Abstract
This data paper contains information about the in vivo model for peritoneal implants used in the paper “Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells” (De Vlieghere et al., 2015) [1]. A double in vivo selection of SK-OV-3 Luc human ovarian cancer cell line was used to create SK-OV-3 Luc IP1 and SK-OV-3 Luc IP2 cell lines. This data paper shows functional activities of the three cell lines in vitro and in vivo. Phase-contrast images show the morphology of these cells, metabolic and luciferase activity has been determined. Survival data of mice peritoneally injected with SK-OV-3 Luc or SK-OV-3 Luc IP2 is available with H&E histology of the peritoneal implants. Tumor growth curves and bioluminescent images of mice inoculated with a different number of SK-OV-3 Luc IP2 cells are also included.
Keywords: in vivo selection, Ovarian cancer, Mouse model, Growth curves, Survival curves
Specifications table
| Subject area | Biology |
| More specific subject area | Cancer biology |
| Type of data | Phase contrast images, graphs, histology, bioluminescence images |
| How data was acquired |
|
| Data format | Analyzed |
| Experimental factors | SK-OV-3-Luc cells were inoculated intraperitoneally in immune deficient female mice Swiss/nu to select for a population that more efficiently forms peritoneal implants. |
| Experimental features | The created cell line SK-OV-3 Luc IP1 and SK-OV-3 Luc IP2 are compared with the parental cell line SK-OV-3 Luc |
| Data source location | Ghent, Belgium |
| Data accessibility | The data is available with this article |
Value of the data
-
•
This data shows intraperitoneal tumor formation with low cell inoculation after in vivo selection of SK-OV-3.
-
•
This method can be applied to other cancer cell lines to increase metastasis take rate even with lower inoculation numbers.
-
•
These data provides growth curves, survival data and histology about the SK-OV-3 Luc (IP2) in vivo model for peritoneal implants, providing researchers with a references for their in vivo studies.
1. Data
Intraperitoneal injection (IP) of SK-OV-3 (Luc) cells is an established in vivo model for the development of peritoneal ovarian tumor implants. Usually inoculation numbers of 1–2×106 SK-OV-3 (Luc) are used 2, 3, 4. The IP injection of 1×106 SK-OV-3 Luc cells gives a 100% tumor take with an average survival time of over 2 months (Fig. 3B) [3]. To better mimic the patient situation where an initial low number of aggressive disseminated cells can form extensive peritoneal metastasis a xenograft mouse model with inoculation of low numbers of cancer cells is needed. in vivo selection has been successfully used to increase the metastatic potential of MDA-MB-231 [5]. These data describe the successive in vivo selection of the SK-OV-3 Luc cell line. Tumor implants of SK-OV-3 Luc bearing mice are re-cultured in vitro to increase in vivo peritoneal implant formation. After successive in vivo selection, IP inoculation of 10-to-20X lower number (1×105) of SK-OV-3 Luc IP2 is sufficient to form peritoneal implants this model has been applied successfully [1].
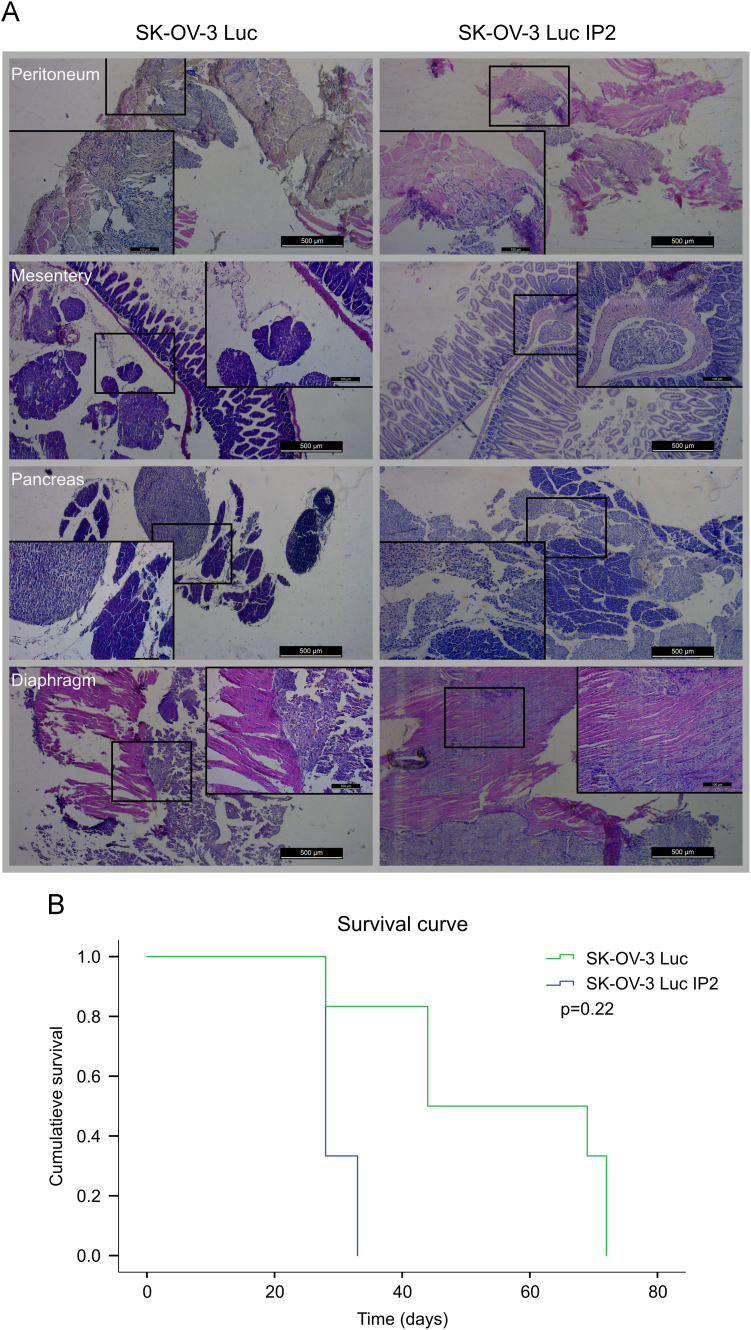
Fig. 3.
(A) H&E staining׳s of peritoneal implants at the site of peritoneum, mesentery, pancreas and diaphragm of SK-OV-3 Luc or SK-OV-3 Luc IP2 tumors. (B) Comparative survival curves of mice IP injected with 1×106 SK-OV-3 Luc and SK-OV-3 Luc IP2 cells.
2. Experimental design, materials and methods
2.1. Cell culture conditions
SK-OV-3 is a human ovarian cancer cell line (ATCC number: HTB-77). SK-OV-3 Luc (Luciferase positive SK-OV-3 cells) were prepared by pFL4.76 plasmid transfection and selection (Promega, Leiden, The Nederlands). SK-OV-3 Luc (IP1/2) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin/streptomycin), and incubated at 37 °C with 10% CO2 in air.
2.2. in vivo selection
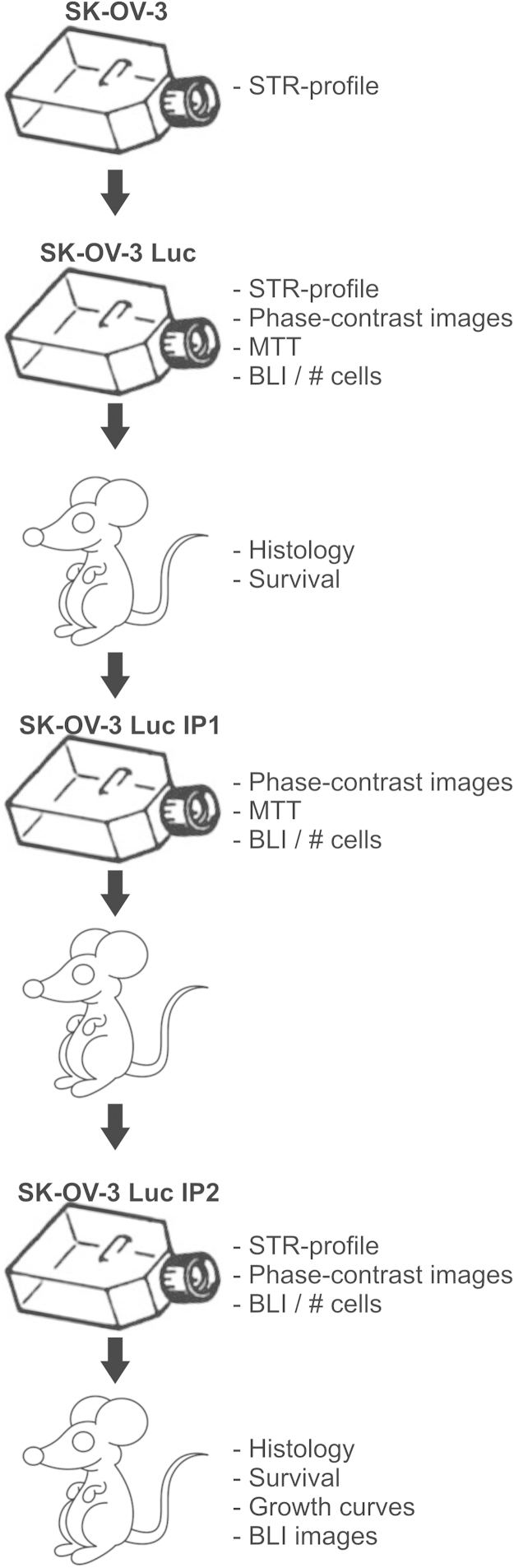
Animal experiments were conducted accordance with the local ethics committee (Ghent University Hospital). 1×106 SK-OV-3-Luc cells were inoculated intraperitoneally in immunodeficient female Swiss/nu mice (Charles River, Chatillon-sur-Chalaronne, France) to isolate populations that form peritoneal implants. Tumor implants were collected in saline. The implants were cut into small pieces (1 mm) and dissociated by de gentleMACS Dissociator (Miltenyi Biotec, Teterow, Germany) in the presences of 1 mg/ml collagenase from Clostridium histolyticum (Sigma-Aldrich) in PBSD+. The mixture was cleared through a cell strainer (70 µm) and the homogenized single cell suspension was expanded in culture by seeding in DMEM 10% FBS. After 24 h, non-adhered cells were removed and the medium was refreshed; the resulting culture was maintained as the parental cell line. Each subsequent intraperitoneal metastatic generation is designated IP1, IP2. Fig. 1 shows a schematic with the different stages of the in vivo selection. Fig. 2A shows phase-contrast images of the three cell lines cultured on plastic
Fig. 1.
Schematic showing the successive stages of the in vivo selection with indication of the different in vitro and in vivo assays conducted.
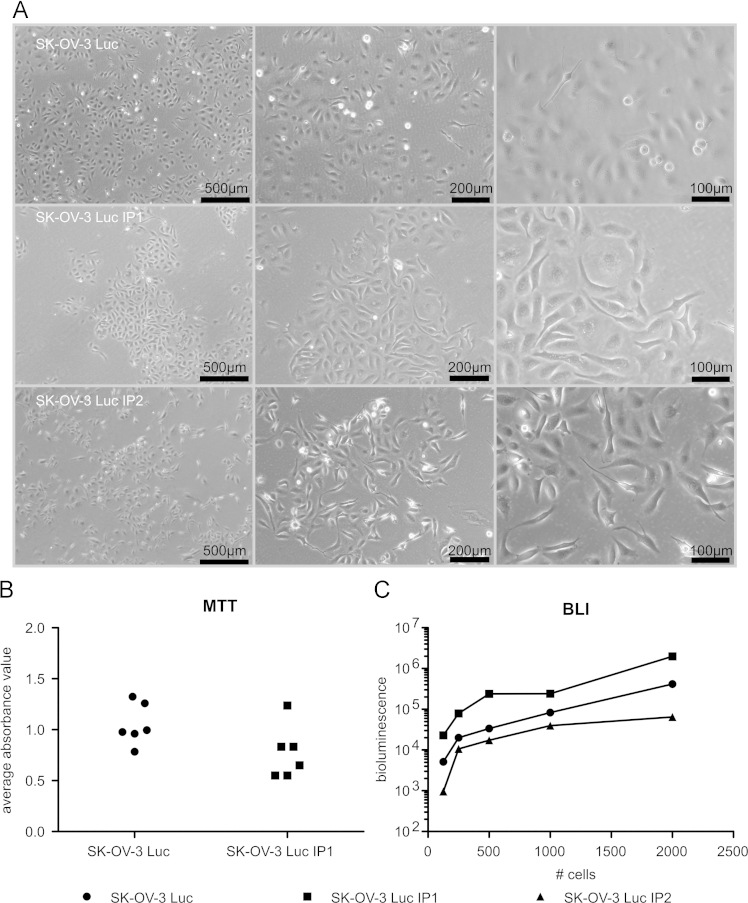
Fig. 2.
(A) Phase-contrast images of SK-OV-3 Luc, SK-OV-3 Luc IP1 and SK-OV-3-Luc-IP2 cultured cell cultured treated culture flasks. (B) Metabolic activity analysis (MTT): the average absorbance value (n=3) of 6 independent metabolic activity analysis 96 h after seeding 4×104 SK-OV-3 Luc or SK-OV-3 Luc IP1. (C) in vitro average bioluminescence (n=3) of SK-OV-3 Luc, SK-OV-3 Luc IP1 and SK-OV-3-Luc-IP2 for 125, 250, 500, 1000 and 2000 cells, 2 h after seeding.
2.3. Sort tandem repeat (SRT) profiling
SRT-profiling was conducted on the SK-OV-3, SK-OV-3 Luc and SK-OV-3 Luc IP2 cell lines to confirm the identity of the cell lines. 9 DNA sites were profiled and the alleles were identical between the three cell lines and to the profile published by ATCC (Amelogenin: X; CSF1PO: 11; D13S317: 8,11; D16S539: 12; D5S818: 11; D7S820: 13,14; THO1: 9,9.3; TPOX: 8,11; vWA: 17,18) [6].
2.4. Metabolic activity analysis: 3-4,5-Dimethylthiazolyl-2-2,5-Diphenyltetrazolium Bromide (MTT)
A single cell suspension of 4×104 SK-OV-3 Luc or SK-OV-3 Luc IP1 cells per well, were seeded in a 96-well plate. After 96 h, MTT analysis was performed. Briefly, the culture medium was replaced by 100 μl culture medium containing 1 mg/ml MTT. Following 2 h incubation at 37 °C, MTT-containing medium was removed and 150 μl of dimethylsulfoxide (DMSO) was added to dissolve formazan crystals. Absorbance was measured at 570 nm and background measured at 650 nm, with a plate reader (Paradigm, Molecular Devices). Fig. 2B shows the average absorbance value (n=3) of 6 independent metabolic activity analysis 96 h after seeding 4×104 SK-OV-3 Luc or SK-OV-3 Luc IP1.
2.5. in vitro bioluminescence
A single cell suspension of SK-OV-3 Luc, SK-OV-3 Luc IP1 and SK-OV-3-Luc-IP2 cells were seeded in a 96-well plate in different cell numbers (125, 250, 500, 1000 and 2000 cells per well). Cells were allowed to adhere for 2 h, just before luciferase activity was measured with IVIS (PerkinElmer), 150 µg/ml D-Luciferin, firefly (Perkin-Elmer) was added. Fig. 2C shows the average bioluminescence value of three replicates.
2.6. in vivo survival analysis
Animal experiments were conducted in accordance with the local ethics committee (Ghent University Hospital). Immune deficient female Swiss/nu mice were inoculated intraperitoneally with 1×106 SK-OV-3-Luc or SK-OV-3-Luc-IP2 cells. Mice were monitored and from the first visual signs of advanced carcinomatosis (decrease in weight or increase in abdominal circumference) the mice had reached their end-point and were sacrificed. Peritoneal organs were embedded in paraffin before standard hematoxylin and eosin (H&E) staining was conducted. Fig. 3A shows H&E staining of the peritoneal tumor implants, B shows comparative survival curves.
2.7. in vivo growth curves
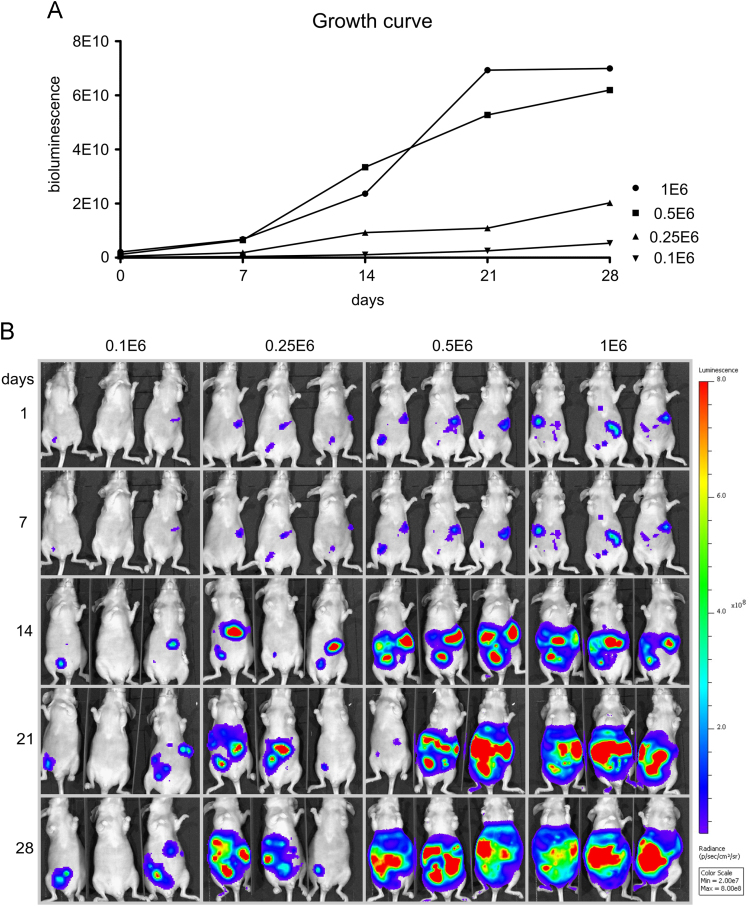
Immune deficient female Swiss/nu mice were inoculated intraperitoneally with 1×106, 0.5×106, 0.25×106 and 0.1×106 SK-OV-3 Luc IP2 cancer cells. Tumor growth was monitored by weekly bioluminescence imaging (BLI). Fig. 4 shows the different growth curves (A) and the bioluminescence images (B).
Fig. 4.
Growth curves (A) and BLI (B) of mice inoculated with 1×106, 0.5×106, 0.25×106 or 0.1×106 SK-OV-3 Luc IP2 cells (n=3).
Acknowledgments
This research was supported by grants from “Stichting tegen Kanker” (2012-217), “Vlaamse Liga tegen Kanker” and Fund for Scientific Research-Flanders. Elly De Vlieghere received a scholarship from IWT (093153). The study sponsors have no role in the design of the study; the collection, analysis and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2015.12.037.
Contributor Information
Marc Bracke, Email: Marc1.Bracke@UGent.be.
Olivier De Wever, Email: Olivier.dewever@ugent.be.
Appendix A. Supplementary material
Supplementary material
References
- 1.De Vlieghere E., Gremonprez F., Verset L., Marien L., Jones C.J., De Craene B. Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells. Biomaterials. 2015;54:148–157. doi: 10.1016/j.biomaterials.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Cho H., Lai T.C., Kwon G.S. Poly(ethylene glycol)-block-poly(epsilon-caprolactone) micelles for combination drug delivery: evaluation of paclitaxel, cyclopamine and gossypol in intraperitoneal xenograft models of ovarian cancer. J. Control Release. 2013;166(1):1–9. doi: 10.1016/j.jconrel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo P.E., Boustta M., Poujol S., Jarlier M., Bressolle F., Teulon I. Intraperitoneal administration of novel doxorubicin loaded polymeric delivery systems against peritoneal carcinomatosis: experimental study in a murine model of ovarian cancer. Gynecol. Oncol. 2011;122(3):632–640. doi: 10.1016/j.ygyno.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 4.Mikula-Pietrasik J., Sosinska P., Naumowicz E., Maksin K., Piotrowska H., Wozniak A. Senescent peritoneal mesothelium induces a pro-angiogenic phenotype in ovarian cancer cells in vitro and in a mouse xenograft model in vivo. Clin. Exp. Metastas. 2015 doi: 10.1007/s10585-015-9753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minn A.J., Gupta G.P., Siegel P.M., Bos P.D., Shu W., Giri D.D. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ATCC: The Global Bioresource Center. 〈http://www.lgcstandards-atcc.org/Products/All/HTB-77.aspx#specifications〉
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material