Abstract
Eukaryotes tune the transcriptional activity of their genome by altering the nucleosome core particle through multiple chemical processes. In particular, replacement of the canonical H2A histone with the variants macroH2A and H2A.Z has been shown to affect DNA accessibility and nucleosome stability; however, the processes by which this occurs remain poorly understood. In this study, we elucidate the molecular mechanisms of these variants with an extensive molecular dynamics study of the canonical nucleosome along with three variant-containing structures: H2A.Z, macroH2A, and an H2A mutant with macroH2A-like L1 loops. Simulation results show that variant L1 loops play a pivotal role in stabilizing DNA binding to the octamer through direct interactions, core structural rearrangements, and altered allosteric networks in the nucleosome. All variants influence dynamics; however, macroH2A-like systems have the largest effect on energetics. In addition, we provide a comprehensive analysis of allosteric networks in the nucleosome and demonstrate that variants take advantage of stronger interactions between L1 loops to propagate dynamics throughout the complex. Furthermore, we show that posttranslational modifications are enriched at key locations in these networks. Taken together, these results provide, to our knowledge, new insights into the relationship between the structure, dynamics, and function of the nucleosome core particle and chromatin fibers, and how they are influenced by chromatin remodeling factors.
Introduction
Eukaryotes package their genetic code in highly ordered chromatin fibers. The fundamental unit of these structures is the nucleosome core particle (NCP), a complex of ∼147 base pairs of DNA that are wrapped around eight histone proteins (Fig. 1) (1). Although they have minimal sequence homology, each core histone has a structural motif of an N-terminal tail, three helices connected by two loops (α1-L1- α2-L2- α3), and a C-terminal tail (1, 2). In the assembled NCP, histones are structurally divided into a (H3-H4)2 tetramer that is positioned between two H2A-H2B dimers. The only location of interdimer interactions is at the base of the NCP that is formed by the H2A L1 loops, whereas each dimer has two interfaces with the tetramer: the H2A docking domain (DD) and the H3-H4 four-helix bundle. (1, 2, 3, 4).
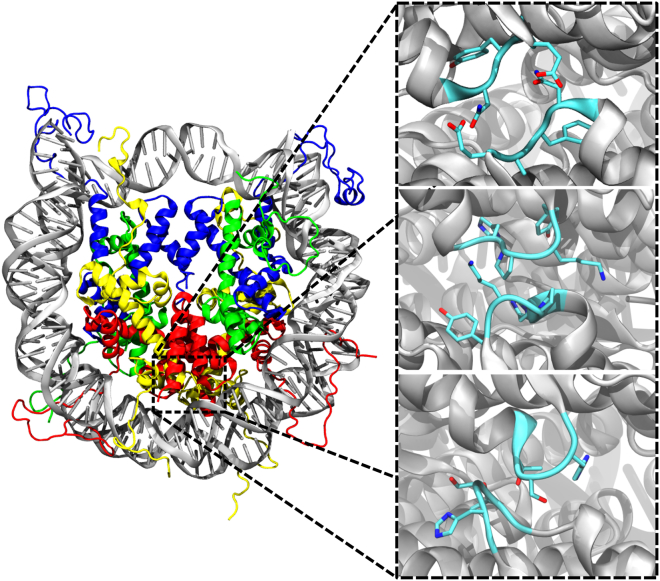
Figure 1.
(Left) The nucleosome core particle: H3 (blue), H4 (green), H2A (yellow), H2B (red), and DNA (gray). (Right) The structures of the three L1 loop sequences considered in this study: canonical (top), macroH2A (middle), and H2A.Z (bottom). The canonical loops possess a net negative charge resulting from Glu41, whereas the macroH2A loops possess a net positive charge from Lys40. The L1 loops of H2A.Z are uncharged, but both macroH2A and H2A.Z loops introduce a larger hydrophobic volume than the canonical. To see this figure in color, go online.
Cells regulate chromatin stability and DNA accessibility by changing the biochemical properties of the NCP (5, 6, 7, 8). One of the primary chromatin remodeling mechanisms is the replacement of H2A or H3 histones with histone variants. (9, 10, 11, 12, 13, 14, 15) These variants have a similar structure and sequence to the canonical histones; however, they diverge at key locations that affect interhistone and DNA-histone contacts. These differences alter the structure and stability of the NCP and are therefore implicated in modulating transcriptional activity. For example, the H2A variant macroH2A exists in large populations in the inactive X chromosome of females but is sparse in active genes (11, 16, 17). In contrast, the H2A.Z variant has been linked to both transcriptional activation and repression and is enhanced in regulatory regions of the genome such as promoters and enhancers (18, 19).
Histone variants influence chromatin through diverse mechanisms and structure/function relationships. macroH2A is unique among variants in that it possesses multiple domains, including the histone domain, a 38-residue linker sequence, and a large macro-domain (3, 20). On its own, the histone domain is sufficient for reducing transcriptional activity in vivo and increasing the stability of the nucleosome complex, even though the crystal structure of an NCP containing this domain shows that variant incorporation causes only minor NCP rearrangements (11, 21). The primary sequence is ∼65% identical to canonical H2A and differs largely from H2A in two important regions: the L1 loops and the docking domain. The canonical 38NYAE41 L1 loop possesses a net negative charge, whereas in contrast the macroH2A L1 38HPKY41 sequence has a net positive charge and an increased hydrophobicity. Substitutions of the L1 loops in canonical H2A with a macroH2A sequence (the L1-mutant) creates nucleosomes with in vitro stabilities and in vivo enrichments that are nearly identical to NCPs containing the complete macroH2A histone domain (11, 21). Therefore, the L1 loops appear to be pivotal in dictating macroH2A’s abilities to affect intranucleosomal functions. Meanwhile, changes to the docking domains show little effect on in vitro stability, but increase in vivo enrichment (11, 21).
The role and mechanisms of the H2A.Z variant remains less well defined, with some experiments showing that H2A.Z increases NCP stability whereas others have found that it destabilizes the system. Similar to macroH2A, a comparison of the H2A.Z and canonical-containing NCP crystal structures show nearly identical overall conformations with the exception of two features (4). First, the structure of the L1 loops is altered, resulting in increased contacts between the two H2A/H2B dimers that likely helps to stabilize the histone octamer. Second, H2A.Z has fewer hydrogen bonds between the docking domain and H3, which could destabilize the dimer/tetramer interface. This combination of stabilizing one area of the NCP whereas destabilizing another may account for the disparate experimental results and the multiple functions H2A.Z appears to have (22).
Experiments have revealed a wealth of information about how histone variants affect NCP and chromatin function, but several questions still remain. For example, how do seemingly minor structural rearrangements affect the stability of the nucleosome? To what extent do changes in the L1 loops propagate through the complex? Do variants influence NCP function through only structural means, or do they take advantage of altered dynamics as well? To address these problems, we have performed extensive molecular dynamics (MD) simulations of four complete NCP systems that include 1) canonical H2A histones, 2) the macroH2A histone-fold domains, 3) L1-mutant H2A histones, and 4) H2A.Z histones. Our results indicate that different sequences in the L1 loops perturb the dynamic and energetic properties in this region of variant containing NCPs. These effects propagate throughout the complex and create subtle, yet important, rearrangements that alter the NCP structure and dynamics through both direct effects and modified allosteric networks. This allows histone variants to influence both the global dynamics and energetics of the NCP, and it likely contributes to large-scale structural changes such as DNA breathing and nucleosome opening, as well as inter-NCP interactions in chromatin fibers (23, 24).
Materials and Methods
System and simulation details
Simulations of the canonical-, macroH2A-, and H2A.Z-containing nucleosomes were initialized from their crystal structures (PDB: 1KX5, 1U35, and 1F66, respectively) (2, 3, 4). The L1-mutant structure was formed using the crystal structure of 1KX5, with the H2A L1 loops mutated from the canonical 38NYAE41 to the macroH2A 38HPKY41 sequence. The missing histone tail residues in the variant systems were constructed using 1KX5 as a model. To ensure that differences between system dynamics are purely results of H2A composition, non-H2A residues were mutated to match 1KX5. Furthermore, the 146 base pairs of DNA in the macroH2A and H2A.Z crystal structures were replaced with the 147 base pairs of 1KX5. Previous computational studies have suggested that H3, H4, and H2B orthologs have little effect on nucleosome stability (25), and comparative analysis of the crystal structures shows that variants do not significantly alter the DNA superhelical pathway (see Fig. S1 in the Supporting Material) (12). Further trajectory analysis confirms that these subtle changes to the variant systems have no discernible effect on the net system equilibration beyond noise (for further discussion and comparative analysis, see the Supporting Material).
The systems were neutralized and solvated in a TIP3P box of 150 mM NaCl that extended at least 10 Å from the solute, creating systems of ∼250,000 atoms. Each system was simulated three times (see the Supporting Material for more details). The simulations were done in the NAMD engine (v2.9) using the AMBER12SB fixed point-charge force field (26, 27). Monovalent ions were modeled according to Joung and Cheatham (28). Production simulations were done in the NPT ensemble using standard techniques (29, 30, 31, 32, 33, 34, 35, 36). Coordinates were stored every 2 ps. Visualizations were made using VMD and PyMOL (37, 38, 39).
Allosteric pathways calculations
Allosteric effects were computed with multiple techniques (for specific details, see the Supporting Material). Per-residue differences in dynamics were determined by calculating the Kullback-Leibler (KL) divergence of dihedral angle populations (40). For these calculations, the canonical populations were used as a reference set. Spurious results were filtered using bootstrapping techniques. Residue-residue correlations were calculated by utilizing the largest mutual information method (41, 42). Residue contacts were determined to be when protein Cα or nucleic C1′ atoms were within 10 Å in at least 70% of the configurations (43). The mapping of allosteric networks was conducted using the weighted implementation of suboptimal pathways (WISP) approach (44). The edge-betweenness centrality of residues in the optimal networks were calculated with the NetworkX Python package, with the significance determined by a hypergeometric distribution (see the Supporting Material) (45, 46, 47).
Interaction energies
Interaction energies between the two H2A histone L1 loops were calculated using cpptraj (48). A cutoff distance of 15 Å was used for both van der Waals and electrostatic interactions. Because the L1 loops interact with both protein and DNA, an intermediate dielectric value of 5 was considered (εDNA = 8, εprotein = 4) (49, 50). Hydrogen bonds were defined by a separation distance of 3.5 Å and an angle of 30°.
DNA binding and complex assembly energies were calculated using the MMPBSA.py function of AMBERTOOLS (v.14) (51). The level of theory was restricted to the generalized born implicit solvent (igb = 5, radii = mbondi2) (52). Coordinates from every 100 ps of production simulation were used. Although the superhelical form of DNA is highly irregular without histones (30), the octamer fold can form without the presence of DNA (53). Thus, to balance accuracy and computational efficiency, a hybrid two-trajectory molecular mechanics/generalized born surface area (MM/GBSA) approach was taken in which the coordinates for the protein constituents were extracted from the nucleosome simulations, but the unbound DNA coordinates were taken from a separate simulation of 147 bp of linear B-form DNA in a 150 mM NaCl environment (see the Supporting Materialsfor complete discussion). Error bars in the energies and all other measures were defined by the standard error of the mean, where the number of independent points was determined by the statistical inefficiency of the data set, as computed with the PyMBAR package (54).
Results
We performed three independent 250 ns MD simulations for four complete NCP systems: 1) canonical, 2) macroH2A histone-fold, 3) L1-mutant, and 4) H2A.Z containing NCPs. In each set of simulations, ∼50 ns was required for the root-mean square deviations (RMSDs) of the complexes to stabilize (Figs. S3–S6) and the tails to collapse from their initial elongated states, resulting in 200 ns of production time per simulation (600 ns per system). These results are consistent with previous MD simulations of the canonical NCP that demonstrated that the overall complex is stable on the hundreds of ns timescale and that the histone tails form strong interactions with the nucleosomal DNA (23, 55, 56). Comparisons between the canonical and variant systems demonstrate that variants have both subtle and large-scale effects on the structure and dynamics of the L1 loops, DNA-histone interactions, and allosteric networks throughout the NCP. Further comparison of our H2A.Z simulations with a control in which no substitutions were made to the 1F66 crystal structure demonstrated that the changes we introduced to match the 1KX5 structure did not significantly affect structural or dynamical properties beyond the equilibration period (see the Supporting Material for further details and discussion).
Altered dynamics of L1 loops
Modifications of the L1 loop sequences in histone variants alter their dynamics and energetics. In the canonical NCP simulations, an average of 0.5 hydrogen bonds were formed between the L1 loops, primarily between the carboxamide nitrogen of asparagine and the carboxylate group of the symmetric glutamate. This is consistent in the L1-mutant (∼0.4) but is reduced to ∼0.2 in macroH2A. In these two systems, the most prevalent hydrogen bonds were formed between the phenol oxygen of tyrosine and the hydrogen-free nitrogen of the symmetric histidine. In the H2A.Z simulations, L1-L1 hydrogen bonds were almost nonexistent (Table S1).
Although hydrogen bonds form most frequently in the canonical loops, the net L1 loop interaction energies are more favorable in the variants (Table 1). The close proximity of the negatively charged glutamates in the canonical NCP creates a disfavored electrostatic interaction. However, the L1-mutant and macroH2A systems avoid an analogous situation through Lys-DNA interactions, which separates the like-charges and creates a more favorable electrostatic configuration. Meanwhile, the lack of charge in the H2A.Z L1 loops also creates a more favorable electrostatic interaction than the canonical NCP. In addition, the L1 loop rearrangement in the macroH2A and L1-mutant systems is further stabilized by van der Waals interactions. In total, the interaction energies of the L1 loops in the L1-mutant and macroH2A structures are substantially favored over those of the canonical ΔUtotal values of −11.2 ± 2.4 and −8.6 ± 2.3 kcal/mol. The H2A.Z L1 loop conformations are also more favorable than in the canonical system (Utotal = −4.4 ± 2.3 kcal/mol).
Table 1.
Net Interaction Energies between the H2A L1 Loops Show that macroH2A and H2A.Z Loops Have More Favorable Interactions than Canonical L1 Loops
| System | Uelect | UvdW | Utot | Uelect | UvdW | Utot |
|---|---|---|---|---|---|---|
| Canonical NCP | 6.3 ± 2.0 | −9.4 ± 0.8 | −3.1 ± 2.2 | − | − | − |
| L1-mutant | −0.9 ± 0.4 | −13.4 ± 0.8 | −14.3 ± 0.9 | −7.2 ± 2.0 | −4.0 ± 0.8 | −11.2 ± 2.4 |
| MacroH2A NCP | 0.4 ± 1.1 | −12.1 ± 0.9 | −11.7 ± 1.0 | −5.9 ± 2.3 | −2.7 ± 0.9 | −8.6 ± 2.3 |
| H2A.Z NCP | −0.6 ± 0.1 | −6.9 ± 0.6 | −7.5 ± 0.6 | −6.9 ± 2.0 | 2.4 ± 1.0 | −4.4 ± 2.3 |
U is defined as the difference in energies between each variant and the canonical system. Negative values show favorability in the variants. All values are reported in kcal/mol.
The different net charges of the L1 loops influence their interactions with the nucleosomal DNA. In the canonical L1 loops, the negative charge located on Glu41 causes a repulsive force to the negatively charged DNA. However, in the macroH2A and L1-mutant systems, Lys40 introduces a positive charge into the loop that forms a salt bridge with the DNA base pair across the axis of symmetry (Fig. 2). The lysine forming this salt bridge sterically hinders the symmetric lysine residue from doing the same, so the interaction exists in only one dimer. The noninteracting lysine is primarily exposed to solvent while intermittently forming a hydrogen bond with a neighboring histidine. Because the L1 loops of H2A.Z are uncharged, they are not capable of forming similar interactions, and therefore did not make any direct contacts with the DNA. Taken with the results of the L1-L1 loop dynamics, we observe that the macroH2A-like loop sequences stabilize both protein-protein and protein-DNA interactions when compared with both the canonical and H2A.Z histones.
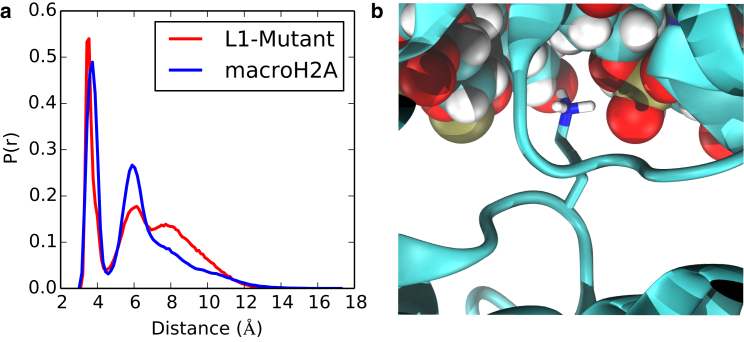
Figure 2.
(a) Distance populations for Lys40 to DNA phosphate show an interaction that is unique to macroH2A-like L1 loops. (b) A representative configuration of the Lys side chain stretching across the molecule to interact with the dimer’s nonassociated DNA. This orientation sterically hinders the symmetric loop from forming a similar interaction. This interaction contributes significantly to stabilizing DNA-octamer binding in the macroH2A and L1-mutant systems. To see this figure in color, go online.
Variant presence alters dimer orientations
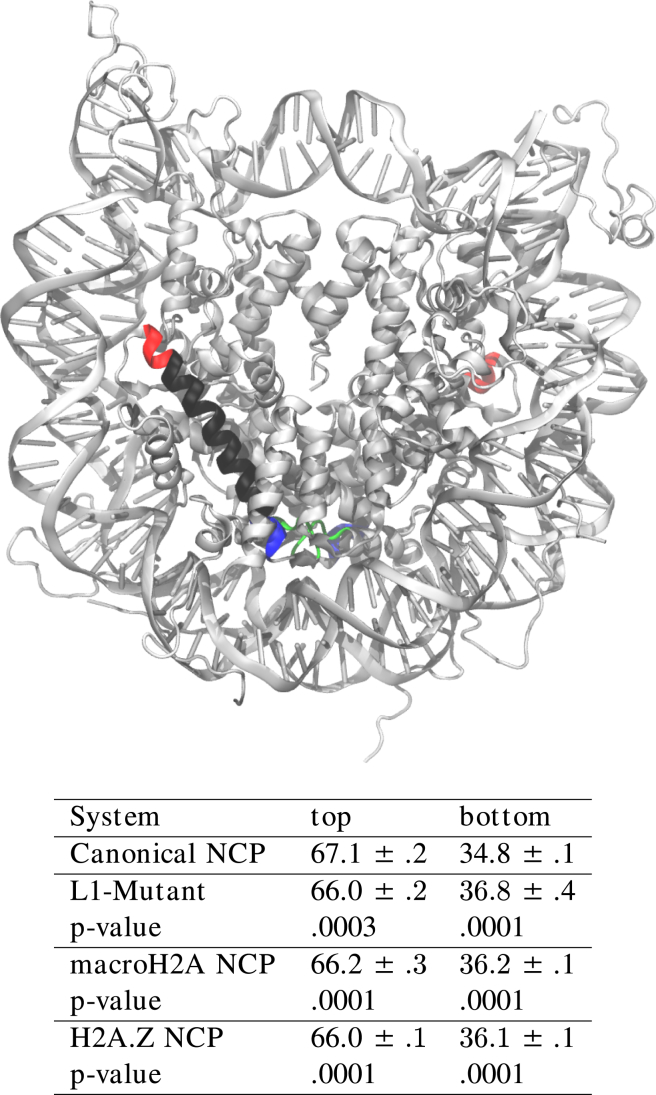
Reorganization of the L1-loops creates perturbations that affect the dimer orientations in the NCP. For example, the H2A α2 helix extends across the dimer, with its N-terminal (the base) at the L1-interface and its C-terminal (the top) solvent-exposed on the far side of the molecule (Fig. 3). Simulation analysis showed that the canonical system exhibited a separation of 67.1 ± 0.2 Å between the tops, and 34.8 ± 0.1 Å between the bottoms of the H2A α2 helices. In contrast, in each of the variant NCPs there is a bulging motion in which the base separation is increased to ∼36.5 Å while the top separation is decreased to ∼66.1 Å. Although these changes in orientation are only on the order of an Å, a t-test indicated that they are all extremely statistically significant (Fig. 3).
Figure 3.
Separation distances for H2A α2 helix locations show a bulging effect in histone variants. The helix is displayed in black whereas the helix top is highlighted in red and the bottom in blue. The L1-loops are shown in green for clarity. Shifts in mean separation are on the order of an Å, but the changes in populations are all incredibly significant. To see this figure in color, go online.
This subtle reorientation of the dimers alters histone-DNA hydrogen bonding. For example, the guanidine group of H2A Arg29 forms a hydrogen bond with the phosphate group of the 23rd base pair of DNA in all systems. In the canonical NCP, this bond forms in 63% of the configurations, whereas in the L1-mutant it is formed 70% of the time. In addition, the frequency of hydrogen bonding between the 22nd base pair phosphate and the backbone amide of H2B Ser33 increases from 50% in the canonical system to 60% in the L1-mutant structure. In H2A.Z, the hydrogen bonding at these locations increases drastically to 73% and 85%, respectively. Interestingly, the macroH2A nucleosome shows a decreased frequency of both of these interactions (52% and 30%, respectively). The reduced hydrogen bonding in the macroH2A nucleosome is likely a result of sequence deviations in the nearby H2A 1 helix (canonical: 30VH31, H2A.Z: 30IH31, macro: 30ML31).
The dimer realignment also affects the hydrogen bonding between protein constituents in the histone core. In the canonical NCP, an average of 14.8 hydrogen bonds are formed between a single dimer and the tetramer, which is in agreement with the ∼15 observed in the crystal structure. This increases to an average of 16.5 in the L1-mutant. The macroH2A and H2A.Z nucleosomes display an average of 14.7 and 13.8 hydrogen bonds, which are substantially more frequent than the ∼8 observed in the initial configurations. This increase in hydrogen bonding is a result of dimer reorientation, which increases the interaction between residues in the four-helix bundle.
Histone H2A L1 sequence influences dynamics throughout the nucleosome
The L1 loops not only influence dimer reorientation, but they also perturb the local dynamics of residues throughout the nucleosome. Calculations of the KL divergence between the canonical and variant systems showed the expected disparity in dihedral sampling of residues within the L1 loop region (Fig. 4). However, they also highlighted significant changes in the dynamics of residues that are distant from these loops. In both the L1-mutant and macroH2A systems, the dimer and tetramer constituents of the docking domains have statistically significant KL divergence values, indicating that their local dynamics are different in these systems relative to the canonical NCP. Although this was expected in the macroH2A and H2A.Z systems because of their sequence deviations, the L1-mutant is sequentially identical to the canonical system in these areas. Therefore, the observed difference in dynamics must be because of allosteric networks that are shifted by the L1 mutation. On the other hand, the dynamics in the histone core of H2A.Z only show small differences from the canonical system, most notably along the H2B α2 helix, whereas the largest divergences are in the DNA.
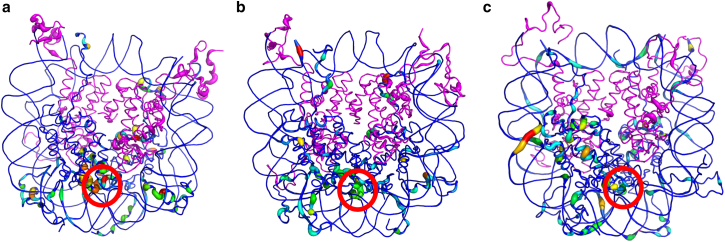
Figure 4.
Per-residue Kullback-Leibler divergence values for difference in sampled dihedral angles for the (a) L1-mutant, (b) macroH2A, and (c) H2A.Z nucleosomes, referenced against populations from the canonical system. The dimers and DNA residues are represented in a rainbow spectrum. Narrow and blue regions display similar dynamics to the canonical nucleosome, and regions displaying larger divergence values are wider and increase from blue to green to red. The tetramer is shown in magenta, where the tube radius is wider for residues with larger deviations from canonical-like dynamics. Divergence values are large near the L1-L1 interface (red circle), but significant differences are also observed in faraway regions. To see this figure in color, go online.
The L1-mutant and macroH2A variants also have increased dynamical correlations between the H2A L1 loops and key portions of the NCP (Table 2; Fig. S9). The strengthened interactions in the L1 loops increase the L1-L1′ correlations from 0.42 in the canonical system to >0.67 in each of the variants. In both the canonical and H2A.Z systems, the average correlation between the L1 loops and either docking domain (symmetric: DD, opposing dimer: DD′) was 0.36 to 0.38. However, in the L1-mutant the average L1-DD and L1-DD′ correlations increased to 0.51 for both measurements, which were further increased to 0.56 and 0.60 in the macroH2A structure. Although the variants had an increased correlation between the L1 loops and DNA near the base of the nucleosome, the correlations between the L1 loops and DNA extremities were similar in all four systems.
Table 2.
Average Correlations between L1 Loops and Relevant Regions of the Nucleosome Core Particle for Each System
| System | L1-L1′ | L1-DD | L1-DD′ | L1-DNA |
|---|---|---|---|---|
| Canonical NCP | 0.42 | 0.38 | 0.36 | 0.49 |
| L1-mutant | 0.70 | 0.51 | 0.51 | 0.48 |
| MacroH2A NCP | 0.73 | 0.56 | 0.60 | 0.48 |
| H2A.Z NCP | 0.67 | 0.38 | 0.38 | 0.44 |
The L1-L1′ and L1-DD(′) correlations are significantly stronger in the systems possessing the macroH2A L1 loops, whereas L1 correlations to the DNA extremities are unchanged. The associated docking domain is abbreviated as DD, and the docking domain of the opposing dimer is abbreviated as DD′.
Variant presence alters allosteric pathways
The origins of the altered dynamics and correlations in NCPs with variants were probed by computing the optimal and suboptimal correlation pathways using with the WISP algorithm (44). Allosteric networks were calculated between the L1 loops and the DNA entry and exit sites, and the tetramer components of the docking domains for each system. The results revealed that not only are there several networks of dynamically coupled residues in the canonical NCP, but that these networks are both modified and strengthened by macroH2A, H2A.Z, and the L1-mutant. The shifts are because of both changes in the NCP hydrogen bonding networks from subtle repositioning of the H2A histones, as well as increased interactions of the L1 loops with one another and with the nucleosomal DNA.
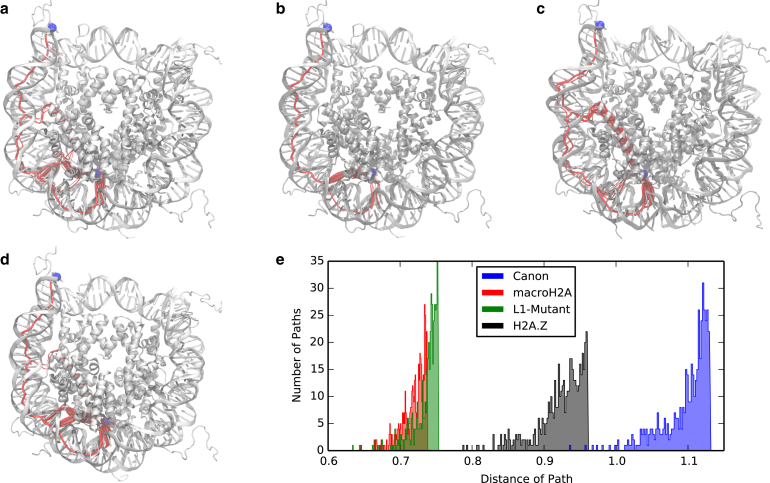
In the L1-to-symmetric-DNA-end pathways, the canonical system utilizes three main routes for information transfer (Fig. 5). In the first, networks primarily pass through neighboring H2B Ser33-DNA and H2A α1 helix Arg29-DNA hydrogen bond interactions and into the DNA, whereas in the second the networks enter the DNA through the H2A Arg42-DNA hydrogen bond near the intradimer interaction site. The third route for propagation extends along the H2A α2 helix, which passes dynamic information into the DNA base pairs via a Thr76-DNA interaction. The pathways of H2A.Z are similar to the canonical but with more pathways accessing the H2A α1 Arg29-DNA interaction than that of H2B Ser33-DNA. In the L1-mutant, the increased prevalence of the Arg29-DNA hydrogen bond heavily biases information transfer through this network and increases the strength of this pathway. The decreased Arg29-DNA interaction in the macroH2A nucleosome causes information to be transferred primarily via the H2A Arg42-DNA hydrogen bond, with a significant number of pathways also traversing the H2A α2 helix.
Figure 5.
The 500 suboptimal pathways between the L1 loops and symmetrically associated DNA entry for the (a) canonical NCP, (b) L1-mutant, (c) macroH2A, and (d) H2A.Z projected on simulation snapshots. Also shown is the histogram of pathway distances (e). The L1 and DNA sites are represented as blue spheres, and the pathways are outlined in red with the wider pathways representing those of shorter distance. Pathways in the variants are shorter, and thus stronger, than in the canonical NCP. To see this figure in color, go online.
The effects of L1-L1′ communication transfer are most apparent in the networks between an L1 loop and the DNA end of the opposite symmetry. In the canonical nucleosome, there exist no pathways between L1 loops, therefore networks must pass through indirect routes that include the DNA and histone tails. However, in all of the variant structures information is readily exchanged between the L1 loops, allowing the pathways to immediately cross into the opposite symmetry dimer (Figs. S14–S17). Once information is passed into this dimer, it follows the typical pathways for L1-to-symmetric-DNA-end propagation. This results in allosteric networks that are not only stronger, but more direct in the histone variants.
Pathways between the L1 loop and docking domains in the same dimer are similar in all systems, but there is a large disparity in the pathways between L1 loops and the docking domain of the other dimer constituent. In the canonical NCP, the majority of paths pass from the L1 loops through the H2B α2 helix into the tetramer portion of the docking domain via the four helix bundle of H2B-H4. The L1-mutant structure shows an increased number of contacts in this region, creating a more diverse set of pathways between bundle helices. The macroH2A nucleosome displays an alternate route in which pathways instead access the docking domain region via protein-DNA interactions. The H2A.Z system uniquely passes information along the H2B α2 helix of the opposing dimer. Pathways in H2A.Z also access the protein-DNA type route of macroH2A and the four helix bundle route of the canonical and L1-mutant systems, but at a reduced frequency.
PTM locations are enriched at allosteric hotspots
Beyond these specific pathways, dynamic networks exist throughout the NCP. To discern the importance of individual residues on these global networks, the edge-betweenness centrality of nucleosome residues was computed (Fig. S10) (46). In the canonical NCP, a majority of the optimal pathways rely heavily on the DNA base pairs and neighboring histone tail lysine and arginine residues to propagate communication throughout the system. In H2A.Z, an increased number of shortest pathways access the L1 loops and the H4 α2 helix, but there remains a heavy reliance on the DNA and histone tails. In the L1-mutant and macroH2A nucleosomes, dynamic traffic to the four-helix bundle increases. Furthermore, pathways in these systems access L1 residues more frequently than any other region.
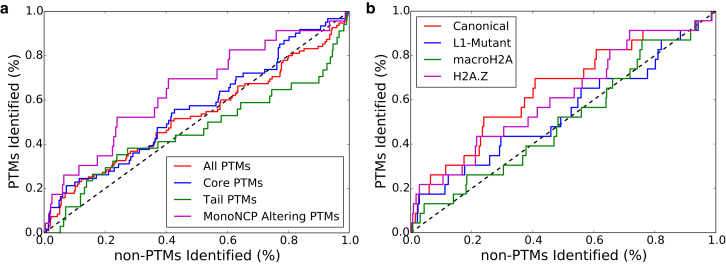
Interestingly, we find that residues with the highest edge-betweenness scores are more likely to be the sites of posttranslational modifications (PTMs). Based on the distribution of centrality scores, we classify residues in the upper 10th percentile as hotspots for communication (see the Supporting Material). A comparison of known PTM sites with these allosteric hotspots indicates that PTMs are enriched at these locations, with an enrichment factor of 254% (p-value of 0.0155). When we compare our hot spot analysis with known PTM sites (7, 57, 58), we observe a significant population of PTM targets (Fig. 6). Although PTMs in the histone core are identified more frequently than those in the tails, the most significant subset contains PTMs that have been implicated in affecting mononucleosome stabilities (monoNCP PTMs) (7). In relation to the types of PTMs, methylation sites are linked with allosteric hotspots more frequently than phosphorylations or acetylations, likely because of their presence at DNA entry/exit sites and between turns of superhelical DNA.
Figure 6.
(a) Receiver operator curves (ROC) for subsets of PTM sites in the canonical nucleosome as identified by edge-betweenness centrality ranking. The largest enrichment can be seen in the PTM subset of monoNCP altering PTMs. The core PTMs are also more frequently identified than the tails. (b) ROC for monoNCP altering PTMs across the variant systems. The canonical and H2A.Z systems are shown to depend greater on monoNCP altering PTMs than the L1-mutant and macroH2A systems for distributing dynamic information. To see this figure in color, go online.
An overall correlation between allosteric hotspots and PTM locations is maintained in the nucleosome variants; however, the specific details differ between the systems (Table S2). For example, all four systems show the importance of PTM sites in the H3 histone near the DNA extremities, whereas histone H4 monoNCP PTM sites in the four-helix bundle are accessed more frequently in variant networks. In general, the canonical system displays the greatest reliance on monoNCP PTMs, then the H2A.Z nucleosome, and finally the L1-mutant and macroH2A systems, respectively.
Structural stability in variant nucleosomes
To quantify global NCP dynamics, a full correlation analysis (FCA) was performed on the C atoms of the histone core α-helices (59). Two of the dominant motions identified in this analysis corresponded to the nucleosome opening motions described by Böhm et al. (24). Projections into the phase-space described by these and other FCA modes showed that all nucleosome systems had similar global dynamics on the hundreds of nanoseconds timescale (see Fig. S7). However, given that the dynamics of nucleosome opening likely occur on the millisecond timescale, our simulations are far too short to effectively explore the effects of histone variants on large-scale NCP dynamics.
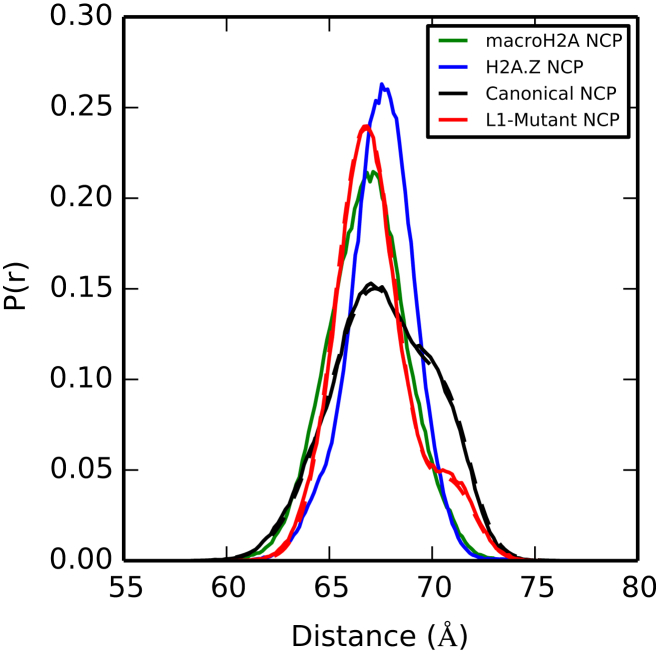
In contrast to the nucleosome opening motions, the DNA end-to-end separation distance does depend on the identity of the H2A histone (Fig. 7). This distance was defined as the separation between the center of masses of the innermost terminal nucleotide of each extremity, which were well behaved and displayed no base flipping. The sampling in the canonical system can be divided into two states: the prominent compact state centered at ∼67 Å and the open state centered at 71 Å. The L1-mutant sampled both the open and closed states; however, the percentage of time spent in the open state was reduced from 14% to 11% of the simulation. Both the macroH2A and H2A.Z systems only sampled the closed state.
Figure 7.
Distance populations for DNA end-to-end spread. The canonical system exists in two states: one centered around 67 Å (compact) and one centered around 71 Å (open). Although the L1-mutant samples both states, the amount of time spent in the open state is drastically reduced. The macroH2A and H2A.Z nucleosomes exist only in the compact state. Fits are represented in dotted lines. To see this figure in color, go online.
Results of an MM/GBSA analysis indicate that the overall DNA binding energetics are also altered by H2A variants. The DNA binding affinities to the L1-mutant and macroH2A octamers were 31.0 ± 9.1 and 5.7 ± 9.7 kcal/mol more favorable than binding to the canonical NCP (Table 3). There are two primary contributors to this shift: direct interactions with the L1 loops (ΔΔGL1) and the changes in the DNA configuration (ΔΔGDNA). In the L1-mutant and macroH2A systems, ΔΔGL1 was largely a result of removing the negatively charged Glu41 from the canonical loop and introduction of the Lys40-DNA interaction, which combine for an increase in binding free energy on the order of 10 kcal/mol. The reorientation of the dimers in the macroH2A-like systems also influences a favorable shift in DNA conformation relative to the canonical system (ΔΔGDNA = −9.3 ± 5.6 and −17.0 ± 1.8 kcal/mol for the L1-mutant and macroH2A nucleosomes, respectively). However, in macroH2A a number of small shifts in the remainder of the NCP oppose binding and therefore make it more comparable with the canonical system, which does not occur in the L1-mutant NCP. The H2A.Z nucleosome does not exhibit the same favorability for DNA binding when compared with the canonical octamer, but instead shows a disfavoring shift of 5.6 ± 8.9 kcal/mol. The removal of the negative charge on Glu41 creates a favorable shift of 5.8 ± 0.1 kcal/mol in ΔΔGL1, but this is balanced by the nearly identical free energy penalty in the DNA rearrangement term ΔΔGDNA.
Table 3.
MM/GBSA Calculated Binding Energies for DNA Binding Affinity to the Histone Core in Each of the NCP Systems
| System | ΔGbinding | ΔΔGbinding | ΔΔGL1 | ΔΔGDNA | ΔGassembly | ΔΔGassembly |
|---|---|---|---|---|---|---|
| Canonical NCP | −428.6 ± 5.6 | − | − | – | −618.6 ± 5.8 | – |
| L1-mutant | −459.6 ± 7.2 | −31.0 ± 9.1 | −8.7 ± 0.1 | −9.3 ± 5.6 | −679.6 ± 7.5 | −60.0 ± 9.5 |
| MacroH2A NCP | −434.5 ± 7.9 | −5.7 ± 9.7 | −10.7 ± 0.1 | −17.0 ± 1.8 | −637.1 ± 7.9 | −18.5 ± 9.8 |
| H2A.Z NCP | −423.0 ± 6.9 | 5.6 ± 8.9 | −5.8 ± 0.1 | 5.8 ± 5.4 | −616.3 ± 6.9 | 2.3 ± 9.0 |
ΔΔG’s are referenced against the canonical NCP. The L1 loop sequence and DNA conformations of the variant structures contribute significantly toward favorable binding of DNA in the macroH2A-like systems, relative the canonical NCP. All values are reported in kcal/mol.
Similarly, another MM/GBSA analysis revealed that macroH2A variants modify the energetics of complex assembly. The ΔGassembly of the L1-mutant and macroH2A nucleosomes were −60.0 ± 9.5 and −18.5 ± 9.8 kcal/mol more favorable than the canonical system. The favorability in the macroH2A-like systems is a result of favorable DNA binding coupled with stronger protein-protein interactions. Calculations for ΔΔGassembly of H2A.Z agree with previous calculations on a static NCP structure that suggested this complex is slightly less stable than the canonical nucleosome (25); however, our study builds off the results of Vijayalakshmi et al. by demonstrating that dynamic rearrangements bring the stabilities of these systems closer into agreement (Table 3).
Discussion
The simulations and analysis presented in this study detail a series of mechanisms by which the histone variants macroH2A and H2A.Z influence the dynamics of the nucleosome core particle. The subtle structural rearrangements these variants cause leverage the tightly packed nature of the histone core to influence the global energetics and dynamics of the complex, thus influencing gene expression. Dynamic effects appear to be particularly important, as they allow for the propagation of information through allosteric networks that span large distances. Although our simulations are only able to probe the sub-μs timescale, the dynamic differences observed at the dimer-tetramer and DNA/histone interfaces will likely be amplified on the ms timescale and result in these variants having altered nucleosome opening and DNA breathing motions.
These results also offer, to our knowledge, new insights into biochemical experiments that probed the mechanism of macroH2A. For example, Nusinow et al. showed that the L1-mutant is enriched in the inactive female X chromosome at nearly the same rate as the complete histone-domain of macroH2A (11). Point mutations demonstrated that enrichment was significantly increased by the two mutations that introduce additional bulk into the L1 loops, N38H and E41Y, whereas it was decreased by the Y39P mutation, which decreases the size of the L1 loop. Based on our results, we believe that larger side chains may help encourage the α2 bulging motion observed in each of the variant simulations, and therefore make the NCP more variant-like.
In another set of experiments, Chakravarthy et al. demonstrated that mutations to the L1 loops modulate the salt-dependent stability of the histone octamer (21). They showed that in both the L1-mutant and macroH2A-containing system, the histone octamer is stable down to 0.5 M NaCl, whereas the canonical and H2A.Z-containing structures dissociate into dimer and tetramer constituents in solutions below 1.1 M. In agreement with this, we observe a significantly more favorable interaction between the H2A L1 loops in the variant structures than in the canonical structure. Because the L1-L1 interface is the only location of dimer-dimer interaction, stability in this region translates to octamer stability.
The mechanisms of H2A.Z remain more elusive. Stability studies have been nonconclusive as some indicate that H2A.Z enhances stability (60), whereas others suggest that it destabilizes the nucleosome (25). Our simulations show H2A.Z nucleosome stabilities that are in agreement with the canonical system, despite their differing dynamics. These systems were constructed with identical sequences, except for H2A composition. Therefore, our findings support a mechanism that suggests that H2A.Z by itself has little-to-no effect on NCP stability. Instead, H2A.Z presence may be combined with other factors, such as PTMs or H3 variant presence, to alter particle stability (22, 61). Furthermore, the altered dynamics and locations of allosteric networks and hotspots between H2A.Z and canonical nucleosomes may result in different responses to these chromatin remodeling factors. The dimer reordering may also act to recruit transcriptional machinery to chromatin possessing large populations of H2A.Z, such as transcriptional starting sites.
Finally, we present a comprehensive analysis of the dynamic networks in the nucleosome. We observe that these networks are strongly affected by the dynamics of the L1 loops that are allosterically linked to a wide number of important regions in the nucleosome core. Using only small changes in their structure, variants are able to modify these networks to affect the function of the NCP. We hypothesize that this is a general mechanism that other chromatin remodeling factors may also utilize. For example, the finding that PTMs are enriched at residues with increased allosteric activity suggests that these perturbations may take advantage of dynamic networks to amplify their effects on chromatin and influence global NCP dynamics. In addition, by altering these networks, variants may be able to tune the responses of nucleosomes to specific PTMs. Future work to study the disparate effects of chromatin remodeling factors on dynamics in the nucleosome is required to fully understand the mechanisms of in vivo gene expression and regulation.
Author Contributions
S.B. performed the simulations. S.B. and J.W. designed the experiments, analyzed data, and wrote the manuscript.
Acknowledgments
The authors thank S. Chakravarthy for valuable discussions concerning the work in this study.
This research was supported by the National Institute of General Medical Sciences of the National Institutes of Health (Grant No. R15GM114758). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant No. ACI-1053575. In addition, this research used resources of the National Energy Research Scientific Computing Center, which is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Editor: Lois Pollack
Footnotes
Supporting Materials and Methods, twenty-five figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(15)04756-6.
Supporting Material
References
- 1.Luger K., Mäder A.W., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Davey C.A., Sargent D.F., Richmond T.J. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J. Mol. Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarthy S., Gundimella S.K., Luger K. Structural characterization of the histone variant macroH2A. Mol. Cell. Biol. 2005;25:7616–7624. doi: 10.1128/MCB.25.17.7616-7624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suto R.K., Clarkson M.J., Luger K. Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat. Struct. Mol. Biol. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- 5.Osley M.A., Fleming A.B., Kao C.F. Histone ubiquitylation and the regulation of transcription. Results Probl. Cell Differ. 2006;41:47–75. doi: 10.1007/400_006. [DOI] [PubMed] [Google Scholar]
- 6.Luger K., Dechassa M.L., Tremethick D.J. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 2012;13:436–447. doi: 10.1038/nrm3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman G.D., Poirier M.G. Post-translational modifications of histones that influence nucleosome dynamics. Chem. Rev. 2015;115:2274–2295. doi: 10.1021/cr500350x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 9.Bönisch C., Hake S.B. Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res. 2012;40:10719–10741. doi: 10.1093/nar/gks865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaytan A.K., Landsman D., Panchenko A.R. Nucleosome adaptability conferred by sequence and structural variations in histone H2A-H2B dimers. Curr. Opin. Struct. Biol. 2015;32:48–57. doi: 10.1016/j.sbi.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusinow D.A., Sharp J.A., Panning B. The histone domain of macroH2A1 contains several dispersed elements that are each sufficient to direct enrichment on the inactive X chromosome. J. Mol. Biol. 2007;371:11–18. doi: 10.1016/j.jmb.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarthy S., Bao Y., Luger K. Structural characterization of histone H2A variants. Cold Spring Harb. Symp. Quant. Biol. 2004;69:227–234. doi: 10.1101/sqb.2004.69.227. [DOI] [PubMed] [Google Scholar]
- 13.Pehrson J.R., Fried V.A. MacroH2A, a core histone containing a large nonhistone region. Science. 1992;257:1398–1400. doi: 10.1126/science.1529340. [DOI] [PubMed] [Google Scholar]
- 14.Choo J.H., Kim J.D., Kim J. Allele-specific deposition of macroH2A1 in imprinting control regions. Hum. Mol. Genet. 2006;15:717–724. doi: 10.1093/hmg/ddi485. [DOI] [PubMed] [Google Scholar]
- 15.Buschbeck M., Uribesalgo I., Di Croce L. The histone variant macroH2A is an epigenetic regulator of key developmental genes. Nat. Struct. Mol. Biol. 2009;16:1074–1079. doi: 10.1038/nsmb.1665. [DOI] [PubMed] [Google Scholar]
- 16.Chadwick B.P., Willard H.F. Cell cycle-dependent localization of macroH2A in chromatin of the inactive X chromosome. J. Cell Biol. 2002;157:1113–1123. doi: 10.1083/jcb.200112074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costanzi C., Stein P., Pehrson J.R. Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos. Development. 2000;127:2283–2289. doi: 10.1242/dev.127.11.2283. [DOI] [PubMed] [Google Scholar]
- 18.Guillemette B., Bataille A.R., Gaudreau L. Variant histone H2A.Z is globally localized to the promoters of inactive yeast genes and regulates nucleosome positioning. PLoS Biol. 2005;3:e384. doi: 10.1371/journal.pbio.0030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott D.W., Ivanova V.S., Ausió J. Characterization of the stability and folding of H2A.Z chromatin particles: implications for transcriptional activation. J. Biol. Chem. 2001;276:41945–41949. doi: 10.1074/jbc.M108217200. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarthy S., Patel A., Bowman G.D. The basic linker of macroH2A stabilizes DNA at the entry/exit site of the nucleosome. Nucleic Acids Res. 2012;40:8285–8295. doi: 10.1093/nar/gks645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarthy S., Luger K. The histone variant macro-H2A preferentially forms “hybrid nucleosomes.”. J. Biol. Chem. 2006;281:25522–25531. doi: 10.1074/jbc.M602258200. [DOI] [PubMed] [Google Scholar]
- 22.Marques M., Laflamme L., Gaudreau L. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics. 2010;5:267–272. doi: 10.4161/epi.5.4.11520. [DOI] [PubMed] [Google Scholar]
- 23.Roccatano D., Barthel A., Zacharias M. Structural flexibility of the nucleosome core particle at atomic resolution studied by molecular dynamics simulation. Biopolymers. 2007;85:407–421. doi: 10.1002/bip.20690. [DOI] [PubMed] [Google Scholar]
- 24.Böhm V., Hieb A.R., Langowski J. Nucleosome accessibility governed by the dimer/tetramer interface. Nucleic Acids Res. 2011;39:3093–3102. doi: 10.1093/nar/gkq1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijayalakshmi M., Shivashankar G.V., Sowdhamini R. Simulations of SIN mutations and histone variants in human nucleosomes reveal altered protein-DNA and core histone interactions. J. Biomol. Struct. Dyn. 2007;25:207–218. [PubMed] [Google Scholar]
- 26.Hornak V., Abel R., Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joung I.S., Cheatham T.E., 3rd Molecular dynamics simulations of the dynamic and energetic properties of alkali and halide ions using water-model-specific ion parameters. J. Phys. Chem. B. 2009;113:13279–13290. doi: 10.1021/jp902584c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryckaert J.-P., Ciccotti G., Berendsen H.J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977;23:327–341. [Google Scholar]
- 30.Bishop T.C. Molecular dynamics simulations of a nucleosome and free DNA. J. Biomol. Struct. Dyn. 2005;22:673–686. doi: 10.1080/07391102.2005.10507034. [DOI] [PubMed] [Google Scholar]
- 31.Mura C., McCammon J.A. Molecular dynamics of a kappaB DNA element: base flipping via cross-strand intercalative stacking in a microsecond-scale simulation. Nucleic Acids Res. 2008;36:4941–4955. doi: 10.1093/nar/gkn473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karatasos K. Self-association and complexation of the anti-cancer drug doxorubicin with PEGylated hyperbranched polyesters in an aqueous environment. J. Phys. Chem. B. 2013;117:2564–2575. doi: 10.1021/jp312125c. [DOI] [PubMed] [Google Scholar]
- 33.Gumbart J., Schulten K. Molecular dynamics studies of the archaeal translocon. Biophys. J. 2006;90:2356–2367. doi: 10.1529/biophysj.105.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darden T., York D., Pedersen L. Particle mesh Ewald: an N log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 35.Feller S.E., Zhang Y.H., Brooks B.R. Constant-pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- 36.Olsson M.H.M., Søndergaard C.R., Jensen J.H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 37.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 38.Stone, J. 1998. An efficient library for parallel ray tracing and animation. M.S. thesis. University of Missouri-Rolla, Rolla, MO.
- 39.Schrödinger, L. L. C. 2010. The PyMOL Molecular Graphics System. http://www.pymol.org. Accessed January 21, 2014.
- 40.McClendon C.L., Hua L., Jacobson M.P. Comparing conformational ensembles using the Kullback-Leibler divergence expansion. J. Chem. Theory Comput. 2012;8:2115–2126. doi: 10.1021/ct300008d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarabelli G., Grant B.J. Kinesin-5 allosteric inhibitors uncouple the dynamics of nucleotide, microtubule, and neck-linker binding sites. Biophys. J. 2014;107:2204–2213. doi: 10.1016/j.bpj.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lange O.F., Grubmüller H. Generalized correlation for biomolecular dynamics. Proteins. 2006;62:1053–1061. doi: 10.1002/prot.20784. [DOI] [PubMed] [Google Scholar]
- 43.Michaud-Agrawal N., Denning E.J., Beckstein O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Wart A.T., Durrant J., Amaro R.E. Weighted implementation of suboptimal paths (WISP): an optimized algorithm and tool for dynamical network analysis. J. Chem. Theory Comput. 2014;10:511–517. doi: 10.1021/ct4008603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagberg, A. A., D. A. Schult, and P. J. Swart. 2008. Exploring network structure, dynamics, and function using NetworkX. Gӓel Varoquaux, Travis, Vaught, and Jarrod Millman, eds. Proceedings of the 7thPython in Science Conference (SciPy2008), 11–15.
- 46.Brandes U. A faster algorithm for betweenness centrality. J. Math. Sociol. 2001;25:163–177. [Google Scholar]
- 47.Rice J.A. 3rd ed. Duxbury Press; Belmont, CA: 2007. Mathematical Statistics and Data Analysis. [Google Scholar]
- 48.Roe D.R., Cheatham T.E., 3rd PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9:3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 49.Cuervo A., Dans P.D., Fumagalli L. Direct measurement of the dielectric polarization properties of DNA. Proc. Natl. Acad. Sci. USA. 2014;111:E3624–E3630. doi: 10.1073/pnas.1405702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schutz C.N., Warshel A. What are the dielectric “constants” of proteins and how to validate electrostatic models? Proteins. 2001;44:400–417. doi: 10.1002/prot.1106. [DOI] [PubMed] [Google Scholar]
- 51.Miller B.R., 3rd, McGee T.D., Jr., Roitberg A.E. MMPBSA.py: an efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 52.Onufriev A., Bashford D., Case D.A. Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins. 2004;55:383–394. doi: 10.1002/prot.20033. [DOI] [PubMed] [Google Scholar]
- 53.Wood C.M., Nicholson J.M., Baldwin J.P. High-resolution structure of the native histone octamer. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2005;61:541–545. doi: 10.1107/S1744309105013813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shirts M.R., Chodera J.D. Statistically optimal analysis of samples from multiple equilibrium states. J. Chem. Phys. 2008;129:124105. doi: 10.1063/1.2978177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas M., Langowski J., Bishop T.C. Atomistic simulations of nucleosomes. WIREs Comput. Mol. Sci. 2013;3:378–392. [Google Scholar]
- 56.Biswas M., Voltz K., Langowski J. Role of histone tails in structural stability of the nucleosome. PLOS Comput. Biol. 2011;7:e1002279. doi: 10.1371/journal.pcbi.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khare S.P., Habib F., Galande S. HIstome—a relational knowledgebase of human histone proteins and histone modifying enzymes. Nucleic Acids Res. 2012;40:D337–D342. doi: 10.1093/nar/gkr1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hornbeck P.V., Kornhauser J.M., Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lange O.F., Grubmüller H. Full correlation analysis of conformational protein dynamics. Proteins. 2008;70:1294–1312. doi: 10.1002/prot.21618. [DOI] [PubMed] [Google Scholar]
- 60.Hoch D.A., Stratton J.J., Gloss L.M. Protein-protein Förster resonance energy transfer analysis of nucleosome core particles containing H2A and H2A.Z. J. Mol. Biol. 2007;371:971–988. doi: 10.1016/j.jmb.2007.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin C., Felsenfeld G. Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev. 2007;21:1519–1529. doi: 10.1101/gad.1547707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.