Abstract
Objectives:
To test the possible association between oxytocin and melatonin levels with the severity of social and cognitive dysfunctions, and to study the correlation between these parameters in children with autism.
Methods:
A case-control study was carried out in the Department of Chemistry and Biochemistry, College of Medicine, Al-Nahrain University, Baghdad, Iraq. The study was performed on 60 male autistic patients recruited from the Pediatric Department of Al-Sader General Hospital, Baghdad, Iraq between November 2014 and April 2015. The levels of oxytocin and melatonin were measured in the serum of these autistic male patients, and categorized as mild, moderate, and severe (20 patients each), and was compared with 26 age- and gender-matched control subjects.
Results:
The data indicated that the levels of oxytocin (44.72 ± 36.1 µIU/mL) and melatonin in patients (23.08 ± 10.41 pg/mL) were significantly lower (p<0.05) than that of age-matched (102.1 ± 34.31 µIU/mL) and gender-matched controls (53.05 ± 38.38 pg/mL). These parameters were remarkably associated with the severity of the disease that was indicated by the significant decrease in the levels of oxytocin (47 ± 25.47 µIU/mL) and melatonin in moderate (20 ± 6.14 pg/mL), and patients with severe oxytocin (27.92 ± 10.23 µIU/mL) and patients with severe melatonin (21.69 ± 7.02 pg/mL) when compared with mild autistic patients with oxytocin (59.22 ± 27.32 µIU/mL) and melatonin (27.55 ± 14.71 pg/mL). These 2 parameters showed a significant positive correlation with each other in moderate (r=0.513; p=0.021), and severe patients (r=0.598; p=0.005).
Conclusion:
Receiver operating characteristic analysis revealed that oxytocin can be considered as a good diagnostic marker in severe autistic patients while melatonin can be considered as a good diagnostic marker in all autistic subgroups. This study proves the possibility of using oxytocin and melatonin in the diagnosis, and as markers of autism severity.
Autism is a neurodevelopmental disorder that is characterized by deficits in social interaction, social communication, restricted and repetitive interests, and behavioral patterns. Symptoms of autism manifest in children usually by the age of 3.1 It is also characterized by impaired language and social skills, and restricted areas of interest. Additional features may include poor eye contact, repetitive behavior, sensory modulatory dysfunction, and varying levels of cognition and motor disturbances.1 One of the most problems in autistic patients is that the clinical signs usually appear and manifest at the age of 3 years, but prospective studies of infants at risk have demonstrated that deficits in social responsiveness, communication, and play could be present early, at the age of 6-12 months.2 Many parameters including hormones, neurotransmitters, and immunological mediators may be involved in the severity and pathogenesis of autism. Oxytocin is involved in the regulation of repetitive and affiliative behaviors, which is the key features of autism; it is believed that oxytocin may play a role in autism. Some clinical studies3,4 report that oxytocin may be considered a promising therapy for psychiatric diseases, such as depression, schizophrenia, anxiety disorders, and autism. Studies3,4 show that children with autism may have lower plasma oxytocin compared with age- and gender-matched control subjects. Another study5 also assumed that the oxytocin and arginine vasopressin systems in the brain are promising pharmacotherapeutic targets to enhance social behavior and to reverse social deficits. Melatonin (MT) is a neurohormone that affects and regulates the circadian sleep-wake rhythm. Melatonin is mainly synthesized by the pinealocytes in the pineal gland.6 Melatonin is synthesized from tryptophan, which is subjected to hydroxylation followed by a decarboxylation to form serotonin, which is in turn, acetylated and then O-methylated by to yield MT.2 Melatonin regulates many physiological functions with pleiotropic effects on the immune system. Despite the large number of research that prove the role of MT as an immunomodulatory compound, it still unclear how it regulates the immune system activity.7 Whereas some authors illustrated that MT may be considered as an immunostimulant, many other studies7 have also described anti-inflammatory properties. Carrillo-Vico et al’s7 review supports the idea of MT as an immune buffer, acting as a stimulant under basal, or immunosuppressive conditions, or may act as an anti-inflammatory mediator in the presence of exacerbated immune responses, such as acute inflammation.7 Recent studies3,8 showed an abnormal decrease in MT secretion in children subjected to autism. Melatonin is of interest in autism due to its possible role in neurodevelopment and its effect on sleep-wake rhythm in autistic patients. Additionally, central and peripheral changes in serotonin in autism have been widely studied and well-established, and as mentioned, MT synthesized from serotonin in 2 steps in the pineal gland and the gut.8 Previous studies3,9 showed an effect of oxytocin on the level of the precursor of MT; serotonin that may provide an explanation on the suspected effect of oxytocin on the level of MT.9 In this present study, we focused on 2 hormones that was reported to have neurological and immunological effects (oxytocin and MT), and the aim was to investigate the possible roles of these 2 hormones in the diagnosis and prognosis of autism.
Methods
A case-control study was carried out on 60 male patients who had autism recruited from the Pediatric Department of Al-Sader General Hospital, Baghdad, Iraq between November 2014 and April 2015. Their ages ranged between 3 and 13 years (mean ± standard deviation [SD] 7.28 ± 2.89 years). The control group comprised of 26 age- and gender-matched apparently healthy children with mean ± SD age of 6.92 ± 2.59 years. The patients met the diagnostic criteria of autism according to the 5th edition of Diagnostic and Statistical Manual of Mental Disorders,10 and sub-grouped into mild (n=20), moderate (n=20), and severe autistic patients (n=20). The control children were normally developing, healthy, unrelated to the autistic children, and without any of the exclusion criteria, which include: any disorder that may be related to the incidence of autism spectrum disorder (for example, Rett syndrome, focal epilepsy); any neurologic problems involving pathology above the brain stem, except uncomplicated non-focal epilepsy; any inherited medical conditions involving the central nervous system (CNS), even if the link with autism is not fully established; any clinically significant defects in vision or hearing; any conditions that may be attributed to the autism picture (for example, severe nutritional or psychological deprivation); any active administration therapy or other agents; history of upper respiratory tract diseases, previous inflammatory diseases, allergic history; and they had no clinical indications of any infectious disease or neuropsychiatric disorders. All participants had normal results for urine analysis and with inclusion criteria that include that the patients had no associated neurological diseases (such as, cerebral palsy, tuberous sclerosis); no associated metabolic disorders (for example, phenylketonuria) due to these comorbidities associated with autism may affect the results of serum serotonin, and that may influence the levels of MT and all participants were not receiving any medications.
The local Ethical Committee of the College of Medicine, University of Al-Nahrain, Baghdad, Iraq, approved this study. In addition, the parents or the legal guardians of the investigated subjects signed an informed written consent of participation in the study. This study was conducted according to the Helsinki principles.
Sample collection
Five milliliters of blood samples were collected from overnight fasting subjects (autistic children and control) in plain tubes at 9 A.M. Serum was obtained and kept at -20°C until analysis time for measurement of serum MT and oxytocin.
Biochemical assays. Oxytocin measurement
The enzyme-linked immunosorbent assay (ELISA, Cusabio, China) assay kit was utilized. This immunoassay kit allows the in vitro quantitative determination of human oxytocin concentrations in serum. The microtiter plate provided in this kit had been pre-coated with an antibody specific to oxytocin. Standards or samples were then added to the appropriate microtiter plate wells with a horseradish peroxidase (HRP)-conjugated antibody and incubated. Substrate solutions were then added to each well. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution and the color change was measured spectrophotometrically at 450 nm. The oxytocin concentration in the samples was then determined by comparing the optical density (OD) of the samples with the standard curve.
Melatonin measurement
The ELISA employs the quantitative sandwich enzyme immunoassay technique. Antibody specific for MT has been pre-coated onto a microplate. Standards and samples were pipetted into the wells, and any MT present was bound by the immobilized antibody. After removing any unbound substances, a biotin-conjugated antibody specific for MT was added to the wells. After washing, avidin conjugated HRP was added to the wells. Followed by a wash to remove any unbound avidin-enzyme reagent, a substrate solution was added to the wells and color develops in proportion to the amount of MT bound in the initial step. The color development was stopped and the intensity of the color was measured spectrophotometrically at 450 nm. The concentration of MT in the serum was then determined by comparing the samples’ OD with the standard curve.
Statistical analysis
Results were expressed as mean ± SD and all statistical comparisons were carried out by means of independent t-test, and analysis of variance (ANOVA) test with p≤0.05 considered as statistically significant.11 The correlation was performed between all parameters using Pearson correlation test,11 all statistical analysis was carried out using the Statistical Package for Social Sciences version 20 (IBM Corp., Armonk, New York, USA). Receiver operating characteristic (ROC) analysis was performed as a comprehensive way to assess the accuracy of the studied markers. The area under the curve (AUC) provides a useful tool to compare different biomarkers. Whereas an AUC value close to one indicates an excellent diagnostic and predictive marker, a curve that lies close to the diagonal (AUC=0.5) has no diagnostic significance. The AUC close to one is always accompanied by satisfactory values of specificity and sensitivity of the biomarkers.12
Results
Levels of oxytocin, and MT were compared between patients with different severity of autism (mild, moderate, or severe) and age-matched control subjects. Data are presented as a mean±SD of 60 autistic patients divided into 3 subgroups; mild, moderate, and severe (20 patients each) compared with 26 controls, and the significant difference between both groups and subgroups of patients with autism is presented in Table 1. It was noticed that the 2 measured parameters differed significantly either between patients and controls, or between subgroups of patients showing different levels of cognitive and social impairment (mild, moderate, and severe). From Table 1 it was demonstrated that there was a significant (p<0.001) decrease in the level of oxytocin and MT in autistic patients in comparison with controls. Oxytocin levels showed a non-significant (p=0.107) difference between mild autistic patients and control, while significant differences were found between moderate (p=0.029) and severe (p=0.003) autistic patients in comparison with controls. On the other hand, a non-significant (p=0.356) difference was found between moderate and mild autistic patients, and a highly significant (p=0.004) decrease in oxytocin level was shown in severe autistic patients in comparison with moderate autistic patients.
Table 1.
Oxytocin and melatonin levels in autistic and control groups according to a study in Bahrain.
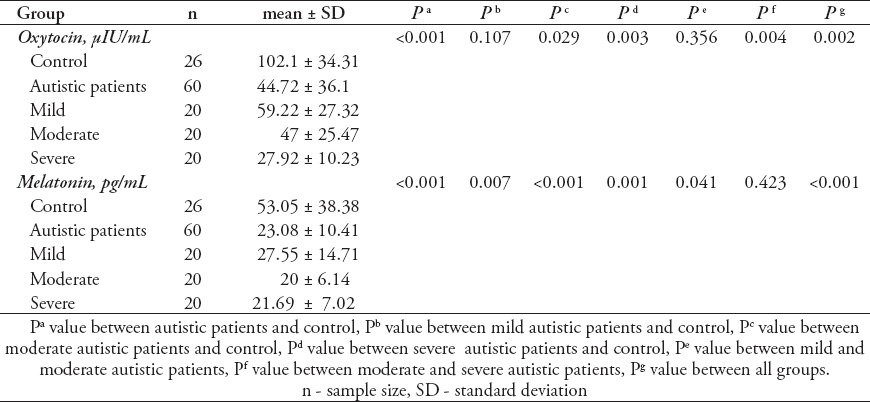
Melatonin levels showed a highly significant difference (p<0.01) between mild (p=0.007), moderate (p<0.001), and severe (p=0.001) autistic patients in comparison with controls. A significant (p=0.041) difference was found between moderate and mild autistic patients, while a non-significant difference was found between severe and moderate autistic patients.
In Table 2 a significant decrease was found in the level of oxytocin in control subjects from unrelated parents in comparison with those with related parents. Autistic patients (n=60) show a non-significant difference between patients from related and unrelated parents, and the only significant difference in the levels of oxytocin and MT was found between patients with related and unrelated parents in moderate autistic patients groups (p=0.024 for oxytocin, p=0.02 for MT). The correlation results between Oxytocin and MT in control, whole autistic patients, mild, moderate, and severe autistic patients were listed in Table 3 that showed a positive significant correlation between oxytocin and MT in moderate autistic patients (r=0.513; p=0.021), and a highly significant positive correlation between oxytocin and MT in severe autistic patients (r=0.598; p=0.005). On the other hand, non-significant correlations were found between oxytocin and MT in control subjects, whole autistic patients, and mild autistic patients. It was demonstrated that more than 76% (46 patients) of autistic patients showed sleep problems. The severe autistic patients showed the highest number patients with sleep problems (18 patients) in comparison with moderate (15 patients), and mild autistic patients (13 patients).
Table 2.
Oxytocin and melatonin levels of control and autistic groups with related and unrelated parents according to a study in Bahrain.
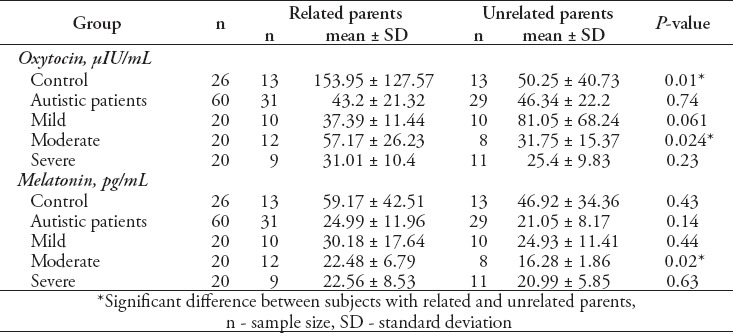
Table 3.
Correlations between oxytocin and melatonin in different study groups.
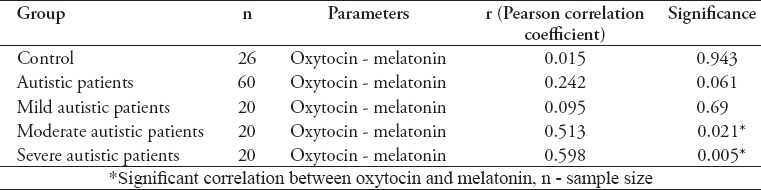
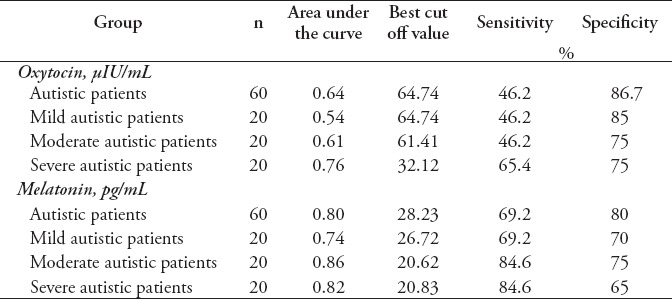
Table 4 demonstrates the ROC analysis data as AUC, cut off values, specificity, and sensitivity of the measured parameters. Oxytocin exhibited AUC values higher than 0.6 in whole autistic patients (AUC=0.64), moderate (AUC=0.61), and severe autistic patients (AUC=0.76) with satisfactory values of accuracy presented as good specificity and sensitivity. Melatonin exhibited AUC values higher than 0.7 in whole autistic patients (AUC=0.8), mild (AUC=0.74), moderate (AUC=0.86), and severe autistic patients (AUC=0.82) with satisfactory values of accuracy presented as good specificity and sensitivity.
Table 4.
Receiver operating characteristic curve of oxytocin and melatonin in all studied groups.
Discussion
Many biomarkers might be involved in the etiology, pathogenesis, diagnosis, and prognosis of autism. Most of them have an effect on behavior, cognition, and other neurological problems.3 This study depends on information obtained from measuring serum oxytocin and MT that reflect the physiological activity and pathology in various organs, including the CNS. In humans, approximately 500 mL of cerebrospinal fluid is absorbed into the blood daily, making blood a suitable source of neurodevelopmental or neurodegenerative disease biomarkers.13 Hormones and neurotransmitter assessment can be a useful tool in any clinical practice; immune system and nervous system activity must be considered and examined as a single system functioning in parallel. It is well-established that neurological and immunological abnormalities exist in autistic individuals; however, the relationship between hormonal, neural, and immune function has not been emphasized enough.14 The current study involves the assessment of the levels of 2 hormones, oxytocin and MT that have a complex neurological and immunological effect on the neurotransmitters15 and other immunological mediators.7
Oxytocin is involved in the regulation of affiliative and repetitive behaviors, which are important features of autism.14 Oxytocin releasing occurs into blood and within distinct brain regions in response to stress and some social stimuli, and it also believed to have an anti-depressant-like effect.15 Some clinical trials suggest that oxytocin can be a promising drug for psychiatric diseases, such as depression, anxiety disorders, schizophrenia, and autism.16 Therapeutically, oxytocin may also have a potential role in the treatment of major depressive disorders; on the other hand oxytocin administered into blood does not readily cross the blood-brain barrier.16
Table 1 shows that autistic children involved in this study have a significantly lower plasma oxytocin level (p<0.05) compared with age- and gender-matched control subjects. This is in agreement with previous studies that demonstrate a significant lower oxytocin levels in autistics compared with control subjects and suggest that oxytocin may have a crucial role in the symptoms of autism.14,17 Previous studies report that oxytocin has an important role in the regulation of behavior in animals, but has not yet been examined in depth in autistic children.18
The level of oxytocin in autistic children examined in this study showed a non-significant decrease (p>0.05) in mild autistic patients, a significant decrease (p<0.05) in moderate autistic patients, and a highly significant decrease (p<0.01) in severe autistic patients compared with control as shown in Table 1. On the other hand, a non-significant decrease (p>0.05) in moderate autistic patients in comparison with mild autistic patients was shown and a highly significant (p<0.01) decrease was found in severe autistic patients when compared with moderate autistic patients. These results may prove the possible role of oxytocin in the diagnosis of autism, assessment of severity, and also, for prognostic purposes.
The results of this study were in agreement with other studies 9,14 that demonstrated a correlation between low oxytocin level and severity of autism, and also consistent with other previous researches those provide preliminary support for a link between social anxiety severity and plasma oxytocin.19,20 Other studies3,4,5,16,18 suggest that the brain oxytocin and arginine vasopressin systems are promising pharmaco-therapeutic targets to improve social behavior and to reverse social deficits.18
The possible link between autism and low oxytocin level were recently explained by Quattrocki and Friston21 who proposed that oxytocin plays an important role in socio-sexual behavior. They also proposed that oxytocin encodes the saliency or precision of interoceptive signals and enables the neuronal plasticity necessary for acquiring a generative model of the emotional and social. A defect in oxytocin system in infancy could, therefore, help to explain the marked deficits in language, and social communication, as well as the sensory, autonomic, motor, behavioral, and cognitive abnormalities seen in autism.21 Alabdali et al14 reported that genetic evidence may also involve in the explanation of the role of oxytocin in the pathophysiology of autism. In another study,22 a link was found between the autism phenotype and both the oxytocin gene (OXT, located at 20p13), and the oxytocin receptor gene (OXTR, located at 3p26).
In the present study a non-significant difference was found in the level of oxytocin between patients with related and unrelated parents that may indicate the absence of the genetic basis of autism in autistic participants of this study. These results were inconsistent with other studies3,9,12,14 that provide an evidence for the genetic role in the pathogenesis of autism. On the other hand, in this study a significant decrease in oxytocin level was found in moderate autistic patients from unrelated parents when compared with patients from related parents, this may provide information required to be further studied in parallel with a genetic study for oxytocin and oxytocin receptor genes.
Melatonin is an important hormone involved in autism due to its apparent role in neurodevelopment and reports of sleep-wake rhythm disturbances in individuals with autism.2 Most of autistic patients that subjected in present study showed sleep problems. As demonstrated, approximately 76.6% of autistic patients examined in this study showed sleep problems. These sleep problems were parallel with the low level of MT in the autistic patients that was found in this study. The level of MT in autistic patients was significantly (p<0.05) lower than that of control as shown in Table 1.
The possible causes of the reduction in MT level can be attributed either to the central and peripheral alterations in serotonin levels in autism that were widely reported previously2 or to the defect in the synthesis of MT.23 Previous studies8,23 of MT production in autistic disorder were also limited by small sample sizes and were not entirely consistent, but all reported an abnormal MT production,2 which is inconsistent with the results of the current study.
Several studies are in accordance with the results obtained in the present study, some of them owned the cause of decreased MT levels to the defect in the N-acetylserotonin O-methyltransferase (ASMT), which is also known as hydroxyindole-O-methyltransferase, the limiting enzyme in the synthesis of MT.24 The defect in ASMT may be caused by a genetic defect in the enzyme expression.23,24
In the current study, MT level in moderate autistic patients from related parents showed a significant higher level than that of patients from unrelated parents as shown in Table 2. On the other hand, other patient groups showed a non-significant decrease in MT level in patients from unrelated parents in comparison with patients from related parents. This finding requires further studies that include measuring of the serotonin level and genetic assessment of the ASMT gene to illustrate the possible genetic defect.
The important findings in this study were the significant (p<0.05) decrease in the levels of MT in moderate and severe autistic patients in comparison with mild patients. This decrease may provide information regarding the involvement of MT in the severity and participation of MT in the pathogenicity and also the possible use of MT as a marker for diagnosis and prognosis of autism. The results of this study were also in agreement with Alabdali et al14 that stated a decrease in the level of oxytocin in the autistic patients and also, a significant decrease in the level of serotonin, from which MT synthesized.
Interestingly, there was a significant positive correlation between oxytocin and MT in moderate autistic patients (r=0.513; p=0.021) and highly significant positive correlation between oxytocin and MT in severe autistic patients (r=0.598; p=0.005), while mild patients with autism showing a non-significant correlation (p>0.05). This may prove the finding of the involvement of MT and oxytocin in the severity or may be in the pathogenesis of autism, and also provide evidence on the usefulness of oxytocin and MT as markers for diagnosis and prognosis in autistic patients.
The possible explanation of this positive correlation between oxytocin and MT may be due to direct effect of oxytocin on peripheral serotonin levels, which is the precursor of MT,25 while other studies showed a reciprocal relationships between brain oxytocin and serotonin systems during development, Hammock et al9 found that OT and 5-HT were negatively correlated with each other and this relationship was most prominent in children under 11-years-old.
The negative correlation between oxytocin and serotonin levels in the human data can be interpreted in a number of ways. Oxytocin could affect increased gastrointestinal synthesis of serotonin, increased platelet serotonin uptake, or decreased serotonergic release from platelets,14 thus, low level of oxytocin may cause a reduction in serotonin synthesis that in turn cause a decrease in the level of MT. On the other hand, another study26 that conducted on experimental animals found that the nocturnal increase in MT may influence pituitary hormone secretion, and found that MT in physiological concentrations stimulates the release of oxytocin. This may be another explanation of the positive correlation between oxytocin and MT.
Receiver operating characteristic in Table 4 showed that serum oxytocin test in autistic patients was a poor diagnostic and predictive marker (AUC=0.64) with low sensitivity (46.2%), and good specificity (86.7%). Oxytocin test showed increase in the AUC value with the increase in the severity of autism. Oxytocin test showed to be a fair diagnostic and predictive marker (AUC=0.76) in severe autistic patients with an acceptable sensitivity (65.4%) and specificity (75%). On the other hand, Table 4 showed that serum MT test in autistic patients was a good diagnostic and predictive marker (AUC=0.8) with an acceptable sensitivity (69.2%) and good specificity (80%). Melatonin test showed an increase in the AUC value with the increase in the severity of autism. Melatonin test showed to be a good diagnostic and predictive marker (AUC=0.86) in moderate autistic patients with good sensitivity (84.6%) and an acceptable specificity (75%), and also showed to be a good diagnostic and predictive marker (AUC=0.82) in severe autistic patients with good sensitivity (84.6%) and an acceptable specificity (65%).
The major limitations of this case-control study include that the number of children examined was small that affect the statistical power of the study, and the second limitation include that the performance mostly at only a single hospital. So further work is needed on a larger scale from different areas of Iraq to address the indicated limitations.
In conclusion, the levels of oxytocin and MT in autistic patients showed a significant lower levels than that of control and the decrease in the levels of oxytocin and melatonin was in parallel with the severity of autism. This study also revealed that there was a significant positive correlation between these 2 parameters that can be used in future work as a marker for diagnosis and prognosis, and to assess the severity of autism. The results obtained from ROC curve demonstrated that these parameters can be used in future as good markers for diagnosis and prognosis, and to assess the severity of autism.
Acknowledgment
The authors gratefully acknowledge the staff of the Department of Chemistry and Biochemistry, College of Medicine, Al-Nahrain University, Baghdad, Iraq for their facilities in performing this study, and the Head and staff members of the Pediatric Department, as well as the Head and staff members of the laboratories at Al-Sader General Hospital, Baghdad, Iraq.
Footnotes
Related Articles.
Ebtissam Z. Murshid. Dental knowledge of educators and healthcare providers working with children with autism spectrum disorders. Saudi Med J 36: 1477-1485.
Al-Odaib AN, Al-Sedairy ST. An overview of the Prince Salman Center for Disability Research scientific outcomes. Saudi Med J 2014; 35 Suppl 1: S75-S90.
Almaramhy HH, Allama AM. Indicators for surgical intervention in thoracic empyema in children. Saudi Med J 2015; 36: 1061-1066.
References
- 1.Brentani H, Paula CS, Bordini D, Rolim D, Sato F, Portolese J, et al. Autism spectrum disorders: an overview on diagnosis and treatment. Rev Bras Psiquiatr. 2013;35(Suppl 1):S62–S72. doi: 10.1590/1516-4446-2013-S104. [DOI] [PubMed] [Google Scholar]
- 2.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggeri B, Sarkans U, Schumann G, Persico AM. Biomarkers in autism spectrum disorder: the old and the new. Psychopharmacology (Berl) 2014;231:1201–1216. doi: 10.1007/s00213-013-3290-7. [DOI] [PubMed] [Google Scholar]
- 4.Husarova V, Lakatosova S, Pivovarciova A, Bakos J, Durdiakova J, Kubranska A, et al. Brief report: Plasma oxytocin is lower in children with Asperger syndrome and associated with autistic trait attention to detail. Open Journal of Psychiatry. 2013;3:399–402. [Google Scholar]
- 5.Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, et al. Arginine Vasopressin Is a Blood-Based Biomarker of Social Functioning in Children with Autism. PLoS One. 2015;10:e0132224. doi: 10.1371/journal.pone.0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Faria Poloni J, Feltes BC, Bonatto D. Melatonin as a central molecule connecting neural development and calcium signaling. Funct Integr Genomics. 2011;11:383–388. doi: 10.1007/s10142-011-0221-8. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8638–8683. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tordjman S, Anderson GM, Bellissant E, Botbol M, Charbuy H, Camus F, et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology. 2012;37:1990–1997. doi: 10.1016/j.psyneuen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Hammock E, Veenstra-VanderWeele J, Yan Z, Kerr TM, Morris M, Anderson GM, et al. Examining autism spectrum disorders by biomarkers: example from the oxytocin and serotonin systems. J Am Acad Child Adolesc Psychiatry. 2012;51:712–721. doi: 10.1016/j.jaac.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington (DC): American Psychiatric Publishing; 2013. [Google Scholar]
- 11.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15:625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]
- 12.Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 13.Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129:3042–3050. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 14.Alabdali A, Al-Ayadhi L, El-Ansary A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J Neuroinflammation. 2014;11:4. doi: 10.1186/1742-2094-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slattery DA, Neumann ID. Oxytocin and Major Depressive Disorder: Experimental and Clinical Evidence for Links to Aetiology and Possible Treatment. Pharmaceuticals (Basel) 2010;3:702–724. doi: 10.3390/ph3030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzaki M, Matsushita H, Tomizawa K, Matsui H. Oxytocin: a therapeutic target for mental disorders. J Physiol Sci. 2012;62:441–444. doi: 10.1007/s12576-012-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, et al. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor a mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Horm Behav. 2014;65:57–65. doi: 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Lukas M, Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. 2013;21:219–247. doi: 10.1097/HRP.0b013e3182a75b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Masry N, Soliman A, Abdel Moety H. Alterations of prolyl endopeptidase, oxytocin and vasopressin activity in the plasma of autistic children. Current Psychiatry. 2010;17:31–37. [Google Scholar]
- 21.Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovey D, Zettergren A, Jonsson L, Melke J, Anckarsäter H, Lichtenstein P, et al. Associations between oxytocin-related genes and autistic-like traits. Soc Neurosci. 2014;9:378–386. doi: 10.1080/17470919.2014.897995. [DOI] [PubMed] [Google Scholar]
- 23.Talarowska M, Szemraj J, Zajączkowska M, Gałecki P. ASMT gene expression correlates with cognitive impairment in patients with recurrent depressive disorder. Med Sci Monit. 2014;20:905–912. doi: 10.12659/MSM.890160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kripke DF, Nievergelt CM, Tranah GJ, Murray SS, McCarthy MJ, Rex KM, et al. Polymorphisms in melatonin synthesis pathways: possible influences on depression. J Circadian Rhythms. 2011;9:8. doi: 10.1186/1740-3391-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marazziti D, Baroni S, Giannaccini G, Betti L, Massimetti G, Carmassi C, et al. A link between oxytocin and serotonin in humans: Supporting evidence from peripheral markers. Eur Neuropsychopharmacol. 2012;22:578–583. doi: 10.1016/j.euroneuro.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Roszczyk M, Juszczak M. Forskolin-stimulated vasopressin and oxytocin release from the rat hypothalamo-neurohypophysial system in vitro is inhibited by melatonin. Endokrynol Pol. 2014;65:125–131. doi: 10.5603/EP.2014.0018. [DOI] [PubMed] [Google Scholar]


