Abstract
Background:
Breast cancer (BC) is the second most commonly diagnosed cancer after lung cancer. Survival of BC patients is affected by intermediate events. This study was aimed to investigate the disease course of primary nonmetastatic BC patients with first recurrence of the tumor (FRT) as the intermediate event using the illness- death model.
Methods:
This retrospective cohort study was conducted on 529 Iranian females with BC underwent surgery, from 1995 to 2013. Patients, tumor and treatment characteristics were collected from medical records of the patients. The illness-death model were used to investigate the relationship between these factors and survival time. Data were analyzed using version 3.1.1 of R software.
Results:
The risk of FRT in patients who had tumors size in the range of 2–5 cm and >5 cm was 1.3 and 3.5 times higher than that of patients with tumor size ≤2 cm, respectively (P<0.001). Furthermore, risk of death in patients aged ≥50 years was 1.6 times higher compared to patients aged less than 50 years (P =0.012). Risk of death after metastasis in patients with tumor size >5 cm was 2.1 times higher than patients with tumor size ≤2 cm (P =0.019).
Conclusions:
The stage of the disease and tumor size have statistically significant effects on patients’ survival before occurrence of the FRT. Furthermore, illness-death model was found to be a useful tool in modeling the disease course of BC patients.
Keywords: Breast cancer, Intermediate events, Illness- death model, Survival analysis
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer after lung cancer by 1.7 million new cases (11.9% of all cancer types) (1). BC is the most frequent cause of cancer death among women (2) and the frequently diagnosed cancer among them in 140 of 184 countries worldwide (1). In Iran, as a developing country, BC has the first rank among women’s cancers (3) with 8090 new cases annually resulting in more than 1300 deaths per year (4). According to the cancer registry reports by Iran ministry of health in 2008, Age-Standardized incidence Rate (ASR) was 33.21 per 100,000 female populations (5).
Metastasis is the leading cause of death from cancers (6). BC Patients experience more than one type of event in the disease process such as local metastasis, distant metastasis, death and etc.(7). It is important to investigate the disease course of primary non-metastatic BC patients with first recurrence of the tumor (FRT), as an intermediate event. Death is considered as the most important endpoint in patients with metastatic cancer (8, 9) and metastasis is the second most important endpoint which is often use in recurrent models (10). Usually separate survival analyses are used for each endpoint and also for the intermediate events.
However, these separate survival analyses are not completely satisfying since they fail to exhibit the relations between different types of the endpoints (11). FRT as an intermediate event is not prevalent in survival studies because of the complexity of survival model calculations. However, such problems can be solved by fitting advanced survival models such as the illness-death (disability) model (12). The illness-death model is a type of multi-state (MS) models which is use for describing chronic diseases with possible intermediate events. MS models provide a framework that allow for the analysis of event history data (13). To the best of our knowledge, there are few studies considering the effect of the FRT on death as an intermediate event, in Iran.
Therefore, the present study was undertaken with the following objectives:
1) To understand the effect of the time of metastasis on the further disease progress.
2) To study the effect of different factors on the transition intensities between the courses of BC.
Materials and Methods
This retrospective cohort study was conducted on 564 BC patients underwent surgery in Darol-Aitam-e Mahdieh center from 1995 to 2013 in Hamadan Province, west of Iran. Data were obtained from patients’ medical records and phone call interview. Patients who met the following criteria were excluded from the study:
Having many missing data in their clinical and demographic records at diagnosis
Having surgery other than radical mastectomy, removal of the breast and lymph nodes involved, breast conserving therapy and removal of breast (one fourth of the breast) as the first treatment followed by chemotherapy and radiotherapy.
Pathological, clinical and demographic factors were collected from patients’ medical records. Factors which had been missed in the most of the records as well as those with registration errors were ignored. All patients had undergone biopsy and their cancer was approved by a pathologist. Therefore, 529 patients and 9 factors including age of patients at diagnosis time (<50 and ≥50 years), tumor size (less than 2 cm, 2– 5 cm, and greater than 5 cm), family history, radiotherapy, type of tumor (Ductal, Lobular, Medullar), type of surgery (Lumpectomy, Radical Mastectomy, Segmental Mastectomy, Simple Mastectomy), number of involved lymph nodes (less than 2, 3–6, more than 7) and stage of the disease (I, II, III) were included in the model, based on the American Joint Committee on Cancer classification (14). Hormone therapy data were not available, whereas all patients had received chemotherapy. Additionally, the time (years) elapsed since the cancer diagnosed until death and/or FRT were measured as outcome variables.
Illness-death Model:
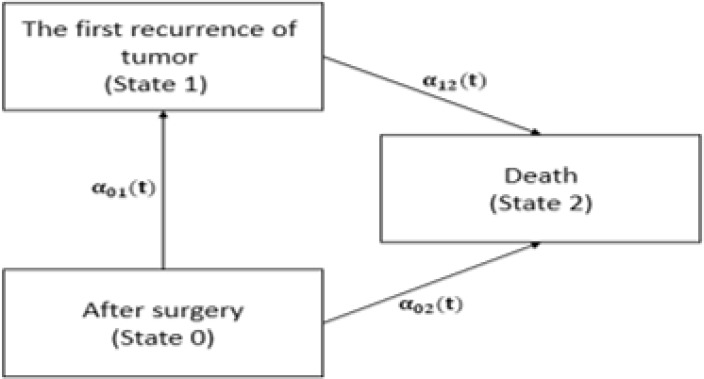
The illness-death model is a very useful tool for describing the course of BC. Fig. 1 shows the schematic form of disability model for BC. We defined the death as dying from BC and the FRT as both distant and local metastasis. In this model the Cox transition intensity, a common choice in survival analyses and MS models, was used to investigate the effects of various factors on the intensity of the transitions between different states. Three multivariable Cox transition intensities were fitted with different starting and ending points. Starting and ending points for the first transition were time of surgery (state 0) and experience of FRT (state 1), for the second transition were time of surgery (state 0) and experience of death (state 2), and for the third transition were time of FRT (state 1) and experience of death (state 2). We denoted the time of surgery and the time of FRT as t and d; respectively, and thus the time of surgery to FRT, or time to entry into the intermediate second state denoted as m, m= t−d.
Fig. 1:
Disability model for breast cancer
For the kth patient with covariate vector xk, the hazard of transition i → j, denoted by λij(t), is:
which may simplified as (15):
Where λij,0(t) is the baseline hazard of transition i → j, βij′ and γij are regression coefficients that relates time and covariate vector to intensity of transitions, respectively.
In MS models, we need to estimate transition intensities and transition probabilities to show the impact of FRT on survival of BC patients. We obtained transition probabilities by Kolmogorov backward differential equations (16) and estimate transition intensities adjusted for patients’ demographic characteristics and pathological factors by generalized partial likelihood (17). We considered a three-state model described in Fig. 1.
The state of a subject at time t is represented by the value X (t) taken by a stochastic process X. Subjects were included in different states as follow: in state 0 (after surgery) if they had a surgery to remove the tumor, in state 1 if they had FRT as an intermediate event, and in state 2 if they were dead as an absorbing state. Time of death and of the FRT is exactly observed, unless they are right censored. Right censoring for tumor recurrence occurred if at the time of the analysis a subject is still without tumor recurrence and experience right censoring for death if at the time of the analysis a subject is still alive (17). The transition intensities λ01(t), λ02(t) represents hazard rates of FRT and death for a subject who had surgery; respectively, and λ12(t) is hazard rate of death for a subject who had FRT. Kaplan-Meier estimator have been used to derive the survival curve.
The statistical evolution was performed by version 3.1.1 of R software (18), a free software environment for statistical computing and graphics, and msm, survival, mstate and mvna packages of R. In all the statistical tests, P<0.05 was considered significant and a P between 0.05 and 0.10 was considered as border line significant.
Results
Of 529 studied patients 194 (36.4%) patients died by the end of the study. Age of patients ranged from 23 to 80 years (47.1±10.7) and 67% of them (354 patients) have less than 50 years. Furthermore, 471(89%) patients had family history of BC and the majority of them had tumors less than ≤ 2 cm (60%, 320 patients) and involved lymph nodes number less than 2 (45%, 239 patients). The most frequent tumor type in studied patients was Ductal (88%, 470 patients) and 400 patients (76%) had radical mastectomy surgery (Table 1).
Table 1:
Demographic, clinical and tumor-related characteristics for all BC patients (n=529)
| Factors | Category | N (%) | Median† | Hazard Ratio (95% CI) | P |
|---|---|---|---|---|---|
| Age (yr) | < 50 | 354 (67) | 9.1 | 1 | -- |
| ≥ 50 | 175 (33) | 7.4 | 1.4 * (1.07–1.9) | 0.01 | |
| Family history of BC | Yes | 354 (67) | 8.9 | 1 | -- |
| No | 58 (11) | 9.1 | 1.1 (0.6–1.8) | 0.6 | |
| Stage of disease | I | 234 (44) | 8.9 | 1 | -- |
| II | 121 (23) | 8.1 | 1.3 (0.9–1.8) | 0.1 | |
| III | 142 (27) | 9.1 | 1.3 ** (0.9–1.9) | 0.07 | |
| Missing | 32 (6) | -- | -- | -- | |
| Tumor size (cm) | ≤ 2 | 320 (60) | 8.9 | 1 | -- |
| 2– 5 | 178 (33) | 9.3 | 1.2 (0.8–1.6) | 0.2 | |
| > 5 | 31 (7) | 5.8 | 2.01 * (1.2–3.1) | 0.003 | |
| Number of involved lymph nodes | ≤ 2 | 239 (45) | 9.1 | 1 | -- |
| 3–6 | 113 (21) | 13.7 | 1.2 (0.8–1.7) | 0.3 | |
| ≥ 7 | 85 (16) | 9.4 | 1.5 * (1.02–2.3) | 0.03 | |
| Missing | 92 (18) | -- | -- | -- | |
| Metastasis status | Distant/ Local | 138 (26) | 6.0 | 1 | -- |
| No | 391 (74) | NA | 1.03 (0.8–1.3) | 0.7 | |
| Radiotherapy | Before surgery | 81 (15) | 5.5 | 1 | -- |
| After surgery | 448 (85) | 9.1 | 0.7 ** (0.5–1.05) | 0.09 | |
| Type of tumor | Ductal | 470 (88) | 8.9 | 1 | -- |
| Lobular | 32 (7) | 11.4 | 0.8 (0.4–1.5) | 0.5 | |
| Medullar | 27 (5) | NA | 0.9 (0.4–1.9) | 0.8 | |
| Type of surgery | Lumpectomy | 27 (5) | 9.3 | 1 | -- |
| Radical Mastectomy | 400 (76) | 8.9 | 1.3 (0.7–2.7) | 0.3 | |
| Segmental Mastectomy | 40 (7) | 9.3 | 1.5 (0.6–3.4) | 0.3 | |
| Simple Mastectomy | 62 (12) | 9.2 | 1.1 (0.4–2.5) | 0.8 |
Median of survival time (year)
Statistically significant at 0.05/
Statistically significant at 0.1
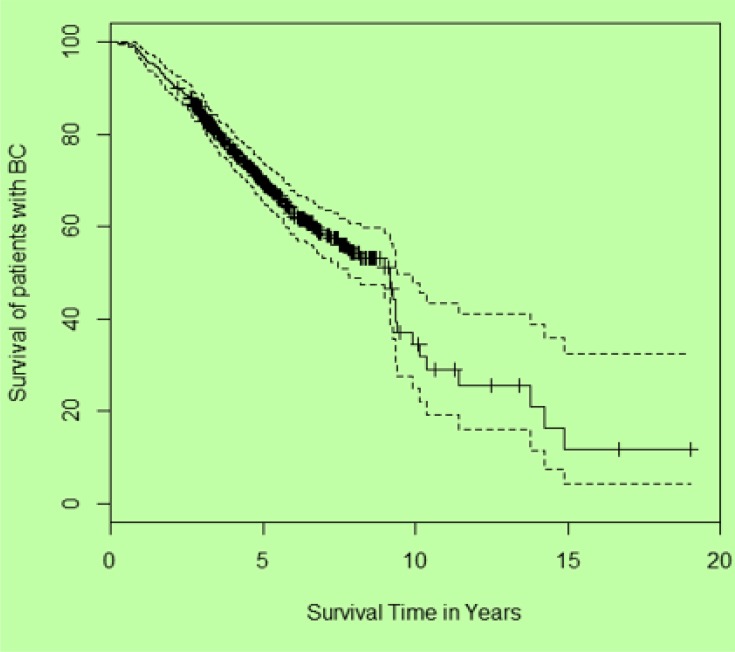
The 5-year survival rate was 68.5% and Kaplan-Meier estimates showed that about 50% of the patients survived for 9 years or more (Fig. 2).
Fig. 2:
The crude overall survival for BC patients Ffi
Crude hazard ratios for BC patients in relation to demographic, clinical and tumor-related characteristics are shown in Table 1. The risk of death for ≥50-year-old patients was 1.4 times higher in comparison with younger patients (HR=1.4 (95%CI: 1.07–1.9), P=0.01).
Patients with tumor sizes greater than 5 cm had better prognosis compared to those with tumor sizes less than 2 cm (HR=2 (95%CI:1.2–3.1), P=0.003). Additionally, the risk of death in patients who had less than two involved lymph nodes was 1.5 times higher in comparison with patients with more than seven involved lymph nodes (HR=1.5 (95%CI:1.02–2.3), P=0.03). In the beginning of the present study, all patients had no metastasis but some of them became metastatic during the study. This factor was entered as a time-dependent variable in the final model. Patients without metastasis had better prognosis than patients who had distant/local metastasis (HR=1.03 (95%CI: 0.8–1.3), P=0.7); however, the hazard ratio was not significant (Table 1).
Of 529 studied subjects at state 0, 125 patients entered to state 1 and 116 patients entered to state 2 and the rest had no events. Furthermore, of 125 patients who were entered to state 1, 77 patients entered to state 2.
The risks of FRT (transition 1) in patients with tumor sizes 2–5 cm and >5 cm were 1.3 and 3.5 times higher compared to patients with tumors ≤2 cm, respectively (HR=1.3 (95%CI: 0.9–2.02) & 3.5 (95%CI: 2.08–5.9), P<0.001). Patients who were in stage II were in risk of FRT 1.9 times more than patients were in stage I (HR=1.9 (95%CI: 1.2–2.9), P=0.002). Additionally, risk of death (transition 2) in patients who were ≥50 years old was 1.6 times higher compared to younger patients (HR=1.6 (95%CI:1.1–2.4), P=0.01). Risk of death (transition 3) in patients who experienced FRT (transition1) and had tumor sizes greater than 5 cm was 2.1 times higher compared to patients who had tumors ≤2 cm (HR=2.1 (95%CI:1.1–4.0), P=0.01). Risk of death and FRT in patients who had more than seven lymph nodes was 1.6 and 1.5 times higher compared to patients who had less than 2 nodes, respectively (HR=1.6 (95%CI: 1.0–2.7) & 1.5 (95%CI: 1.0–2.5), P=0.08). Effects of other variables were not statistically significant in any of the transitions. The disease stage became IV for patients who experienced FRT, so variable dropped from the model (Table 2).
Table 2:
Estimated effects in Cox models for each transition
| State 0 → 1 | State 0 → 2 | State 1 → 2 | |||||
|---|---|---|---|---|---|---|---|
| Factors | Category | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Age (yr) | < 50 | REF | -- | REF | -- | REF | -- |
| ≥ 50 | 1.05 (0.7–1.5) | 0.8 | 1.6 (1.1–2.4) | 0.01 * | 1.1 (0.6–1.7) | 0.6 | |
| Family history | No | REF | -- | REF | -- | REF | -- |
| Yes | 1.1 (0.6–2.1) | 0.6 | 0.9 (0.5–1.7) | 0.9 | 1.5 (0.6–3.6) | 0.2 | |
| Stage of disease | I | REF | -- | REF | -- | -- | -- |
| II | 1.9 (1.2–2.9) | 0.002 * | 1.1 (0.7–1.9) | 0.5 | -- | -- | |
| III | 1.3 (0.8–2.07) | 0.2 | 1.4 (0.9–2.2) | 0.08 | -- | -- | |
| Tumor size (cm) | ≤ 2 | REF | -- | REF | -- | REF | -- |
| 2– 5 | 1.3 (0.9–2.02) | <0.001 * | 1.1 (0.7–1.6) | 0.6 | 1.4 (0.8–2.5) | 0.1 | |
| > 5 | 3.5 (2.08–5.9) | <0.001 * | 1.2 (0.5–2.7) | 0.5 | 2.1 (1.1–4.0) | 0.01 * | |
| Number of involved lymph nodes | ≤ 2 | REF | -- | REF | -- | REF | -- |
| 3–6 | 1.03 (0.5–1.7) | 0.9 | 1.3 (0.8–2.1) | 0.2 | 0.9 (0.4–2.2) | 0.9 | |
| ≥ 7 | 1.6 (1.0–2.7) | 0.08 ** | 1.5 (1.0–2.5) | 0.08 ** | 1.2 (0.6–2.6) | 0.5 | |
| Radiotherapy | Before surgery | REF | -- | REF | -- | REF | -- |
| After surgery | 0.7 (0.4–1.1) | 0.1 | 0.7 (0.4–1.1) | 0.3 | 0.6 (0.3–1.1) | 0.1 | |
| Type of tumor | Ductal | REF | -- | REF | -- | REF | -- |
| Lobular | 0.5 (0.2–1.2) | 0.1 | 0.8 (0.3–1.7) | 0.6 | 1.2 (0.5–3.2) | 0.6 | |
| Medullar | 0.8 (0.3–2.1) | 0.7 | 1.2 (0.5–2.5) | 0.6 | 0.3 (0.04–2.4) | 0.2 | |
| Type of surgery | Lumpectomy | REF | -- | REF | -- | REF | -- |
| Radical Mastectomy | 0.9(0.4–2.0) | 0.8 | 1.7(0.6–4.7) | 0.2 | 1.1(0.4–3.0) | 0.7 | |
| Segmental Mastectomy | 1.2 (0.4–3.2) | 0.6 | 2.1 (0.6–6.7) | 0.2 | 0.8 (0.2–2.9) | 0.7 | |
| Simple Mastectomy | 1.04 (0.4–2.5) | 0.9 | 0.9 (0.2–3.1) | 0.9 | 1.2 (0.4–3.9) | 0.6 | |
Significant at 0.05/
Significant at 0.1
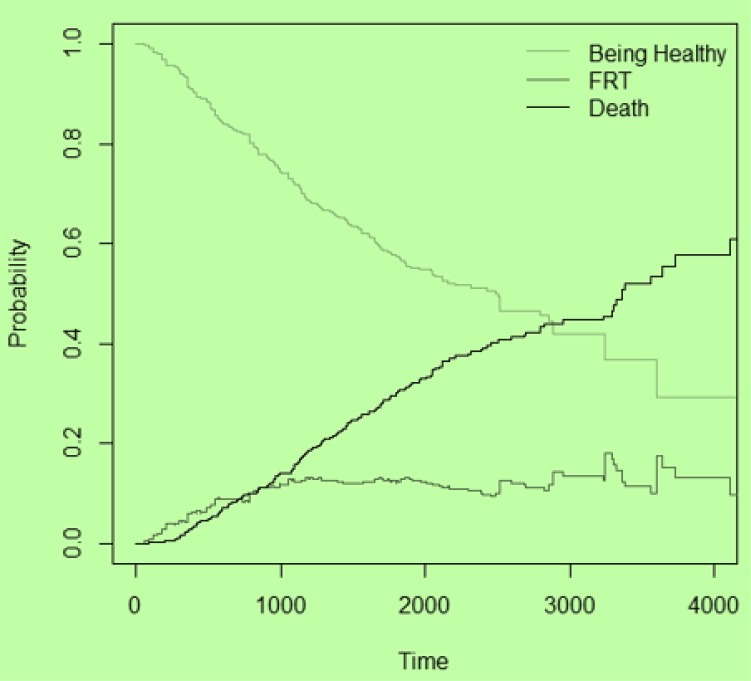
Figure 3 illustrates the transition probability of being healthy decreased over time and became stable after about 8 years. Conversely, probability of death and recurrence of tumor increased by different slopes and became constant after about 13.5 years; however, the probability of recurrence had a dramatic increase for the first 2.5 years, and then showed a little decrease with a slight slope.
Fig. 3:
The transition probabilities for each event
Discussion
In many medical fields, intermediate events play an important role in study of the disease courses. Probability and hazard of occurrence of intermediate events could obtain by using MS models (12, 17, 19). MS models have been applied to demonstrate the progression between different stages of HIV disease (20) and to investigate the survival of patients affected by metastasis as an intermediate event (21). In the present study metastasis is consider as an intermediate event which may have a noticeable effect on survival of BC patients.
Descriptive statistics showed that mean (SD) age at diagnosis time was about 47.1(10.7) years which is similar to those found in Korea and southern China studies (22, 23). Normally, stage of disease increases with age in irregular screening programs (24). Therefore, setting appropriate mechanisms for detection of BC patients in early stages of the disease is critical. However, in some developing countries, the current screening methods are inappropriate and non-effective (25). Thus, well-designed and effective screening and health care programs as well as the women awareness of warning signs of BC such as painless lump, retraction of nipple, and bloody discharge are necessary to early diagnosis of BC (25).
MS model showed that when a patient experiences FRT, the shape of relations between prognostic factors become different. It is important to note that in analyzing two transitions, which are ended to death (transitions 2 and 3), we have different risk sets that may cause different significant prognostic factors in each transition. In transition 2 patients were tumor free and in transition 3 patients experienced FRT. The most important factors affecting death in transition 2 were patients’ age at diagnosis time and the number of involved lymph nodes. Tumor size was the most important risk factor for death in metastatic patients. Similar findings have been observed by other authors (26). In the present study, the presence of FRT in terms of time-dependent covariate was not a significant prognostic factor in overall survival. However, it seems that MS model can declared the effect of FRT better than time-dependent model by comparing parameter estimation changes. Several studies demonstrated that recurrence of tumor significantly decrease the survival time. Karimi et al. indicated that risk of death for patients who experience relapse is 3.2 times more than those who did not (27). De Bock et al showed that the risk of death in patients with Loco-regional recurrence was three times higher than patients without recurrence (28).
Based on American Cancer Society population-based studies, BC death rate is increased with age (24). Likewise, our results showed that ≥50-year-old patients are at more risk for death than younger patients. In contrast, Gao et al. indicated that age could not change the risk of death in BC patients and Karimi et al. showed that age at diagnosis time had no remarkable effect on patients’ survival (27, 29).
It is indicated that several factors including age at diagnosis time, stage of disease, tumor size and type, number of involved lymph nodes have important effects on disease course of BC patients with or without metastasis in each transition of MS model (26, 27, 30). In the present study, stage of the disease and tumor size was the most important prognostic factors, which affect FRT. In Karimi et al. study patients in stage III or IV had worse prognosis than those at stage I and II (27). Age of patients at diagnosis time was the most important factor effecting survival. The number of involved lymph nodes was the most important factor that affected on both FRT and death. There were limitations in this study including incomplete records, missing hormone therapy information and lack of access to other data centers.
According to transition probability plot, patients who experienced recurrence of tumor after surgery had high death likelihood in first 2.5 years after surgery. The risk of FRT can be reduced by appropriate medical cares at this period of time after surgery. Another fact that is obvious in transition probability plot is that the likelihood of tumor recurrence was more than death in the first few days after surgery. On the contrary, probability of death increased with a positive slope after it.
Conclusion
The findings of the present study showed that the stage of the disease has significant effect on death before occurrence of the FRT and the effect of tumor size was significant before and after it. Additionally, Number of involved lymph nodes is the most important factor affecting both FRT and death. Furthermore, illness-death model was found to be a useful tool in modeling the disease course of BC patients and effect of the intermediate events.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
These investigations were partially supported by funding from the vice chancellor for research and technology affairs, contract no. 9311286230, Hamadan University of Medical Sciences. We are grateful to Hamadan Mahdieh oncology center’s personnel for their helpful corporation. The authors would like to hereby declare that the investigations undertaken and described in this article are part of the results of the thesis of the first author. The authors declare that there is no conflict of interests.
References
- 1. The international agency for research on cancer (2013). Latest world cancer statistics. World Health Organization. Available from: https://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf
- 2. Beiki O, Hall P, Ekbom A, Moradi T. (2012). Breast cancer incidence and case fatality among 4.7 million women in relation to social and ethnic background: a population-based cohort study. Breast Cancer Res, 14 (1): R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radmard AR. (2010). Five common cancers in Iran. Arch Iran Med, 13 ( 2): 143–6. [PubMed] [Google Scholar]
- 4. Akbari ME, Mozaffar M, Heidari A, Zirakzadeh H, Akbari A, Akbari M, et al. (2011). Recurrence and Survival Effect in Breast Conserving Surgery: What are the Predictive and/or Prognostic Factors? Iran J Cancer Prev, 4 ( 2): 49–54. [Google Scholar]
- 5. Haghighat S, Akbari M, Ghaffari S, Yavari P. (2012). Standardized breast cancer mortality rate compared to the general female population of Iran. Asian Pac J Cancer Prev, 13: 5525–8. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng Get al. (2012). Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res, 18 ( 20): 5701–10. [DOI] [PubMed] [Google Scholar]
- 7. Schmoor C, Schumacher M, Finke J, Beyersmann J. (2013). Competing risks and multistate models. Clin Cancer Res, 19 ( 1): 12–21. [DOI] [PubMed] [Google Scholar]
- 8. Johnson JR, Williams G, Pazdur R. (2003). End points and United States food and drug administration approval of oncology drugs. J Clin Oncol, 21 ( 7): 1404–11. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki C, Blomqvist L, Hatschek T, Carlsson L, Einbeigi Z, Linderholm Bet al. (2013). Impact of the first tumor response at eight weeks on overall survival in metastatic breast cancer patients treated with first-line combination chemotherapy. Med Oncol, 30 ( 1): 415. [DOI] [PubMed] [Google Scholar]
- 10. Dent R, Trudeau M, Pritchard KI, et al. (2007). Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res, 13 ( 15): 4429–34. [DOI] [PubMed] [Google Scholar]
- 11. Putter H, van der Hage J, de Bock GH, Elgalta R, van de Velde CJ. (2006). Estimation and prediction in a multi-state model for breast cancer. Biom J, 48 ( 3): 366–80. [DOI] [PubMed] [Google Scholar]
- 12. Meier-Hirmer C, Schumacher M. (2013). Multi-state model for studying an intermediate event using time-dependent covariates: application to breast cancer. BMC Med Res Methodol, 13 ( 1): 80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putter H, Fiocco M, Geskus RB. (2007). Tutorial in biostatistics: competing risks and multi-state models. Stat Med, 26 ( 11): 2389–430. [DOI] [PubMed] [Google Scholar]
- 14. Egner JR. (2010). AJCC cancer staging manual. JAMA, 304 ( 15): 1726–7. [Google Scholar]
- 15. Iacobelli S, Carstensen B. (2013). Multiple time scales in multi-state models. Stat Med, 32 ( 30): 5315–27. [DOI] [PubMed] [Google Scholar]
- 16. Ross SM. (1996). Stochastic processes: John Wiley & Sons, New York. [Google Scholar]
- 17. Commenges D, Joly P. Multi-state model for dementia, institutionalization, and death (2004). Commun Stat Theory Methods, 33 ( 6): 1315–26. [Google Scholar]
- 18. R Development Core Team (2012). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 19. Andersen PK, Perme MP. Inference for outcome probabilities in multi-state models (2008). Lifetime data anal, 14 (4): 405–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira Rde V, Shimakura SE, Campos DP, Victoriano FP, Ribeiro SR, Veloso VGet al. (2013). Multi-state models for defining degrees of chronicity related to HIV-infected patient therapy adherence. Cad Saude Publica, 29 ( 4): 801–11. [DOI] [PubMed] [Google Scholar]
- 21. Zare A, Mahmoodi M, Mohammad K, Zeraati H, Hosseini M, Naieni KH. (2013). Survival analysis of patients with gastric cancer undergoing surgery at the iran cancer institute: a method based on multi-state models. Asian Pac J Cancer Prev, 14: 6369–73. [DOI] [PubMed] [Google Scholar]
- 22. Kim M-J, Ro JY, Ahn S-H, Kim HH, Kim S-B, Gong G. (2006). Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum pathol, 37( 9): 1217–26. [DOI] [PubMed] [Google Scholar]
- 23. Xue C, Wang X, Peng R, Shi Y, Qin T, Liu Det al. (2012). Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci, 103 ( 9): 1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Cancer Society (2012). Breast cancer facts and figures 2011–2012. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf
- 25. Montazeri A, Vahdaninia M, Harirchi I, Harirchi AM, Sajadian A, Khaleghi F, et al. (2008). Breast cancer in Iran: need for greater women awareness of warning signs and effective screening methods. Asia Pac Fam Med, 7 ( 1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. (1999). Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat, 56( 1): 67–78. [DOI] [PubMed] [Google Scholar]
- 27. Karimi A, Delpisheh A, Sayehmiri K, Saboori H, Rahimi E. (2014). Predictive factors of survival time of breast cancer in kurdistan province of Iran between 2006–2014: a Cox regression approach. Asian Pac J Cancer Prev, 15 ( 19): 8483–8. [DOI] [PubMed] [Google Scholar]
- 28. De Bock G, Putter H, Bonnema J, Van Der Hage J, Bartelink H, Van De Velde C. (2009). The impact of loco-regional recurrences on metastatic progression in early-stage breast cancer: a multistate model. Breast Cancer Res Treat, 117 ( 2): 401–8. [DOI] [PubMed] [Google Scholar]
- 29. Gao N, Xu H, Liu C, Xu H, Chen G, Wang X, et al. (2014). Nestin: predicting specific survival factors for breast cancer. Tumor Biol, 35( 3): 1751–5. [DOI] [PubMed] [Google Scholar]
- 30. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. (2012). Factors associated with mortality after breast cancer metastasis. Cancer Causes Control, 23 ( 1): 103–12. [DOI] [PubMed] [Google Scholar]