Abstract
Diabetes mellitus (DM), an endocrine disorder, will be one of the leading causes of death world-wide in about two decades. Cellular injuries and disorders of energy metabolism are two key factors in the pathogenesis of diabetes, which also become the important causes for the process of diabetic complications. AMPK is a key enzyme in maintaining metabolic homeostasis and has been implicated in the activation of autophagy in distinct tissues. An increasing number of researchers have confirmed that autophagy is a potential factor to affect or induce diabetes and its complications nowadays, which could remove cytotoxic proteins and dysfunctional organelles. This review will summarize the regulation of autophagy and AMPK in diabetes and its complications, and explore how AMPK stimulates autophagy in different diabetic syndromes. A deeper understanding of the regulation and activity of AMPK in autophagy would enhance its development as a promising therapeutic target for diabetes treatment.
Abbreviations: ACC, carboxylase; AdipoR, adiponectin receptors; ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, 5′-monophosphate-activated protein kinase; ATP, adenosine triphosphate; CaMKK, Ca2+ calmodulin-dependent protein kinase kinase; DEPTOR, DEP domain-containing mTOR-interacting protein; DM, Diabetes mellitus; DN, Diabetic nephropathy; ERK, extracellular signal-regulated kinase; FoxO, forkhead box class O; GFRs, glomerular filtration rates; IKK, IκB kinase; JLDG, Jinlida granule; JNK, janus kinase; LC3, light chain 3; LKB1, liver kinase B1; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin (mTOR) complex 1; PKC, protein kinase C; PRAS40, proline-rich Akt substrate 40 kDa; RAPTOR, regulator associated protein of mTOR; SOGA, suppressor of glucose form autophagy; SQSTM1, sequestosome 1; STZ, streptozotocin; TSC, tuberous sclerosis complex; ULK1, Unc-51-like kinase 1; VPS34, vacuolar protein-sorting 34
KEY WORDS: Diabetes, Autophagy, AMP-activated protein kinases, Diabetic complications
Graphical abstract
This review summarizes the regulation of autophagy and AMPK in diabetes and its complications, and explores how AMPK stimulates autophagy in different diabetic syndromes. A deeper understanding of the regulation and activity of AMPK in autophagy would enhance its development as a promising therapeutic target for diabetes treatment.
1. AMP-activated protein kinases (AMPK) and autophagy
1.1. Introduction of AMPK
The heterotrimeric protein, AMPK, plays a pivotal regulatory role in cellular energy homeostasis and metabolism. This serine/threonine kinase is formed by a catalytic subunit α and two regulatory subunits β and γ1, 2, 3. Each subunit has its own isoforms and binds different ligands. As an example, for decreasing the activity of AMPK, adenosine triphosphate (ATP) could be bound with in an opposite manner, while adenosine diphosphate (ADP) could bind with the γ subunit for protecting AMPK via Thr-172 dephosphorylation1, 4. These AMPK subunit isoforms could combine or interact with each other, which might form about 12 heterotrimers in different tissues5, 6. Furthermore, activated AMPK, as an energy sensor, could block cellular proliferation and synthesis of cholesterol or fatty acid7, but stimulate glucose uptake and autophagy8, 9. Due to its significant sensor role in modulating pathways in catabolic processes, AMPK inhibition is correlated with endocrine metabolic conditions, including diabetes and its induced complications. Signaling pathways affected by inhibition AMPK have been studied extensively in diabetes and its complications, such as autophagy, mitochondrial biogenesis and energy regulation. In the present review, we will focus on the important role of AMPK and autophagy in diabetes and its complications.
1.2. Introduction of autophagy
Autophagy, a kind of intracellular recycling system, is the basic catabolism activated by lysosomes or autolysosomes, including degradation of dysfunctional or unnecessary cellular organelles and nutrients2, 10. It has been reported that autophagy plays an important role in the regulation of catabolic nutrients; when nutrients are abundant, insulin secretion would be stimulated. But when nutrients are lacking autophagy might be evoked at a cellular level11. Autophagy could be induced by oxidative stress or any other cellular injuries and loss. There are three types of autophagy, involving macroautophagy, microautophagy and chaperone-mediated autophagy. The macroautophagy involving fusion with lysosomes is the common autophagy, which is also what we will review below.
Autophagy could be regulated by several factors and protein sensors, including amino acids or insulin. Autophagy induced by another condition of nutrient starvation like glucose deprivation is less common12, but they are all associated with signaling pathways. Generally, besides the positive effects supported by few researches, negative effect, inhibited by mammalian target of rapamycin (mTOR) complex 1 (mTORC1) seems to be more related to glucose deprivation of autophagy13. It should be clarified that mTORC1 is a signaling complex, which involves mTOR, RAPTOR (regulator associated protein of mTOR), PRAS40 (proline-rich Akt substrate 40 kDa), DEPTOR (DEP domain-containing mTOR-interacting protein) and LST8 homolog (mTOR associated protein)14. The upstream regulatory factors respond to ATP depletion (AMPK, P53), reactive oxygen species [janus kinase (JNK), extracellular signal-regulated kinase (ERK)] and directly induction of glucose deprivation [forkhead box class O (FoxO), IκB kinase (IKK)]. Furthermore, the direct up-regulatory kinase could be serine/threonine protein kinase Unc-51-like kinase 1 (ULK1) and Beclin1-BCL2 complex. For instance, ULK1 could be phosphorylated at Ser317 and Ser777 to induce autophagy. And Beclin1 could also be phosphorylated to block the forming of the Beclin1-BCL2 complex, which would inhibit autophagy. Likewise, microtubule-associated protein 1 A/1B-light chain 3 (LC3) is an autophagosomal marker and one of the important measurement indicators, which could reflect the status of autophagic activity induced by starvation5. Thus, AMPK and autophagy will be summarized with regard to the mechanism of upstream and downstream modulation in classic pathways.
1.3. The mechanism of AMPK-regulated autophagy
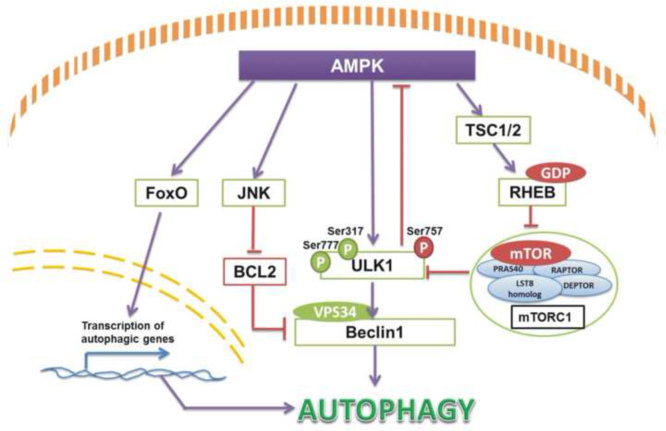
AMPK is a major activator of autophagy in the catabolic process of oxidative stress and energy starvation. Their modulation has been concluded in a few classic pathways. In previous studies autophagy was just thought to play a dual role in either avoiding cell death in some conditions or inducing autophagic death15. After a few years, it has been linked with protein kinase-AMPK activation directly and indirectly. AMPK could activate autophagy by directly activating ULK1. Specifically, AMPK and mTORC1 are both initiators of autophagy via ULK1 activation9. AMPK could active ULK1 by phosphorylation not only at Ser317, Ser777, but also at Ser467, Ser555, Ser637 and Thr5748, while ULK1 is inhibited by mTORC1 phosphorylation at Ser317 and Ser777. To some extent, AMPK activity could be suppressed by mTORC1 via increased phosphorylation of ULK1 at Ser7579, 14. AMPK could activate autophagy indirectly by tuberous sclerosis complex 2 (TSC2) phosphorylation and subsequent mTORC1 inhibition16 (Fig. 1). Moreover, activation of AMPK also could stimulate JNK1 following dissociation of Beclin-1 from BCL-2 to form vacuolar protein-sorting 34 (VPS34) complex17 and turn on autophagy. Other studies also reported that AMPK modulates FoxO transcription factor expression, which leads to expression of autophagy-associated genes14. Thus, AMPK is a key junction as both an upstream energy sensor and a downstream autophagy activator, especially in endocrine disordered diseases such as diabetes.
Figure 1.
Autophagy stimulated by AMPK activation contains four normal pathways found till now. When energy is insufficient, AMPK could phosphorylate JNK, ULK1, TSC1/2 and FoxO transcription factor to activate autophagy in many tissues generally. And the upstream pathways in autophagy activity regulation also contain BCL2 inhibition, Beclin1 stimulation and mTORC1 blocking.
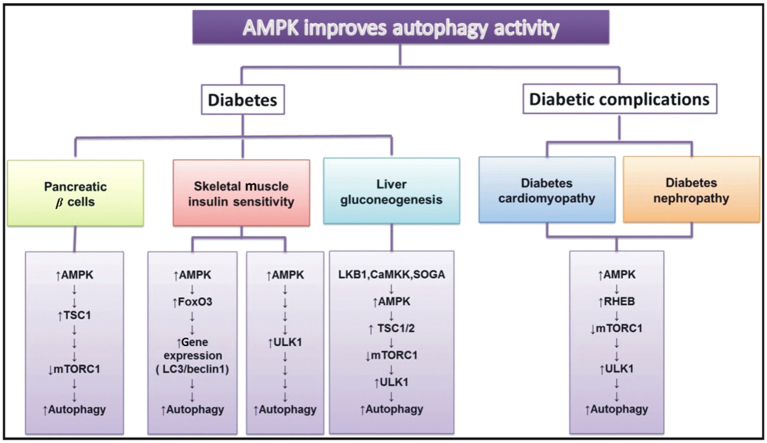
2. The regulation of autophagy by AMPK in diabetes and its complications
2.1. AMPK and autophagy in pancreatic β cells
The pancreatic β cell in pancreatic islets is a significant glucose regulatory cell regard to insulin secretion. Pancreatic islets are also correlated with lipid metabolism. When disordered pancreatic islet or dysfunctional pancreatic β cells occur, it is always related to abnormal glucose and lipid levels18. Additionally, Dong and Czaja or Singh et al.19, 20 have reported that intracellular lipid droplets might be one substrate of autophagy. In the process of lipid overloading, autophagy could metabolize lipid by moving the unnecessary part. Because activated autophagy might not only alleviate endoplasmic reticulum stress, but also restart the process of lipophagy for pancreatic islets protection21. Cellular level studies in pancreatic β cells also show that autophagy plays a pivotal role. The relative pathway might contain LC3, PKC (protein kinase C), JNK as well as others22, 23, 24. Moreover, in normal conditions, metformin could suppress Min6 β cell proliferation and promote cell apoptosis through an AMPK independent and autophagic mechanism. However, as an effective anti-diabetic drug, metformin could protect pancreatic β cells in particular from the apoptosis induced by palmitic acid22. Another Chinese traditional medicine Jinlida granule (JLDG) has also been shown to reduce lipid accumulation in pancreatic β cells and lead to autophagy via protection of AMPK activation. The study demonstrated that JLDG might not only inhibit lipogenesis by downregulation of acetyl coenzyme A carboxylase (ACC) and so on, but also reduce the expression of mTOR and stimulate expression of tuberous sclerosis complex 1 (TSC1) and LC3 to induce the autophagy18. Except the protective mechanism, Chen et al.23 have argued that as an adaptive response, β cell autophagy could inhibit increased insulin resistance. Take the diabetic fatty mouse model as an example: it has been found that autophagy was necessary to maintain the normal architecture of islets and intracellular insulin metabolism by increasing the insulin degradation rate in β bells of the Rab3A−/− null mouse. Altered autophagy might also induce loss of pancreatic β cell mass in diabetes23, 25. Nonetheless, decreasing beclin1 expression could protect Min6 cells to some extent. It has been reported that a certain degree of autophagy is essential for Min6 cell survival26. So the mechanism and regulation is between autophagy and autophagic cell death. But whether autophagy could boost cell death or regulate cell survival of pancreatic β cells and diabetes therapy need to be elucidated. The evidence nonetheless indicates that autophagy could play an essential protective role in glucose starvation via AMPK stimulation.
2.2. AMPK and autophagy in muscle insulin sensitivity
Skeletal muscle insulin sensitivity can be improved by physical exercise, which reduces the risk of diabetes and cardiovascular disease27. Intensity and duration of exercise might determine the status of AMPK activation, which has a strong relationship with the increasing status of insulin sensitivity24. Likewise, it has been found that autophagy could modulate glucose homeostasis in skeletal muscle and increase insulin sensitivity. So there is a link between AMPK and autophagy in exercise and insulin sensitivity in skeletal muscle. Firstly, one study has shown that mutation of AMPK sucrose non-fermenting 1 (SNF1) gene might induced defective energy metabolism in the yeast and ATG1 and ATG13, which are autophagy related protein kinases, are also involved in this regulation18, 27. Liu et al.10 have also reported that muscle-specific AMPKα2-deficient mice, which lost AMPKα2, could impair activation of autophagy in muscle exercise, suggesting that stimulation of AMPK is essential for muscle autophagy induced by physical exercise. The possible pathway might include the Beclin1-BCL2 complex. Furthermore, BCL2 mutant mice research has proved that autophagy is a critical regulator in metabolism related exercise, and this is correlated with insulin resistance. For a detailed pathway, AMPK phosphorylated FoxO3 to induce the expression of LC3, beclin1 in skeletal muscle, which is associated with autophagy28. AMPK could interact with ULK1 directly to stimulate autophagy as described above10. Additionally, dihydromyricetin, which is a natural flavonoid, has been shown to improved skeletal muscle insulin resistance by stimulation of autophagy through AMPK-beclin1 or LC3 pathways. And the AMPK inhibitor compound C has been used to prove that the autophagy was induced by AMPK, because the blocking of AMPK suppressed the activation of autophagy and the improvement in skeletal muscle insulin resistance29. Therefore, several study results have demonstrated that AMPK activates autophagy to increase insulin sensitivity in skeletal muscle, and AMPK is a necessary sensor in these modulation processes.
2.3. AMPK and autophagy in liver gluconeogenesis
Liver, another glucose metabolic organ, can play a role in suppressing hyperglycemia. There are two mechanisms to decrease glucose production in liver to influence metabolism and diabetes. One is to activate glycolysis or increase the synthesis of glycogen, protein and lipid, and another is to inhibit glycogenolysis, gluconeogenesis, proteolysis or lipolysis30. AMPK and autophagy can participate in glucose regulation of liver. On one hand, AMPK could suppress ACC to be a rate-limiting step for lipogenesis by liver via accelerating oxidation of long chain fat acids and inhibiting insulin-mediated lipid synthesis. On the other hand, activation of AMPK could lead to autophagy improvement, which would be decreased in liver hyperglycemia30, 31. So autophagy might be related to lipid synthesis in liver. There are two possible ways to affect lipid synthesis with regard to AMPK, including liver kinase B1 (LKB1) and Ca2+ calmodulin-dependent protein kinase kinase (CaMKK). To be specific, LKB1 might stimulate AMPK to translocate to cytosol. In mouse hepatocytes, adaptor protein containing pleckstrin homology domain, phosphotyrosine, interaction, pH domain and leucine zipper motif 1 (APPL1) could combine with adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) at N-terminal domains, which induced LKB1 cytosolic localization for AMPK activation32, 33. Moreover, AdipoR1 and AdipoR2 binding could promote APPL1 homodimerization34. For the CaMKK pathway, it binds the similar significant sequence and shares structural homology with LKB1 to be the upregulator for AMPK, which could be activated by adiponectin trimers30, 35, 36, 37. In addition, adiponectin could also decrease autophagy via AMPK in the liver. This inhibition of autophagy was known as suppressor of glucose form autophagy (SOGA)38, 39. One relative study reported that SOGA knockdown might activate autophagy or proteolysis to prevent hepatocytes glucose production by adiponectin stimulation40, 41. Another experiments conducted by Kundu et al.42 demonstrated that hyperglycemia impairs AMPK phosphorylation, which depended on LKB1 and CaMKK activation. Inactive AMPK also inhibited autophagy through mTOR upregulation and accumulation of matrix protein. In addition, the downstream regulation is similar with the normal autophagy activation pathway like AMPK-ULK143. So LKB1, CaMKK and SOGA could be the more significant discovery as the present potential sensors for AMPK and autophagy glucose production or lipid synthesis in hepatocytes, especially in hyperglycemic liver. Autophagy plays a positive protective role in liver glucose and lipid regulation in metabolic diseases as well.
2.4. AMPK and autophagy in diabetic cardiomyopathy
Diabetic cardiomyopathy has become a major cause of diabetes-related morbidity and mortality. It is characterized by ventricular dysfunction that develops in diabetic patients without coronary artery disease or hypertension. Autophagy has been known as an important myocardial adaptive response to conserve energy. Dieter et al.14 reported that improving cardiac autophagy in patients with diabetes might prevent the impact of diabetic cardiomyopathy development. Restoration of autophagy could block the accumulation of dysfunctional organelles and cytotoxic protein aggregates. Autophagy is strongly controlled by the mammalian target of rapamycin (mTOR)-dependent signaling pathway. Most cardiac studies confirm that mTOR is a negative regulator of autophagy as well (via mTORC1-ULK1/2 phosphorylation or mTORC2-Akt-FoxO phosphorylation). Inhibition of mTOR is linked to autophagy induction in isolated cardiomyocytes and myocardial tissue44, 45. The AMPK-mTOR pathway has been considered an important mechanism in autophagy regulation in response to energy stress and glucose starvation. In mammals, compromised cellular energy production inhibits mTOR through activation of AMPK and subsequently, phosphorylation of the TSC. Since mTOR negatively regulate autophagy, it is likely that diabetes activates TSC-mTOR signaling through inactivation of AMPK, which inhibits the ULK kinase complex, preventing the initiation of autophagy. Nonetheless, over activation of autophagy in diabetes has also been found to be harmful to development of cardiomyopathy and cardiac function to induce excessive cell stress46. Whether the autophagy in cardiomyocytes should be a protective or detrimental mechanism is still a debate at this time47. Therefore, it might be better to balance the status of autophagy activation in diabetic cardiomyopathy. Besides autophagy of cardiomyocytes, myocardial fibroblasts have also been mentioned in the rapamycin-regulated process of diabetic cardiomyopathy. However, most studies of pathological myocardial fibroblasts focused on cell apoptosis, extracellular matrix increasing, expression of collagen increasing and so on. There are no original reports about autophagy dysfunction of myocardial fibroblasts in diabetic cardiomyopathy. Additionally, according to the pathological process of hepatic fibrosis and renal fibrosis, autophagy is involved in increasing extracellular matrix and collagen degradation. Thus autophagy dysfunction of myocardial fibroblasts in cardiomyopathy or myocardial fibrosis might be a potential mechanism of diabetic cardiomyopathy, and a critical point and a target for diabetic cardiomyopathy treatment44.
2.5. AMPK and autophagy in diabetic nephropathy
Diabetic nephropathy (DN) is a serious complication of diabetes. In the early stages of diabetes, patients exhibit hyperfiltration with high glomerular filtration rates (GFRs) and infrequent occurance of microalbuminuria. Glomerular damage, along with proteinuria, and subsequent tubulointerstitial lesions induced by diabetes finally lead to end stage renal disease48. Several studies have suggested that autophagy is involved in the pathogenesis of DN. AMPK as a regulator of autophagy has also been suggested to be involved in the pathogenesis of DN. It has been reported that podocytes and proximal tubular cells are the main cells affected in DN. Autophagy plays a crucial role in maintaining the function of podocytes and proximal tubular cells. Fang et al.49 reported that in high-glucose conditions in cultured podocytes and streptozotocin (STZ)-induced type 1 diabetic rats, autophagy was suppressed with a decrease of the expression of Beclin-1, ATG12-5, and LC3, and lead to the impaired filtration barrier function of podocytes. Interestingly, Cina et al.50 reported that mTORC1 is highly activated and may be involved in the mechanisms of autophagy inhibition in podocytes of diabetic mice and patients. In addition, several studies have reported that AMPK activation by AICAR or adiponectin shows podocyte protective effects against various nephrotoxic conditions. It seems that autophagy activation is intricate in AMPK-mediated podocyte protection. AMPK can activate autophagy via two independent mechanisms: suppression of mTORC1 activity and direct control of ULK1 phosphorylation. Between these two independent mechanisms of AMPK on the regulation of autophagy, suppression of mTORC1 activity might contribute to the protection of AMPK in podocytes of DN. Furthermore, the results from Yamahara et al.51 suggested that hyperactivation of mTORC1 signaling in proximal tubular cells was involved in obesity-mediated autophagy suppress. However, proximal tubular cells are correlated with SIRT1 (sirtuin type 1) or p62/SQSTM1 (sequestosome 1) protein in autophagy. As an example, in proximal tubular cells accumulation of p62/SQSTM1 protein occurred in type 2 diabetes patients, which suggested that deficiency of autophagy might affect diabetic kidney disease in humans48, 51. These findings lead us to hypothesize that autophagy is altered in diabetic kidneys, and autophagy deficiency should contribute to the pathogenesis of diabetic nephropathy, related with not only podocytes but also proximal tubular cells. Therefore, if pharmacological AMPK activation really acts as an autophagy activator, a drug that stimulates AMPK may be a potential therapy for diabetic nephropathy52. Several studies have reported that AMPK activation shows renoprotective effects in diabetic nephropathy. Autophagy may be involved in AMPK mediated renoprotective action.
3. Conclusions and perspective
It has been found for diabetes mellitus that autophagy is a significant factor with AMPK, an important regulatory signaling pathway. The autophagy induced by cellular lesions could affect a variety of tissues, glucose or lipid metabolism and insulin secretion. Hence, these conditions might lead to serious chronic disease as diabetes complications (Fig. 2). At times, diabetes itself may not be such a serious status. But continuing or additional tissue damage could lead to a critical mass of cell injuries and imbalance homeostasis. Accordingly, autophagy induced by AMPK is involved in calorie production and lipogenesis mainly with other protein signals, such as mTOC1, LKB1, ULK and LC3. The crosstalk between autophagy and AMPK might be a possible therapeutic target. In future research, blocking other regulatory pathways of autophagy like mTOC1 might be significant, including AMPK upstream and downstream regulation. Likewise, in recent decades, medicine, therapeutic approaches and pathogenesis would tend to depend on individuals. Therefore, combining with genetic and protein levels in AMPK and autophagy stimulation pathways might be a tendency for diabetes and complications regulatory analysis.
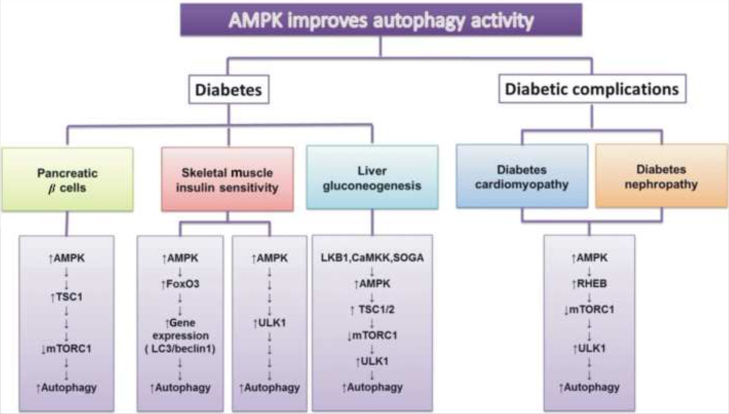
Figure 2.
Activation of autophagy could occur in several diabetes-related diseases, including pancreatic β cells, muscle insulin sensitivity and liver gluconeogenesis related syndromes or diabetes cardiomyopathy and diabetes nephropathy. Subsequently, mTORC1 and TSC1 expression were included in the regulation of pancreatic β cells. In skeletal muscle, ULK1 or FoxO3 and LC3 might affect insulin sensitivity as well as in liver gluconeogenesis, which has a strong association with autophagy activation. Additionally, LKB1, CAMKK and SOGA are the special sensors involved in liver gluconeogenesis studied recently. And AMPK-RHEB-TOC1 is the significant signaling pathway in diabetes cardiomyopathy and nephropathy. All of these are linked with energy sensor, AMPK.
Acknowledgments
This work was supported by funding from the National Natural Science Foundation of China (No. 81170745). And this work also conducted in Key Lab of Traditional Medicine for Diabetes of Jilin Province and Preclinical Pharmacology R&D Center of Jilin Province.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Mackenzie RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:55–64. doi: 10.2147/DMSO.S48260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouyang CH, You JY, Xie ZL. The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol. 2014;71:71–80. doi: 10.1016/j.yjmcc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 4.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishan S, Richardson DR, Sahni S. Adenosine monophosphate-activated kinase and its key role in catabolism: structure, regulation, biological activity, and pharmacological activation. Mol Pharmacol. 2015;87:363–377. doi: 10.1124/mol.114.095810. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 7.Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS. AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology. 2012;153:2130–2141. doi: 10.1210/en.2011-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu XL, Niu YM, Yuan HR, Huang J, Fu L. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism. 2015;64:658–665. doi: 10.1016/j.metabol.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Goginashvili A, Zhang ZR, Erbs E, Spiegelhalter C, Kessler P, Mihlan M. Insulin secretory granules control autophagy in pancreatic β cells. Science. 2015;347:878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 12.Moruno F, Pérez-Jiménez E, Knecht E. Regulation of autophagy by glucose in mammalian cells. Cells. 2012;1:372–395. doi: 10.3390/cells1030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravikumar B, Stewart A, Kita H, Kato K, Duden R, Rubinsztein DC. Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Hum Mol Genet. 2003;12:985–994. doi: 10.1093/hmg/ddg109. [DOI] [PubMed] [Google Scholar]
- 14.Kubli DA, Gustafsson A.B. Cardiomyocyte health: adapting to metabolic changes through autophagy. Trends Endocrinol Metab. 2014;25:156–164. doi: 10.1016/j.tem.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan WL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou MH, Xie ZL. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 2013;9:624–625. doi: 10.4161/auto.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang DK, Tian M, Qi Y, Chen G, Xu LJ, Zou X. Jinlida granule inhibits palmitic acid induced-intracellular lipid accumulation and enhances autophagy in NIT-1 pancreatic β cells through AMPK activation. J Ethnopharmacol. 2015;161:99–107. doi: 10.1016/j.jep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Dong HQ, Czaja MJ. Regulation of lipid droplets by autophagy. Trends Endocrinol Metab. 2011;22:234–240. doi: 10.1016/j.tem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh R, Kaushik S, Wang YJ, Xiang YQ, Novak I, Komatsu M. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachar-Wikstrom E, Wikstrom JD, Ariav Y, Tirosh B, Kaiser N, Cerasi E. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013;62:1227–1237. doi: 10.2337/db12-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang YL, Huang W, Wang J, Xu ZP, He JY, Lin XH. Metformin plays a dual role in MIN6 pancreatic β cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10:268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZF, Li YB, Han JY, Wang J, Yin JJ, Li JB. The double-edged effect of autophagy in pancreatic β cells and diabetes. Autophagy. 2011;7:12–16. doi: 10.4161/auto.7.1.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg GR, Jorgensen SB. The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini Rev Med Chem. 2007;7:521–528. doi: 10.2174/138955707780619662. [DOI] [PubMed] [Google Scholar]
- 25.Marsh BJ, Soden C, Alarcón C, Wicksteed BL, Yaekura K, Costin AJ. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine β-cells. Mol Endocrinol. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto K, Hanson PT, Tran H, Ford EL, Han ZQ, Johnson JD. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J Biol Chem. 2009;284:27664–27673. doi: 10.1074/jbc.M109.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dagon Y, Mantzoros C, Kim YB. Exercising insulin sensitivity: AMPK turns on autophagy! Metabolism. 2015;64:655–657. doi: 10.1016/j.metabol.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez AM, Csibi A, Raibon A, Cornille K, Gay S, Bernardi H. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem. 2012;113:695–710. doi: 10.1002/jcb.23399. [DOI] [PubMed] [Google Scholar]
- 29.Shi LY, Zhang T, Liang XY, Hu Q, Huang J, Zhou Y. Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway. Mol Cell Endocrinol. 2015;409:92–102. doi: 10.1016/j.mce.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord. 2014;15:137–147. doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 32.Deepa SS, Dong LQ. APPL1: role in adiponectin signaling and beyond. Am J Physiol Endocrinol Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deepa SS, Zhou LJ, Ryu J, Wang CH, Mao XM, Li C. APPL1 mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCζ signaling pathway. Mol Endocrinol. 2011;25:1773–1785. doi: 10.1210/me.2011-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CH, Xin XB, Xiang RH, Ramos FJ, Liu ML, Lee HJ. Yin-yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J Biol Chem. 2009;284:31608–31615. doi: 10.1074/jbc.M109.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-κB activation in vascular endothelial cells. FEBS Lett. 2008;582:1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 36.Zhou LJ, Deepa SS, Etzler JC, Ryu J, Mao XM, Fang QC. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 38.Forbes JM. The physiological deadlock between AMPK and gluconeogenesis: SOGA, a novel protein, may provide the key. Am J Pathol. 2010;177:1600–1602. doi: 10.2353/ajpath.2010.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao MT, Klionsky DJ. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab. 2011;13:119–120. doi: 10.1016/j.cmet.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowerd RB, Asmar MM, Alderman JM, Alderman EA, Garland AL, Busby WH. Adiponectin lowers glucose production by increasing SOGA. Am J Pathol. 2010;177:1936–1945. doi: 10.2353/ajpath.2010.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camacho RC, Pencek RR, Lacy DB, James FD, Donahue EP, Wasserman DH. Portal venous 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside infusion overcomes hyperinsulinemic suppression of endogenous glucose output. Diabetes. 2005;54:373–382. doi: 10.2337/diabetes.54.2.373. [DOI] [PubMed] [Google Scholar]
- 42.Kundu S, Pushpakumar S, Khundmiri SJ, Sen U. Hydrogen sulfide mitigates hyperglycemic remodeling via liver kinase B1-adenosine monophosphate-activated protein kinase signaling. Biochim Biophys Acta. 2014;1843:2816–2826. doi: 10.1016/j.bbamcr.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puri P, Chandra A. Autophagy modulation as a potential therapeutic target for liver diseases. J Clin Exp Hepatol. 2014;4:51–59. doi: 10.1016/j.jceh.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, Zorzato F. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DH, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Investig. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellor KM, Bell JR, Young MJ, Ritchie RH. Delbridge LMD. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol. 2011;50:1035–1043. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Levine B, Yuan JY. Autophagy in cell death: an innocent convict? J Clin Investig. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15–R30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang L, Zhou Y, Cao HD, Wen P, Jiang L, He WC. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8:e60546. doi: 10.1371/journal.pone.0060546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cinà DP, Onay T, Paltoo A, Li CJ, Maezawa Y, De Arteaga J. Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol. 2012;23:412–420. doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamahara K, Kume S, Koya D, Tanaka Y, Morita Y, Chin-Kanasaki M. Obesity-mediated autophagy insufficiency exacerbates proteinuria-induced tubulointerstitial lesions. J Am Soc Nephrol. 2013;24:1769–1781. doi: 10.1681/ASN.2012111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]