Abstract
Schizophrenia and depression are prevalent psychiatric disorders, but their underlying neural bases remains poorly understood. Neuroimaging evidence has pointed towards the relevance of functional connectivity aberrations in default mode network (DMN) hubs, dorso-medial prefrontal cortex and precuneus, in both disorders, but commonalities and differences in resting state functional connectivity of those two regions across disorders has not been formally assessed. Here, we took a transdiagnostic approach to investigate resting state functional connectivity of those two regions in 75 patients with schizophrenia and 82 controls from 4 scanning sites and 102 patients with depression and 106 controls from 3 sites. Our results demonstrate common dysconnectivity patterns as indexed by a significant reduction of functional connectivity between precuneus and bilateral superior parietal lobe in schizophrenia and depression. Furthermore, our findings highlight diagnosis-specific connectivity reductions of the parietal operculum in schizophrenia relative to depression. In light of evidence that points towards the importance of the DMN for social cognitive abilities and well documented impairments of social interaction in both patient groups, it is conceivable that the observed transdiagnostic connectivity alterations may contribute to interpersonal difficulties, but this could not be assessed directly in our study as measures of social behavior were not available. Given the operculum's role in somatosensory integration, diagnosis-specific connectivity reductions may indicate a pathophysiological mechanism for basic self-disturbances that is characteristic of schizophrenia, but not depression.
Keywords: Transdiagnostic analysis, Resting state functional connectivity, fMRI, Default mode network, Schizophrenia, Major depression
Highlights
-
•
Social impairments are well known in schizophrenia (SCZ) and major depressive disorder (MDD).
-
•
We investigated resting state functional connectivity of the default mode network in both patient groups.
-
•
Results demonstrate common fMRI dysconnectivity patterns of precuneus and superior parietal lobe.
-
•
Findings also highlight diagnosis-specific dysconnectivity of the parietal operculum in SCZ.
-
•
Transdiagnostic connectivity alterations may contribute to the social difficulties of both patient groups.
1. Introduction
Impairments of the ability to engage successfully in social interactions are well documented in schizophrenia (SCZ) and major depressive disorder (MDD) (e.g., Billeke and Aboitiz, 2013, Fiszdon et al., 2013, Ladegaard et al., 2014, Lee et al., 2013, Savla et al., 2012, Schilbach, 2015, Schneider et al., 2012). In SCZ, these impairments of social interaction have been related to disturbances of self-related and other-related reasoning, i.e., social cognition (e.g., Frith and Corcoran, 1996). In particular, SCZ patients are thought to attribute more meaning to their social surroundings than usual, reflected in so-called positive symptoms such as delusions and paranoia (Frith, 2004). Alternatively, alterations of social cognition in SCZ have been described as a “loss of natural evidence” for being in a world intersubjectively shared with others, thereby leading to alienation and social withdrawal, which may culminate in a psychotic crisis, in which lost intersubjective meaning is replaced by a ‘private’ world of delusions (Blankenburg, 1971, Fuchs, 2007, Klosterkotter et al., 2001). The latter changes have been related to the so-called negative symptom dimension of schizophrenia, which is also known to be highly relevant for prognosis and socio-economic outcome (Fulford et al., 2013, Salvatore et al., 2007).
In depressed patients, impairments of social interaction have similarly been related to attributional biases, which become manifest in abnormally increased, self-defeating, introspective thoughts and self-referential concerns (Marchetti et al., 2012). As in SCZ, disturbances of self-perception and self-referential thought can adversely affect interpersonal relations in MDD and make successful participation in social interaction difficult (Fuchs, 2001, Schilbach et al., 2014). Furthermore, it has been recognized that negative interpersonal experiences throughout the life span constitute an important risk factor for the development of depression and have, thus, become a key target of psychotherapeutic interventions (McCullough, 2003). Since social interactions are normally experienced as intrinsically rewarding (Schilbach et al., 2010), unsuccessful or reduced social interactions can further contribute to depressive symptomatology, but are also known to negatively affect the course of SCZ (Akdeniz et al., 2014, Hooker et al., 2014, Lee et al., 2014, Thomas et al., 2014).
Taken together, impairments of social interaction are well documented in both MDD and SCZ and can therefore be considered as a transdiagnostic symptom. The neurobiology that may underlie these transdiagnostic impairments, i.e., symptoms which fall onto a dimension that cuts across different nosological categories, in otherwise highly dissimilar disorders, however, remains poorly understood. In particular, when considering widespread dysconnectivity in both MDD and SCZ (Hamilton et al., 2013, Stephan et al., 2009), it is not clear whether transdiagnostically observed social impairments are subserved by distinct or common patterns of dysconnectivity. This question of similarities and differences in the neurobiological substrates of transdiagnostic social impairments is of particular relevance in light of current efforts of redefining psychiatric nosology in terms of neurobehavioral systems by taking a dimensional approach to the study of the genetic, neural, and behavioral features of mental disorders (Buckholtz and Meyer-Lindenberg, 2012, Cuthbert and Insel, 2013, Morris and Cuthbert, 2012).
Previous neuroimaging evidence indicates that aberrations of introspective processes relevant for social interactions are related to changes of functional connectivity (FC) in key nodes of the very robust “default mode network” (DMN) in particular related to social processing (Bastos-Leite et al., 2015, Das et al., 2012, Liston et al., 2014, Meda et al., 2012, Nixon et al., 2014, Schilbach et al., 2014, Yu et al., 2012). The DMN is classically defined as brain regions that show relative neural deactivation when focusing on the external environment and relative activation for internally focused tasks including autobiographic memory retrieval and conceiving the perspectives of others (Buckner et al., 2008). Recently, a large-scale neuroimaging meta-analysis characterized the overlap of the DMN, emotional processing and social-cognitive networks (Schilbach et al., 2012). The spatial convergence was identified in two cortical midline regions, namely the dorsomedial prefrontal cortex (DMPFC) and precuneus including the posterior cingulate cortex (PRC/PCC) representing the anterior and the posterior hubs of the DMN, respectively (Fig. 1). Functionally, the DMPFC is involved in the generation of stimulus-independent thoughts about another person's mental states (Frith, 2008), while the PRC is frequently associated with internally directed attention (Shannon and Buckner, 2004). In order to assess dysconnectivity patterns that might be relevant for transdiagnostic social impairments, we used seed-based FC analyses of these two hubs of the DMN in groups of SCZ and MDD patients relative to cohorts of matched controls.
Fig. 1.
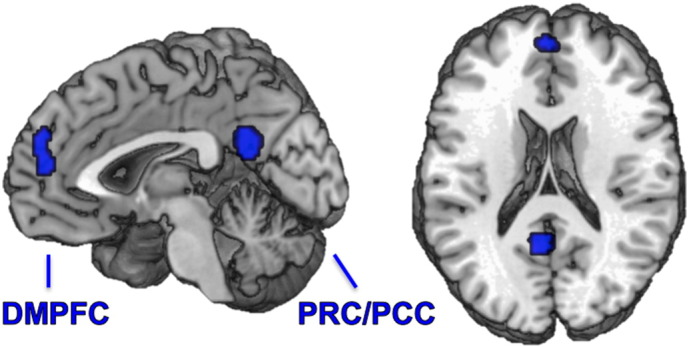
Meta-analytically defined default mode network. Taken from Schilbach et al. (2012), precuneus including posterior cingulate cortex: PRC/PCC MNI: x − 4, y − 54, z 24, dorso-medial prefrontal cortex: DMPFC MNI: x − 2, y 52, z 14.
As the current study is based on the idea that transdiagnostic impairments of social interaction in SCZ and MDD may rely on common circuit dysfunction, we hypothesized to find similarities in functional dysconnectivity patterns, which may represent a common neurophysiological basis of the social deficits observed in both disorders.
2. Material & methods
2.1. Meta-analytically informed seed definition
For the current study, the seed regions of interest for the whole-brain FC analysis were derived from a quantitative meta-analysis on the statistical convergence of task-related neural deactivations in 533 experiments and increased activation in social-cognitive as well as in emotional tasks in 74 and 1474 experiments, respectively (Schilbach et al., 2012).
2.2. Sample description
In order to obtain a sufficient number of participants for robust statistics, samples of SCZ and MDD patients recruited in the hospital setting were pooled over different measurement sites (Table 1) with each subsample being matched to a site-specific group of healthy controls (HC). As pooling over different MR scanners may introduce a systematic confound when comparing BOLD contrasts between scanners, every patient subsample was complemented with a group of closely matched HC of the same site. Thereby, group comparisons were based on a matched contribution of participants from every MR scanner minimizing the influence of MR scanner and EPI sequence parameters on group differences.
Table 1.
Scanning parameters across sites.
| Schizophrenia | ||||
|---|---|---|---|---|
|
Aachen Siemens TrioTim TR: 2200 ms TE: 30 ms Number of slices: 36 Slice-thickness: 3.2 mm Gap: 3.84 mm Flip-angle: 77∞ Orientation: axial In-plane resolution: 3.1 × 30.1 mm2 |
Aachen #2 Siemens TrioTim TR: 2000 ms TE: 28 ms Number of slices: 34 Slice-thickness: 3.3 mm Gap: 3.6 mm Flip-angle: 77∞ Orientation: axial In-plane resolution: 3.6 × 3.6 mm2 |
Göttingen Siemens TrioTim TR: 2000 ms TE: 30 ms Number of slices: 33 Slice-thickness: 3 mm Gap: 0.6 mm Flip-angle: 70∞ Orientation: axial In-plane resolution: 3 × 3 mm2 |
Utrecht Philips Achieva 3 T TR: 609 ms TE: 32.4 ms Number of slices: 40 Flip-angle: 10∞ Voxel-size: 4 × 4 × 4 mm FOV: 224 × 256 × 160 Matrix: 64 × 64 × 40 Orientation: coronal |
Lille IRM Philips 3 T TR: 1000 ms TE: 9.6 ms Number of slices: 45 Slice-thickness: 3.4 Flip-angle: 9 Voxel-size: 3.219 × 3.219 × 3.4 Orientation: SAG |
| Major depressive disorder | ||||
|
Aachen Siemens TrioTim TR: 2200 ms TE: 30 ms Number of slices: 36 Slice-thickness: 30.2 mm Gap: 3.84 mm Flip-angle: 77∞ Orientation: axial In-plane resolution: 30.1 × 30.1 mm2 |
Munich Philips Achieva 3 T TR: 2000 ms TE: 35 ms Number of slices: 32 Slice-thickness: 4 mm Gap: 0 Flip-angle: 82∞ Orientation: AC/PC In-plane resolution: 2.3 × 20.3 mm2 |
Göttingen Siemens TrioTim TR: 2000 ms TE: 30 ms Number of slices: 33 Slice-thickness: 3 mm Gap: 0.6 mm Flip-angle: 70∞ Orientation: axial In-plane resolution: 3 × 3 mm2 |
||
Patient and control groups for every site in both diseases were independently matched for age, sex and within scanner movements using the following procedure. For each subsample, 1,000,000 random samples of patient–control combinations were drawn and t-tests were computed for three movement parameters (see below) and age and chi-square tests for sex using MATLAB. Then, the biggest subsample of patients and controls with a p-value > 0.2 in all tests was chosen to rule out any trend towards group difference regarding the applied parameters. Finally, the same matching criteria were used for the whole cohort in SCZ and MDD. Thereby, we assume that group differences in resting-state connectivity would neither be driven by systematically different head motion in the scanning process nor by differences in age or sex distribution. The SCZ sample included 75 patients and 82 controls from 4 scanning sites (Table 2) and the MDD group consisted of 102 patients and 106 controls from 3 sites (Table 3). Diagnosis was confirmed by clinical examination of the attending psychiatrist in accordance with the International Classification of Diseases (ICD-10). The two patient groups did not differ in terms of disease duration (mean duration: SCZ 9.03 ± 8.22; MDD 10.63 ± 10.20; Mann–Whitney U test: p = 0.72).
Table 2.
Group characteristics for schizophrenia (SCZ); SD: standard deviation.
| SCZ patients | Healthy controls | Statistical comparison | |
|---|---|---|---|
| Participants | n = | n = | |
| Entire group | 75 | 82 | |
| Aachen | 13 | 13 | |
| Aachen #2 | 13 | 15 | |
| Utrecht | 10 | 10 | |
| Göttingen | 30 | 35 | |
| Lille | 9 | 9 | |
| Sex | Male/female | Male/female | Chi2 test |
| Entire group | 54/23 | 59/23 | 0.9946 |
| Aachen | 8/5 | 8/5 | 1.0000 |
| Aachen #2 | 11/2 | 12/3 | 0.7505 |
| Utrecht | 5/5 | 5/5 | 1.0000 |
| Göttingen | 24/6 | 28/7 | 1.0000 |
| Lille | 11/3 | 11/3 | 1.0000 |
| Age | Mean ± SD | Mean ± SD | ttest/ranksum |
| Entire group | 33.46 ± 9.61 | 33.79 ± 10.36 | 0.8372/0.9579 |
| Aachen | 38.54 ± 8.52 | 38.62 ± 10.29 | 0.9836/1.0000 |
| Aachen #2 | 33.38 ± 10.40 | 33.40 ± 11.36 | 0.9971/1.0000 |
| Utrecht | 32.68 ± 9.29 | 35.90 ± 12.99 | 0.5314/0.6769 |
| Göttingen | 31.27 ± 9.59 | 32.46 ± 9.44 | 0.6166/0.3936 |
| Lille | 34.44 ± 9.58 | 30.33 ± 8.26 | 0.3441/0.1371 |
Table 3.
Group characteristics for major depression disorder (MDD). SD: standard deviation.
| MDD patients | Healthy controls | Statistical comparison | |
|---|---|---|---|
| Participants | n = | n = | |
| Entire group | 102 | 106 | |
| Göttingen | 49 | 51 | |
| Aachen | 30 | 30 | |
| Munich | 23 | 25 | |
| Sex | Male/female | Male/female | Chi2 |
| Entire group | 49/53 | 51/55 | 0.9915 |
| Göttingen | 27/22 | 29/22 | 0.8593 |
| Aachen | 11/19 | 11/19 | 1.0000 |
| Munich | 11/12 | 11/14 | 0.7904 |
| Age | Mean ± SD | Mean ± SD | ttest/ranksum |
| Entire group | 37.75 ± 13.29 | 36.99 ± 12.92 | 0.6784/0.6356 |
| Göttingen | 34.00 ± 10.55 | 34.04 ± 10.92 | 0.9855/0.8306 |
| Aachen | 36.10 ± 12.21 | 36.10 ± 12.58 | 1.0000/0.8648 |
| Munich | 47.87 ± 15.14 | 44.08 ± 14.78 | 0.3849/0.4029 |
Due to recruitment taking place in a hospital setting and in light of around a decade long disease history, patients were medicated following established medication regimes. 80% of the SCZ patients were treated with second generation antipsychotics (SGA) and only a minority of 6.7% was receiving first generation antipsychotics (FGA) or a combination of the two (8%, Table 4). 50% of the MDD cohort was prescribed selective serotonin reuptake inhibitors (SSRI), serotonin–norepinephrine reuptake inhibitors (SNRI) or norepinephrine–dopamine reuptake inhibitors (NDRI), while 16.7% were additionally taking Tricyclic antidepressants (TCA). TCA alone were taken by 15.7% of MDD patients and 5.9% were treated with SGA in combination with the two former agents or with reuptake inhibitors only (5.9%, Table 5). It is important to note that systematically different medication for SCZ and MDD should have different within disease group effects on FC. This source of variance should rather make it more difficult to statistically detect similarities in connectivity aberrations over diseases, than representing a major confound to the transdiagnostic analysis. In addition, this more naturalistic setting should also allow better generalization of the FC results to the overall population of patients with depression and schizophrenia.
Table 4.
Overview of medication in the schizophrenia cohort. SGA: second generation antipsychotics; FGA: first generation antipsychotics.
| Schizophrenia | n = | SGA | FGA | SGA & FGA | Missing data |
|---|---|---|---|---|---|
| Aachen (n) | 13 | 12 | – | – | 1 |
| Aachen #2 (n) | 13 | 12 | 1 | – | 0 |
| Göttingen (n) | 30 | 25 | – | 4 | 1 |
| Utrecht (n) | 10 | 3 | 3 | 2 | 2 |
| Lille (n) | 9 | 8 | 1 | – | 0 |
| Overall (%) | 75 | 80% | 6.7% | 8% | 5.3% |
Table 5.
Overview of medication in the major depression cohort (MDD). SSRI: selective serotonin reuptake inhibitors; SNRI: serotonin–norepinephrine reuptake inhibitors; NDRI: norepinephrine–dopamine reuptake inhibitor; TCA: tricyclic antidepressants; SGA: second generation antipsychotics.
| MDD | n = | SSRI/SNRI/NDRI | SSRI/SNRI/NDRI & TCA | TCA | SSRI/SNRI/NDRI & SGA | SSRI/SNRI/NDRI & TCA & SGA | Other/none |
|---|---|---|---|---|---|---|---|
| Aachen (n) | 30 | 19 | 4 | 1 | 2 | 3 | 1 |
| Munich (n) | 23 | 9 | 8 | 4 | 2 | – | – |
| Göttingen (n) | 49 | 24 | 5 | 11 | 2 | 3 | 2/2 |
| Overall (%) | 102 | 51% | 16.7% | 15.7% | 5.9% | 5.9% | 4.9% |
Healthy subjects for group comparisons did not have a history of neurological or psychiatric disorders and all subjects gave written informed consent to participate in the study as approved by the local ethics committees. The ethics committee at the HHU Düsseldorf also approved joint re-analysis of the data. All participants were instructed to lie still during the scanning session, keep their eyes closed (except for the participants from Göttingen) and to let their mind wander, but not to fall asleep. The latter was confirmed during a post-scan debriefing interview.
2.3. Resting state fMRI data: imaging & preprocessing
For each subject resting state EPI images were acquired using standard blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence]. Prior to further processing (using SPM8, www.fil.ion.ucl.ac.uk/spm) the first four images were discarded allowing for magnetic field saturation. The EPI images were corrected for head movement by affine registration in a two-pass procedure realigning EPI volumes to its mean image. The mean EPI image for each subject was spatially normalized to the MNI single subject template using the “unified segmentation” approach (Ashburner and Friston, 2005). The ensuing deformation field was applied to the individual EPI volumes and smoothed with a 5-mm FWHM Gaussian kernel.
In recent years, it has repeatedly been shown that head motion is a crucial confounding factor in resting-state analysis leading to spurious connectivity estimates as well as artificial group differences (for review see Power et al., 2015). In order to minimize the influence of motion artifacts on group comparisons, we conducted a matching of within scanner movements between patient and control subgroups. The following three head motion parameters were derived from the individual realignment parameters: i) framewise displacement (FD) represents the volume by volume movements (Van Dijk et al., 2012), ii) root mean squared (RMS) movements indicating variance over voxels (Satterthwaite et al., 2013) and iii) DVARS as a combination of the former with D referring to temporal derivative of time courses and VARS referring to RMS variance over voxels (Power et al., 2012). In these three parameters, neither patient and control subsamples nor the overall disease cohorts showed group differences (t-tests with p > 0.2) indicating reasonably similar head motion in the scanning process of patients and healthy controls (Tables 6+7). In order to reduce spurious correlations between BOLD time courses through confounds such as physiological noise and motion, variance that could be explained by the following nuisance variables was removed from each voxel's time series: i) the six motion parameters derived from the image realignment and ii) their first derivative. According to published evaluations, motion regressors entered the model as first and second-order terms resulting in 24 movement regressors (Satterthwaite et al., 2013). In light of evidence suggesting that group comparisons may be distorted by correcting for the global mean signal (Gotts et al., 2013, Saad et al., 2012), no global signal regression was performed in the current study. Finally, data was band pass filtered preserving BOLD frequencies between 0.01 and 0.08 Hz (Fox and Raichle, 2007).
Table 6.
Comparison of within-scan motion parameters for schizophrenia (SCZ); DVARS: temporal derivative of time courses cf. Power 2012, FD: frame wise displacement cf. Van Dijk et al., 2012, RMS: variance over voxels cf. Satterthwaite et al., 2013. SD: standard deviation.
| SCZ patients |
Healthy controls |
Statistical comparison p-values |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | t-Test/ranksum | |
| DVARS | |||
| Entire group | 1.40 ± 0.30 | 1.35 ± 0.32 | 0.2695/0.1893 |
| Aachen | 1.58 ± 0.26 | 1.54 ± 0.24 | 0.6894/0.7196 |
| Aachen #2 | 1.20 ± 0.22 | 1.11 ± 0.16 | 0.2721/0.4336 |
| Utrecht | 1.80 ± 0.22 | 1.85 ± 0.18 | 0.5690/0.4274 |
| Göttingen | 1.22 ± 0.18 | 1.17 ± 0.19 | 0.2776/0.1388 |
| Lille | 1.62 ± 0.12 | 1.59 ± 0.23 | 0.7763/0.4894 |
| FD | |||
| Entire group | 0.28 ± 0.13 | 0.26 ± 0.10 | 0.1754/0.4405 |
| Aachen | 0.34 ± 0.17 | 0.30 ± 0.11 | 0.5297/0.9183 |
| Aachen #2 | 0.30 ± 0.15 | 0.26 ± 0.09 | 0.3639/0.7125 |
| Utrecht | 0.29 ± 0.11 | 0.25 ± 0.04 | 0.2769/0.8501 |
| Göttingen | 0.22 ± 0.07 | 0.23 ± 0.09 | 0.6033/0.8487 |
| Lille | 0.40 ± 0.12 | 0.33 ± 0.15 | 0.2953/0.1903 |
| RMS | |||
| Entire group | 0.21 ± 0.10 | 0.19 ± 0.07 | 0.1384/0.3902 |
| Aachen | 0.25 ± 0.13 | 0.22 ± 0.08 | 0.5226/0.6816 |
| Aachen #2 | 0.22 ± 0.11 | 0.19 ± 0.07 | 0.2936/0.4611 |
| Utrecht | 0.20 ± 0.08 | 0.17 ± 0.03 | 0.3041/0.7913 |
| Göttingen | 0.16 ± 0.05 | 0.16 ± 0.07 | 0.6251/0.8590 |
| Lille | 0.29 ± 0.10 | 0.23 ± 0.10 | 0.2178/0.1615 |
Table 7.
Comparison of within-scanner motion parameters for major depression disorder (MDD); DVARS: temporal derivative of time courses cf. Power 2012, FD: frame wise displacement cf. Van Dijk et al., 2012, RMS: variance over voxels cf. Satterthwaite et al., 2013. SD: standard deviation.
| MDD patients |
Healthy controls |
Statistical comparison p-values |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | t-Test/ranksum | |
| DVARS | |||
| Entire group | 1.31 ± 0.30 | 1.29 ± 0.26 | 0.6299/0.8420 |
| Göttingen | 1.22 ± 0.21 | 1.18 ± 0.17 | 0.2894/0.4819 |
| Aachen | 1.52 ± 0.32 | 1.55 ± 0.26 | 0.6464/0.6414 |
| Munich | 1.22 ± 0.30 | 1.20 ± 0.19 | 0.7448/0.8365 |
| FD | |||
| Entire group | 0.26 ± 0.14 | 0.25 ± 0.11 | 0.3909/0.6490 |
| Göttingen | 0.26 ± 0.15 | 0.23 ± 0.11 | 0.2773/0.4606 |
| Aachen | 0.28 ± 0.14 | 0.31 ± 0.11 | 0.5243/0.4376 |
| Munich | 0.24 ± 0.10 | 0.21 ± 0.08 | 0.2905/0.3119 |
| RMS | |||
| Entire group | 0.19 ± 0.10 | 0.18 ± 0.08 | 0.3891/0.6195 |
| Göttingen | 0.19 ± 0.11 | 0.17 ± 0.08 | 0.3067/0.4441 |
| Aachen | 0.21 ± 0.10 | 0.22 ± 0.08 | 0.5895/0.4119 |
| Munich | 0.17 ± 0.07 | 0.15 ± 0.06 | 0.3054/0.3119 |
2.4. Resting state fMRI data: individual & group level analyses
To quantify resting state FC of the meta-analytically defined DMPFC and PRC/PCC volumes of interest (VOI) for each subject, their time-series were extracted as the first eigenvariate of the gray matter voxels within the VOI. Linear Pearson correlations were computed for both VOIs with all other voxels' time-series in every subject and correlation coefficients were Fischer's Z transformed for group comparison. A group-level ANOVA was calculated to directly compare group effects for SCZ and MDD patients in one model including regressors for both seed regions in each of the 4 groups resulting in 8 regressors (SCZ[DLPFC + PRC/PCC] + HCscz[2VOIs] + MDD[2VOIs] + HCmdd[2VOIs]).
Disorder-related changes in connectivity of both seeds were tested within each patient group relative to the respective control group, always in conjunction with the main effect of the respective seed's positive correlation in each control group (e.g., PRC/PCC[HCSCZ > SCZ] ∩ PRC/PCC[HCSCZ] ∩ PRC/PCC[HCMDD]). Within each group, conjunctions over both seeds' FC changes were performed to analyze brain-wide FC aberrations. The same was done in both patient groups to assess transdiagnostic FC changes across SCZ and MDD.
Finally, disorder-specific differences in connectivity changes were explored by testing for an interaction effect between FC aberration and patient group, i.e., whether a given FC decrease in one patient group is bigger than the one observed in the other group (e.g., PRC/PCC[HCSCZ > SCZ > HCMDD > MDD]), again in conjunction with the group-specific pattern of FC change. All results were corrected for multiple testing at the cluster level with a cluster-forming threshold of p < 0.001 and thresholded at p < 0.05 (cFWE).
3. Results
3.1. Within-diagnosis connectivity differences
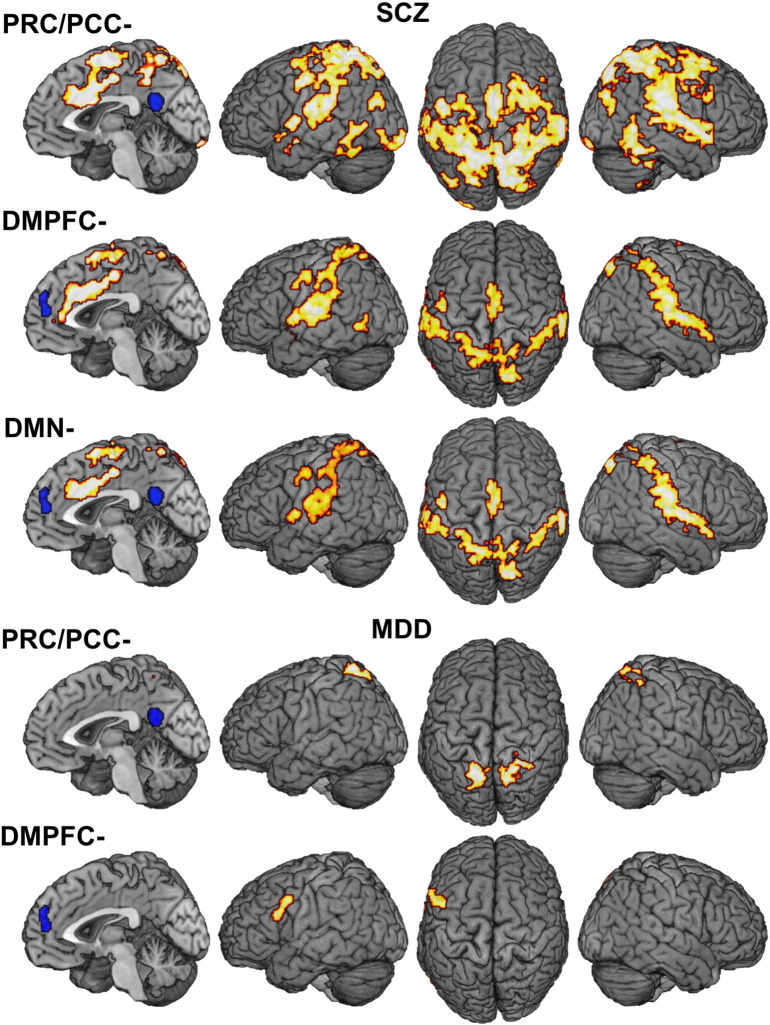
SCZ patients relative to healthy controls HC showed widespread connectivity reduction of the PRC/PCC to many brain areas including inferior (IPL) and superior parietal lobule (SPL), medial and lateral premotor cortex (PMC), midcingulate cortex (MCC), the rolandic operculum (OP), superior temporal sulcus (STS) and occipital cortex, fusiform gyrus, primary sensorimotor cortex, cerebellum and the insula (Fig. 2A; MNI-coordinates in Table 8). For the DMPFC, connectivity reduction in SCZ was found in a similar but less extensive network comprising SPL, IPL, STS, OP, left lateral and medial PMC, MCC, perigenual anterior cingulate cortex (pACC,) insula and left postcentral gyrus. The conjunction between FC reduction of the anterior and posterior hubs of the DMN revealed aberrant connectivity of MCC, supplementary motor area (SMA), bilateral SPL, OP and STS, posterior insula and secondary somatosensory cortex (SII). In MDD the PRC/PCC showed reduced FC with bilateral SPL (Fig. 2B). Reduced connectivity in MDD was also observed for the anterior node of the DMN with the DMPFC being less connected to left area 44. FC increases were not observed in any of the patient as compared to the respective control groups.
Fig. 2.
Results of within-diagnosis analyses: A) FC reduction of PRC/PCC and DMPFC in patients with schizophrenia relative to healthy controls and their overlap. B) FC reduction of PRC and DMPFC in patients with depression relative to healthy controls.
Table 8.
Results of within-diagnosis analyses. Suprathreshold clusters at a height threshold of p = 0.05 cluster-level corr. and a cluster-forming threshold of puc < 0.001. MNI coordinates of principally activated voxels for each cluster are given; assignment to anatomical locations carried by using the SPM Anatomy toolbox.
| Macroanatomical location | x | y | z | k | T |
|---|---|---|---|---|---|
| [SCZ < CON]PRCFig. 2A | |||||
| Superior parietal lobule | 12 | − 38 | 48 | 17022 | 6.64 |
| Right fusiform gyrus | 52 | − 50 | − 24 | 1185 | 5.32 |
| Left fusiform gyrus | − 50 | − 48 | − 12 | 500 | 5.00 |
| Left V3 | − 24 | − 100 | − 14 | 336 | 5.05 |
| Left rolandic operculum | − 58 | 0 | 6 | 219 | 4.79 |
| Left inferior parietal lobule | − 38 | − 74 | 20 | 200 | 4.53 |
| Left anterior insula | − 32 | 24 | 2 | 137 | 4.83 |
| Right cerebellum | 32 | − 44 | − 52 | 136 | 5.07 |
| Left precuneus | − 20 | − 48 | 20 | 103 | 5.39 |
| Right precuneus | 26 | − 56 | 16 | 102 | 4.56 |
| [SCZ < CON]DMPFCFig. 2B | |||||
| Right insula lobe | 36 | 4 | 10 | 2473 | 5.42 |
| Left superior temporal gyrus | − 58 | − 22 | 12 | 1802 | 5.73 |
| Right anterior cingulate cortex | 2 | 18 | 28 | 1413 | 5.38 |
| Right precuneus | 12 | − 76 | 54 | 1105 | 4.72 |
| Posterior-medial frontal cortex | 0 | − 14 | 66 | 351 | 4.32 |
| Left postcentral gyrus | − 50 | − 8 | 46 | 141 | 4.14 |
| Left insula lobe | − 40 | 4 | − 10 | 134 | 5.09 |
| Left middle temporal gyrus | − 50 | − 58 | − 4 | 118 | 4.10 |
| Left precuneus | − 10 | − 42 | 58 | 101 | 4.10 |
| [MDD < CON]PRCFig. 2C | |||||
| Right superior parietal cortex | 20 | − 52 | 64 | 453 | 4.96 |
| Left superior parietal cortex | − 16 | − 62 | 62 | 315 | 4.83 |
| [MDD < CON]DMPFCFig. 2D | |||||
| Left precentral gyrus | − 58 | 12 | 32 | 312 | 4.64 |
3.2. Transdiagnostic alterations in functional connectivity profiles
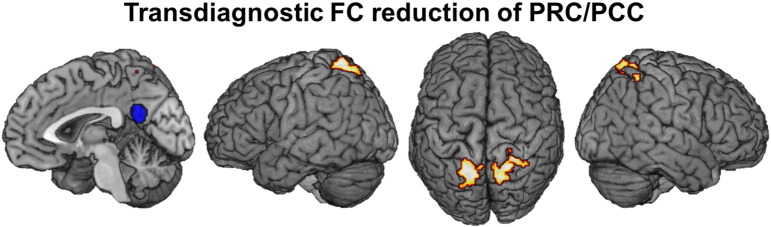
The analysis of common connectivity alterations in both patient groups revealed a transdiagnostic reduction of FC between the PRC/PCC and bilateral SPL relative to their respective healthy control groups (Fig. 3; Table 9).
Fig. 3.
Transdiagnostic similarities in functional connectivity alterations: Reduction of FC between PRC/PCC and bilateral superior parietal cortex across both diagnostic groups relative to healthy controls.
Table 9.
Results of transdiagnostic analyses. Suprathreshold clusters at a height threshold of p = 0.05 cluster-level corr. and a cluster-forming threshold of puc < 0.001. MNI coordinates of principally activated voxels for each cluster are given; assignment to anatomical locations carried by using the SPM Anatomy toolbox.
| Macroanatomical location | x | y | z | k | T |
|---|---|---|---|---|---|
| [PAT < CON]PRC | |||||
| Right superior parietal cortex | 20 | − 52 | 64 | 389 | 4.91 |
| Left superior parietal cortex | − 16 | − 54 | 68 | 291 | 4.83 |
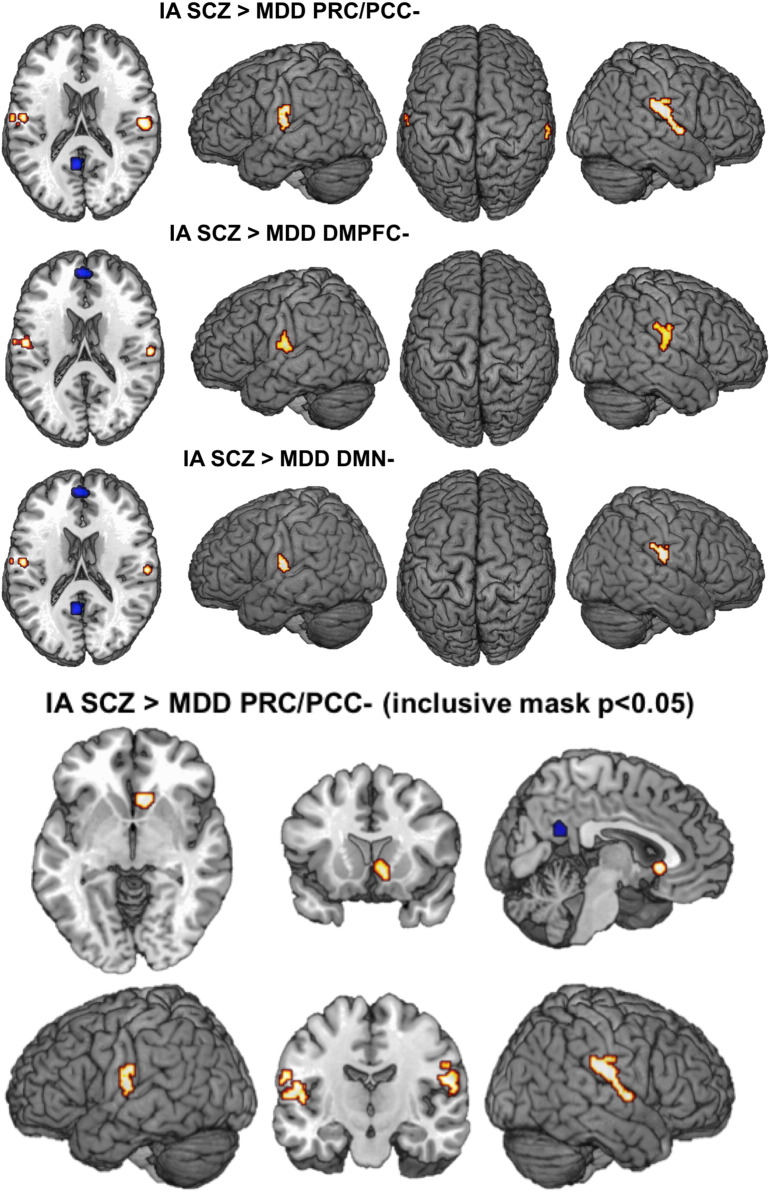
| [SCZ < CON] relative to [MDD < CON]PRC/PCC | |||||
| Right rolandic operculum | 58 | − 18 | 18 | 346 | 4.52 |
| Left rolandic operculum | − 52 | − 12 | 14 | 121 | 3.95 |
| [SCZ < CON] relative to [MDD < CON]PRC/PCC inclusively masked p < 0.05 | |||||
| Right rolandic operculum | 58 | − 18 | 18 | 361 | 4.53 |
| Left rolandic operculum | − 50 | − 14 | 14 | 133 | 4.41 |
| Right caudate nucleus | 8 | 18 | − 4 | 110 | 4.75 |
| [SCZ < CON] relative to [MDD < CON]DMPFC | |||||
| Right rolandic operculum | 62 | − 20 | 22 | 112 | 3.92 |
| Left rolandic operculum | − 52 | − 12 | 14 | 110 | 4.67 |
| [SCZ < CON] relative to [MDD < CON]DMN | |||||
| Right rolandic operculum | 62 | − 20 | 22 | 67 | 3.92 |
| Left rolandic operculum | − 52 | − 12 | 14 | 56 | 3.95 |
3.3. Diagnosis-specific alterations in functional connectivity profiles
Differential changes in FC were found for SCZ patients, who showed a more pronounced connectivity reduction of the PRC/PCC with bilateral parietal OP than MDD patients (Fig. 4). Further investigation of the interaction effect between PRC/PCC-based FC reductions in SCZ as compared to MDD revealed an additional difference with less FC between PRC/PCC and the ventral striatum in SCZ as compared to MDD patients. The analysis of disorder-specific DMPFC connectivity differences showed a significant reduction of FC in SCZ to a smaller proportion of bilateral parietal OP.
Fig. 4.
Transdiagnostic differences in functional connectivity alterations: A) More pronounced reduction of FC from PRC/PCC and DMPFC with bilateral operculum and B) the ventral striatum in SCZ < CON relative to MDD < CON.
4. Discussion
In this study, we assessed transdiagnostic aberrations of resting state FC in SCZ and MDD patients using seed-based resting state analyses of the DMN (Schilbach et al., 2012). This investigation was based on the clinical insight that in spite of obvious phenomenological differences between disorders, both SCZ and MDD are also characterized by transdiagnostic symptoms (e.g., impairments of social interaction abilities), which may point towards common circuit dysconnectivity.
The results of our study, indeed, demonstrate shared, region-specific FC reductions of the DMN across both SCZ and MDD. This transdiagnostically observed dysconnectivity pattern included the precuneus and bilateral superior parietal cortex. Furthermore, we found evidence for differential connectivity alterations of the DMN in SCZ relative to MDD with more pronounced reductions of connectivity to both anterior and posterior nodes of the DMN in SCZ.
4.1. Within-diagnosis functional connectivity differences: Schizophrenia
In line with previous research, our results demonstrate significant alterations of FC of the DMN in SCZ (Bastos-Leite et al., 2015, Fischer et al., 2014, Gong et al., 2014, Guo et al., 2014, Rolland et al., 2015). In particular, the PRC/PCC has been implicated as a ‘hot spot’ for structural as well as FC aberrations in SCZ, which are not only observed across the entire clinical spectrum (Wang et al., 2014), but also appear to be related to relevant genetic variants (Gong et al., 2014). From a cognitive psychology perspective, it has been argued that FC of the PRC/PCC and other parietal areas may be particularly relevant for self-oriented processing and for providing a stable egocentric representation of space (Land, 2014, Lou et al., 2010). Furthermore, it has been argued that this network relevant for self-awareness might be particularly affected in SCZ (Bluhm et al., 2009, Guo et al., 2014).
Dysconnectivity of the anterior hub of the DMN, i.e., the DMPFC, has also previously been associated with a familial risk for SCZ and its intrinsic connectivity is known to carry measurable consequences for social functioning (Dodell-Feder et al., 2014). Consistent with these previous findings, our results demonstrate a significant reduction of FC for DMPFC with pACC, MCC, bilateral insula and STS, all of which are regions that are involved in cognitive control and self-monitoring processes and could, therefore, contribute to social abilities (Bernhardt et al., 2014, Meyer et al., 2013). In particular, it has been demonstrated that DMPFC serves an important modulatory role by influencing activity in other brain regions when task requirements make it necessary (Wheelock et al., 2014). Therefore, alterations of DMPFC-based intrinsic connectivity as observed in SCZ patients may adversely affect cognitive processing by disallowing for such modulations to take place (Becerril and Barch, 2013).
4.2. Within-diagnosis functional connectivity differences: Depression
Within the MDD group connectivity reductions of the posterior node of the DMN, i.e., PRC/PCC, was found to bilateral SPL, which constitutes a striking parallel to the findings in the SCZ group. While alterations of SPL activity at rest are less often described in the literature to be associated with MDD (Liu et al., 2012), the structural connection between bilateral SPL was found to discriminate depressed patients from controls in a classification analysis using diffusion tractography (Korgaonkar et al., 2012). With regard to symptom severity, an increase in PRC/PCC activation was found for depressed patients responding to treatment with fluoxetine (Mayberg et al., 1999) as well as for deep brain stimulation (Lozano et al., 2008).
Reduced connectivity in MDD was also observed for the anterior node of the DMN, as indicated by a significant reduction of DMPFC connectivity with left inferior frontal gyrus. These two regions and their co-activation patterns are known to be relevant for planning, carrying out and responding to actions, in particular when they occur in a social context (Schilbach et al., 2011), which can be disturbed in MDD. Consistently, both regions have also previously been found to show aberrant activation patterns in patients relative to controls for tasks that are typically associated with depressive symptomatology (Seidel et al., 2012).
4.3. Transdiagnostic similarities in functional connectivity alterations
Based on the assumption that phenotypic commonalities could rely on common brain circuit dysfunction (Buckholtz and Meyer-Lindenberg, 2012), we conducted an analysis of transdiagnostic similarities in connectivity alterations across SCZ and MDD patients relative to the respective control groups. This analysis demonstrated a significant reduction of FC between PRC/PCC and bilateral SPL across both patient groups. Interestingly, the interaction of medial and lateral parietal networks has been shown to be important for regulating the balance between internally and externally directed attention (Leech et al., 2011). More specifically, the brain regions implicated (PRC/PCC and SPL) have both been associated with top-down control of attention and indirectly with sensorimotor integration and are thus thought to provide a coherent self-representation across space and time (Schedlbauer et al., 2014). While SPL activity change has been linked to spatial shifts of attention (Kelley et al., 2008), precuneus has been linked to non-spatial shifts of attention (Giesbrecht et al., 2003). Further evidence has demonstrated strong reciprocal relations between activity in these two regions, which have been discussed as contributing to a finely tuned interplay between stimulus-oriented and stimulus-independent cognition (e.g., Dosenbach et al., 2008).
Fittingly, both SCZ and MDD are associated with a dysbalance between internally and externally directed attention and mental state attribution: While patients with MDD are known to attribute internally when making sense of external occurrences and tend to do so in a self-defeating manner, patients with SCZ often use an externalizing bias in the attribution of causation of social events (Janssen et al., 2006). Social difficulties in both disorders could, therefore, be related to such negative self-referential thoughts, distorted attributional biases and the subsequent development of anxiety. Also, patients from both diagnostic groups often withdraw from social contexts, which may lead to a further consolidation of disorder-specific symptomatology. It is tempting to speculate that these social impairments could be related to common dysconnectivity patterns of posterior nodes of DMN that are present in both patient groups, but unfortunately no measures of social behavior were available in our study to directly investigate this putative association.
4.4. Disorder-specific differences in functional connectivity alterations
Finally, we explored disorder-specific differences in FC by testing whether observed connectivity alterations are more pronounced in one patient group than in the other. This analysis revealed a specifically reduced connectivity pattern in SCZ patients for PRC/PCC and DMPFC with the parietal operculum. In general, disruption of the parietal operculum has already been demonstrated in SCZ (Andreou et al., 2015, Fornito et al., 2011) and is well in line with cognitive functions associated with this structure, such as interoceptive awareness, multisensory integration and body perception (Ebisch et al., 2014, Ionta et al., 2011, Tsakiris, 2010). In light of these findings, dysconnectivity of the parietal operculum may represent a disorder-specific aspect of SCZ that could be linked to alterations of a basic sense of self. Furthermore, it is conceivable that activity alterations of this region feed into or trigger higher-order self-processing disturbances and subsequent misattributions of mental states, which could be related to activity changes of medial parietal or dorsomedial prefrontal cortex (Menon et al., 2011, Nekovarova et al., 2014, Rotarska-Jagiela et al., 2010). Taken together, the reduced coupling of OP with the DMN in SCZ may represent an important and disorder-specific pathophysiological mechanism for self-disturbances in SCZ.
Furthermore, disorder-specific connectivity differences in SCZ as compared to MDD were also found for the ventral striatum, which has been strongly linked to reward processing (Weiland et al., 2013). In schizophrenia, ventral striatal activity has furthermore been related to the concept of “aberrant salience”, according to which a deregulated state of activity can lead to “an aberrant assignment of salience to elements of one's experience” and whereby hallucinations “reflect a direct experience of the aberrant salience of internal representations” (Kapur, 2003). Accordingly, clinical studies of visual and auditory hallucinations have demonstrated alterations of ventral striatal connectivity (Rolland et al., 2015). Our findings corroborate these findings by documenting that ventral striatal dysconnectivity is significantly associated with SCZ rather than MDD.
4.5. Limitations
The design of the current study, which draws upon the possibility of data pooling over several measurement sites as well as two psychiatric disorders, includes the following important drawbacks. From a methodological point of view, combining data from multiple sites entails the risk of finding spurious effects due to systematic differences between sample recruitment and the measurement of behavioral and imaging data or solely due to the comparison of different scanners. To minimize the influence of measurement site on group comparisons, one should control for systematic differences in confounding factors like age, gender or within scanner movement between sites. Another approach would be to explicitly modeling measurement site as a variable of no interest, which becomes difficult when dealing with more than two sites, as the actual amount of difference will certainly be unequal between sites. Therefore, and to retain as much variance in the data as possible we applied the former strategy and independently matched groups of patients and controls within every site and disease for age, sex and within scanner movements. As there were no significant differences of these factors within each subsample as well as in the overall cohorts for both diseases, group comparisons should not significantly be driven by site differences. Moreover, due to the data pooling procedure implemented in our study with the aim of maximizing sample size, we were not able to collect representative data on the state of social impairments in both patient groups. This fact clearly limits the generalizability of the presented FC decrease as to their specificity for social impairments. Even though, transdiagnostic dysconnectivity between PRC/PCC and SPL could be shown for both MDD and SCZ, its co-variation with severity of social impairments in general, as well as with particular disabilities in social behavior, remains to be shown. As resting-state FC is a very indirect measure of these social-cognitive processes, connectivity modeling based on task-based studies would be needed to directly relate the transdiagnostic findings observed in our study to individual clinical symptomatology, attributional styles and real-life social functioning. Furthermore, future research could use connectivity measures to evaluate network dysfunction during the course of illness with the aim of predicting the effects of various treatment options.
5. Conclusions
Taken together, our study provides first-time evidence for commonalities in transdiagnostic functional connectivity alterations of the DMN in SCZ and MDD. These findings at the neural level parallel descriptions of phenotypic commonalities across both disorders, which exist next to disorder-specific disturbances and have emphasized impairments of social interaction (for a review see Schilbach, 2015). In light of these findings, it is tempting to speculate that phenotypic overlap could be related to common brain circuit dysfunction. Unfortunately, no measures of social behavior were available in the patient samples here described to further investigate this possible relationship. In terms of the neurofunctional alterations present in both disorders groups, previous research can be taken to suggest that medial and superior parietal cortex dysconnectivity may lead to a disturbance of the finely tuned and flexible balance between internally and externally directed attention. In addition to this, dysconnectivity of the DMN could also be related to well known differences in attributional style that patients use to make sense of the environment.
Furthermore, our analysis demonstrates that disorder-specific differences also exist as indexed by more strongly reduced coupling of the parietal operculum with the DMN in SCZ as compared to MDD. In light of previous research, which has convincingly demonstrated that the operculum plays an important role in somatosensory integration and body perception and that SCZ is associated with a disruption of activity in this brain region, this finding of opercular dysconnectivity with the DMN may represent an important and disorder-specific pathophysiological mechanism for self-disturbances in SCZ. In particular, one could speculate that activity alterations of the operculum may trigger additional self-processing disturbances and could thereby lead to subsequent misattributions of mental states.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
L.S. was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) and the Max-Planck-Society (MPG). S.B.E. was supported by grants from the National Institute of Health, the DFG, the Helmholtz Initiative on Systems Biology and the European EFT program. L.K. and B.D. were supported by DFG, IRTG-1328 and JARA-Brain.
References
- Akdeniz C., Tost H., Meyer-Lindenberg A. The neurobiology of social environmental risk for schizophrenia: an evolving research field. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49:507–517. doi: 10.1007/s00127-014-0858-4. [DOI] [PubMed] [Google Scholar]
- Andreou C., Nolte G., Leicht G., Polomac N., Hanganu-Opatz I., Lambert M. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr. Bull. 2015;41(4):930–939. doi: 10.1093/schbul/sbu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bastos-Leite A., Ridgway G., Silveira C., Norton A., Reis S., Friston K. Dysconnectivity within the default mode in first-episode schizophrenia: a stochastic dynamic causal modeling study with functional magnetic resonance imaging. Schizophr. Bull. 2015;41(1):144–153. doi: 10.1093/schbul/sbu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril K., Barch D. Conflict and error processing in an extended cingulo-opercular and cerebellar network in schizophrenia. Neuroimage Clin. 2013;3:470–480. doi: 10.1016/j.nicl.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B., Valk S., Silani G., Bird G., Frith U., Singer T. Selective disruption of sociocognitive structural brain networks in autism and alexithymia. Cereb. Cortex. 2014;24(12):3258–3267. doi: 10.1093/cercor/bht182. [DOI] [PubMed] [Google Scholar]
- Billeke P., Aboitiz F. Social cognition in schizophrenia: from social stimuli processing to social engagement. Front. Psychiatry. 2013;4:4. doi: 10.3389/fpsyt.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg W. Parodos; Berlin: 1971. The Loss of Natural Self-Evidence: A Contribution to the Study of Sypmtom-Poor Schizophrenias. [Google Scholar]
- Bluhm R., Miller J., Lanius R., Osuch E., Boksman K., Neufeld R. Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. doi: 10.1016/j.pscychresns.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Buckholtz J., Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cuthbert B., Insel T. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Calhoun V., Malhi G. Mentalizing in male schizophrenia patients is compromised by virtue of dysfunctional connectivity between task-positive and task-negative networks. Schizophr. Res. 2012;140:51–58. doi: 10.1016/j.schres.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Delisi L., Hooker C. The relationship between default mode network connectivity and social functioning in individuals at familial high-risk for schizophrenia. Schizophr. Res. 2014;156:87–95. doi: 10.1016/j.schres.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch S., Mantini D., Northoff G., Salone A., De Berardis D., Ferri F. Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophr. Bull. 2014;40:1072–1082. doi: 10.1093/schbul/sbt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Whitfield-Gabrieli S., Roth R., Brunette M., Green A. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: effects of cannabis and THC. Schizophr. Res. 2014;158(1–3):176–182. doi: 10.1016/j.schres.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszdon J., Fanning J., Johannesen J., Bell M. Social cognitive deficits in schizophrenia and their relationship to clinical and functional status. Psychiatry Res. 2013;205:25–29. doi: 10.1016/j.psychres.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M., Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frith C. Schizophrenia and theory of mind. Psychol. Med. 2004;34:385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- Frith C.D. Social cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C., Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychol. Med. 1996;26:521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Fuchs T. Melancholia as a desynchronization: towards a psychopathology of interpersonal time. Psychopathology. 2001;34:179–186. doi: 10.1159/000049304. [DOI] [PubMed] [Google Scholar]
- Fuchs T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology. 2007;40:229–235. doi: 10.1159/000101365. [DOI] [PubMed] [Google Scholar]
- Fulford D., Niendam T., Floyd E., Carter C., Mathalon D., Vinogradov S. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr. Res. 2013;147:125–131. doi: 10.1016/j.schres.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht B., Woldorff M.G., Song A.W., Mangun G.R. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gong X., Lu W., Kendrick K., Pu W., Wang C., Jin L. A Brain-wide association study of DISC1 genetic variants reveals a relationship with the structure and functional connectivity of the precuneus in schizophrenia. Hum. Brain Mapp. 2014;35(11):5414–5430. doi: 10.1002/hbm.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts S., Saad Z., Jo H., Wallace G., Cox R., Martin A. The perils of global signal regression for group comparisons: a case study of autism spectrum disorders. Front. Hum. Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Kendrick K., Yu R., Wang H., Feng J. Key functional circuitry altered in schizophrenia involves parietal regions associated with sense of self. Hum. Brain Mapp. 2014;35:123–139. doi: 10.1002/hbm.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen M.C., Gotlib I.H. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol. Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C., Benson T., Gyurak A., Yin H., Tully L., Lincoln S. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J. Abnorm. Psychol. 2014;123:190–204. doi: 10.1037/a0035223. [DOI] [PubMed] [Google Scholar]
- Ionta S., Heydrich L., Lenggenhager B., Mouthon M., Fornari E., Chapuis D. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron. 2011;70:363–374. doi: 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Janssen I., Versmissen D., Campo J., Myin-Germeys I., van Os J., Krabbendam L. Attribution style and psychosis: evidence for an externalizing bias in patients but not in individuals at high risk. Psychol. Med. 2006;36:771–778. doi: 10.1017/S0033291706007422. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kelley T.A., Serences J.T., Giesbrecht B., Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cereb. Cortex. 2008;18:114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkotter J., Hellmich M., Steinmeyer E., Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Cooper N.J., Williams L.M., Grieve S.M. Mapping inter-regional connectivity of the entire cortex to characterize major depressive disorder: a whole-brain diffusion tensor imaging tractography study. Neuroreport. 2012;23:566–571. doi: 10.1097/WNR.0b013e3283546264. [DOI] [PubMed] [Google Scholar]
- Ladegaard N., Larsen E., Videbech P., Lysaker P. Higher-order social cognition in first-episode major depression. Psychiatry Res. 2014;216:37–43. doi: 10.1016/j.psychres.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Land M. Do we have an internal model of the outside world? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130045. doi: 10.1098/rstb.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Altshuler L., Glahn D., Miklowitz D., Ochsner K., Green M. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am. J. Psychiatry. 2013;170:334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Ku J., Kim J., Jang D., Yoon K., Kim S. Aberrant neural responses to social rejection in patients with schizophrenia. Soc. Neurosci. 2014:1–12. doi: 10.1080/17470919.2014.907202. [DOI] [PubMed] [Google Scholar]
- Leech R., Kamourieh S., Beckmann C., Sharp D. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Chen A., Zebley B., Drysdale A., Gordon R., Leuchter B. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ma X., Wu X., Li F., Zhang Y., Zhou F. Resting-state abnormal baseline brain activity in unipolar and bipolar depression. Neurosci. Lett. 2012;516:202–206. doi: 10.1016/j.neulet.2012.03.083. [DOI] [PubMed] [Google Scholar]
- Lou H., Luber B., Stanford A., Lisanby S. Self-specific processing in the default network: a single-pulse TMS study. Exp. Brain Res. 2010;207:27–38. doi: 10.1007/s00221-010-2425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano A.M., Mayberg H.S., Giacobbe P., Hamani C., Craddock R.C., Kennedy S.H. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E., Sonuga-Barke E., De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol. Rev. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- Mayberg H., Liotti M., Brannan S., McGinnis S., Mahurin R., Jerabek P. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCullough J.J. Treatment for chronic depression using Cognitive Behavioral Analysis System of Psychotherapy (CBASP) J. Clin. Psychol. 2003;59:833–846. doi: 10.1002/jclp.10176. [DOI] [PubMed] [Google Scholar]
- Meda S., Gill A., Stevens M., Lorenzoni R., Glahn D., Calhoun V. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol. Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M., Schmitz T.W., Anderson A.K., Graff A., Korostil M., Mamo D. Exploring the neural correlates of delusions of reference. Biol. Psychiatry. 2011;70:1127–1133. doi: 10.1016/j.biopsych.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Meyer M., Masten C., Ma Y., Wang C., Shi Z., Eisenberger N. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc. Cogn. Affect. Neurosci. 2013;8:446–454. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S., Cuthbert B. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekovarova T., Fajnerova I., Horacek J., Spaniel F. Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory. Front. Behav. Neurosci. 2014;8:171. doi: 10.3389/fnbeh.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon N., Liddle P., Nixon E., Worwood G., Liotti M., Palaniyappan L. Biological vulnerability to depression: linked structural and functional brain network findings. Br. J. Psychiatry. 2014;204:283–289. doi: 10.1192/bjp.bp.113.129965. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland B., Amad A., Poulet E., Bordet R., Vignaud A., Bation R. Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr. Bull. 2015;41(1):291–299. doi: 10.1093/schbul/sbu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knochel V., Uhlhaas P.J., Vogeley K., Linden D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Saad Z., Gotts S., Murphy K., Chen G., Jo H., Martin A. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore G., Dimaggio G., Lysaker P. An intersubjective perspective on negative symptoms of schizophrenia: implications of simulation theory. Cogn. Neuropsychiatry. 2007;12:144–164. doi: 10.1080/13546800600819921. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savla G.N., Twamley E.W., Delis D.C., Roesch S.C., Jeste D.V., Palmer B.W. Dimensions of executive functioning in schizophrenia and their relationship with processing speed. Schizophr. Bull. 2012;38:760–768. doi: 10.1093/schbul/sbq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer A.M., Copara M.S., Watrous A.J., Ekstrom A.D. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci. Rep. 2014;4:6431. doi: 10.1038/srep06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L. Toward a second-person neuropsychiatry. Philos. Trans. R. Soc. B. 2015 doi: 10.1098/rstb.2015.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Wilms M., Eickhoff S., Romanzetti S., Tepest R., Bente G. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 2010;22:2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S., Cieslik E., Shah N., Fink G., Vogeley K. Eyes on me: an fMRI study of the effects of social gaze on action control. Soc. Cogn. Affect. Neurosci. 2011;6:393–403. doi: 10.1093/scan/nsq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Bzdok D., Timmermans B., Fox P., Laird A., Vogeley K. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Muller V., Hoffstaedter F., Clos M., Goya-Maldonado R., Gruber O. Meta-analytically informed network analysis of resting state FMRI reveals hyperconnectivity in an introspective socio-affective network in depression. PLoS One. 2014;9:e94973. doi: 10.1371/journal.pone.0094973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D., Regenbogen C., Kellermann T., Finkelmeyer A., Kohn N., Derntl B. Empathic behavioral and physiological responses to dynamic stimuli in depression. Psychiatry Res. 2012;200:294–305. doi: 10.1016/j.psychres.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Seidel E., Satterthwaite T., Eickhoff S., Schneider F., Gur R., Wolf D. Neural correlates of depressive realism—an fMRI study on causal attribution in depression. J. Affect. Disord. 2012;138:268–276. doi: 10.1016/j.jad.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon B.J., Buckner R.L. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N., Ribaux D., Phillips L. Rumination, depressive symptoms and awareness of illness in schizophrenia. Behav. Cogn. Psychother. 2014;42:143–155. doi: 10.1017/S1352465812000884. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Xia M., Lai Y., Dai Z., Cao Q., Cheng Z. Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophr. Res. 2014;156:150–156. doi: 10.1016/j.schres.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Weiland B., Welsh R., Yau W., Zucker R., Zubieta J., Heitzeg M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M., Sreenivasan K., Wood K., Ver Hoef L., Deshpande G., Knight D. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage. 2014;102(Pt 2):904–912. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Allen E., Sui J., Arbabshirani M., Pearlson G., Calhoun V. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Curr. Top. Med. Chem. 2012;12:2415–2425. doi: 10.2174/156802612805289890. [DOI] [PMC free article] [PubMed] [Google Scholar]