Abstract
Objectives:
To investigate the effect of non-elastic/elastic abdominal binders on intra-vesical pressure (IVP), physiological functions, and clinical outcomes in laparotomy patients at the perioperative stage.
Methods:
This prospective study was conducted from May to October 2014 at the Trauma Surgery Department, Daping Hospital, Chongqing, China. Laparotomy patients were randomly divided into non-elastic abdominal binder group (28 patients), and elastic abdominal binder group (29 patients). Binders were applied for 14 days following the operation, or until discharge. Demographic information, Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE-II) scores (prior to the operation, on the first day after operation, the day IVP measurement was stopped, and one day before discharge), and outcomes were recorded. The IVP was measured before the operation to postoperative day 7.
Results:
There were no significant differences in the demographic information, outcomes, SOFA or APACHE-II scores between the 2 groups. Initial out-of-bed mobilization occurred earlier in the elastic binder group (3.2 ± 2.0 versus 5.0 ± 3.7 days, p=0.028). A greater increase in IVP was observed in the non-elastic binder group than in the elastic binder group (2.9 ± 1.1 versus 1.1 ± 0.7 mm Hg, p=0.000).
Conclusion:
Elastic binders have relatively little effect on IVP and are more helpful at promoting postoperative recovery than non-elastic binders. Therefore, elastic binders are more suitable for clinical use.
The role of abdominal binders (ABs) in the postoperative recovery process following abdominal surgery should not be ignored. Bouvier et al1 conducted a questionnaire survey of French surgeons, and found that 94% of respondents tended to apply ABs after abdominal surgery. The reported aim of this application of ABs was to prevent wound dehiscence (83%), and reduce postoperative pain and discomfort (66%).1 A series of prospective studies confirmed that ABs not only significantly relieve pain and restlessness, but also improve out-of-bed mobilization.2-4 Additionally, psychological support after the use of ABs is important for promoting rehabilitation.5,6 Additionally, ABs can help support the application of other treatment devices.7 The adverse effects of ABs on the body should also be noted. Lasithiotakis et al8 analyzed one case of a spontaneous non-traumatic transdiaphragmatic intercostal hernia and reported that the high intra-abdominal pressure (IAP) caused by long-term abdominal binder wear can cause slimming and loosening of the diaphragm and intercostal muscles, thus, weakening their resistance to the rapidly increased pressure of the thoracic-abdominal cavity. Furthermore, the esophageal partial hiatal hernia and short segment acid reflux caused by elevated IAP, may be the main factors contributing to the occurrence of esophagogastric junction adenoma in patients without acid reflux symptoms.9,10 The use of ABs limits abdominal compliance (Cab) and, thus, can cause elevated IAP, potentially increasing the risk of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS).11 If wearing ABs induces iatrogenic IAH or further increases IAP, it will inevitably cause the patient’s condition to deteriorate, and thus, affect the patient’s prognosis. Currently, no reports are available that clearly indicate which type of AB is suitable for patients with IAH, or a high risk of it. In addition, current comparative studies of elastic and non-elastic ABs lack objective evidence regarding their advantages and disadvantages, except for patient reports of feeling more comfortable wearing elastic binders than non-elastic binders.1 Therefore, through this study, we aim to clearly define the effects of different abdominal binder types on IAP, physiology and clinical outcomes, and to find the binder type that has only a small effect on IAP, minimizes the possibility of IAH, assists in postoperative recovery, and provides guidance for future clinical work.
Methods
This prospective clinical study was conducted from May to October 2014 at the Department of Trauma Surgery, Daping Hospital, Chongqing, China. Consecutive patients treated during the study period were enrolled. Patients were included in the study if they were age ≥18 years, and had elective or emergency laparotomy for gastrointestinal tumor, obstruction, stoma closure, or abdominal trauma. Patient or his/her family members who refuses to participate in the experiment was excluded.
Abdominal binders
The 2 types of ABs used in this study are medical devices that can be purchased. Non-elastic ABs are made from non-elastic cotton material (Chongqing Tianjiquan Yikang Medical Device Company, Chongqing, China), it has a length of 117 cm and a width of 30.5 cm, and 6 fixed belts are located at the junction of the dorsal side of the abdomen with lengths of 57 cm and widths of 7 cm. To wear a non-elastic AB, the patient should first tightly draw the ventral side of the binder across the abdominal wall and place the fixing bands in the same manner, finally, the last pair of fixation bands should be fixed using a fixed-type knot above the abdomen (Figure 1). Elastic ABs (Chongqing Tianji Quanyi Medical Device Company, Chongqing, China) have lengths of 83 cm and a width of 21.5 cm. The abdominal and back sides are made of inelastic cotton material. Four elastic rubber bands at the 2 ends of the dorsal side are connected to the abdominal side, which has a nylon piercer. The ductility of each elastic rubber band is one cm/0.24 kg. The binding degree of the binders is adjusted by changing the position between the piercers (Figure 1). The binding degree of the 2 ABs should protect incisions, and should not lead to pain or dyspnea. The binder is removed before surgery after measuring the baseline value of IVP. A non-elastic/elastic AB is immediately wrapped around the patient after the operation, and is maintained until discharge, or 14 days after surgery.
Figure 1.
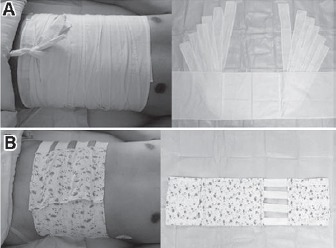
Methods in wearing: A) non-elastic; and B) elastic abdominal binders.
The IVP measurement
Based on the guidelines published by the World Society of the Abdominal Compartment Syndrome (WSACS) in 2013,12 all patients received standard IVP measurements to define their IAP under different states. The IVP manometry device modified by Malbrain was used,13 and was assembled and connected to the patient’s catheter under sterile conditions. Patients were placed in a complete supine position, and 20 ml sterile saline was injected into the bladder via the catheter after emptying the urine. The midaxillary line was set as the zero reference plane, and the IVP value was read at end-expiration using central venous pressure monitoring equipment (Medifix, B. Braun Melsungen AG, Melsungen, Germany), and is expressed in mmHg (1 mmHg=1.4 cmH2O). The monitoring frequency was once every 4 hours (6 times per day). Each measurement was repeated within a 3-minute interval, and the average of those 2 measurements was used as the measurement value.
Study procedure
During the study period, patients who met the inclusion criteria were randomly divided into non-elastic and elastic abdominal binder groups. Demographic information, such as gender, age, height, and weight was recorded. The SOFA and APACHE-II scores were recorded before the operation, on the first day after the operation, on the day when IVP measurement was stopped, and on the day prior to discharge. Treatment outcome-related data (hospitalization time, cost, initial flatus/defecation/food intake after operation, time of initiation of out-of-bed activity, and time of stitch removal) were recorded. The IVP was first measured in the absence of the AB before and immediately after surgery, after awakening from anesthesia, and one to 7 days after the operation (measurement was stopped if the catheter was removed, or if the patient was transferred, discharged, or died during this period). After installing the AB, 5 minutes was allowed to pass to stabilize the patient’s physiological status, and then IVP was measured with the incision plane as the center. This study was approved by the ethics committees of Daping Hospital. All patients signed an informed consent form. The study was conducted in accordance with the principles of the Helsinki Declaration. Both doctors and patients were required to maintain confidentiality regarding the survey results. Investigators were not allowed to interfere with the physician’s clinical decisions, or to give the patient any information that may have affected the results.
Statistical analysis
Measurement data were expressed as the means ± standard deviation (SD). Continuous variables were compared using the t test. Frequencies were compared using the Pearson Chi-Square test or Fisher’s exact test. The repeated measurement data were compared using a general linear model. A paired-samples t-test was used to compare IVP before and after wearing the AB at each time point. The Statistical Package for Social Sciences version 13 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A p<0.05 was considered statistically significant.
Results
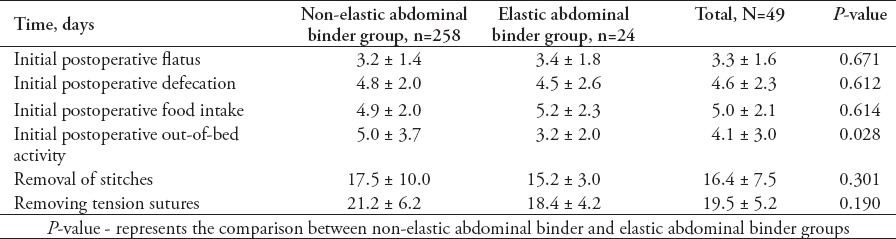
Fifty-seven patients were recruited, including 28 in the non-elastic AB group (49.1%), and 29 in the elastic binder group (50.9%). There were no significant differences in age, gender, body mass index (BMI), or number of patients who were treated with tension sutures between the 2 groups (Table 1). Indwelling urethral catheters were present in 57 patients from before the operation to the first day after the operation (before surgery, immediately after surgery, after awakening from anesthesia, first day after operation). Indwelling urethral catheters were placed in 7 patients in the non-elastic AB group and 6 patients in the elastic binder group within 7 days after the operation (p=0.698). The IVP was higher after wearing the AB in both groups (Table 2), and IVP was much higher in the non-elastic group than in the elastic group (Table 3). The following observations were made for the measurements obtained before the application of the AB: there was no significant difference in IVP from before the operation to postoperative day one between the 2 groups (p=0.403), and IVP did not change during that time period (p=0.298); IVP was not significantly different between the 2 groups from before the operation to postoperative day 7 (p=0.147), and IVP gradually decreased over that time period (p=0.036). The following observations were made for the measurements taken after wearing the AB: IVP was higher in the non-elastic group than in the elastic group from before the operation to the first day after the operation (p=0.004), IVP was higher after awakening from anesthesia and on the first day after the operation than before the operation (p before operation versus awakening from anesthesia = 0.001, p before operation versus first after surgery = 0.001); from before the operation to postoperative day 7, IVP was higher in the non-elastic group than in the elastic group (p=0.035), and during the first 7 postoperative days, IVP increased and then gradually decreased compared with its value before the operation (p=0.000). During the observation period, there was no significant difference between the 2 groups in SOFA (p=0.129) or APACHE-II (p=0.768) scores. The SOFA score changed significantly over time (p=0.045). The SOFA score was highest on the first day after the operation with an average score of 1.2 ± 2.3 points, first day after the operation versus before the operation: 1.2 ± 2.3 versus 0.7 ± 1.8 points (p=0.007); first day after the operation versus the day IVP measurement was stopped: 1.2 ± 2.3 versus 0.7 ± 1.2 points (p=0.044); and first day after the operation versus one day before discharge: 1.2 ± 2.3 versus 0.4 ± 1.0 points (p=0.01). The SOFA score then gradually diminished until discharge (the day IVP measurement was stopped versus one day before discharge: 0.7 ± 1.2 versus 0.4 ± 1.0 points (p=0.045). Similarly, APACHE-II score was not remarkably different between the 2 groups (p=0.768), however, following the operation, it tended to increase and then gradually decrease in the entire study cohort (p=0.051). The highest average APACHE-II score was observed on the first day after the operation (6.3 ± 4.8 points). Disruption of the wound was not found after the operation in either group and no treatment outcomes, except the time of initial out-of-bed mobilization were significantly different between the 2 groups (Tables 4 & 5).
Table 1.
Demographic information of patients included in a study in China.
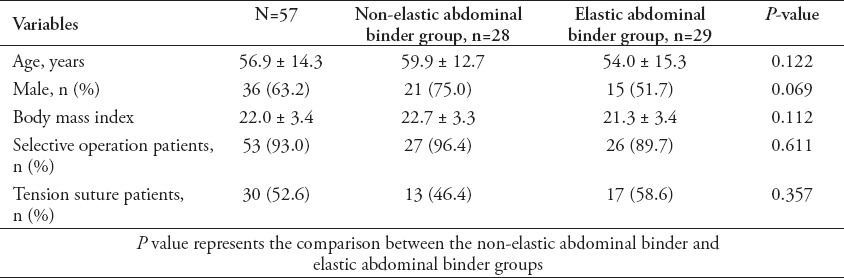
Table 2.
Comparison of intra-vesical pressure before operation to 7 days after operation with or without abdominal binder in non-elastic and elastic binder groups (mmHg) of patients included in a study in China.
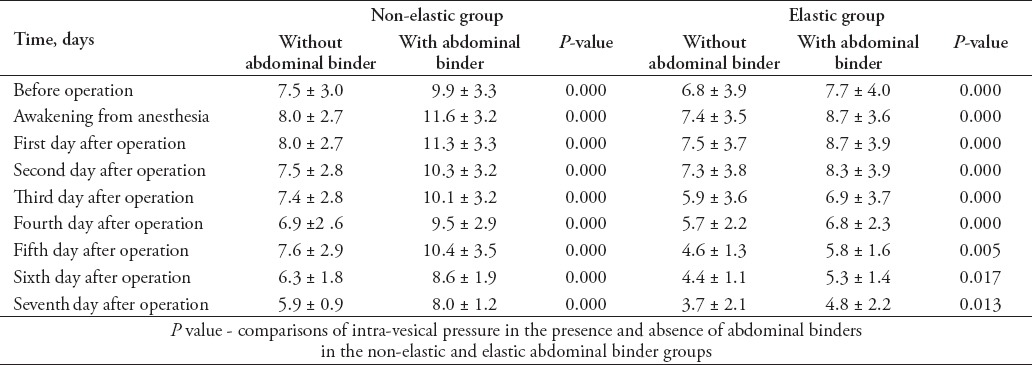
Table 3.
Comparison of incremental intra-vesical pressure between non-elastic and elastic abdominal binder groups (mmHg) of patients included in a study in China.
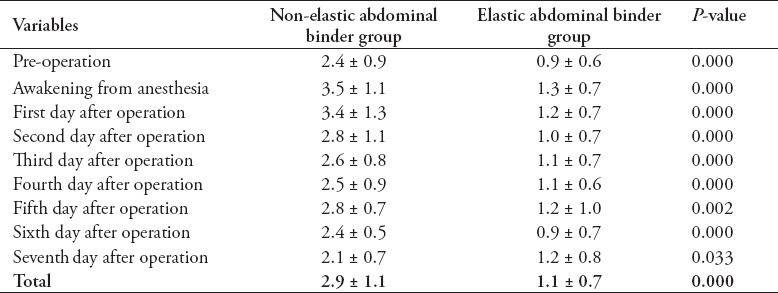
Table 4.
Basic medical information and treatment outcomes of patients included in a study in China.
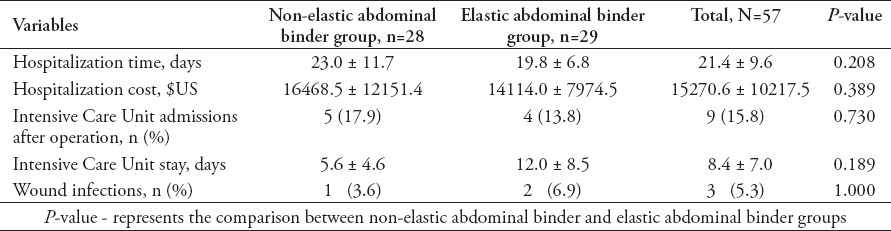
Table 5.
Postoperative related time information and treatment outcomes of patients included in a study in China.
Discussion
Studies have reported that European countries may be more inclined to use elastic ABs,1,14 while Chinese physicians prefer using non-elastic ABs in clinical practice. The main reasons for this preference of Chinese physicians are as follows: physicians believe that binders with less ductility may be better at protecting incisions, and non-elastic ABs can cause more obvious compression of the abdominal wall, which is also important for preventing and mitigating incision edema. Clinically, because of the bias of Chinese physicians toward applying non-elastic ABs, the value of the postoperative application of elastic ABs is unclear. According to the “Enhanced Recovery After Surgery (ERAS)” concept, early activity after surgery can prevent deep vein thrombosis, hypostatic pneumonia, muscle atrophy and other complications caused by a lack of activity and helps with wound healing.15 Accordingly, the 2013 ERAS Guidelines stated that early activity within one to 3 days after the operation is crucial for ERAS. In this study, the average time for initial out-of-bed mobilization in the elastic group was 3.2 ± 2.0 days, which was earlier than in the non-elastic group (5.0 ± 3.7 days, p=0.028) and met the above criteria. In addition, the elastic group had a shorter hospital stay, lower cost of treatment, and sooner stitch removal than the non-elastic group, although these differences were not statistically significant. Although this study did not follow the rules of ERAS, we believe that a standardized and rational treatment process combined with early physical activity promotes patient recovery following abdominal surgery.
As IAH and ACS are being studied more frequently, scholars are increasingly recognizing the impact of Cab on IAP. The Cab (mL/mmHg) is a measure of the ease of abdominal expansion (determined by the elasticity of the abdominal wall and diaphragm); thus, under the condition of IAP, Cab is measured as the change (mL) in intra-abdominal volume (IAV). When Cab increases, the impact of changes in IAV on IAP decreases, and vice versa.16 Siddins et al17 observed that after removal of adhesive drapes, IAP was reduced due to increases in abdominal wall compliance and abdominal workspace. There are 2 main aspects of the impact of ABs on IAP, such as: 1) the mechanical restriction provided by the abdominal binder reduces abdominal wall compliance and reshaping capacity,16 and 2) the compression force of the AB on the abdominal wall may be partially conducted into the abdominal cavity, causing IAP changes. Therefore, scholars have designated the use of ABs as a risk factor for decreased Cab.16
The aim of ABs is to protect incisions and reduce postoperative complications, but their adverse impact on IAP should also be considered. The use of ABs should be avoided in patients with IAH or risk factors for IAH, and if a binder must be used, the application time should be minimal. To protect the wound and avoid iatrogenic injury, it is essential not to wrap the AB too tightly, and to closely monitor IVP and avoid other factors that can affect IAP, IAV, and Cab.16 Elastic ABs have better ductility than non-elastic binders and can move with the patient’s abdominal movements, so their impact on Cab is lower than those of non-elastic binders. Moreover, different fixation methods have different effects on IAP, with the “imbricated” multilayer fixation method of non-elastic binders having a stronger compression and restriction effect than the single-layer Velcro strap fixation method of elastic ABs. Thus, the effects of elastic ABs on IAP and Cab are lower than those of non-elastic binders.
This study found that disease severity scores (SOFA and APACHE-II scores) first increase after the operation, and then decrease over time. This corresponds to the perioperative stress response, which presents after surgery and impacts the patient’s body, and is then gradually eliminated. It is worth noting that the trend in IAP is approximately the same as the trends in disease severity scores during the perioperative period. The invasive nature of laparotomy and the increase in disease severity after surgery are risk factors for a pathological increase in IAP.12 With increased IAP, the diaphragm may move toward the cephalic end of the body, causing pressure to leave the abdominal cavity, and resulting in increased intrathoracic and intracranial pressure and dysfunction of the respiratory and nervous systems. Similarly, vascular compression caused by elevated IAP can lead to renal ischemia and increased cardiac preload.18,19 On the other hand, elevated IAP may further damage physiological function, as indicated by changes in relevant scores. Physiological status gradually stabilizes, and when it does, the increases in IAP and vascular compression, which promote each other, gradually fade. After awakening from anesthesia, IAP was higher than before the operation in both groups. Abdominal surgery can cause organ swelling and evacuation dysfunction. Hemoperitoneum and pneumatosis can cause changes in IAV and eventually increase IAP.12,20 The combined effect of the above factors may explain why postoperative IAP is higher than preoperative IAP.
This study has the following limitations. Due to the limited sample size, no significant differences in the prevention of wound infection or dehiscence were observed between the 2 groups. Although using a larger sample size would increase the possibility of obtaining positive findings, the empirical conclusion that non-elastic ABs protect the incision after the operation better than elastic ABs could not be confirmed based on the current evidence. Subsequent prospective clinical studies with large sample sizes that investigate patients treated with non-elastic/elastic ABs, and patients without ABs may help clarify whether ABs help protect the incision, and which type of AB is more suitable. In addition, we did not quantify the binding degree of the ABs when evaluating the advantages and disadvantages of these binders in terms of postoperative recovery; therefore, we cannot directly analyze the relationship between different types of ABs and increased levels of IAP. However, the restrictive effect of ABs on the abdomen and the degree of compression of the incision that they provide vary with each individual, and patients’ sensitivity to pain may be directly related to their comfort with wearing ABs. Based on ethical principles, we did not design a uniform standard for the binding degree of ABs in order to avoid causing additional damage to the patients. The data obtained in this study are sufficient to determine the impact of ABs made of different materials on patients’ IVP and postoperative recovery.
In conclusion, due to its ductility and binding manner, elastic ABs have less of an effect on IAP than non-elastic binders and are better at promoting postoperative recovery. When using ABs, clinical biomarkers, such as IAP, should be closely monitored, and the treatment plan should be adjusted in a timely manner to avoid iatrogenic injury.
Footnotes
References
- 1.Bouvier A, Rat P, Drissi-Chbihi F, Bonnetain F, Lacaine F, Mariette C, et al. Abdominal binders after laparotomy: review of the literature and French survey of policies. Hernia. 2014;18:501–506. doi: 10.1007/s10029-014-1264-2. [DOI] [PubMed] [Google Scholar]
- 2.Cheifetz O, Lucy SD, Overend TJ, Crowe J. The effect of abdominal support on functional outcomes in patients following major abdominal surgery: a randomized controlled trial. Physiother Can. 2010;62:242–253. doi: 10.3138/physio.62.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larson CM, Ratzer ER, Davis-Merritt D, Clark JR. The effect of abdominal binders on postoperative pulmonary function. Am Surg. 2009;75:169–171. [PubMed] [Google Scholar]
- 4.Szender JB, Hall KL, Kost ER. A randomized-clinical trial examining a neoprene abdominal binder in gynecologic surgery patients. Clin Exp Obstet Gynecol. 2014;41:525–529. [PMC free article] [PubMed] [Google Scholar]
- 5.Christoffersen M, Olsen B, Rosenberg J, Bisgaard T. Randomized clinical trial on the postoperative use of an abdominal binder after laparoscopic umbilical and epigastric hernia repair. Hernia. 2015;19:147–153. doi: 10.1007/s10029-014-1289-6. [DOI] [PubMed] [Google Scholar]
- 6.Strigård K, Stark B, Bogren A, Gunnarsson U. Ventral hernia and patient experience of an elastic girdle. ANZ J Surg. 2015;85:525–528. doi: 10.1111/ans.12924. [DOI] [PubMed] [Google Scholar]
- 7.David M, Pracca F, Simini F. Non-invasive negative pressure system to treat abdominal hypertension. In: Jobbagy A, editor. 5th European Conference of the International Federation for Medical and Biological Engineering. Montevideo (Uruguay): Springer; 2012. pp. 211–214. [Google Scholar]
- 8.Lasithiotakis K, Venianaki M, Tsavalas N, Zacharioudakis G, Petrakis I, Daskalogiannaki M, et al. Incarcerated spontaneous transdiaphragmatic intercostal hernia. Int J Surg Case Rep. 2011;2:212–214. doi: 10.1016/j.ijscr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YY, McColl KE. Disruption of the gastroesophageal junction by central obesity and waist belt: role of raised intra-abdominal pressure. Dis Esophagus. 2015;28:318–325. doi: 10.1111/dote.12202. [DOI] [PubMed] [Google Scholar]
- 10.Lee YY, Wirz AA, Whiting JG, Robertson EV, Smith D, Weir A, et al. Waist belt and central obesity cause partial hiatus hernia and short-segment acid reflux in asymptomatic volunteers. Gut. 2014;63:1053–1060. doi: 10.1136/gutjnl-2013-305803. [DOI] [PubMed] [Google Scholar]
- 11.Malbrain ML, Roberts DJ, De Laet I, De Waele JJ, Sugrue M, Schachtrupp A, et al. The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 1: definitions and pathophysiology. Anaesthesiol Intensive Ther. 2014;46:392–405. doi: 10.5603/AIT.2014.0062. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malbrain ML, De Laet IE, De Waele JJ, Kirkpatrick AW. Intra-abdominal hypertension: definitions, monitoring, interpretation and management. Best Pract Res Clin Anaesthesiol. 2013;27:249–270. doi: 10.1016/j.bpa.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Rothman JP, Gunnarsson U, Bisgaard T. Abdominal binders may reduce pain and improve physical function after major abdominal surgery - a systematic review. Dan Med J. 2014;61:A4941. [PubMed] [Google Scholar]
- 15.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 16.Malbrain ML, De Laet I, De Waele JJ, Sugrue M, Schachtrupp A, Duchesne J, et al. The role of abdominal compliance, the neglected parameter in critically ill patients - a consensus review of 16. Part 2: measurement techniques and management recommendations. Anaesthesiol Intensive Ther. 2014;46:406–432. doi: 10.5603/AIT.2014.0063. [DOI] [PubMed] [Google Scholar]
- 17.Siddins M, Boland J, Riederer M, Kanchanabat B, Rao MM, Hewett P. Influence of adhesive drapes on intraperitoneal volume and pressure during laparoscopy. ANZ J Surg. 2002;72:553–556. doi: 10.1046/j.1445-2197.2002.02481.x. [DOI] [PubMed] [Google Scholar]
- 18.Balogh ZJ, Lumsdaine W, Moore EE, Moore FA. Postinjury abdominal compartment syndrome: from recognition to prevention. Lancet. 2014;384:1466–1475. doi: 10.1016/S0140-6736(14)61689-5. [DOI] [PubMed] [Google Scholar]
- 19.Cheatham ML. Intra-abdominal pressure: Why are you not measuring it. Crit Care Med. 2014;42:467–469. doi: 10.1097/CCM.0000436120.32758.e4. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs F, Bruyere M, Senat MV, Purenne E, Benhamou D, Fernandez H. Are standard intra-abdominal pressure values different during pregnancy? PLoS One. 2013;8:e77324. doi: 10.1371/journal.pone.0077324. [DOI] [PMC free article] [PubMed] [Google Scholar]


