Abstract
Objectives:
To assess glucagon-like peptide 1 (GLP-1) secretion after oral glucose tolerance tests (OGTTs) in subjects with newly diagnosed type 2 diabetes mellitus (T2DM), impaired glucose tolerance (IGT), and normal glucose tolerance (NGT) to clarify changes in GLP-1 secretion during the course of T2DM.
Methods:
In this cross sectional study, 80 subjects were divided into the NGT, IGT, and T2DM groups after undergoing a 75 g OGTT from March to December 2014 at the School of Medicine, First Affiliated Hospital, Shihezi University, Xinjiang, China. Plasma total GLP-1 was measured at 0, 30, 60, 120, and 180 minutes. Homeostasis model assessment of insulin resistance (HOMA-IR), islet β-cell function (HOMA-β), Gutt index, Matsuda index, incremental GLP-1 (ΔGLP-1), and areas under the curves of GLP-1 (AUCglp-1), glucose (AUCg), and insulin (AUCins) were calculated.
Results:
Plasma total GLP-1 at 30-120 minutes and ΔGLP-1 at 30-120 minutes were lower in the T2DM group than in the IGT and NGT groups (p<0.05). Peak GLP-1 levels were 35% lower in the T2DM group than in the NGT group. Plasma total GLP-1, ΔGLP-1, and AUCglp-1 correlated negatively with HOMA-IR and AUCg, and positively with HOMA-β, Gutt index, Matsuda index, and AUCins (p<0.05).
Conclusion:
The GLP-1 secretion after 75 g OGTT was impaired in newly diagnosed T2DM patients, inversely proportional to IR and hyperglycemia, and positively correlated with β-cell function and insulin sensitivity.
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder with multiple pathophysiological abnormalities. Currently, T2DM is one of the most common chronic diseases in almost every country. China has the largest population of diabetes patients. The prevalence of diabetes among adults in China is 11.6%, which is equivalent to 114 million patients.1 Glucagon-like peptide-1 (GLP-1) is secreted by L-cells in the distal parts of the intestines in response to nutrient ingestion.2 The GLP-1 regulates blood glucose levels deriving from several mechanisms. It stimulates insulin secretion from the pancreatic β-cells in a glucose-dependent manner and suppresses glucagon secretion.3,4 The GLP-1 also affects gastrointestinal motility, enhances satiety, promotes weight loss, and increases β-cell mass.5-9 Therefore, impaired GLP-1 secretion may contribute to the initiation and development of DM. As a consequence of these properties, GLP-1 based therapies (GLP-1 agonists and dipeptidyl peptidase-4 inhibitors) are currently playing a cornerstone role in the treatment of T2DM. Since GLP-1 plays an important role in the pathophysiology of DM, the assessment of GLP-1 secretory responses in individuals with different glycometabolism states is of great interest. The outcomes of GLP-1 secretion after the administration of an oral glucose load in individuals with and without diabetes are controversial. Toft-Nielsen et al10 reported a 53% reduction in integrated incremental GLP-1 concentrations in T2DM patients relative to healthy controls, while participants with impaired glucose tolerance (IGT) had an intermediate GLP-1 response. However, other studies11,12 have reported that GLP-1 secretion was not reduced in response to an oral glucose tolerance test (OGTT), or meal test in patients with T2DM. The mode of GLP-1 secretion in Asians may be different. Thus far, limited data are available on comparisons of GLP-1 levels among Chinese individuals with newly diagnosed T2DM, IGT, and normal glucose tolerance (NGT). Therefore, this study was conducted to measure GLP-1 levels during a standard OGTT in Chinese subjects with newly diagnosed T2DM, IGT, and NGT, in order to characterize the changes in GLP-1 secretion during the course of T2DM development. We also investigated the relationship between GLP-1 secretion, insulin resistance (IR), and insulin β-cell function.
Methods
This cross sectional study was carried out between March and December 2014. Data were collected from the Endocrinology and Metabolism Department at the School of Medicine, First Affiliated Hospital, Shihezi University, Shihezi, Xinjiang, China. A total of 80 participants, aged 35-71 years, and suspected to have T2DM were considered eligible for this study. All participants were of Han Chinese ethnicity and had not been treated with any hypoglycemic or hypolipidemic agents. Individuals with malignancy, serious hepatic diseases, gastrointestinal diseases, or other major diseases were excluded. On the basis of the results of a 75 g OGTT, participants were divided into T2DM group (n=35), IGT group (n=22), and NGT group (n=23). The diagnoses of IGT and T2DM were based on the World Health Organization diagnostic criteria.13 Before the study, informed consent was obtained from every participant. This study was conducted in accordance with the principles of the Helsinki Declaration. It was approved by the Human Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Shihezi University.
Anthropometric and biochemical measurements
In all participants, anthropometric and body composition measurements were performed before breakfast. Body mass index (BMI) was calculated as weight divided by height squared. Waist and hip circumferences were measured for the calculation of the waist-to-hip ratio (WHR). A standard 75 g OGTT was conducted for all subjects after overnight fasting (longer than 10 hours). In brief, 75 g glucose was ingested within 5 minutes. Blood samples were collected in the fasting state, and 30, 60, 120, and 180 minutes after taking 75 g glucose. Blood samples were withdrawn from the cubital vein and collected in evacuated sample tubes containing preservatives (such as, EDTA-containing tubes for biochemical measurements, EDTA and dipeptidyl peptidase-4 inhibitor-containing tubes for GLP-1 determination). The tubes were kept on ice until centrifugation. Separated plasma samples were frozen and stored at -70°C until further analysis. In addition, one ml fresh whole blood samples were collected for the measurement of hemoglobin A1c (HbA1c) levels using an automatic HbA1c analyzer (D10, Bio-Rad, California, USA). Plasma glucose (at each OGTT time point), and fasting plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were analyzed enzymatically using an autoanalyzer (Hitachi, Tokyo, Japan). Fasting plasma C-peptide (FCP) and insulin (at each OGTT time point) were detected using electrochemiluminescence (E170, Roche, Switzerland). Plasma total GLP-1 (at each OGTT time point) was determined using an enzyme-linked immunosorbent assay kit, as per the manufacturer’s instructions (Westang Biological Technology, Shanghai, China). The intra- and inter-assay coefficients of variation were less than 10.3%, and the sensitivity was typically less than 0.1 pmol/L.
Statistical analysis and calculations
Data analyses were carried out using Statistical Package for Social Sciences version 17 (SPSS Inc., Chicago, IL, USA). The results were expressed as the mean ± standard deviation (SD), or as median and interquartile range, if the data were not normally distributed. Normal distribution of the data was tested. One-way analysis of variance followed by the Student-Neuman-Keuls method was used to test between-group differences in normally distributed data. Comparisons of the constituent ratios were assessed using the chi-square test. The rank sum test was used for nonparametric data, and the Kruskal-Wallis test was used for multiple comparisons. Spearman rank correlation analysis was used to explore the correlations between total GLP-1 levels (at each OGTT time point), incremental GLP-1 (ΔGLP-1) levels, and area under the curve (AUC)glp-1 with homeostatic model assessment (HOMA)-IR, HOMA-β, Matsuda index, Gutt index, AUCg, and AUCins. Multivariate linear regression was also carried out to adjust for confounding factors for GLP-1 levels (at each OGTT time point). P-values less than 0.05 were considered to indicate a statistically significant difference. The AUCs were calculated using the trapezoidal method. The ΔGLP-1 levels were calculated by subtracting the fasting GLP-1 level from the GLP-1 levels at 30, 60, 120, and 180 minutes. The HOMA-IR was used to evaluate IR, and HOMA-β was used to estimate basal insulin secretion.14 Meanwhile, Matsuda index15 and Gutt indices16 were chosen for assessment of insulin sensitivity. Formulas are as follows:
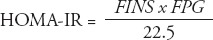
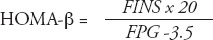
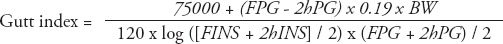
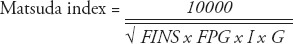
where: FPG = fasting plasma glucose level, 2hPG = 2-hour postprandial blood glucose level of OGTT, G = mean plasma glucose level of OGTT, FINS (µU/mL) = fasting insulin level, 2hINS = 2-hour insulin level of OGTT, I (µU/mL) = mean insulin level of OGTT, BW (Kg) = body weight. Plasma glucose levels were mg/dl in Matsuda index formula, and mmol/l in other formulas.
Results
General clinical characteristics
The general characteristics of the subjects in the 3 groups is shown in Table 1. Age and gender distribution did not significantly differ among the groups (p>0.05 for all). Systolic blood pressure (SBP), diastolic blood pressure (DBP), and LDL-C levels were significantly higher in the T2DM group than in the NGT group (p<0.05 for all). The TG, TC, FPG, and HbA1c levels were higher in the T2DM than in the IGT and NGT groups (p<0.05 for all). Compared with the NGT group, the T2DM exhibited low HDL-C level (p<0.05). The HOMA-IR was higher and HOMA-β, Gutt and Matsuda indices were lower in the T2DM group than in the IGT and NGT groups p<0.05 for all). Moreover, HOMA-β, Gutt and Matsuda indices were lower in the IGT than in the NGT group (p<0.05 for all). Other variables, such as height, weight, waist circumference, hip circumference, BMI, WHR, FCP, and FINS were similar in all groups (p>0.05 for all).
Table 1.
General clinical characteristics of the subjects included in a study in China (x̄ ± standard deviation).
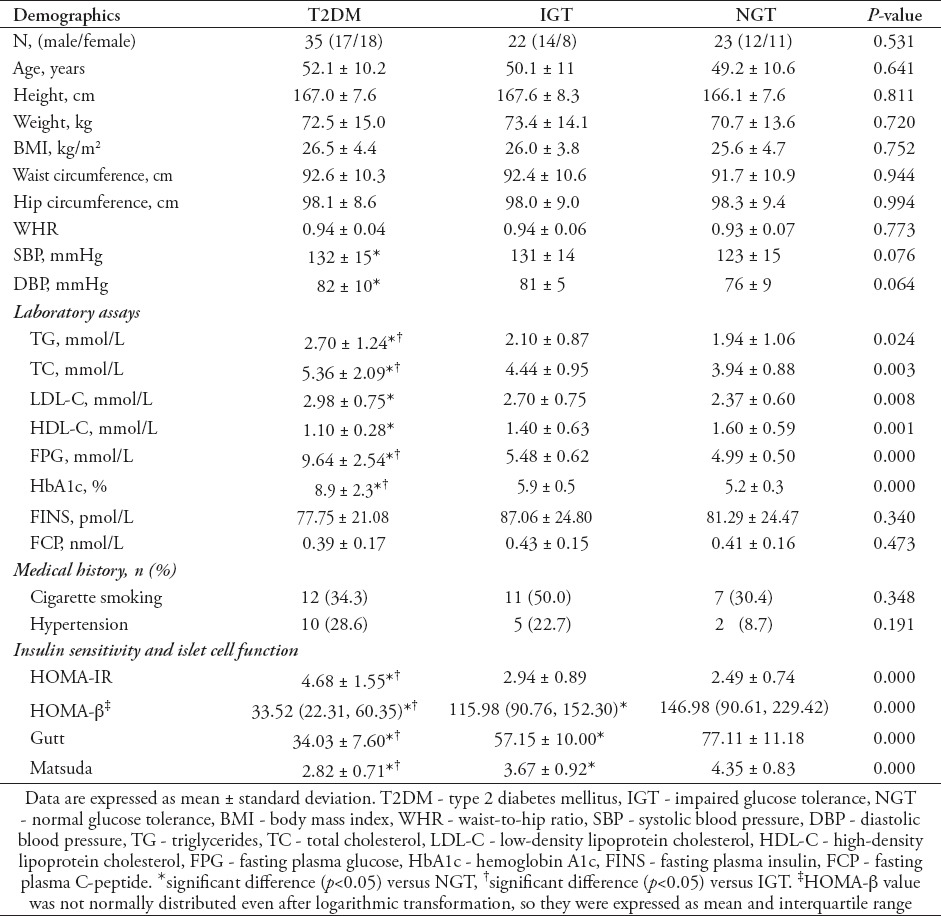
Total GLP-1, glucose, and insulin levels during OGTT
The fasting and 180 minutes plasma total GLP-1 level in the T2DM group was lower than in the NGT group; GLP-1 levels at 30-120 min were lower in the T2DM group than in the IGT and NGT groups (Figure 1A). However, these levels did not differ between the IGT and NGT groups (p>0.05). In each group, peak GLP-1 secretion occurred at approximately 30 minutes during OGTT. The peak level of total GLP-1 in the T2DM group was 35% lower than that in the NGT group. Glucose and insulin levels markedly differed among the 3 groups (p<0.05 for all, Figures 1B and 1C). In addition, ΔGLP-1 levels were compared in this study (Figure 1D). The ΔGLP-130 min, ΔGLP-160 min, and ΔGLP-1120min levels were lower in the T2DM group than in the IGT and NGT groups. The ΔGLP-1180 min level in the T2DM group was lower than that in the NGT group (p<0.05). No significant differences in ΔGLP-1 levels were observed between the IGT and NGT groups (p>0.05). We also compared AUCglp-1 among the 3 groups (Figure 1E). The AUCglp-1 was obviously lower in the T2DM group than in the IGT and NGT groups (p<0.05). Furthermore, there was a 32.8% reduction in AUCglp-1 in the T2DM group relative to the NGT group.
Figure 1.
Comparisons of glucagon-like peptide-1 (GLP-1), glucose and insulin levels, incremental (∆) GLP-1 levels, and AUCglp-1 during OGTT among the 3 groups: A) GLP-1 levels, B) glucose levels, C) insulin levels, D) incremental GLP-1 levels from 30 to 180 minutes, and E) AUCglp-1. *significant difference (p<0.05) versus normal glucose tolerance (NGT), †significant difference (p<0.05) versus impaired glucose tolerance (IGT). T2DM - type 2 diabetes mellitus
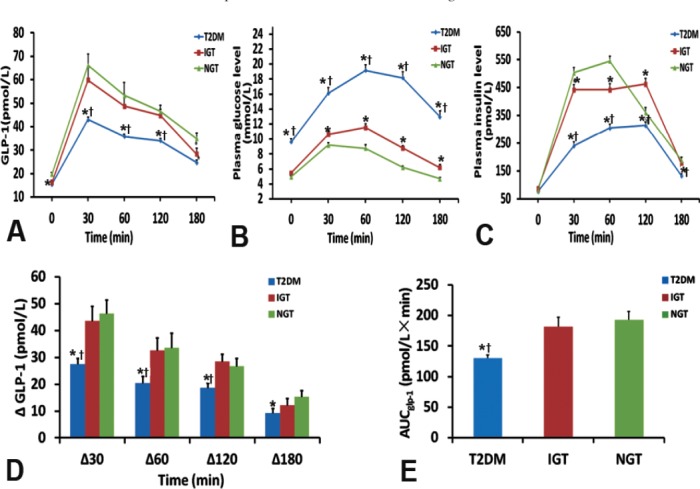
Correlation of total GLP-1, ΔGLP-1, and AUCglp-1 with HOMA-IR, HOMA-β, Gutt index, Matsuda index, AUCg, and AUCins
The total GLP-1 levels at each OGTT time point, ΔGLP-1 levels, and AUCglp-1 correlated with HOMA-IR, HOMA-β, Gutt index, Matsuda index, AUCg, and AUCins in all subjects are shown in Table 2. The FGLP-1, GLP-130 min, GLP-160min, GLP-1120min, ΔGLP-130min, ΔGLP-160min, ΔGLP-1120min, and AUCglp-1 were inversely proportional with HOMA-IR (p<0.05). The FGLP-1, GLP-130min, GLP-1120min, GLP-1180min, and AUCglp-1 positively correlated with HOMA-β (p<0.05). All variables, but ΔGLP-1180min, positively correlated with Gutt index (p<0.05). The FGLP-1, GLP-130min, GLP-160min, GLP-1120min, ΔGLP-130min, ΔGLP-160min, ΔGLP-1120min and AUCglp-1 positively correlated with Matsuda index (p<0.05). Every variable negatively correlated with AUCg (p<0.05). The FGLP-1, GLP-130min, GLP-1120min, GLP-1180min, ΔGLP-130min, and AUCglp-1 were positively proportional with AUCins (p<0.05). These results indicated that increased IR, decreased insulin sensitivity, and pancreatic β-cell function may contribute to defective GLP-1 secretion, and vice versa.
Table 2.
Analysis of the correlation of GLP-1, ΔGLP-1, AUCglp-1 with HOMA-IR, HOMA-β, Gutt index, Matsuda index, AUCg, and AUCins.
Multivariate linear regression analysis
To determine whether total GLP-1 levels at each OGTT time point were associated with various clinical factors, multivariate linear regression models were used. The total GLP-1 levels (at each OGTT time point) were selected as the dependent variables, and age, BMI, WHR, SBP, DBP, TG, TC, LDL-C, HDL-C, FPG, FINS, FCP, and HbA1c were set as the independent variables. The FGLP-1 was found to be negatively correlated with FPG (t = -2.866, p=0.005), and DBP (t = -2.436, p=0.017). The GLP-130min and GLP-1120min negatively correlated with FPG (t = -2.810, p=0.006 and t = -3.849, p=0.000). The GLP-160min and GLP-1180min negatively correlated with HbA1c (t =-2.585, p=0.012 and t = -3.191, p=0.002).
Discussion
We assessed GLP-1 responses to 75 g OGTT among subjects with different glucose tolerances to determine whether GLP-1 secretion was related to IR and pancreatic islet β-cell function. We found that the GLP-1 levels at each OGTT time point, ΔGLP-1 levels, and AUCglp-1 were lower in the T2DM group than in the IGT and NGT groups, but that these levels did not differ between the IGT and NGT groups. Compensatory GLP-1 secretion may occur in the prediabetic state, and decompensation may result in overt diabetes. The GLP-1 secretion was correlated with hyperglycemia, IR, and islet β-cell dysfunction. Glucotoxicity and IR may impair GLP-1 secretion, and low GLP-1 levels may aggravate β-cell dysfunction, IR, and hyperglycemia. This study provided evidence that Chinese patients with newly diagnosed T2DM had decreased GLP-1 secretion.
It was reported that the GLP-1 levels after a glucose load are lower in patients with impaired fasting glucose (IFG) + IGT and DM than in subjects with NGT.17 However, the authors of that study17 tested GLP-1 levels at only 2 time points (in the fasting state and 2 hours after glucose load). Their results were in partial agreement with our present study. In the present study, we performed a standard 75 g OGTT and measured GLP-1 levels at 5 successive time points. We found a reduction of GLP-1 secretion in T2DM patients. However, GLP-1 secretion did not differ between subjects with IGT and NGT. This indicates that impaired GLP-1 secretion may be a phenomenon typically observed in T2DM. However, recent studies have reported either no abnormalities at all, or increased GLP-1 secretion in T2DM patients.11,18-20 The possible reasons for this are as follows: first, hypoglycemic agents, especially metformin, have been shown to increase GLP-1 secretion.21 Bile acid sequestrants, such as colesevelam, have been reported to enhance GLP-1 secretion.22 Researchers should consider these types of confounding factors when investing GLP-1 secretion.
In this study, we selected patients with newly diagnosed, untreated T2DM; thus, their GLP-1 levels represented the natural secretion state in these patients. Second, some subject-specific factors have been found to be significantly related to GLP-1 secretion, such as, age, body weight, and BMI.20,23 Therefore, the T2DM group should be matched to healthy controls, as well, as possible. In this study, the general characteristics (age, gender, body weight, BMI) were matched very well. Third, genetic background may influence GLP-1 secretion. There are several genes that were reported to affect the physiology of GLP-1.24,25 Recently, Sleddering et al26 determined that the GLP-1 levels after a glucose load were higher in young healthy South Asians living in the UK than in their Caucasian counterparts. Our study provided evidence that Chinese patients with newly diagnosed T2DM had impaired GLP-1 secretion as compared with that in matched subjects with IGT and NGT. Whether the GLP-1 secretion pattern in the Han Chinese population differs from that in other ethnicities is unknown. Further studies are required to assess differences in GLP-1 secretion among various races. We observed that in each group, peak GLP-1 secretion occurred at approximately 30 minutes during OGTT, which is consistent with previous findings.27 Peak GLP-1 level was almost 35% lower in the T2DM group than in the NGT group. Furthermore, AUCglp-1 was 32.8% lower in the T2DM group than in the NGT group, which was more than that reported by Toft-Nielsen et al.10 However, there was no difference in peak GLP-1 and AUCglp-1 between the IGT and NGT groups. We hypothesized that compensatory GLP-1 secretion may occur in the prediabetic state. With disease progression, the function of the L cells in the intestines may decline, leading to a decrease in GLP-1 level. The exact mechanism underlying it remains to be investigated in future studies.
The present results also suggested that the GLP-1 levels and incremental GLP-1 levels during OGTT, as well as AUCglp-1 were correlated to plasma glucose level, pancreatic islet β-cell function and IR. This indicates that hyperglycemia, IR, and decreased pancreatic β-cell function may contribute to defective GLP-1 secretion, and vice versa. Some authors have suggested that reduced incretin effect was not a primary event in the development of T2DM, but rather a consequence of the diabetic state.17 Glucotoxicity and IR may impair GLP-1secretion, and low GLP-1 levels may aggravate β-cell dysfunction, IR, and hyperglycemia. In addition, GLP-1 levels negatively correlated with DBP. It is well known that hypertension is an important component of metabolic disorder and correlated with hyperglycemia and IR. Thus, glycometabolic disorder, IR, β-cell dysfunction, and defective GLP-1 secretion form a vicious cycle in T2DM.
A limitation of this study is its small sample size, due to which the correlation of GLP-1 levels with age and BMI may not have been as apparent as in previous reports.11,20 Another limitation is that glucagon and non-esterified fatty acid levels were not measured; both of these have been reported to be confounding factors for GLP-1 secretion.20
In conclusion, GLP-1 secretion in response to 75 g OGTT was impaired in patients with newly diagnosed, untreated T2DM, as compared with that in matched subjects with IGT and NGT. The GLP-1 secretion was inversely proportional to IR and hyperglycemia, and positively correlated with pancreatic islet β-cell function and insulin sensitivity. This study provided the GLP-1 secretion mode of newly diagnosed T2DM. These findings are meaningful for the prevention and treatment of T2DM.
Acknowledgment
The authors gratefully acknowledge the volunteers whose availability made this work possible. We also appreciate the efforts of the staff at the Department of Medical Laboratory of the First Affiliated Hospital, School of Medicine, Shihezi University, China.
Footnotes
References
- 1.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014;76:535–559. doi: 10.1146/annurev-physiol-021113-170315. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316–3323. doi: 10.2337/db13-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J Diabetes Investig. 2013;4:108–130. doi: 10.1111/jdi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Deacon CF, Holst JJ, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58:2688–2698. doi: 10.1007/s00125-015-3754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1–16. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63:9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24:85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 11.Calanna S, Christensen M, Holst JJ, Laferrere B, Gluud LL, Vilsboll T, et al. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–972. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Tang X, Cao H, Lu Q, Li N, Liu Y, et al. Impaired secretion of total glucagon-like peptide-1 in people with impaired fasting glucose combined impaired glucose tolerance. Int J Med Sci. 2012;9:574–581. doi: 10.7150/ijms.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabe D, Kuroe A, Watanabe K, Iwasaki M, Hamasaki A, Hamamoto Y, et al. Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications. 2015;29:413–421. doi: 10.1016/j.jdiacomp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35:959–964. doi: 10.2337/dc11-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10–18. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Liu H, Chen J, Li Y, Qu S. Multiple Factors Related to the Secretion of Glucagon-Like Peptide-1. Int J Endocrinol 2015. 2015:651757. doi: 10.1155/2015/651757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beysen C, Murphy EJ, Deines K, Chan M, Tsang E, Glass A, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55:432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 23.Geloneze B, de Oliveira Mda S, Vasques AC, Novaes FS, Pareja JC, Tambascia MA. Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Metabolism. 2014;63:922–929. doi: 10.1016/j.metabol.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet. 2009;18:2388–2399. doi: 10.1093/hmg/ddp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müssig K, Staiger H, Machicao F, Kirchhoff K, Guthoff M, Schäfer SA, et al. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes. 2009;58:1715–1720. doi: 10.2337/db08-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM. Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism. 2014;63:226–232. doi: 10.1016/j.metabol.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsboll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:737–745. doi: 10.1210/jc.2010-2435. [DOI] [PubMed] [Google Scholar]


