Abstract
OBJECTIVES
We assessed retention and predictors of attrition (recorded death or loss to follow-up) in antiretroviral treatment (ART) clinics in Tanzania, Uganda and Zambia.
METHODS
We conducted a retrospective cohort study among adults (≥18 years) starting ART during 2003–2010. We purposefully selected six health facilities per country and randomly selected 250 patients from each facility. Patients who visited clinics at least once during the 90 days before data abstraction were defined as retained. Data on individual and programme level risk factors for attrition were obtained through chart review and clinic manager interviews. Kaplan–Meier curves for retention across sites were created. Predictors of attrition were assessed using a multivariable Cox-proportional hazards model, adjusted for site-level clustering.
RESULTS
From 17 facilities, 4147 patients were included. Retention ranged from 52.0% to 96.2% at 1 year to 25.8%–90.4% at 4 years. Multivariable analysis of ART initiation characteristics found the following independent risk factors for attrition: younger age [adjusted hazard ratio (aHR) and 95% confidence interval (95%CI) = 1.30 (1.14–1.47)], WHO stage 4 ([aHR (95% CI): 1.56 (1.29–1.88)], >10% bodyweight loss [aHR (95%CI) = 1.17 (1.00–1.38)], poor functional status [ambulatory aHR (95%CI) = 1.29 (1.09–1.54); bedridden aHR1.54 (1.15–2.07)], and increasing years of clinic operation prior to ART initiation in government facilities [aHR (95%CI) = 1.17 (1.10–1.23)]. Patients with higher CD4 cell count were less likely to experience attrition [aHR (95%CI) = 0.88 (0.78–1.00)] for every log (tenfold) increase. Sites offering community ART dispensing [aHR (95% CI) = 0.55 (0.30–1.01) for women; 0.40 (0.21–0.75) for men] had significantly less attrition.
CONCLUSIONS
Patient retention to an individual programme worsened over time especially among males, younger persons and those with poor clinical indicators. Community ART drug dispensing programmes could improve retention.
Keywords: ART, HIV, retention, sub-Saharan Africa
Introduction
At the end of 2013, two-thirds of the estimated 35 million people globally living with HIV lived in sub-Saharan Africa (UNAIDS 2014). The number of people receiving antiretroviral treatment (ART) reached about 13 million in 2013. Sub-Saharan Africa achieved the greatest increase in ART coverage by reaching 9 million people, to about 37% coverage (UNAIDS 2014).
Corresponding with efforts to expand access to ART, there has been an increasing emphasis on attaining the high levels of retention and adherence necessary to achieve good clinical outcomes (Bangsberg et al. 2001; Hogg et al. 2002; Paterson et al. 2000; Nachega et al. 2007). Retention is a critical determinant of adherence as patients must actively attend and participate in an ART care programme to receive their medication and to have their HIV clinical indicators monitored. Therefore, retention is a key indicator of programme quality (Giordano et al. 2007).
However, retention of patients in ART care remains a major challenge in sub-Saharan programmes. Results from a meta-analysis of 32 studies from programmes in sub-Saharan Africa showed that only 80% of patients started on ART were still in care after 1 year, 77% after 2 years and 72% after 3 years (Rosen et al. 2007; Fox & Rosen 2010). Loss to follow-up (LTFU) and recorded death were the major causes of non-retention or attrition.
This study is the first component of a study examining retention and adherence to antiretroviral treatment among adults in three countries in sub-Saharan Africa. In this manuscript, we report the results of a retrospective medical chart review of adult ART patients from ART programme sites in Tanzania, Uganda and Zambia. The objectives of the study were to characterise the level of retention of patients on ART across multiple and different programme settings and to examine the relationship between individual and programme level characteristics and retention proportions.
Methods
Design and study setting
A retrospective review of 4500 randomly selected medical records of ART patients from Tanzania, Uganda and Zambia was conducted. In each country, six sites were purposefully chosen to explore the impact that different programme characteristics may have on retention and adherence outcomes. This process resulted in study sites from different levels in the health system (ranging from primary/community-based health centres to national referral hospitals), from different types of health facilities (public sector, nongovernmental organisations (NGOs) or faith-based organisations), from urban-rural locations, and with different ART provision experiences and adherence strategies.
Inclusion criteria for site selection
Only sites with a minimum cohort size of 300 patients at the time of the protocol development (2006) were considered to fulfil the sample size requirement of 250 patients per study site. The site-selection process was conducted in consultation with country-specific stakeholders including Ministries of Health (MOH) and United States Government (USG) partner organisations.
Inclusion and exclusion criteria for medical chart review
Patients who were at least 18 years of age at ART initiation, who initiated ART treatment (a combination of 3 antiretroviral drugs) at the site when free drugs were available and who had started ART at least 6 months prior to the data collection were included. Patients involved in other ART-related research were excluded.
Data collection and sampling
During the period from April to July 2010, a retrospective chart review of 4500 medical charts was conducted. During study start-up, the study team noticed that one facility in Tanzania consisted of two clinics that served adults on ART: an adult-only clinic and a family clinic where adults may seek care and treatment with their families. One hundred and 25 patients from each of the adult-only and the family clinics were selected.
The study team worked with sites to develop sampling frames consisting of patient identification numbers (IDs). The study data analyst used these sampling frames to generate the 250 random numbers using a computerised random number generator in Microsoft Excel that indicated which patient charts would be abstracted. Data abstractors at each site pulled the randomly selected charts and screened them for eligibility. They recorded on a screening log if a chart was missing, ineligible, or eligible and abstracted. Replacement numbers were generated for those charts that were either ineligible or were missing after a minimum of three attempts to locate the chart over three consecutive weeks. Three sources of data were used: patient medical files, pharmacy logbooks and laboratory registers.
The study sites varied greatly in terms of how specific their sampling frames could be. Some sites had sampling frames consisting only of adult patients who had started on ART at that site. Other sites could only produce sampling frames with all patients (adults and children) ever registered in HIV care. The ability to focus the sampling frame depended on the site’s data management systems.
Study teams underwent 1 week of data abstraction training and consisted of 2–3 data abstractors (mainly data entry staff and nurses from the facility) and one supervisor (typically the sister-in-charge or lead doctor managing the clinic). To better understand the programme factors related to retention and adherence, each study-site ART programme manager was interviewed about their ART clinic model in June 2011.
Data management and analysis
Data were double-entered in a study database using Epi-Data Entry 3.1 (EpiData Association, Odense, Denmark, Europe) at the in-country research organisation and then transferred to the central data office at FHI 360 for further cleaning and consistency checks. Consistency checks across the different sources of data were performed. Data analysis followed a statistical analysis plan, which was finalised before the completion of the study database. All analyses were performed using Stata versions 10 and 12 (Stata Corporation, College Station, TX, USA).
Attrition from the programme was defined as having no clinic, pharmacy or laboratory visit in the 3 months prior to data collection among patients whose charts did not indicate being transferred out or death. The primary outcome variable was time to discontinuation from the programme, defined as the time from start of first antiretroviral (ARV) drug treatment prescribed at the health facility until the last visit. The time to discontinuation was analysed using survival data methods (Altman & Bland 1998) where lost to follow-up and known deaths were considered the event of interest. Patients who were retained or transferred out were censored.
Retention proportions were estimated using the Kaplan–Meier method (Pocock et al. 2002). The analysis of risk factors for attrition was performed using Cox-proportional hazards regression, with a shared frailty term to account for clustering of outcomes at each site (Hougaard 1995). The proportional hazards assumption of predictors was assessed using graphical methods. A multiple Cox-regression model was constructed using a hierarchical approach. This approach included first individual level (P-value <0.1) and subsequently programme level (P-value <0.2) characteristics. Variables that were missing in more than 30% of subjects or were not available at all sites or were present in a small minority of patients (<10%) were excluded from the multiple Cox-regression model. The model was simplified using Akaike’s Information Criterion, retaining predictors and clinically plausible interaction terms that increased model fit, with a penalisation for increasing model complexity (Collet 2003). Confidence intervals for effects were estimated using the Wald method and P-values using likelihood ratio tests. The final Cox-regression model was corrected for missing data in the predictors of attrition using Multivariate Imputation by Chained Equations (Little 1992; Royston 2004). Ten data sets were imputed with baseline predictors presumed to be missing at random, conditional on site and individual level predictors. Estimates were combined across imputed data sets according to Rubin’s rules (Little 1992).
Sample size
A random sample of 250 ART-treated patients in each site was needed to allow the estimation of the retention proportion to be measured with a precision of 5% at site level, assuming the retention proportion at 6 months after ART initiation was at least 80%.
Ethics statement
The study was approved by the Institutional Review Board (IRB) of the U.S. Centers for Disease Control and Prevention (US), FHI 360’s Protection of Human Subjects Committee (US), the Muhimbili University of Health and Allied Sciences’ Research and Publications Committee (Tanzania), the National HIV/AIDS Research Committee (Uganda), the Uganda National Council for Science & Technology, the Tropical Diseases Research Centre’s Ethics Review Committee (Zambia), the Ministry of Health (Zambia), the Massachusetts General Hospital’s IRB (US) and Universitair Ziekenhuis Antwerpen (Belgium).
As the study was a retrospective chart review with minimal risks, and requesting consent would mean inclusion of only retained patients, the IRBs waived informed consent requirements to abstract participant charts. Written informed consent was required prior to interviewing the ART clinic managers.
Role of the funding source
This research was supported by a contract with FHI 360 by the U.S. Centers for Disease Control and Prevention (CDC) and the Health Resources & Services Administration (HRSA) with funds from the President’s Emergency Plan for AIDS Relief (PEFPAR). CDC provided technical input into the study design, data collection, data analysis, data interpretation and writing of the manuscript.
Results
A total of 7755 patient medical files were screened for eligibility at the participating sites. Of these, 1951 were ineligible, 1310 files were missing, and 4494 files were abstracted. During preparation for analysis, 84 duplicate files were excluded and another 22 were excluded for not meeting the eligibility criteria. One site in Zambia used an incorrect sampling frame (excluding patients who were known dead or lost to follow-up) which led to the exclusion of that site’s 241 patients, leaving 4147 patients for the final analysis.
Characteristics of the study population
Patients started ART between 2003 and 2010. The median age at ART initiation was 36 years (interquartile range (IQR): 30–42), and 2670 (64.4%) were female. Three quarters of patients (3141 or 75.7%) had a baseline CD4 cell count, with the median CD4 cell count of 134 cells/µl (IQR: 63–206). About half of patients (2197 or 53.0%) were in WHO stage 3 or 4. A vast majority were started on one of the four traditional non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens (3598 or 86.8%) and about 11% on a tenofovir-based regimen. Other characteristics, stratified by country, are presented in Table 1.
Table 1.
Patient characteristics at ART initiation in multicountry retention study
| Characteristic | Tanzania (n = 1458) |
Uganda (n = 1472) |
Zambia (n = 1217) |
Total number of participants (N = 4147) |
|---|---|---|---|---|
| Demographics | ||||
| Age (years): median (IQR) | 37 (31–43) | 35 (30–41) | 35 (30–42) | 36 (30–42) |
| Age 18–29 years: n (%) | 266 (18.2) | 344 (23.4) | 291 (23.9) | 901 (21.7) |
| Age ≥30 years: n (%) | 1191 (81.7) | 1122 (76.2) | 923 (75.8) | 3236 (78.0) |
| Gender: n (%) | ||||
| Male | 484 (33.2) | 504 (34.2) | 488 (40.1) | 1476 (35.6) |
| Female | 974 (66.8) | 968 (65.8) | 728 (59.8) | 2670 (64.4) |
| Distance from clinic: n (%) | ||||
| <10 km | 631 (43.3) | 1073 (72.9) | 212 (17.4) | 1916 (46.2) |
| 10–25 km | 251 (17.2) | 185 (12.6) | 15 (1.2) | 451 (10.9) |
| >25 km | 461 (31.6) | 139 (9.4) | 13 (1.1) | 613 (14.8) |
| Missing | 115 (7.9) | 75 (5.1) | 977 (80.3) | 1167 (28.1) |
| ART related | ||||
| Year of ART initiation: n (%) | ||||
| 2003–2004 | 36 (2.5) | 54 (3.7) | 51 (4.2) | 141 (3.4) |
| 2005 | 232 (15.9) | 226 (15.4) | 188 (15.5) | 646 (15.6) |
| 2006 | 278 (19.1) | 229 (15.6) | 278 (22.8) | 785 (18.9) |
| 2007 | 287 (19.7) | 337 (22.9) | 336 (27.6) | 960 (23.2) |
| 2008 | 360 (24.7) | 296 (20.1) | 212 (17.4) | 868 (20.9) |
| 2009–2010 | 265 (18.2) | 330 (22.4) | 152 (12.5) | 747 (18.1) |
| Years of clinic operation prior to ART initiation: n (%) | ||||
| <1 year | 286 (19.6) | 183 (12.4) | 72 (5.9) | 541 (13.1) |
| ≥1 to <2 years | 312 (21.4) | 196 (13.3) | 196 (16.1) | 704 (17.0) |
| ≥2 to <3 years | 272 (18.7) | 325 (22.1) | 305 (25.1) | 902 (21.8) |
| ≥3 to <4 years | 341 (23.4) | 304 (20.7) | 277 (22.8) | 922 (22.2) |
| ≥4 to <5 years | 196 (13.4) | 207 (14.1) | 211 (17.3) | 614 (14.8) |
| ≥5 years | 51 (3.5) | 257 (17.5) | 156 (12.8) | 464 (11.2) |
| Prior ART experience: n (%) | 112 (7.7) | 85 (5.8) | 55 (4.5) | 252 (6.1) |
| Prior exposure to NVP for PMTCT: n (%) | 0 (0.0) | 86 (8.9) | 94 (12.9) | 180 (6.7) |
| Starting ART regimen: n (%) | ||||
| D4T-3TC-NVP | 991 (68.0) | 540 (36.7) | 404 (33.2) | 1935 (46.7) |
| D4T-3TC-EFV | 86 (5.9) | 23 (1.6) | 79 (6.5) | 188 (4.5) |
| ZDV-3TC-NVP | 128 (8.8) | 542 (36.8) | 240 (19.7) | 910 (21.9) |
| ZDV-3TC-EFV | 230 (15.8) | 257 (17.5) | 96 (7.9) | 583 (14.1) |
| TDF-3TC/FTC-NVP/EFV | 7 (0.5) | 93 (6.3) | 354 (29.1) | 454 (11.0) |
| PI based | 2 (0.1) | 13 (0.9) | 8 (0.7) | 23 (0.6) |
| Other | 1 (0.1) | 1 (0.1) | 33 (2.7) | 35 (0.8) |
| Missing/non-sensical | 13 (0.9) | 3 (0.2) | 3 (0.3) | 19 (0.5) |
| CTX prophylaxis: n (%) | 981 (67.3) | 1186 (80.6) | 724 (59.5) | 2891 (69.7) |
| Clinical characteristics | ||||
| WHO clinical stage at start: n (%) | ||||
| WHO stage 1–2 | 301 (20.6) | 639 (43.4) | 394 (32.8) | 1334 (32.2) |
| WHO stage 3 | 539 (37.0) | 508 (34.5) | 553 (45.4) | 1600 (38.6) |
| WHO stage 4 | 325 (22.3) | 160 (10.9) | 112 (9.2) | 597 (14.4) |
| Missing | 293 (20.1) | 165 (11.2) | 158 (13.0) | 616 (14.9) |
| Functional status: n (%) | ||||
| Working/active | 748 (51.3) | 904 (61.4) | 488 (40.1) | 2140 (51.6) |
| Ambulatory | 261 (17.9) | 73 (5.0) | 352 (28.9) | 686 (16.5) |
| Bedridden | 31 (2.1) | 13 (0.9) | 71 (5.8) | 115 (2.8) |
| Missing | 418 (28.7) | 482 (32.7) | 306 (25.1) | 1206 (29.1) |
| Laboratory parameters | ||||
| CD4 (cells/µl): median (IQR) | 133 (59–206) | 136 (65–202) | 134 (67–217) | 134 (63–206) |
| Missing: n (%) | 337 (23.1) | 303 (20.6) | 366 (30.1) | 1006 (24.3) |
| TLC (cells/µl): median (IQR) | 1700 (1100–3000) | 1400 (980–1970) | 1200 (500–1900) | 1400 (900–2230) |
| Missing: n (%) | 903 (61.9) | 1151 (78.2) | 695 (57.1) | 2749 (66.3) |
| Haemoglobin (g/dl) | 10.2 (8.9–11.8) | 11.9 (10.5–13.2) | 10.6 (9.0–12.1) | 11.0 (9.5–12.5) |
| Missing: n (%) | 683 (46.8) | 604 (41.0) | 512 (42.1) | 1799 (43.4) |
| Opportunistic infections | ||||
| Weight loss >10%: n (%) | 310 (21.3) | 96 (6.5) | 435 (35.7) | 841 (20.3) |
| Chronic diarrhoea >1 month: n (%) | 159 (10.9) | 41 (2.8) | 282 (23.2) | 482 (11.6) |
| Fever >1 month: n (%) | 287 (19.7) | 80 (5.4) | 246 (20.2) | 613 (14.8) |
| Oral candidiasis: n (%) | 103 (7.1) | 76 (5.2) | 90 (7.4) | 269 (6.5) |
| Wasting syndrome: n (%) | 135 (9.3) | 34 (2.3) | 40 (3.3) | 209 (5.0) |
| Pulmonary TB: n (%) | 171 (11.7) | 151 (10.3) | 169 (13.9) | 491 (11.8) |
ART, antiretroviral treatment; IQR, interquartile range; PMTCT, prevention mother-to-child transmission; NVP, nevirapine; 3TC, lamivudine; D4T, stavudine; ZDV, zidovudine; EFV, efavirenz; TDF, tenofovir, FTC, emtricitabine; PI, protease inhibitor; CTX, cotrimoxazole; TLC, total lymphocyte count; TB, tuberculosis.
Working/active: able to perform usual work in or out of the house; ambulatory: able to perform activities of daily living but not able to work; bedridden: not able to perform activities of daily living.
Wasting syndrome: weight loss of >10%, unexplained chronic diarrhoea >1 month and unexplained fever >1 month.
Data are missing for age and gender when the total number of patient was less than 4147.
Characteristics of the patients whose charts were missing could not be described as the sampling frame consisted of patient identifiers only, and although date of birth and sex were recorded on the study screening logs, the information could not be collected for patients whose charts were missing.
Programme characteristics
Eighteen sites were included in the analysis, seven sites in Tanzania (the facility consisting of two different models of care was considered as two separate sites), six sites in Uganda and five sites in Zambia (one site was excluded because of using an incorrect sampling frame).
Half of the health facilities were government facilities. Non-governmental facilities were either faith-based or run by a non-religious non-governmental organisation (NGO). However, the level and type of health facilities were not evenly distributed across the three countries (with more primary health facilities and NGO-supported facilities in Uganda). Two-thirds of the sites were located in an urban setting. At the time of data extraction, 8 sites had less than 2000 ART patients (range: 350–1967), 6 sites had between 2000 and 4000 patients (range: 2095–3989) and 4 had more than 4000 patients on ART (range: 4807–7471). Other characteristics, stratified by country, are presented in Table 2. Four programmes used community-based distribution of ARV drugs for stable patients. An overview of the modalities of community-based ARV distribution among the study sites is given in Table 3.
Table 2.
Site characteristics in multicountry retention study
| Characteristic | Tanzania (n = 7) |
Uganda (n = 6) |
Zambia (n = 5) |
Total number of sites (N = 18) |
|---|---|---|---|---|
| General information | ||||
| Level of health facility | ||||
| National referral hospital | 2 | 1 | 1 | 4 |
| Provincial/regional Hospital | 2 | 0 | 2 | 4 |
| District hospital | 3 | 1 | 2 | 6 |
| Primary/community-based health care | 0 | 4 | 0 | 4 |
| Type of health facility | ||||
| Government | 4 | 1 | 4 | 9 |
| Mission facility | 3 | 1 | 1 | 5 |
| Non-religious NGO | 0 | 4 | 0 | 4 |
| Setting | ||||
| Urban | 4 | 5 | 3 | 12 |
| Rural/periurban | 3 | 1 | 2 | 6 |
| ART-related information | ||||
| Year ART was started at facility | ||||
| 2003 | 1 | 2 | 2 | 5 |
| 2004 | 3 | 2 | 3 | 8 |
| 2005 | 2 | 2 | 0 | 4 |
| 2006 | 0 | 0 | 0 | 0 |
| 2007 | 1 | 0 | 0 | 1 |
| Number of adults currently on ARVs | ||||
| <2000 | 6 | 1 | 1 | 8 |
| 2000–4000 | 1 | 4 | 1 | 6 |
| >4000 | 0 | 1 | 3 | 4 |
| Home-based care | ||||
| No | 0 | 3 | 4 | 7 |
| Yes | 7 | 3 | 1 | 11 |
| Physician-based care | ||||
| No | 2 | 1 | 0 | 3 |
| Yes | 5 | 5 | 5 | 15 |
| ARV-dispensing characteristics | ||||
| Buddy needed for ART initiation | ||||
| No | 0 | 0 | 3 | 3 |
| Yes | 7 | 6 | 2 | 15 |
| Three counselling sessions needed for ART initiation | ||||
| No | 1 | 2 | 1 | 4 |
| Yes | 6 | 4 | 4 | 14 |
| Visit frequency after 6 months on ARVs | ||||
| Monthly | 5 | 1 | 0 | 6 |
| Every 2 months | 0 | 4 | 4 | 8 |
| Every 3 months | 2 | 1 | 1 | 4 |
| Community-based distribution of ARVs | ||||
| No | 7 | 3 | 4 | 14 |
| Yes | 0 | 3 | 1 | 4 |
Table 3.
Community-based ARV drug distribution among study clinics in Tanzania, Uganda and Zambia
| Community distribution of ARVs: any dispensing of ARVs happening outside the regular clinic, covering models where patients are only picking up their ARV drugs from a mobile point to models with mobile health posts with clinical check-up and adherence counselling | |
|---|---|
| Programme and type of facility | Activities |
| Programme 1: Non-governmental organisation |
Mobile clinic at community drug dispensing points on specific days ARV drug and non-ARV drug pickup Clinical investigation (patient monitoring), phlebotomy and adherence counselling Referral of complicated cases |
| Programme 2: Faith-based organisation |
Mobile clinics at peripheral (non-ART) health centres and makeshift community clinics on specific days ARV drug and non-ARV drug pickup Clinical investigation (patient monitoring), phlebotomy and adherence counselling In addition: ARV drug distribution door to door to stable patients by community ART and TB treatment supporters for specific patients (patients whose work/study schedule does not allow them to visit the clinic) |
| Programme 3: Government | Mobile clinics at peripheral (non-ART) health centres on specific days ARV drug and non-ARV drug pickup Referral for clinical investigations, phlebotomy and adherence counselling |
| Programme 4: Faith-based organisation |
Mobile clinics at peripheral (non-ART) health centres on specific days ARV drug and non-ARV drug pickup Clinical investigation (patient monitoring), phlebotomy and adherence counselling |
Retention proportions
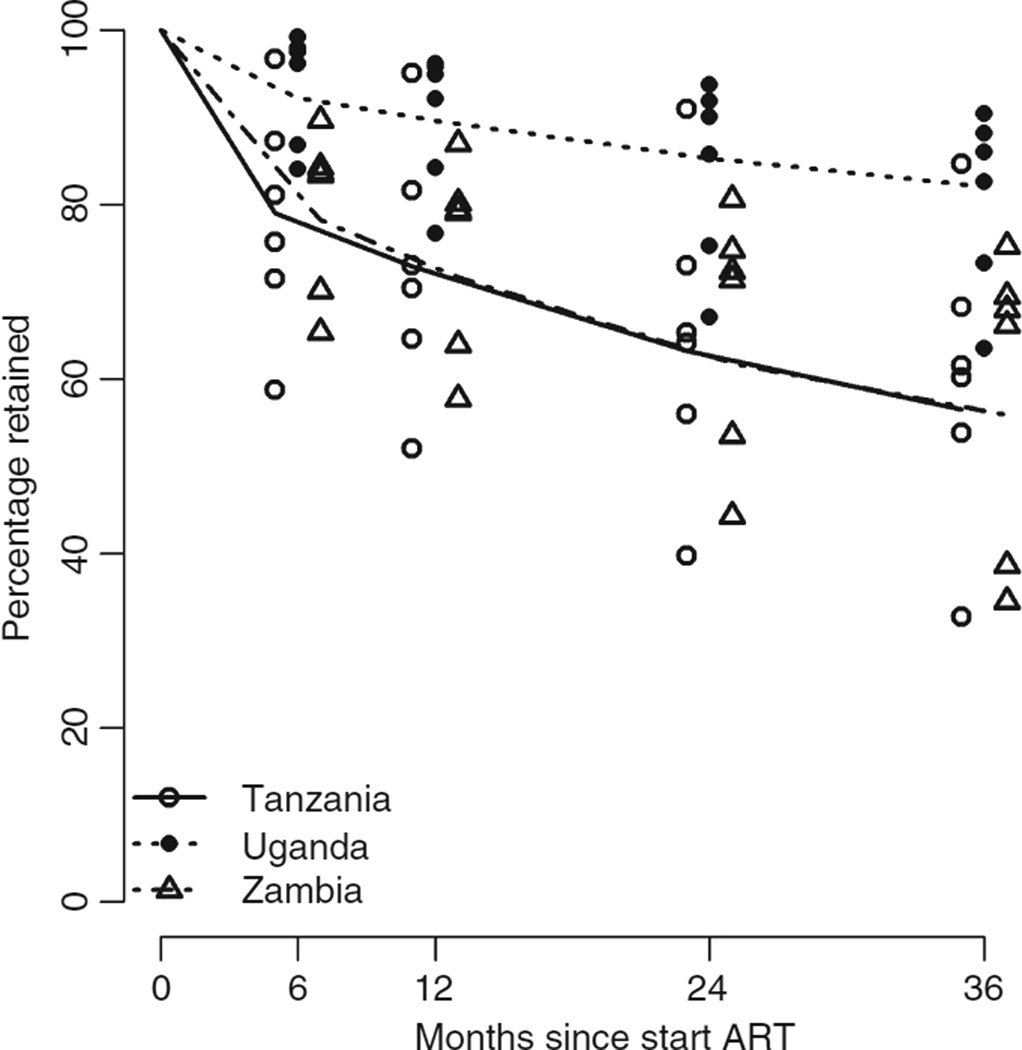
The total period of follow-up by 4147 patients was 8378.5 years, with a median follow-up time of 1.7 years per person (IQR: 0.7–3.2). The retention proportion per site ranged from 58.7% to 99.2% at 6 months, from 52.0% to 96.2% at 1 year, from 39.7% to 93.8% at 2 years, from 32.7% to 90.4% at 3 years and from 25.8% to 90.4% at 4 years (Figure 1). Among the 1312 non-retained patients, 260 (19.8%) were known to have died. The remaining 1052 were lost to follow-up.
Figure 1.
Kaplan-Meier estimates by site in Tanzania, Uganda and Zambia.
Predictors for attrition
During univariate analysis, significant associations for attrition were found for the following baseline characteristics: younger age (<30 years), male sex, further distance to the clinic, increasing years of clinic operation prior to ART initiation, a higher WHO stage, weight loss of >10% of body mass, wasting syndrome (weight loss of >10%, unexplained chronic diarrhoea >1 month and unexplained fever >1 month), a lower CD4 cell count, a lower total lymphocyte count, a lower haemoglobin count and a poorer functional status (Table 4).
Table 4.
Individual predictors of attrition in multicountry retention study
| N | Retention proportion |
Hazard ratio (95% CI) |
P-value† | |||
|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | ||||
| Total | 4147 | |||||
| Demographics | ||||||
| Age at start ART | ||||||
| 18–29 years | 901 | 77.4 | 69.7 | 62.8 | 1 | 0.001 |
| ≥30 years | 3236 | 79.1 | 71.7 | 67.0 | 0.81 (0.71, 0.92) | |
| Sex | ||||||
| Female | 2670 | 80.6 | 73.6 | 68.5 | 1 | <0.001 |
| Male | 1476 | 75.1 | 67.1 | 61.4 | 1.26 (1.13, 1.41) | |
| Distance to clinic (/10 km) | – | – | – | 1.03 (1.01, 1.05) | 0.007 | |
| ART-related and other treatment related predictors | ||||||
| Prior ART experience | ||||||
| No | 3895 | 78.8 | 71.4 | 66.1 | 0.86 (0.68, 1.08) | |
| Yes | 252 | 76.9 | 68.5 | 63.5 | 1 | 0.187 |
| Prior exposure to NVP for PMTCT | ||||||
| No | 1753 | 79.9 | 73.0 | 67.3 | 1 | 0.326 |
| Yes | 180 | 92.1 | 84.5 | 80.0 | 0.78 (0.53, 1.13) | |
| Missing | 738 | 75.1 | 67.1 | 61.4 | 0.92 (0.77, 1.11) | |
| Years since ART started at programme (/year) | – | – | – | 1.10 (1.05, 1.15) | <0.001 | |
| TB treatment | ||||||
| No | 3327 | 80.3 | 73.4 | 68.9 | 1 | 0.131 |
| Yes | 386 | 75.8 | 67.6 | 59.0 | 1.11 (0.92, 1.33) | |
| Missing | 434 | 68.8 | 58.8 | 51.1 | 1.17 (0.99, 1.39) | |
| CTX prophylaxis | ||||||
| No | 693 | 80.4 | 73.3 | 68.5 | 0.96 (0.82, 1.12) | |
| Yes | 2891 | 76.3 | 69.2 | 62.7 | 1 | 0.810 |
| Missing | 563 | 72.8 | 63.4 | 57.7 | 1.02 (0.86, 1.20) | |
| Clinical Characteristics at ART start | ||||||
| WHO stage at start ART | ||||||
| I & II | 1334 | 86.9 | 80.0 | 73.6 | 1 | <0.001 |
| III | 1600 | 78.5 | 70.8 | 65.8 | 1.20 (1.03, 1.39) | |
| IV | 597 | 62.5 | 55.5 | 52.4 | 1.98 (1.66, 2.37) | |
| Missing | 616 | 76.6 | 68.8 | 62.9 | 1.29 (1.07, 1.55) | |
| Functional status | ||||||
| Working/active | 2140 | 84.0 | 77.8 | 72.8 | 1 | <0.001 |
| Ambulatory | 686 | 66.2 | 54.6 | 48.4 | 1.69 (1.45, 1.97) | |
| Bedridden | 115 | 51.4 | 47.4 | 44.7 | 2.61 (2.00, 3.41) | |
| Missing | 1206 | 78.9 | 71.5 | 66.0 | 1.28 (1.10, 1.50) | |
| CD4 (log) | – | – | – | 0.64 (0.49, 0.84) | <0.001 | |
| TLC (cells/µl) | ||||||
| <1200 cells/µl | 581 | 72.7 | 65.0 | 58.2 | 1.21 (1.00, 1.46) | |
| ≥1200 cells/µl | 817 | 76.6 | 68.7 | 63.7 | 1 | <0.001 |
| Missing | 2749 | 80.6 | 73.4 | 68.3 | 0.86 (0.74, 1.01) | |
| Haemoglobin (g/dl) | ||||||
| <10 g/dl | 803 | 69.3 | 61.9 | 57.3 | 1.43 (1.23, 1.67) | |
| ≥10 g/dl | 1545 | 84.1 | 77.3 | 72.1 | 1 | <0.001 |
| Missing | 1799 | 78.2 | 70.3 | 64.7 | 0.93 (0.81, 1.08) | |
| Weight loss >10% | ||||||
| No | 3306 | 80.3 | 73.2 | 67.8 | 1 | <0.001 |
| Yes | 841 | 72.1 | 63.8 | 58.7 | 1.32 (1.13, 1.54) | |
| Chronic diarrhoea >1 month | ||||||
| No | 3665 | 79.3 | 71.8 | 66.2 | 1 | 0.651 |
| Yes | 482 | 74.0 | 67.4 | 64.1 | 1.04 (0.87, 1.26) | |
| Fever >1 month | ||||||
| No | 3534 | 79.9 | 72.7 | 67.0 | 1 | 0.062 |
| Yes | 613 | 71.7 | 63.1 | 59.8 | 1.16 (0.99, 1.36) | |
| Oral candidiasis | ||||||
| No | 3878 | 79.1 | 71.4 | 66.4 | 1 | 0.051 |
| Yes | 269 | 72.2 | 69.3 | 60.7 | 1.24 (1.00, 1.54) | |
| Wasting syndrome | ||||||
| No | 3938 | 79.7 | 72.2 | 66.8 | 1 | <0.001 |
| Yes | 209 | 59.1 | 54.0 | 50.5 | 2.0 (1.56, 2.57) | |
| Pulmonary TB | ||||||
| No | 3656 | 79.0 | 71.4 | 66.2 | 1 | 0.965 |
| Yes | 491 | 76.3 | 69.9 | 64.2 | 1.00 (0.85, 1.19) | |
ART, antiretroviral treatment; CI, confidence interval; PMTCT, prevention mother-to-child transmission; NVP, nevirapine; CTX, co-trimoxazole; TLC, total lymphocyte count; TB, tuberculosis.
Wasting syndrome: weight loss of >10%, unexplained chronic diarrhoea >1 month and unexplained fever >1 month.
Data are missing for age and gender when the total number of patient was less than 4147.
P-value from univariate Cox regression models describing the effects of each individual predictor without correction for other factors, adjusting for site using shared frailty methods.
All programme characteristics described in Table 2 were examined for association with attrition. Univariate analysis found that the level and type of health facility and the dispensing of ARV drugs at the community level were significant predictors of attrition. Participants from primary/community-based facilities experienced lower proportions of attrition. The same was true of faith-based and NGO-based facility participants. Facilities that had community-based ARV drug dispensing also experienced lower proportions of attrition. We confirmed the effects of programme characteristics identified in the analyses across countries by assessing these effects in each country separately. During the stratified analysis by country, attrition was found to be significantly worse for governmental programmes in Tanzania and Zambia, but not in Uganda. Country was also associated with retention, with considerably less attrition found in Uganda compared with Tanzania and Zambia (Table 5). This was explained by the fact that three of the four sites with community-based ARV distribution were based in Uganda. Country was not formally considered as a site-level predictor as sites were not necessarily representative for the country as a whole, and including country as site-level predictor in the final model was not possible due to computational difficulties.
Table 5.
Programme level predictors of attrition in multicountry retention study
| Nr Sites |
Nr Patients |
Retention proportion |
Hazard ratio (95% CI) | P-value* | |||
|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | |||||
| Country | |||||||
| Tanzania | 7 | 1458 | 71.0 | 62.7 | 58.3 | 1 | 0.005 |
| Uganda | 6 | 1472 | 90.5 | 85.3 | 81.5 | 0.35 (0.19, 0.62) | |
| Zambia | 5 | 1217 | 73.2 | 64.3 | 56.8 | 1.05 (0.57, 1.92) | |
| General health facility characteristics | |||||||
| Level of health facility | |||||||
| National referral hospital | 4 | 749 | 76.9 | 67.3 | 61.8 | 1 | 0.043 |
| Provincial/regional hospital | 4 | 980 | 71.7 | 63.5 | 55.6 | 1.25 (0.57, 2.74) | |
| District hospital | 6 | 1432 | 75.2 | 67.1 | 63.0 | 1.17 (0.57, 2.39) | |
| Primary health centre/community based | 4 | 986 | 91.8 | 87.8 | 83.4 | 0.37 (0.17, 0.82) | |
| Type of health facility | |||||||
| Government | 9 | 2188 | 71.0 | 61.6 | 55.1 | 1 | 0.007 |
| Mission (faith based) | 5 | 973 | 85.5 | 80.5 | 75.9 | 0.44 (0.24, 0.79) | |
| Non-religious NGO | 4 | 986 | 88.8 | 83.0 | 79.1 | 0.35 (0.19, 0.67) | |
| Setting | |||||||
| Rural/periurban | 6 | 1441 | 74.2 | 66.7 | 62.7 | 1 | 0.383 |
| Urban | 12 | 2706 | 81.0 | 73.7 | 67.8 | 0.74 (0.38, 1.45) | |
| Number of adults on ARVs | |||||||
| <2000 | 8 | 1703 | 74.4 | 66.7 | 62.7 | 1 | 0.726 |
| 2000–4000 | 6 | 1474 | 83.7 | 77.3 | 72.1 | 0.74 (0.36, 1.53) | |
| >4000 | 4 | 970 | 78.4 | 70.1 | 61.1 | 0.93 (0.41, 2.11) | |
| Home-based care | |||||||
| No | 7 | 1732 | 77.2 | 68.7 | 62.0 | 1 | 0.625 |
| Yes | 11 | 2415 | 79.7 | 73.1 | 69.0 | 0.85 (0.44, 1.63) | |
| Physician-based care | |||||||
| No | 3 | 711 | 72.5 | 63.3 | 58.4 | 1 | 0.308 |
| Yes | 15 | 3436 | 79.9 | 72.8 | 67.5 | 0.66 (0.28, 1.51) | |
| ARV-dispensing Characteristics | |||||||
| Buddy needed for ART initiation | |||||||
| No | 3 | 744 | 67.0 | 56.6 | 46.6 | 1 | 0.078 |
| Yes | 15 | 3403 | 81.2 | 74.6 | 70.5 | 0.50 (0.23, 1.11) | |
| Three counselling sessions needed for ART initiation | |||||||
| No | 4 | 871 | 81.6 | 74.1 | 69.0 | 1 | 0.614 |
| Yes | 14 | 3276 | 77.9 | 70.5 | 65.1 | 1.22 (0.57, 2.64) | |
| Refill frequency (after 6 months) | |||||||
| Monthly | 6 | 1223 | 74.0 | 65.8 | 61.6 | 1 | 0.856 |
| Every 2 months | 8 | 1978 | 80.9 | 74.0 | 67.6 | 0.83 (0.40, 1.72) | |
| Every 3 months | 4 | 946 | 80.0 | 72.5 | 68.6 | 0.82 (0.34, 1.96) | |
| ARV drug dispensing in community | |||||||
| No | 14 | 3190 | 74.4 | 66.0 | 60.2 | 1 | 0.004 |
| Yes | 4 | 957 | 92.6 | 88.2 | 84.1 | 0.32 (0.17, 0.61) | |
| Sampling | |||||||
| Percentage of selected patient charts not found | |||||||
| <10% | 6 | 1465 | 63.6 | 53.5 | 45.6 | 1 | <0.001 |
| ≥10% to <20% | 6 | 1475 | 88.1 | 82.4 | 78.6 | 0.31 (0.21, 0.54) | |
| >20% | 6 | 1207 | 85.1 | 78.3 | 73.7 | 0.37 (0.22, 0.62) | |
ART, antiretroviral treatment; ARV, antiretroviral; CI, confidence interval.
P-value from Cox regression models describing the effects of each programme level characteristic, correcting for imbalances in patient characteristics between sites and adjusting for site using shared frailty methods.
According to the predefined statistical analysis plan, distance to the clinic was not included in the multivariable Cox-regression model building because this variable was missing in two of the research sites. Other known predictors (Coetzee et al. 2004) excluded due to missing data include lymphocyte count, haemoglobin level (more than 30% missing data), oral candidiasis and wasting (present in <10% of the patients). The final multiple Cox-regression model, retaining only significant predictors and interactions, and correcting for missing data using multiple imputations, revealed that patients of younger age (<30 years) were at higher risk of attrition compared with older patients (≥30 years) [adjusted hazard ratio (aHR) for attrition, (95% confidence interval) (95% CI) = 1.30 (1.14–1.47)]. Patients with baseline WHO clinical stage of 3 or 4 had higher proportions of attrition compared with patients in stages 1 and 2 [aHR (95%CI) = 1.12 (0.95–1.35) and 1.56 (1.29–1.88), respectively]. The same was true for ambulatory (able to perform daily activities but not working) and bedridden (not able to perform daily activities) (WHO 2006) patients compared with working or active patients (able to perform usual work in or out of the house) [aHR (95%CI) = 1.29 (1.09–1.54) and 1.54 (1.15–2.07), respectively]. Patients with a loss of more than 10% of body mass were at greater risk of attrition [aHR (95% CI) = 1.17 (1.00–1.38)]. The probability of attrition decreased proportionally with an increase in CD4 count [aHR (95%CI) = 0.88 (0.78–1.00) for every log (tenfold) increase].
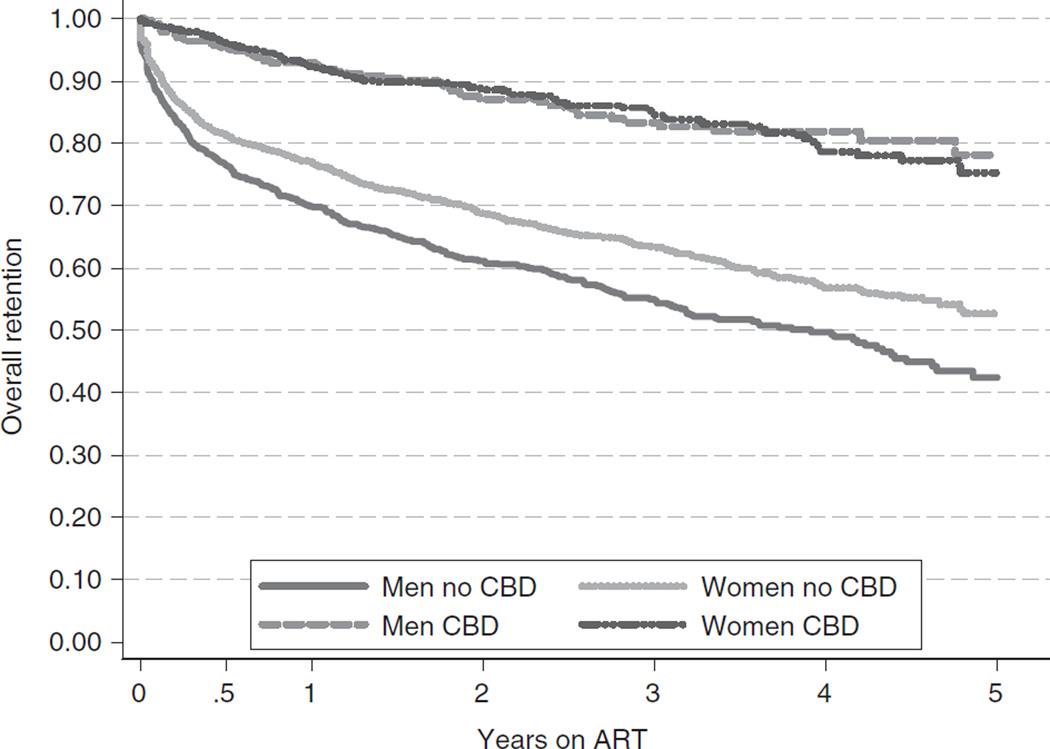
At the programme level, community-based dispensing of ARV drugs was significantly related to less attrition [aHR (95%CI) = 0.55 (0.30–1.01) for women and 0.40 (0.21–0.75) for men] (Figure 2). This effect was particularly strong among males, with males and females at facilities that offered community-based distribution having similar attrition proportions [aHR (95%CI) = 0.95 (0.67–1.33)]. At sites without community distribution of ART, however, males had a higher attrition risk than female [aHR (95% CI) = 1.33 (1.18–1.50)]. In addition, government run facilities compared with faith-based or NGO facilities were found to significantly predict attrition. No significant difference in retention was observed between government and faith-based or NGO facilities during the first year of operation. Attrition significantly increased over time in government facilities [aHR/year (95%CI) = 1.17 (1.10–1.23)], but not in faith-based or NGO facilities [aHR/year (95% CI) = 1.03 (0.95–1.11)] resulting in an overall lower retention in government hospitals (Table 6).
Figure 2.
Kaplan-Meier estimates by Community-Based Distribution (CBD) of ARVs in Tanzania, Uganda and Zambia.
Table 6.
Final multivariate model for predictors of attrition in multicountry retention study*
| Adjusted hazard ratio (95% CI) |
|
|---|---|
| Individual characteristics | |
| Age at start ART: <30 years (vs. ≥30 years) | 1.30 (1.14, 1.47) |
| WHO stage at start ART | |
| III vs. I & II | 1.12 (0.95, 1.31) |
| IV vs. I & II | 1.56 (1.29, 1.88) |
| Weight loss >10% | 1.17 (1.00, 1.38) |
| CD4 count (/log [tenfold] increase) | 0.88 (0.78, 1.00) |
| Functional status | |
| Ambulatory vs. working/active | 1.29 (1.09, 1.54) |
| Bedridden vs. working/active | 1.54 (1.15, 2.07) |
| Sex (men vs. women) | |
| In sites without community | 1.33 (1.18, 1.50) |
| ARV drug dispensing | |
| In sites with community | 0.95 (0.67, 1.33) |
| ARV drug dispensing | |
| Years of clinic operation by Type of health facility (/year) | |
| Government facility | 1.17 (1.10, 1.23) |
| Mission facility/non-religious NGO | 1.03 (0.95, 1.11) |
| Programme characteristics | |
| ARV drug dispensing in community by Sex | |
| For women | 0.55 (0.30, 1.01) |
| For men | 0.40 (0.21, 0.75) |
| Type of health facility: Faith-based facility or NGO vs. Government (at start of the programme) |
0.71 (0.42, 1.21) |
ART, antiretroviral treatment; ARV, antiretroviral; CI, confidence interval; NGO: non-governmental organisation.
Working/active: able to perform usual work in or out of the house; ambulatory: able to perform activities of daily living but not able to work; bedridden: not able to perform activities of daily living.
The model was simplified using Akaike’s Information Criterion, retaining predictors and clinically plausible interaction terms that increased model fit, with a penalisation for increasing model complexity.
A significant association was found between attrition and the number of randomly selected patients for whom the patient chart could not be located. Sites that had more missing records had less attrition (Table 5). Correcting the multiple Cox-regression model for the percentage of selected records which were missing, by including this variable as a covariate, did not significantly change the effect estimates for the predictors included in the final model (data not shown).
Discussion
To date, most studies examining retention to ART care and treatment programmes focus on individual pre-ART clinical predictors. This study makes an important contribution to our understanding of ART retention by examining not only retention proportions across three countries and 18 study sites, but by going beyond individual baseline clinical predictors of attrition to examine the potential effect different programme characteristics may have on retention.
Overall, retention proportions varied widely both across countries and study sites (25.8% to 90.4% at year 4 for example). These results are comparable to those from other studies in sub-Saharan Africa settings (Coetzee et al. 2004; Ferradini et al. 2006; Calmy et al. 2006; Weigel et al. 2012). These studies exemplify the challenges of defining retention in different settings and systematically accessing information in clinics with different data collection systems. Retention proportions are also greatly affected by the choice of LTFU definition (Shepherd et al. 2013).
Many of the baseline clinical characteristics predictive of attrition reinforce findings from other studies in sub-Saharan Africa, including younger age (<30 years), being male, having a higher WHO clinical stage, weight loss of >10% of body mass, a lower CD4 cell count and a poorer functional status (Coetzee et al. 2004; Ferradini et al. 2006; Calmy et al. 2006). These findings reaffirm the need for early identification of HIV-infected individuals and early initiation of ART. Increasing years of clinic operation, prior to when a patient initiated ART, was also an independent risk factor for attrition. This finding has been confirmed by other studies (Braitstein et al. 2006; Cornell et al. 2010). However, this effect was mainly observed in government facilities and was not significant in facilities run by faith-based organisations or NGOs. Rapid scaling-up may have considerably increased the workload for government health workers. This in turn may have compromised the organisation of services and quality of care provided. Faith-based and NGO facilities might have had better coping mechanisms and funding to increase staff levels and to adapt their services and monitoring/tracking systems to the increasing numbers of ART patients. The association between attrition and older governmental programmes could also be partly explained by misclassification of LTFU which, in reality, consists of unreported (silent) transfer to care elsewhere (Geng et al. 2010). Initially, only hospital-based referral centres provided ART treatment, but in the setting of rapid scale-up, patients often transferred to closer lower-level facilities (Bedelu et al. 2007; Chan et al. 2010). Retention in an ART programme reflects a number of heterogeneous outcomes including mortality, LTFU and transfer of care (both silent and recorded). Geng et al. (2010) found that among 14 studies where outcomes in some patients LTFU were reported, about 50% were in care elsewhere. This finding highlights the importance of examining not only programme retention to specific ART clinics, but also retention to care regardless of where the services are rendered.
The most important result of this study is that sites offering ARV drug dispensing in the community had significantly greater programme retention. Of particular importance was the effect of community-based ARV drug distribution on retention of men. As well established in the literature (Geng et al. 2010; Ferradini et al. 2006; Cornell et al. 2009), being male is an independent risk factor for attrition. However, in this study, this difference between the sexes was only observed in sites without community distribution of ARV drugs, where males were 30% more likely than females to experience attrition. In sites with community ARV drug distribution, attrition proportions among both men and women were about 50% smaller compared with women in sites without community distribution (the group with the lowest attrition).
Greater retention in community-based ART programmes may be due to fewer patients transferring out because many of the transfers seen to date are from initial centres to community programmes (Bedelu et al. 2007; Chan et al. 2010). As noted above, these urban centres probably had substantial unrecorded (silent) transfers with the rapid scale-up and decentralisation of ART services (Geng et al. 2010).
What are the implications of these data regarding community-based distribution of ARV drugs? Only four sites in this study had a system of community-based ARV drug distribution. These systems varied from models where patients only pick up their ARV drugs from a mobile point to models with mobile health posts with clinical check-up and adherence counselling. However, most of these programmes still depended heavily on the support of clinic-based staff for community-based ARV drug distribution.
Other models of community-based ART distribution, however, are emerging. In Rwanda, for example, Rich et al. reported a retention rate of 92.3% after 2 years using a model with intensive community-based treatment support that included ART distribution and directly observed ART by community health workers (Rich et al. 2012). Other programmes use trained HIV-infected peers (Community Care Coordinators) to visit ART patients monthly and perform a systematic symptom review and dispense ARV drugs (Wools-Kaloustian et al. 2009). In Mozambique, Medecins Sans Frontieres uses a Community ART model with stable ART patients who dispense monthly ART and provide adherence and social support to other ART clients in the community (Decroo et al. 2011). They reported retention rates of 97.5% after a median follow-up time of 13 months. The effectiveness of community pharmacies where ART patients are trained to distribute ART at community distribution points needs to be confirmed by further research (MSF & UNAIDS 2012). Although these models are showing promising impact on retention, their feasibility and scalability still need to be evaluated.
The implementation of the current WHO guidelines (WHO 2013) aims to increase ART coverage and retention to save lives and to decrease HIV transmission. To achieve the ambitious goal of universal coverage in rural Africa, treatment will need to expand to serve communities beyond the reach of current clinics. The potential of decentralisation of ART delivery (through mobile clinics and community pharmacies) and community participation (through community health workers and the patients and their families) need to be explored further.
Besides misclassification of transfer to care to LTFU, there are other limitations to this study. Study sites were not randomly selected, and this could have introduced some selection bias. However, the selection (performed in 2006) was conducted in consultation with country-specific stakeholders and aimed to have a good balance of site characteristics that might influence retention and adherence. The intrinsic differences among countries (for example, in this study the majority of programmes with community-based distribution of ARV drugs and programmes supported by faith-based or non-governmental organisations were found in Uganda) could result in further confounding.
The strength of the current study is the use of consistent data collecting tools across diverse sites and the possibility of controlling for individual patient characteristics. By design, our research allowed studying interactions between programme level and individual characteristics, as illustrated by the differential effects of community-based distribution of ARV drugs between men and women. The design is less suited to study interactions between programme level characteristics or differences between countries.
Other limitations relate mainly to the constraints of retrospective chart review and the challenges of incomplete data (for example, WHO clinical stage and CD4 cell count) and the absence of certain variables at some of the sites (for example, distance to the clinic). The numbers of missing values for these variables are similar to numbers reported elsewhere (May et al. 2010). They highlight the importance of strengthening data collection systems to better respond and assess retention to care and treatment.
Because this was a retrospective chart review, other important structural predictors of retention, such as mode of transport, educational level and income, could not be assessed. The quality and completeness of the data varied among the study sites. Forster et al. (2008) found that poor quality data were associated with poor retention. In this study, the sites with a large proportion of missing records reported better retention proportions. While the significant association between the number of missing records and retention may indicate the presence of bias in this study, correcting for missing data did not change the conclusions. This indicates the robustness of the findings.
Conclusion
Patient retention to an individual programme worsened over time especially among males, younger persons and those with poor clinical indicators. Increased use of community programmes for ART drug dispensing could be considered for broader implementation.
Acknowledgments
We wish to acknowledge the study participants and participating clinics for their critical role in this study. This research was been supported by PEPFAR through CDC and HRSA. The views, opinions and content of this publication are those of the authors and do not necessarily reflect the views, opinions or policies of the CDC, HRSA or any other federal agency or office.
Footnotes
This paper was presented in part at the XIX International AIDS Conference 2012, 22–27 July, Washington DC, USA.
References
- Altman DG, Bland JM. Time to event (survival) data. BMJ. 1998;317:468–469. doi: 10.1136/bmj.317.7156.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. Journal of Infectious Diseases. 2007;196(Suppl. 3):S464–S468. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- Calmy A, Pinoges L, Szumilin E, et al. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- Chan AK, Mateyu G, Jahn A, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Tropical Medicine and International Health. 2010;15(Suppl. 1):90–97. doi: 10.1111/j.1365-3156.2010.02503.x. [DOI] [PubMed] [Google Scholar]
- Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- Collet D. Modelling Surival Data in Medical Research. 2nd. Boca Raton, FL, USA: Chapmann & Hall/CRC Press; 2003. [Google Scholar]
- Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Tropical Medicine and International Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24:2263–2270. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in tete province, mozambique. Journal of Acquired Immune Deficiency Syndromes. 2011;56:e39–e44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- Forster M, Bailey C, Brinkhof MW, et al. Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bulletin of the World Health Organization. 2008;86:939–947. doi: 10.2471/BLT.07.049908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Tropical Medicine and International Health. 2010;15(Suppl. 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Current HIV/AIDS Reports. 2010;7:234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clinical Infectious Diseases. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- Hougaard P. Frailty models for survival data. Lifetime Data Analysis. 1995;1:255–273. doi: 10.1007/BF00985760. [DOI] [PubMed] [Google Scholar]
- Little RJA. Regression with missing X’s: a review. Journal of the American Statistical Association. 1992;87:1227–1237. [Google Scholar]
- May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MSF and UNAIDS. Closer to Home: Delivering Antiretroviral Therapy in the Community: Experience From Four Countries in Southern Africa. Geneva, Switzerland: MSF and UNAIDS; 2012. [Google Scholar]
- Nachega JB, Hislop M, Dowdy DW, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Annals of Internal Medicine. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- Rich ML, Miller AC, Niyigena P, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. Journal of Acquired Immune Deficiency Syndromes. 2012;59:e35–e42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Medicine. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values. Stata Journal. 2004;4:227–241. [Google Scholar]
- Shepherd BE, Blevins M, Vaz LM, et al. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. American Journal of Epidemiology. 2013;178:819–828. doi: 10.1093/aje/kwt030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. The Gap Report. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26:365–373. doi: 10.1097/QAD.0b013e32834ed814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Patient Monitoring Guidelines for HIV Care and ART. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- WHO. Consolidated Guidelines on the Use Of Antiretroviral Drugs For Treating and Preventing HIV Infection. Recommendations for a Public Health Approach 2013. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- Wools-Kaloustian KK, Sidle JE, Selke HM, et al. A model for extending antiretroviral care beyond the rural health centre. Journal of the International AIDS Society. 2009;12:12–22. doi: 10.1186/1758-2652-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]