric allylic substitutions aatalyzed by chiral copper complexes. Angew. Chem. Int. Ed. 2005;44:4435–4439. doi: 10.1002/anie.200500653. [DOI] [PubMed] [Google Scholar]
- 24.Alexakis A, Bäckvall JE, Krause N, Pàmies O, Diéguez M. Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions. Chem. Rev. 2008;108:2796–2823. doi: 10.1021/cr0683515. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Ito S, Sasaki Y, Matsuura K, Sawamura M. Copper-catalyzed enantioselective substitution of allylic carbonates with diboron: an efficient route to optically active α-chiral allylboronates. J. Am. Chem. Soc. 2007;129:14856–14857. doi: 10.1021/ja076634o. [DOI] [PubMed] [Google Scholar]
- 26.Park JK, Lackey HH, Ondrusek BA, McQuade DT. Stereoconvergent synthesis of chiral allylboronates from an E/Z mixture of allylic aryl ethers using a 6-NHC-Cu(I) catalyst. J. Am. Chem. Soc. 2011;133:2410–2413. doi: 10.1021/ja1112518. [DOI] [PubMed] [Google Scholar]
- 27.Guzman-Martinez A, Hoveyda AH. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary β-substituted stereogenic carbon by NHC-Cu-catalyzed substitution reactions. J. Am. Chem. Soc. 2010;132:10634–10637. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito H, Kunii S, Sawamura M. Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization. Nature Chem. 2010;2:972–976. doi: 10.1038/nchem.801. [DOI] [PubMed] [Google Scholar]
- 29.Delvos LB, Vyas DJ, Oestreich M. Asymmetric synthesis of α-chiral allylic silanes by enantioconvergent γ-selective copper(I)-catalyzed allylic silylation. Angew. Chem. Int. Ed. 2013;52:4650–4653. doi: 10.1002/anie.201300648. [DOI] [PubMed] [Google Scholar]
- 30.Brestensky DM, Stryker JM. Regioselective conjugate reduction and reductive silylation of α,β-unsaturated. Tetrahedron Lett. 1989;30:5677–5680. [Google Scholar]
- 31.Lipshutz BH, Chrisman W, Noson K. Hydrosilylation of aldehydes and ketones catalyzed by [Ph3P(CuH)]6. J. Organomet. Chem. 2001;624:367–371. [Google Scholar]
- 32.Lipshutz BH, Noson K, Chrisman W, Lower A. Asymmetric hydrosilylation of aryl ketones catalyzed by copper hydride complexed by nonracemic biphenyl bis-phosphine ligands. J. Am. Chem. Soc. 2003;125:8779–8782. doi: 10.1021/ja021391f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.