Abstract
A quantitative method for clopidogrel using online-SPE tandem LC–MS/MS was developed and fully validated according to the well-established FDA guidelines. The method achieves adequate sensitivity for pharmacokinetic studies, with lower limit of quantifications (LLOQs) as low as 10 pg/mL. Chromatographic separations were performed on reversed phase columns Kromasil Eternity-2.5-C18-UHPLC for both methods. Positive electrospray ionization in multiple reaction monitoring (MRM) mode was employed for signal detection and a deuterated analogue (clopidogrel-d4) was used as internal standard (IS). Adjustments in sample preparation, including introduction of an online-SPE system proved to be the most effective method to solve the analyte back-conversion in clinical samples. Pooled clinical samples (two levels) were prepared and successfully used as real-sample quality control (QC) in the validation of back-conversion testing under different conditions. The result showed that the real samples were stable in room temperature for 24 h. Linearity, precision, extraction recovery, matrix effect on spiked QC samples and stability tests on both spiked QCs and real sample QCs stored in different conditions met the acceptance criteria. This online-SPE method was successfully applied to a bioequivalence study of 75 mg single dose clopidogrel tablets in 48 healthy male subjects.
Abbreviations: ESI, electrospray ionization; IS, internal standard; LC–MS/MS, liquid chromatography tandem mass spectrometry; LLOQ, lower limit of quantification; MRM, multiple reaction monitoring; SPE, solid phase extraction; QC, quality control
KEY WORDS: Clopidogrel, Online-SPE, LC–MS/MS, Back-conversion, Bioequivalence, Deuterated analogue, Real samples stability, FDA guidelines
Graphical abstract
A quantitative method for clopidogrel using online-SPE tandem LC–MS/MS was developed and fully validated according to the well-established FDA guidelines. The method achieves adequate sensitivity for pharmacokinetic studies, with lower limit of quantifications (LLOQs) as low as 10 pg/mL. Adjustments in sample preparation, including introduction of an online-SPE system proved to be the most effective method to solve the analyte back-conversion in clinical samples.

1. Introduction
Clopidogrel, frequently used with aspirin, is an oral antiplatelet agent1. It has been reported to reduce risks in patients with acute coronary syndrome undergoing coronary stenting2 and have benefits to patients with percutaneous coronary intervention3. Clopidogrel is approved for the reduction of atherosclerotic events in patients with stroke, myocardial infarction, cardiovascular disease, and acute coronary syndrome4.
Clopidogrel is primarily metabolized to an inactive carboxylic acid derivative, generated by hydrolysis, and to the pharmacologically active metabolite (AM) via an inactive 2-oxoclopidogrel by a two-step cytochrome P450 oxidation process5. Analytical methods for determining inactive carboxyl metabolite6 and the active metabolite5 have been reported. Also, an LC–MS/MS method for determining the parent compound clopidogrel has been established and validated7, 8. The main application of such methods was the development of generic formulations of clopidogrel according to bioequivalence regulations (EMEA, FDA, etc.); these norms require the measurement of the parent compound, whenever possible from the analytical point of view, since this is the most sensitive to formulation problems9, 10.
Our laboratory developed and validated an analytical method to quantify clopidogrel in plasma samples of subjects enrolled in bioequivalence studies in 2010. The FDA bioanalytical validation rules (2001) were followed. Preliminary use of this method with clinical samples revealed that supernatant fractions following a methanol precipitation step demonstrated increased clopidogrel levels following storage at ambient temperature, subsequently shown to be due to back-conversion from a metabolite. This back-conversion is further discussed in the result and discussion sections. Presently, a new method to determine clopidogrel in plasma was validated with application of an online-SPE system Symbiosis (Spark, Holland). The back conversion problem was solved by directly injecting real samples into the online-SPE system and automatically loading into LC–MS/MS system. In order to evaluate and monitor the back conversion of clopidogrel, pooled real samples (at two concentration levels) were prepared and analyzed in the validation process. In the sample analysis, one level of pooled real samples was prepared and analyzed to further ensure the settlement of the back conversion of clopidogrel.
The online-SPE method is presented in this paper, together with discussion of validation procedures. The method was successfully applied in the bioequivalence study of single administration of 75 mg dose clopoidogrel tablets.
2. Materials and methods
2.1. Chemicals and reagents
Clopidogrel hydrogensulfate standard was obtained from Dr. Reddy׳s Laboratory Co., Ltd. (Hyderabad, India). The internal standard (IS, clopidogrel-d4 hydrogensulfate) was purchased from TRC (Canada). HPLC-grade acetonitrile was from Tedia Company Inc. (Fairfield, USA) and formic acid was from DIKMA Company Inc. (Lake Forest, USA). Acetate ammonium and dimethylsulfoxide (DMSO) were of analytical grade.
2.2. Stock and working standard solutions
The stock solutions of clopidogrel and IS (clopidogrel-d4) were prepared in 50% DMSO. Hydrogensulfate of both clopidogrel and IS were coverted to calculate the real concentrations of clopidogrel and IS.
All of stock solutions were stored at −30 °C and the stock solutions of clopidogrel were used up to 1 month from preparation. Working standard solutions were prepared in 50% DMSO. Working dilutions from stock solutions of the analyte were freshly prepared when needed.
2.3. Calibration curves and quality control samples preparation
The concentrations ranged from 10 to 10,000 pg/mL for clopidogrel. The calibration points were 10, 20, 100, 500, 1000, 5000, 10,000 pg/mL. Working standard solutions of calibration standards (0.1, 0.2, 1, 5, 10, 50 and 100 ng/mL) were prepared in 50% DMSO. Working solutions of calibration standards were then spiked into human plasma (1/9, v/v).
Pooled real samples were used as additional real sample quality control (QC) in both validation and sample analysis. Two levels of pooled real samples were used to monitor the back conversion of clopidogrel (one level in absorption phase and one level in elimination phase). The concentration of real sample QC was determined by the first validation run. The concentration value of real sample QC in validation run 1 was used to calculate accuracy, precision and bias of real sample QCs in the following validation runs. In the sample analysis, one level of real sample QC was applied. The concentration of real sample QC in sample analysis is analyzed before sample analysis was started. The value of real sample QC was used to calculate accuracy, precision and bias in the following sample analysis process.
2.4. Clopidogrel isolation from plasma samples
Aliquots of plasma (100 μL) were transferred in 1.5 mL polypropylene tubes. Working solution of IS (10 ng/mL, clopidogrel-d4 in 50% DMSO, 10 μL) was added. Samples were vortexed for 30 s. Aliquot (100 μL) of plasma samples with IS were aspirated and transferred in 96-well plates and analyzed by online SPE–LC–MS/MS as described below.
2.5. Liquid chromatography system
In preliminary test, the liquid chromatography system Shimadzu Prominence (Shimadzu, Kyoto, Japan) was equipped with two LC-20AD pumps, a DGU-20A3 vacuum degasser, a SIL-HTC autosampler, a CTO-20AD column oven and a controller module.
In order to solve the back conversion problem of clopidogrel, an online SPE system (Symbiosys, Spark, Holland) was used. The system integrated the liquid chromatography unit with solid phase extraction unit including binary pumps (in this method, isocratic pump was used), an autosampler, an automatic cartridge exchanger unit, two high pressure dispensers and a column oven.
The chromatographic separation was achieved on an Eternity-2.5-C18-UHPLC column (50 mm×2.1 mm, 2.5 μm, Kromasil, Sweden) with a C18 guard column (4 mm×3 mm, 5 μm, Phenomenex, USA). The column oven temperature was set at 45 °C. The samples were kept at 4 °C in an autosampler. The mobile phase consisted of 0.04% formic acid, 3 mmol/L ammonium acetate in acetonitrile/water (65:35, v/v). Mobile phase at the first minute was set at 0.15 mL/min, and flow rate returned to 0.35 mL/min in the remaining 4.5 min. Besides, a peak focusing mode was used to obtain the acquire sensitivity. Peak focus mode included mobile phase and focus reagent (water). Focus reagent was set at 200 μL/min for 1 min, propelling by a high pressure dispenser detailed in Fig. 1 11.
Figure 1.
Peak focus mode in clopidogrel method11.
2.6. Online SPE conditions
The online-SPE cartridge was HySphere C8 EC-SE (10 mm×2.0 mm, 10 μm). In the automatic exchanger unit of Symbiosis System, there were two positions. Position 1 was for the sample pretreatment. After SPE procedures, cartridges were transferred into position 2. The pretreated samples were finally eluted by mobile phase into the analytical column and MS. When the former sample was analyzed, the latter sample could be prepared on SPE simultaneously. The detailed online SPE condition was listed in Table 1.
Table 1.
Online SPE condition for clopidogrel method.
| Procedure | Reagent | Flow rate (μL/min) | Volume (μL) |
|---|---|---|---|
| Conditioning | Acetonitrile | 5000 | 1000 |
| Equilibration | Water | 5000 | 2000 |
| Sample load | Water | 2000 | 1000 |
| Extraction 1 | 25% Acetonitrile in water | 5000 | 1000 |
| Extraction 2 | 25% Acetonitrile in water | 5000 | 500 |
| Cartridge wash | Acetonitrile | 5000 | 1000 |
2.7. Mass spectrometry system
Analytical procedures were carried-out on Applied Biosystems-Sciex (Toronto, Ontario, Canada) mass spectrometers. In the preliminary experiment, a triple-quad linear ion trap model AB Sciex 5500 QTrap instrument was used. For the second method, a triple-quad model AB Sciex 5500 mass spectrometry was used with the online SPE system. Both mass spectrometries were equipped with electrospray ion source. The ion transitions were monitored on m/z 322.1→212.1 amu for clopidogrel and m/z 326.1→216.1 amu for IS (clopidogrel-d4). The data were acquired and processed using Analyst 1.5.1 software package.
The eluent from the HPLC column was diverted into waste for a short period (2 min) using a computer controlled switching valve and in 2.1 min, the eluent was loaded into the mass spectrometer. The acquisition duration was 5.5 min.
2.8. Validation procedures
The validation of the online-SPE HPLC–MS/MS method for the determination of clopidogrel in plasma samples was performed in accordance with FDA rules12. In the method validation, real QC samples were introduced into the method validation and sample analysis process as additional QC to monitor the back conversion of clopidogrel.
The method was validated by testing lower limit of quantification (LLOQ) and carryover, specificity, endogenous interference, linearity, intra- and inter-assay precision and accuracy, matrix effect and recovery, and sample stability (back conversion) following the FDA guidance on bio-analytical method validation12.
The analytical range to be validated was chosen on the basis of the expected plasma concentrations13, 14. Spiked plasma samples were prepared for calibration curves (7 points) and quality controls (5 different levels) as described in the previous section. The following validation tests were carried out.
2.8.1. Sensitivity and carryover
LLOQ was the limit for precisely quantifying the compound. Signal–noise ratio (S/N) should be at least 6:1. The carryover was evaluated by injecting blank sample after ULOQ sample of the calibration standard. Carryover in the blank sample following the ULOQ should not be greater than 20% of the LLOQ for clopidogrel and 5% for the IS.
2.8.2. Specificity
Specificity was to evaluate by analyzing blank human plasma from six different sources. QC samples at LLOQ, low middle and high concentrations were analyzed on six separate sources with three replicates at each concentration per occasion to determine interference from difference sources matrix. The average accuracy and precision, for the back-calculated concentrations of the calibration points, should be within ±15%, except for the LLOQ (±20%).
2.8.3. Endogenous interference
Interference from endogenous material was accessed by analyzing blank human plasma from six different sources. The peak area of interference peak should not exceed 20% of the peak area of LLOQ. The result was to demonstrate the lack of chromatographic interference from blank endogenous plasma components.
2.8.4. Linearity, regression model, precision and accuracy
The linearity of clopidogrel was evaluated over the range of 10–10,000 pg/mL. Calibration curves were prepared in replicate (n=2) and analyzed. The accepted correlation coefficient (r), obtained using the appropriate regression model giving the best fitting in the whole range of tested concentrations, must be >0.99, with precision and accuracy, for the back-calculated concentrations of the calibration points, within ±15%, except for the LLOQ (± 20%).
2.8.5. Intra- and inter-assay precision and accuracy of QC
QCs at five concentration levels: LLOQ, low, middle, high and dilution QC (10, 25, 1500, 8000 and 40,000 pg/mL), were analyzed to evaluate intra- and inter-assay precision and accuracy of the method, with six replicates for each of the three consecutive batches. The dilution factor for dilution QC was 5, which meant that the concentration of spiked dilution QC was 40,000 pg/mL and it was diluted for 5 fold by blank plasma. Precision was expressed as CV (%) for replicate measurements and accuracy (%) by the percentage of deviation between nominal and calculated concentrations.
2.8.6. Extraction recovery and matrix effect
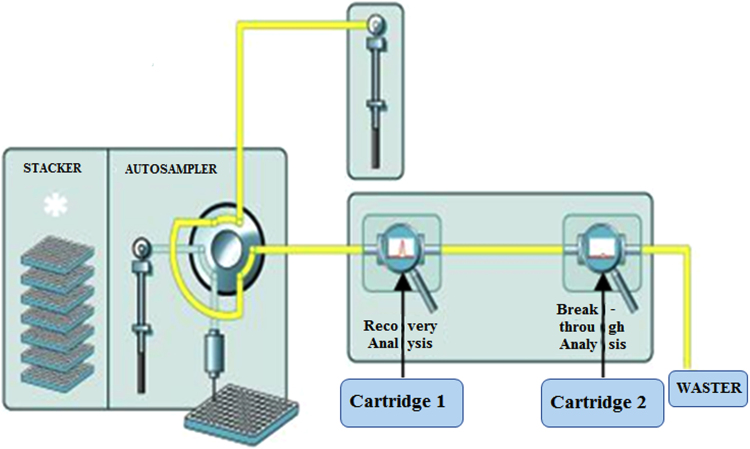
In this study, the analytical system performed online pretreatment. The blank treated samples (supernatant or reconstituted matrix) could not be obtained. Therefore, un-extracted peak area value could not be obtained. Extraction recovery was tested according to the methods provided by instrument vendor. The mechanism can be illustrated in Fig. 2 11. In the test of extraction recovery, two cartridges (Cartridge 1 and 2) were placed on position 1 and 2 to form a tandem mode. QC samples (25, 1500 and 8000 pg/mL, n=6 for each) were analyzed. Every sample analysis could obtain two peak areas as illustrated in Fig. 3. A1 was obtained from cartridge 1 and A2 was obtained from cartridge 2. Recovery (%)=A1/(A1+A2)×100.
Figure 2.
Advance method development (AMD) mode to text extraction recovery11.
Figure 3.
Theoretical two peaks generated by two cartridges tandem mode.
Matrix effect could be evaluated by analyzing plasma QC samples (25, 1500 and 8000 pg/mL, n=6 for each) and IS samples. These peak areas were recorded as A(L), A(M), A(H). Then, chemical QCs were prepared by spiking the same nominal concentration of clopidogrel (25, 1500 and 8000 pg/mL, n=6 for each) and IS using mobile phase as blank matrix. The peak area was recorded as AC (L), AC (M), AC (H) and AC (IS). The matrix effect was evaluated by formula: Matrix effect (%)=A/AC×100.
2.8.7. Sample stability and back conversion test
Pooled real samples were prepared to evaluate and monitor the back conversion. Pooled real sample QC was prepared by pooling the 0.75 h point and 2 h point real samples. Two levels of real sample were prepared as SQC-1 and SQC-2. Therefore, five concentrations of stability concentrations (low, middle, high, SQC-1 and SQC-2) were used for evaluation of sample stability and back conversion.
Stability studies in plasma samples were conducted at five QC levels (low, middle, high, SQC-1 and SQC-2) in different storage conditions. Bench-top stability was evaluated by placing the QC samples at room temperature for 24 h. The freeze-thaw stability was evaluated by analyzing the stability samples at five concentration levels (n=3) after three cycles from −30 °C to room temperature. The autosampler stability was tested by keeping the spiked and pooled stability samples in autosampler at 4 °C. Long term stability was tested by analyzing stability samples (5 levels, n=3) in every 7 days since Day 0. The stock solution stability of clopidogrel and the IS were tested for 24 h at room temperature and 3 months at −30 °C, comparing peak areas to those of freshly prepared solutions.
2.9. Pharmacokinetic assessment
The method was applied to determine the plasma concentrations of clopidogrel from 48 Chinese young healthy subjects for a bioequivalent study of two clopidogrel tablets. The concentration level was evaluated in the range of 24 h. Before sample analysis, one level of real sample QC was prepared by mixing 0.25–4 h back-up real samples. Also, sample analysis QC samples (low, middle and high) were also prepared before sample analysis. Four levels of QC were tested before sample analysis to confirm their accuracy within ±15% of nominal concentrations. The study was approved by the Ethics Committee of Shanghai Xuhui Central Hospital. Each subject was informed of the purpose of the study, and informed consent forms were obtained.
3. Results and discussion
3.1. Selection and optimization of analytical conditions
The molecular ion corresponding to 35Cl (322.1) was chosen as precursor ion for clopidogrel in the MRM method used for quantification, while for clopidogrel-d4, the molecular ion of 326.1 was preferred. Product ion spectrum of clopidogrel was similar to that reported15. For clopidogrel-d4, four isotopes labeled IS had a good selectivity, which could be demonstrated by interference test in the following paragraph. Therefore, we chose 216.1 amu as product ion of clopidogrel-d4. The detailed mass spectrometry parameters are listed in Table 2.
Table 2.
Mass spectrometry parameters for clopidogrel method.
| Ion source | Turbo V™ |
|---|---|
| Source temperature (°C) | 550 |
| Curtain gas (psi) | 30 |
| GS1 (psi) | 70 |
| GS2 (psi) | 60 |
| Ionspray voltage (V) | 1800 |
3.2. Optimization of online-SPE condition
Clopidogrel is a medium polarity molecule. We evaluated C8 and C18 cartridges. The retention times were similar between two types of cartridges. C18 cartridges have a slightly stronger retention but the background signal and interference is also stronger than C8 cartridge. After we evaluated the S/N ratio, we chose the C8 cartridge.
SPE process includes activation, equilibration, sample loading, sample wash and elution. In the equilibration step, we evaluated 1000 μL and 2000 μL water. Water 1000 μL was not able to replace acetonitrile after previous step, which could decrease the recovery of cartridge. Finally we selected 2000 μL water in equilibration. In the sample wash step, we evaluated 5%–50% acetonitrile in water. Matrix effect of 5% aqueous acetonitrile was strong and recovery of analyte decreased in 50% aqueous acetonitrile. Therefore, we chose 25% aqueous acetonitrile as the sample wash solvent. We also discovered that two sample washes could minimize the matrix effect, so we applied two sample wash steps.
In this analytical method, we applied peak focus mode to optimize the peak shape. In this mode, another flow path was introduced after SPE elution, which is similar to gradient elution. We applied water 200 μL as peak focus solvent in this method to optimize the peak shape. Mobile phase 150 μL was used to elude the sample from SPE cartridge.
3.3. Sensitivity and carryover
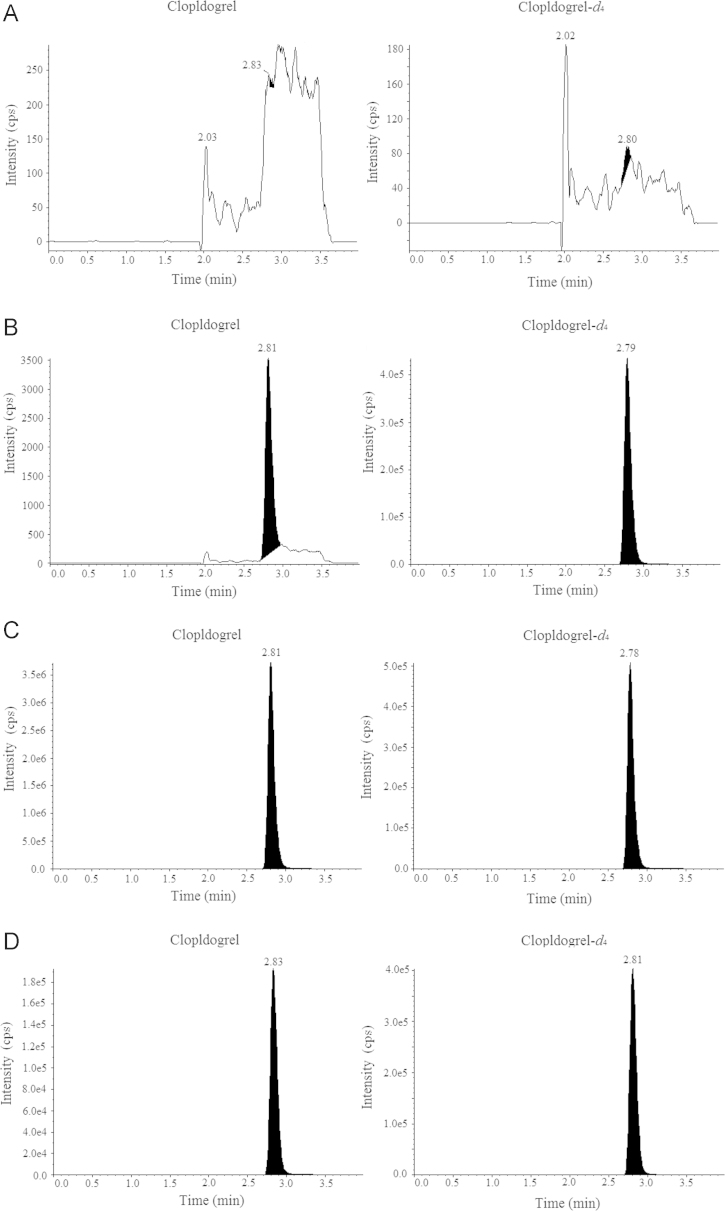
Typical MRM chromatograms for clopidogrel obtained in blank, LLOQ and ULOQ samples are presented in Fig. 4. In the chromatograms of LLOQ, the S/N ratio is >10, demonstrating adequate sensitivity as well as a background free from interfering signals.
Figure 4.
Representative LC–MS/MS chromatograms for clopidogrel and IS (clopidogrel-d4) in human plasma. (A) Blank human plasma; (B) LLOQ of 10 pg/mL of and IS; (C) ULOQ of 10 ng/mL of and IS; (D) human plasma sample.
The carryover of clopidogrel was less than 20% of average peak area of LLOQ. The carryover of IS (clopidogrel-d4) was less than 1% of average peak area.
3.4. Specificity and endogenous interference
Six different sources blank plasma were used to prepared LLOQ, low, middle and high (n=3) to test the specificity of clopidogrel. The average accuracy of clopidogrel was 96.27%–101.1%, with CV of less than 2%. Thus, this method has good specificity with little influence of the matrix from different sources.
Six different sources of blank plasma (n=3) were used for testing the endogenous interference. There were no interfering peaks at either the clopidogrel or the IS retention time.
3.5. Linearity, accuracy and precision
Calibration curves were linear (1/x2 weighted regression model) for the analytical method, with correlation coefficients >0.99. The method for clopidogrel was both accurate and precise for the intra and inter batches for each level (LLOQ, low, middle, high, dilution). The intra-batch precision CV of the assay was no more than 4% at four concentration levels of the QC samples, the CVs for inter-batch precision were no more than 6%. The inter- and intra-batch accuracies of the assay ranged from 90.15%–103.7% and 94.79%–102.7%, respectively. The mean precision and accuracy of five levels are listed in Table 3.
Table 3.
Precision and accuracy for determination of clopidogrel in human plasma (intra-day: n=6; inter-day: n=18, 3 consecutive runs).
| Nominal conc. (pg/mL) | Intra-assay (n=6) |
Inter-assay (n=18) |
||
|---|---|---|---|---|
| Accuracy (%) | CV (%) | Accuracy (%) | CV (%) | |
| 10 | 99.43 | 3.870 | 94.79 | 5.232 |
| 25 | 98.22 | 2.746 | 97.09 | 4.536 |
| 1500 | 100.4 | 0.3078 | 101.0 | 2.383 |
| 8000 | 98.90 | 0.4764 | 99.74 | 0.9858 |
| 40,000 (dilution) | 101.0 | 1.209 | 102.7 | 2.199 |
3.6. Extraction recoveries
The average extraction recoveries of clopidogrel were 99.58%–99.91% with CV of less than 2%. In this study, an online-SPE system was used to solve the sample back conversion problem, which is discussed in the next section. The test method of extraction recoveries in this study was different from the routine practice.
In the routine practice, the extraction recovery was accessed with two steps. First, clopidogrel was quantified by analyzing normal spiked QC samples (25, 1500 and 8000 pg/mL) and IS samples. Each was with six duplicates. These peak areas were recorded as A(L), A(M), A(H). The recovery was calculated by comparing the peak areas of extracted from QC samples with those of spiked concentrations. The spiked concentrations were recorded as A′(L), A′(M), A′(H). Extraction rates were calculated according to the equation: Extraction rate (%)=A/A′×100.
In the online-SPE tandem LC–MS/MS method, the chemical clopidogrel cannot be added to the SPE processed blank samples. Therefore, the routine practice was not feasible in this study. Because of the introduction of online-SPE system, extraction recovery was made up of two parts: sample pretreatment recovery and SPE cartridge recovery. In this study, adding IS was the only step of sample pretreatment and plasma was directly injected into the system, therefore, sample pretreatment recovery was 100%. Cartridge recovery could be evaluated by using advanced method development (AMD) mode, which was recommended by the instrument vendor, Spark Holland.
In AMD mode, the former paralleled two cartridge positions (left and right) switched to be the tandem positions to test the extraction recovery. The spiked QC samples were loaded into the left cartridge to be purified and concentrated. Wash solvents first flushed through left cartridge and then were loaded into the second cartridge (right position) (Fig. 2). Finally, the first and second cartridges were sequentially eluted by the mobile phase, and theoretically two peaks could be observed (Fig. 3). The peak area of first peak represented the absorbing amount of the analyte (clopidogrel) and IS on the first cartridge. The second peak signified the amount of analyte not being absorbed by the first cartridge. Therefore, Recovery (%)=A1/(A1+A2)×100.
3.7. Matrix effect
The mean matrix effect of clopidogrel (3 levels) and IS were 74.01%, 67.91% and 81.23% for clopidogrel and 72.63% for IS with RSD less than 3% indicating mild ion suppression. The matrix effect of clopidogrel and IS was similar with each other.
3.8. Sample stability and back conversion test
In order to evaluate sample stability and back conversion, in this study, we used pooled real samples as real sample QCs (two levels: SQC-1 and SQC-2) and routine spiked QC samples (low, middle and high) to evaluate the sample stability and back conversion problem. The result showed that clopidogrel was stable in ambient temperature for 24 h for both spiked QC samples and real sample QCs with bias of less than 9%. Freeze–thaw stability showed that the bias of the three cycles was less than 5%. Samples (low, middle, high, SQC-1 and SQC-2) in antosampler (4 °C) were stable for at least 60 h. The detailed back conversion test result was shown in Table 4. Long-term stability (five levels) results showed that clopidogrel was stable at −30 °C for at least 77 days (Table 5).
Table 4.
Back-conversion test of pooled real samples (two levels). Mean bias of the ratios concentration after storage vs. first measurement (r.t. for 24 h, 4 °C for 60 h and freeze–thaw 3 cycles).
| Back-conversion test | SQC-1 (Mean) pg/mL | CV (%) | SQC-2 (Mean) pg/mL | CV (%) |
|---|---|---|---|---|
| R.t. for 0 h | 224.3 | 0 | 1315 | 0 |
| Auto-sampler (4 °C) for 60 h | 224.6 | 0.1337 | 1.326 | 0.8365 |
| Freeze–thaw stability (three cycles) | 219.8 | −1.977 | 1322 | 0.5577 |
| R.t. for 24 h | 205.7 | −8.279 | 1.221 | −7.123 |
Table 5.
Long term stability of five concentration levels (low, middle, high, SQC-1 and SQC-2) for 77 days at −30 °C. Mean bias of the ratios concentration after storage vs. first measurement.
| Nominal conc. (pg/mL) | Time interval (day)/CV (%) |
||||||
|---|---|---|---|---|---|---|---|
| 0 day | 21 days | CV (%) | 49 days | CV (%) | 77 days | CV (%) | |
| 25 | 25.39 | 23.49 | −7.482 | 26.17 | 3.045 | 23.79 | −6.327 |
| 1500 | 1499 | 1487 | −0.7783 | 1482 | −1.112 | 1466 | −2.201 |
| 8000 | 7888 | 8041 | 1.931 | 7754 | −1.699 | 7590 | −3.778 |
| 224.3 (SQC-1) | 224.3 | 209.2 | −6.733 | 208.3 | −7.105 | 205.0 | −8.390 |
| 1315 (SQC-2) | 1315 | 1280 | −2.662 | 1245 | −5.323 | 1232 | −6.286 |
At first, we established and validated a protein precipitation method and an LC–MS/MS analyitical method for quantification of clopidogrel. The QC samples in three levels (low, middle and high) passed all the validation criteria according to FDA 2001 guidelines. But in the process of incurred sample reanalysis, we discovered the back conversion problem. We discovered that the concentration in the supernatant of real samples increased remarkably after it was stored in room temperature for 16 h compared with the first analysis (Table 6). But the concentration of spiked QC samples was stable in different conditions.
Table 6.
Back conversion of clopidogrel in real samples after methanol precipitation.
| Sample No. | Supernatant at r.t. (pg/mL) | Supernatant at r.t. after 16 h (pg/mL) |
|---|---|---|
| 1A7 | 331.5 | 2267 |
| 2A7 | 253.2 | 2954 |
| 3A7 | 634.9 | 1725 |
| 4A7 | 3018 | 7143 |
The back conversion problem occurred after plasma samples were pretreated by protein precipitation. Online-SPE system guaranteed that the automatic pretreated samples were directly injected into LC–MS/MS systems, which left no time gap for pretreated samples storage before sample analysis. Besides, we prepared IS by 50% DMSO water and prohibited the use of methanol in the analytical method. The concentration of clopidogrel in real clinical sample was stable in the existence of 50% DMSO water.
In this study, the real samples, clopidogrel were very unstable in existence of methanol, which was in agreement with the data reported by Havard et al.16. Some research papers explained this phenomenon by the back conversion of an unknown metabolite (cloppidogrel carboxylic acid acyl glucuronide)8. Therefore, beside the routine spiked QC samples, pooled real samples were prepared by mixing 0.75 h time point real samples and 2 h time point back-up real samples and naming as SQC-1 and SQC-2. These two real sample stability QCs have the same acceptance criteria with spiked QC: the average bias of back-calculated concentrations of real sample QC should be within ±15% comparing with 0 h. We applied real clinical sample QC to monitor the back conversion, and the concentration of clopidogrel was stable throughout the validation and sample analysis.
For clopidogrel quantification, some research papers reported methods to control the back conversion phenomenon to an acceptable extent5, 8. But different from the researchers reported, we validated that clopidogrel in real samples was stable for at least 24 h in room temperature and back conversion was within ±9% of the first measurement. Silverstro et al.8 reported that clopidogrel in real plasma samples increased by 50% at room temperature for 3 h comparing with first measurement, which was not agreement with our result. In our validation process, we evaluated back conversion ratio of 0, 4 and 24 h at room temperature using two level pooled real samples as SQC-1 and SQC-2. The back conversion ratios were −1.739% and 1.766% at 4 h, respectively. After 24 h in room temperature, the back conversion ratios were −8.279% and −7.123%, respectively. Therefore, back conversion was not occurred in real plasma samples in room temperature. As for the reason of this opposite result, we believe that Silverstro et al. in their method, used methanol as precipitation reagent. Back conversion was very fast in the methanol environment, so slight differences of sample preparation duration might trigger the back conversion and concentration increase.
Therefore, low temperature was not mandatory in the sample preparation process, which was opposite to reported8. The method with application of online-SPE system can solve the back conversion problem with simple sample preparation procedures. Back conversion and stability problem was evaluated throughout the whole validation process with good result.
3.9. Pharmacokinetic assessment
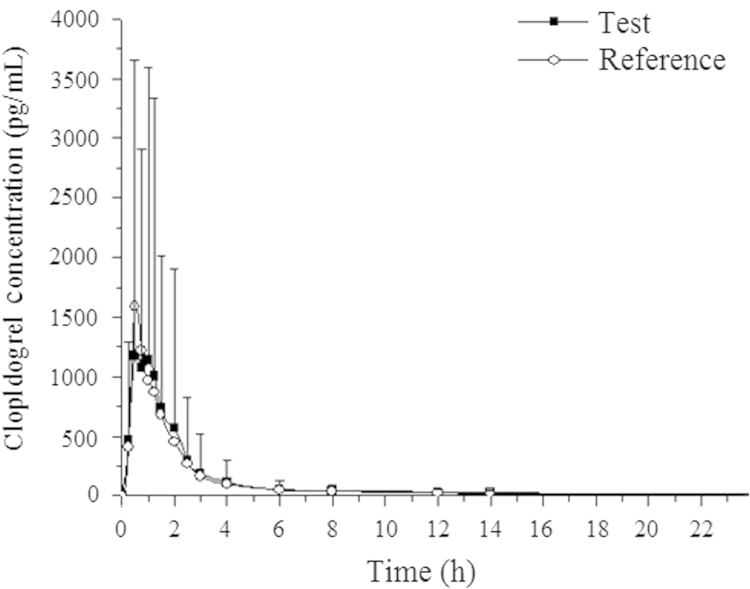
Mean pharmacokinetic curves calculated from plasma samples with volunteer orally treated with two brand tablets of clopidogrel (75 mg) are displayed in Fig. 5. Sample analysis QC as well as real sample QC met the acceptance criteria in all sample analysis runs.
Figure 5.
Mean dose-time curve of clopidogrel after an oral administration of two brand tablets of 75 mg clopidogrel tablets in two formulations to 48 healthy male volunteers.
4. Conclusions
An online SPE tandem mass spectrometry method was developed, validated and used in real sample analysis for determination of clopidogrel, an antiplatelet agent. An unexpected and scarcely met back conversion problem was observed in the method development phase with methanol precipitation.
The application of online SPE system and the avoidance of methanol in this method solved the back conversion problem. In the online SPE tandem mass spectrometry method, plasma samples were processed by SPE cartridges and automatically loaded into analytical column.
In the method validation process, pooled real samples (two levels) were used as real sample QC to evaluate the back conversion in different conditions. Back conversion ratio met the acceptance criteria of routine spiked QC (within 9%). Real QC samples were stable for at least 24 h in room temperature, for at least 60 h in autosampler (4 °C), for at least 77 days in −30 °C. Three freeze–thaw cycles (from −30 °C to room temperature) did not affect the concentration values within acceptance criteria.
Since online-SPE system was not applied into formal method validation process before, extraction recovery, as suggested by instrument vendor Spark Holland, was evaluated by two parts: sample recovery and cartridge recovery. In this study, plasma samples were directly loaded into the system, so the sample recovery was 100%. Cartridge recovery was assessed by using two tandem SPE cartridges. Cartridge recovery in this method was >99%.
Linearity, precision, extraction recovery, matrix effects and stability tests on blank plasma spiked with clopidogrel and stored in different conditions met the acceptance criteria. During the sample analysis of the real samples, the pooled real samples continued followed every sample analysis run with acceptance accuracy and precision. This online-SPE method was successfully applied to a bioequivalence study of 75 mg single dose clopidogrel tablets in 48 healthy male subjects.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Lepäntalo A., Virtanen K.S., Reséndiz J.C., Mikkelsson J., Viiri L.E., Karhunen P.J. Antiplatelet effect of clopidogrel in patients with aspirin therapy undergoing percutaneous coronary interventions-limited inhibition of the P2Y12 receptor. Thromb Res. 2009;124:193–198. doi: 10.1016/j.thromres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Cuisset T., Frere C., Quilici J., Morange P.E., Nait-Saidi L., Carvajal J. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non–ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006;48:1339–1345. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Cowper P.A., Udayakumar K., Sketch M.H., Peterson E.D. Economic effects of prolonged clopidogrel therapy after percutaneous coronary intervention. J Am Coll Cardiol. 2005;45:369–376. doi: 10.1016/j.jacc.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Englberger L., Faeh B., Berdat P.A., Eberli F., Meier B., Carrel T. Impact of clopidogrel in coronary artery bypass grafting. Eur J Cardio-Thorac Surg. 2004;26:96–101. doi: 10.1016/j.ejcts.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi M., Pang H., Kawabata K., Farid N.A., Kurihara A. Quantitative determination of clopidogrel active metabolite in human plasma by LC–MS/MS. J Pharm Biomed Anal. 2008;48:1219–1224. doi: 10.1016/j.jpba.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Lagorce P., Perez Y., Ortiz J., Necciari J., Bressolle F. Assay method for the carboxylic acid metabolite of clopidogrel in human plasma by gas chromatography–mass spectrometry. J Chromatrogr B Biomed Sci Appl. 1998;720:107–117. doi: 10.1016/s0378-4347(98)00452-6. [DOI] [PubMed] [Google Scholar]
- 7.Robinson A., Hillis J., Neal C., Leary A.C. The validation of a bioanalytical method for the determination of clopidogrel in human plasma. J Chromatogr B Biomed Sci Appl. 2007;848:344–354. doi: 10.1016/j.jchromb.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 8.Silvestro L., Gheorghe M.C., Tarcomnicu I., Savu S., Savu S.R., Iordachescu1 A. Development and validation of an HPLC–MS/MS method to determine clopidogrel in human plasma. Use of incurred samples to test back-conversion. J Chromatogr B Biomed Sci Appl. 2010;878:3134–3142. doi: 10.1016/j.jchromb.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Committee for medicinal products for human use (CHMP). Guideline on the investigation of bioequivalence. Doc. Ref: CPMP/QWP/EWP/1401/98 Rev. 1; 2010
- 10.US Food and Drug Administration. Guidance for industry/bioequivalence guidance; October 2002.
- 11.Spark Holland׳s XLC Method Development Guide for Analyst 1.4.1 and Higher. Available from: 〈http://spark.live.addsite.nl/site/download/mMM8quzsaAQ5〉.
- 12.US Food and Drug administration. Guidance for Industry/Bioanalytical method validation; May 2001
- 13.Di Girolamo G., Czerniuk P., Bertuola R., Keller G.A. Bioequivalence of two tablet formulations of clopidogrel in healthy argentinian volunteers: a single-dose, randomized-sequence, open-label crossover study. Clin Ther. 2010;32:161–170. doi: 10.1016/j.clinthera.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.D., Kang W.H., Lee H.W., Park D.J., Ahn J.H., Kim M.J. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther. 2009;31:793–803. doi: 10.1016/j.clinthera.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Lainesse A., Ozalp Y., Wong H., Alpan R.S. Bioequivalence study of clopidogrel bisulfate film-coated tablets. Arzneimittelforschung. 2004;54:600–604. doi: 10.1055/s-0031-1297056. [DOI] [PubMed] [Google Scholar]
- 16.Havard G, Coulombe M-E, Lachance S, Lévesque A, Massé R. AAPS J 2009;11(S2). Annual meeting. Available from: 〈http://www.aapsj.org/abstracts/AM_2009/AAPS2009-003196.pdf〉.