Abstract
Objectives:
To assess the correlation between hormone receptor status (HRS) and age, and its significance as a predictor of outcome in patients with breast cancer (BC).
Methods:
This retrospective review was conducted on 109 patients diagnosed with BC at Salmaniya Medical Complex, Manama, Bahrain from 2010-2013. Patients were divided into 2 age groups; under and over 40 years, and were analyzed for tumor histology, lymph node status, stage, and HRS.
Results:
Younger patients with BC were more likely to be of higher stage, grade, and of larger size. Older women were more likely to be estrogen receptor (ER) positive (72.6% versus 55.3%), and progesterone receptor (PR) positive (71% versus 53.2%) (p=0.03). The human epidermal growth factor receptor (HER)-2 over-expression was seen more in younger women (51% versus 40%) (p=0.2). Younger patients had higher lymph node metastases (88.6% versus 56.1%) (p=0.0004), and higher distant metastases (26.7% versus 6.8%) (p=0.005). The HER-2 over-expression strongly correlated with lymph node status. A total of 63.4% of lymph node positive patients had HER-2 over-expression compared with only 13.3% of lymph node negative patients (p<0.00001).
Conclusion:
Breast cancer is more aggressive and advanced in younger women, a fact that can be significantly attributed to under expression of ER and PR, and over expression of HER-2, which also correlates well with lymph node status, as a measure of aggressiveness. Further studies should evaluate the genetic profile of BC in such population to improve their outcomes.
Breast cancer (BC) is by far the most common invasive cancer in women worldwide. It compromises 25% of invasive cancers in women,1 and 16% of all female cancers.2 Bahrain has a very high incidence rate of BC. In Bahrain, it accounts for 37.2% of all cancers in females, and 20% of all new cancer diagnoses,3 with an age-standardized rate (ASR) per 100,000 women of 44.4 in 2010,4 which is the highest in all of Gulf Cooperation Council (GCC), and among the highest in the world.3 The mean age at diagnosis in Bahrain was 50.7 years in 2014.4 The incidence of BC correlates strongly with age, with the highest incidence rates being observed in older women, and only 6% occurring in women under 40 years old.5 However, BC is usually more aggressive and advanced in the younger age groups.6 The exact reasons remain unclear, and not fully understood. Unfortunately, despite recent advances in the management of BC that allowed for the ASR of incidence to fall from 58.2 per 100,000 in 2000 to 44.4 per 100,000 in 2010,4 mortality rates still remain high. The 5-year survival for BC in Bahrain was 63%4 compared with 80-90% in most developed countries.1 Survival in BC is thought to be dependent on many factors, the most important ones being the histology, tumor size, grade, lymph node status, hormone receptor status (HRS) (estrogen [ER] and progesterone [PR]) and human epidermal growth factor receptor (HER)-2 over-expression, as well as the stage at presentation. Many tools have been used to determine prognosis following surgery for BC, such as the Nottingham Prognostic Index (NPI), which is calculated using 3 pathological criteria: size of lesion; number of involved lymph nodes; and grade of tumor. Older studies have suggested that BC in women under 40 often is of a higher stage and grade, larger size, and has more metastases to the lymph nodes.6-9 Tumors have also been found to be more invasive in younger women with more distant metastases at presentation.7-11 It has also been observed that among the different molecular subtypes of BC; triple negative and HER-2 type BCs are more common in younger women, which studies suggest are more aggressive than the other types.9,10 However, the aggressiveness of these subtypes of tumors is not well explained, and is yet to be fully understood. In this study, we will try to explain the aggressiveness of BC in the younger age group using lymph node status as a measure of aggressiveness, we will be looking at the differences in HRS (ER and PR), and HER-2 over-expression between women under, and over 40 years of age.
Methods
We retrospectively reviewed the data for all BC patients diagnosed at the Salmaniya Medical Complex, Manama, Bahrain from 2010 to 2013. Inclusion criteria were women diagnosed with BC who visited the hospital between 2010 through 2013. The exclusion criteria was a lack of sufficient data as described below. There was a total of 109 patients seen. All types of histologically invasive carcinoma were included. Patients were divided into 2 groups based on age, namely; women under 40, and women over 40 years of age. Data was obtained retrospectively from clinical notes, histology, and radiology reports. Data recorded included patient’s age, tumor type (ductal or other type), size (T1: <20 mm, T2: 20-50 mm, T3: >50 mm, and T4: >50 mm or fixed to chest wall), lymph node involvement (N0: no nodes, N1: <3 nodes, N2: 4-9 nodes, N3: >10 nodes), Stage (I, II, II IV, and unstaged), Grade (I, II, and III), ER and PRs status, HER-2/neu receptor over-expression, and NPI for prognosis. Tumor size was measured along the greatest diameter in the histology specimen. Hormone receptors were considered positive when the receptor concentration was greater than 10% (>30/300). The HER-2 expression was considered positive if it stained +3 by immunohistochemistry, or positive by fluorescence in situ hybridization. All laboratory analysis was carried out in the same setting and conditions, and hence, eliminated any bias introduced by the method. We used lymph node positivity as a measure for tumor aggressiveness, and tried to show a correlation between HRS and lymph node positivity by comparing the status of lymph node positive women with lymph node negative women. The different molecular subtypes of tumors were analyzed in our patients. These subtypes being luminal A (ER and/or PR positive, HER-2 negative), luminal B (ER and/or PR positive, HER-2 negative), HER-2 type (ER and PR negative, HER-2 positive), and triple negative (ER, PR, and HER-2 negative). A review of articles was conducted using PubMed and the Cochrane Library. Only papers from later than 2009 were included in the references. The study followed the rules and regulations set by the Ministry of Health in Bahrain, which closely follows the principles of the Helsinki Declaration. Due to the retrospective nature of the study, no further ethics approval was necessary.
The statistical analysis for different variables were performed using chi-square tests. Significance was set at p<0.05. Data was analyzed using Excel and Statistical Package for Social Sciences version 23 (IBM Corp., Armonk, NY, USA).
Results
A total of 109 patients were included in this study, and divided into 2 age groups, namely; women under 40 (referred to as ‘younger’) (n=47, 43%), and women over 40 (referred as ‘older’ women) (n=62, 57%). However, this does not accurately reflect the age distribution of BC in Bahrain, as lots of patients in the older group had to be excluded due to lack of sufficient data. The mean age at diagnosis in the younger group was 36 years old (range: 28-39), and in the older group it was 55 years old (range: 40-85). Quite a few of the tumor characteristics were significantly different between the 2 age groups. Younger patients were more likely to be of higher stage and grade, larger size, ER and PR receptor positive, and HER-2 receptor negative (Figures 1, 2, & 3) (p<0.05). All these point to a worse prognosis in younger women as shown in Table 1, along with the relevant chi-square test and p-value results. The most common invasive carcinoma in both groups by far was ductal carcinoma (87%, no special type). Other types of carcinomas included: lobular, mucinous, papillary, and medullary. In women under 40, 21% had carcinomas of non-ductal type compared with only 6.5% of women over 40 (p=0.02). Older women were more likely to be ER positive (72.6% versus 55.3%), and PR positive (71% versus 53.2%) than younger women (p=0.03). The HER-2 over-expression was seen more in younger women (51% versus 40%), however this was not statistically significant (p=0.2) (Figure 3). Younger patients were much more likely to have lymph node metastases (88.6% versus only 56.1% in older patients), and this result was very significant (p=0.0004) (Table 2) (Figure 4). This result allowed us to more accurately find a correlation between HRS and lymph node positivity. In younger women, 12 out of 45 (26.7%) had distant metastases at presentation compared with 4 out of 59 (6.8%) in older women (p=0.005) (Table 2). The most common metastases were to the bone and liver. There was a strong correlation noted between ER and PR negativity and lymph node positivity. There was 31% of lymph node positive patients that were ER negative, compared with 13% of lymph node negative patients (p=0.06). Similarly, 35% of lymph node positive patients were PR negative compared with 10% of lymph node negative patients (p=0.01) (Table 3) (Figure 5). The HER-2 positivity was also strongly associated with lymph node involvement, as it is well known that HER-2 positive tumors are more aggressive. Lymph node positive patients (63.4%) had HER-2 over-expression compared with only 13.3% of lymph node negative patients (p=<0.00001) (Table 3) (Figure 5). Luminal A subtype (ER and/or PR positive, HER-2 negative) was more common in older women (51.6% versus 27.6%) (p=0.045). Luminal B was slightly more common in younger women (23.4% versus 21.0%). Triple negative and HER-2 type tumors were much more common in younger women (21.3% versus 8.1%, and 27.7% versus 19.4%) (p=0.046) (Table 4) (Figure 6).
Figure 1.
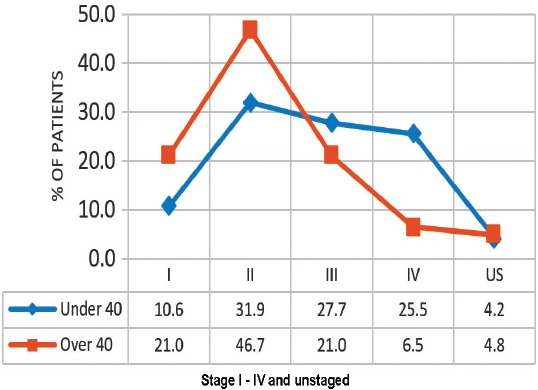
A graph showing that younger patients (under 40) were more likely to be at a higher stage for breast cancer than older patients (over 40) (p=0.012).
Figure 2.
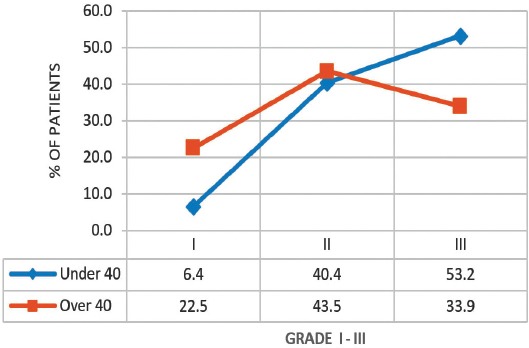
A graph showing that younger patients (under 40) were more likely to be of higher grade for breast cancer than older patients (over 40) (p=0.031).
Figure 3.
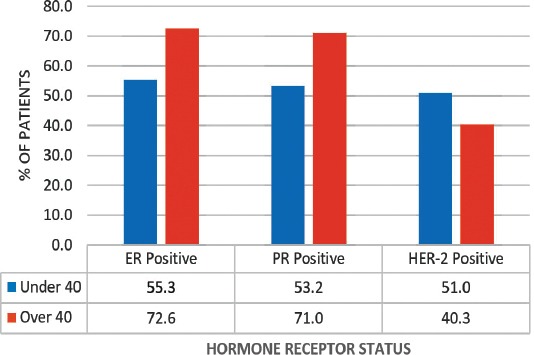
A graph showing that younger patients (under 40) were more likely to be estrogen receptor (ER) positive (p=0.036), progesterone receptor (PR) positive (p=0.034), and human epidermal growth factor receptor (HER)-2 negative (p=0.02).
Table 1.
Characteristics of the study population included in a study on breast cancer in Bahrain.
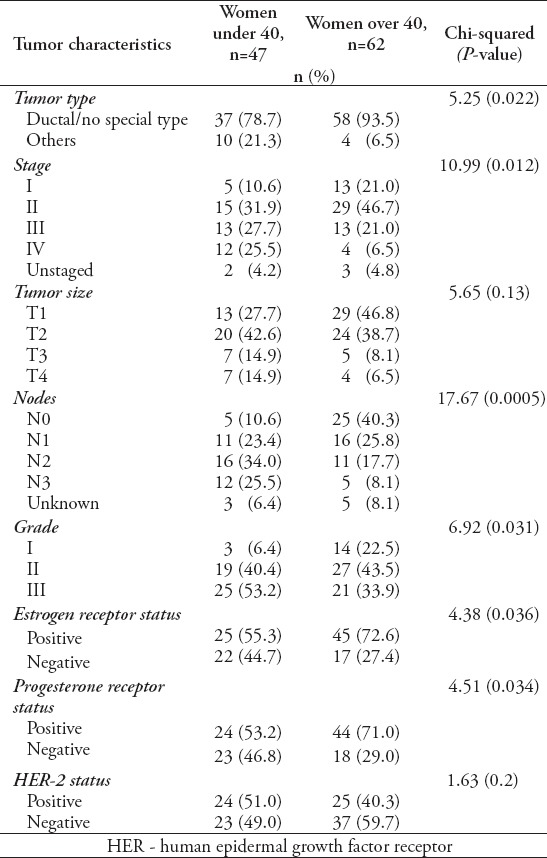
Table 2.
Characteristics of younger women more likely to have lymph node and distant metastases as found in a study in Bahrain (*significant).

Figure 4.
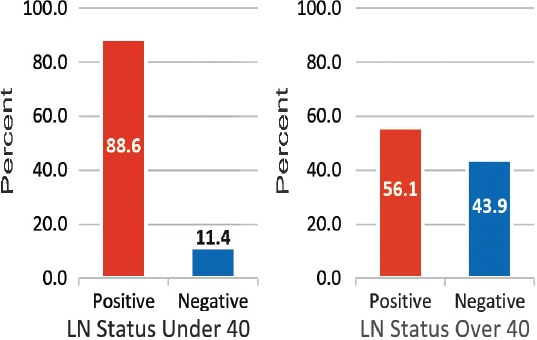
A graph showing that younger patients (under 40) were more likely to have positive lymph nodes (LN) (p=0.0004).
Table 3.
Correlation between lymph node involvement and hormone receptor status.
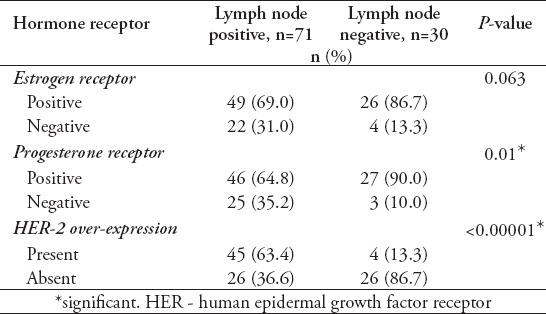
Figure 5.
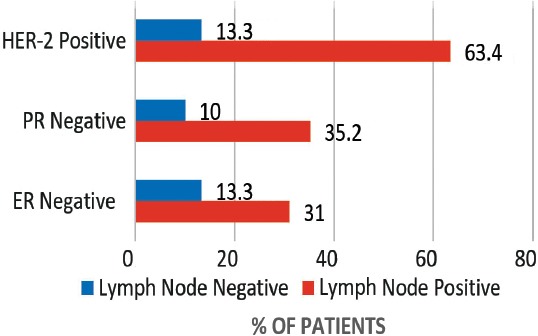
A graph showing the correlation between lymph node involvement and estrogen receptor (ER) negativity (p=0.063), progesterone receptor (PR) negativity (p=0.01), and human epidermal growth factor receptor (HER)-2 positivity (p<0.00001) in all patients (under and over 40).
Table 4.
Comparison of the different molecular subtypes of tumors.
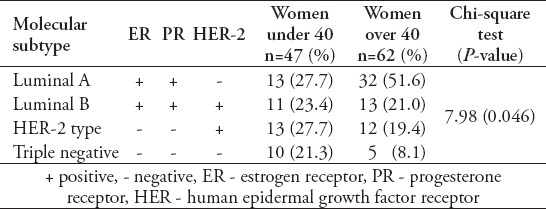
Figure 6.
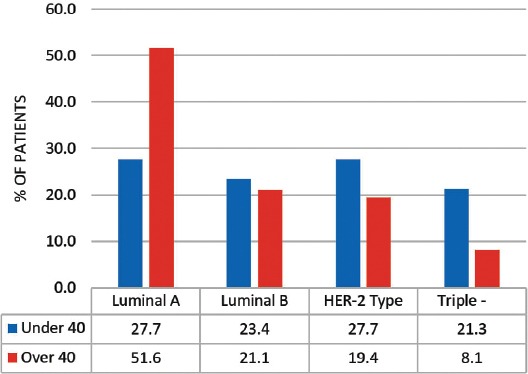
A graph showing the comparison of the different molecular subtypes of tumors between women under and over 40 (p=0.046).
Discussion
Many studies have reported that younger age is an independent factor for poor prognosis in BC.8,10-13 In our study, we have clearly highlighted that younger women with BC in Bahrain have tumors of higher stage, grade, size, and have more lymph node metastases. All these point to a worse prognosis.10-13 Lymph node status is one of the best predictors for prognosis. Our study quite clearly shows that younger women have much higher lymph node positivity than older women, which is again consistent with other studies carried out in different populations.13,14 Using lymph node status as a measure for the aggressiveness of the tumor; we observed a statistically significant correlation with under expression of ER and PR receptors, and/or overexpression of HER-2 and positive lymph node involvement.
Recent advances in molecular biology have shown that there are different molecular subtypes of BC based on their HR status as shown in Table 4, and that breast tumors are more aggressive if they were HER-2 positive (luminal B and HER-2 type), or triple negative for ER, PR, and HER-2.8,9,12,15-23 In this present study, we have shown that these 2 types are more common in younger women as further highlighted by the following studies. The HER2 receptors are amplified in more than 50% of young patients (luminal B + HER-2 type) compared with 40% in older patients.14 A significant proportion of young patients (21.3% versus 8.3%) are triple negative (ER, PR & HER-2 negative) which is considered the most aggressive type of BC. Most younger patients with BC were ER and PR negative, which is also associated with more aggressive behavior.8,12 These reasons, along with the results from our study, help explain the aggressiveness of the tumor in younger women.
Our study is limited by a small sample size. Further studies should include a larger sample size and also evaluate the differences in the genetic profile of patients, especially the genetic abnormalities like BRCA 1 and 2 genes, as these may have a significant effect on the molecular subtype of the tumors.
In conclusion, BC in younger women is more aggressive than in older women, and hence presents at a later stage with a worse prognosis. There is a significant proportion of younger women with triple negative and HER-2 type tumors, which are the 2 most aggressive forms of tumors, and this correlates well with lymph node status as a measure of aggressiveness. Further studies are needed to evaluate the molecular profile and genetic mapping to rule out associated inherited genetic abnormalities, such as BRCA 1 and 2 genes, which are closely linked to the triple negative subtype of BC.21,22 Such knowledge will be integral for proper screening and management of affected patients, and help explore more effective therapeutic targets in these younger patients.
Footnotes
Related Articles.
Saggu S, Rehman H, Abbas ZK, Ansari AA. Recent incidence and descriptive epidemiological survey of breast cancer in Saudi Arabia. Saudi Med J 2015; 36: 1176-1180.
Albasri A, Hussainy AS, Sundkji I, Alhujaily A. Histopathological features of breast cancer in Al-Madinah region of Saudi Arabia. Saudi Med J 2014; 35: 1489-1493.
Zhou J, Yan Y, Guo L, Ou H, Hai J, Zhang C, et al. Distinct outcomes in patients with different molecular subtypes of inflammatory breast cancer. Saudi Med J 20014; 35: 1324-1330.
References
- 1.International Agency for Research on Cancer. World Cancer Report. 2014. [Accessed 2015 Sept. 20]. Available from: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=80&codcch=275 .
- 2.World Health Organization. [Accessed 2015 May 16];Breast cancer: prevention and control. 2015 Available from: http://www.who.int/cancer/detection/breastcancer/en/index1.html . [Google Scholar]
- 3.Kocic B, Filipovic S, Vrbic V, Pejcic I. Breast cancer in women under 40 years of age. J BUON. 2011;16:635–639. [PubMed] [Google Scholar]
- 4.Madouj AN, Eldali A, Zahrani AS. [Accessed 2015 Sep 20];10 year cancer incidence among nationals of the GCC states 1998-2007. Available from: http://www.moh.gov.bh/pdf/publications/GCC%20Cancer%20Incidence%202011.pdf . [Google Scholar]
- 5.Hamadeh RR, Abulfatih NM, Fekri MA, Al-Mehza HA. Epidemiology of Breast Cancer among Bahraini Women: Data from the Bahrain Cancer Registry. Sultan Qaboos Univ Med J. 2014;14:176–182. [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. SEER Cancer Statistics Review. 1975-2009. [Accessed 2015 Sept. 20]. Available from: http://seer.cancer.gov/archive/csr/1975_2009_pops09/results_merged/sect_04_breast.pdf .
- 7.Sariego J. Breast Cancer in the Young Patient. The American Surgeon. 2010;76:1397–1400. [PubMed] [Google Scholar]
- 8.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2009;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 9.El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2009;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2009;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 11.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel CA, Domchek SM. Breast cancer in young women. Breast Cancer Res. 2010;12:212. doi: 10.1186/bcr2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141:507–514. doi: 10.1007/s10549-013-2711-y. [DOI] [PubMed] [Google Scholar]
- 14.Anders CK, Johnson R, Litton J, Philips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeSantis C, Seigel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 16.Choi YH, Ahn JH, Kim SB, Jung KH, Gong GY, Kim MJ, et al. Tissue microarray-based study of patients with lymph node-negative breast cancer shows that HER2/neu overexpression is an important predictive marker of poor prognosis. Ann Oncol. 2009;20:1337–1343. doi: 10.1093/annonc/mdp003. [DOI] [PubMed] [Google Scholar]
- 17.Parise CA, Caggiano V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J Cancer Epidemiol 2014. 2014:469251. doi: 10.1155/2014/469251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21:1848–1855. doi: 10.1158/1055-9965.EPI-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund MJ, Butler EN, Hair BY, Ward KC, Andrews JH, Oprea-Ilies G, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer. 2010;116:2549–2459. doi: 10.1002/cncr.25016. [DOI] [PubMed] [Google Scholar]
- 20.Millar EK, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 21.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 22.Hartman AR, Kalidate RR, Sailer LM, Painter L, Grier CE, Endsley RR, et al. Prevalence of BRCA mutations in an unselected population of triple-negative breast cancer. Cancer. 2012;118:2787–2795. doi: 10.1002/cncr.26576. [DOI] [PubMed] [Google Scholar]
- 23.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]


