Abstract
Objective:
Prior to implementing a trial to evaluate the economic costs and clinical outcomes of pharmacogenetic testing in a large safety net health care system, we determined the number of patients taking targeted medications and their clinical care encounter sites.
Methods:
Using 1-year electronic medical record data, we evaluated the number of patients who had started one or more of 30 known pharmacogenomically actionable medications and the number of care encounter sites the patients had visited.
Results:
Results showed 7039 unique patients who started one or more of the target medications within a 12-month period with visits to 73 care sites within the system.
Conclusion:
Findings suggest that the type of large-scale, multi-drug, multi-gene approach to pharmacogenetic testing we are planning is widely relevant, and successful implementation will require wide-scale education of prescribers and other personnel involved in medication dispensing and handling.
Keywords: Pharmacoepidemiology, genotype, patient safety, pharmacogenetics, pharmacogenomics, genetic polymorphism
Introduction
The implementation of pharmacogenomics is increasingly relevant at the population level. Nearly 70% of Americans take at least one medication and more than 20% take five or more,1 with spending on medications projected to be over US$457 billion by 2019.2 Defined as the study of genetic variation associated with drug response, pharmacogenomics seeks to understand how genes affect the pharmacokinetics and pharmacodynamics of drugs.3 Pharmacogenomics allows drug therapy to be individualized based on genotypes, potentially addressing the 20%–95% of variability in drug disposition that is genetics related.4
Clinical implementation of pharmacogenetic testing may improve patient outcomes by reducing side effects and improving treatment response.5 Currently, drug therapy for many conditions is plagued by unacceptable levels of adverse drug reactions, ineffectiveness, and poor adherence. Each year, adverse drug reactions are responsible for the death of approximately 100,000 patients6 and 5% of hospitalizations.7 Adverse reactions are one of several reasons for poor medication adherence, which ultimately reduces drug efficacy and worsens the societal disease burden.8,9 Genomic science has the potential to change this picture. For many of the most commonly used drugs, the specific genetic variants that result in either toxic adverse reactions or sustained efficacy are now known.
Pharmacogenetically actionable medications are defined as those for which there is sufficient information to guide drug or dosing changes in clinical practice. Over 160 medications now incorporate pharmacogenetic information on their labels,10 and a subset of these have sufficient evidence to guide clinical practice changes based on an individual patient’s pharmacogenetic test results. For example, the Clinical Pharmacogenetic Implementation Consortium (CPIC) has produced 35 evidence-based guidelines for gene and drug pairs to help clinicians understand how genetic test results should be used to optimize both individual medications (e.g. codeine and warfarin) and classes of drugs (e.g. tricyclic antidepressants).11 CPIC guidelines suggest alternative medications or higher or lower doses based on pharmacogenetic test results. However, only a limited number of institutions in the United States, Canada, and Europe have begun to implement clinical pharmacogenetic testing to guide medication prescribing.11
In order to implement pharmacogenetic testing within a health care system, it is important to determine how many patients and clinics might be affected. Such knowledge is critical to identifying how and where to best direct resources for testing patients and educating providers about pharmacogenomics initiatives, particularly in resource constrained settings. Empirical data to answer these questions are somewhat limited. One study showed that 64% of patients seen in the primary care clinics of a large academic medical center had been prescribed at least one known pharmacogenomically actionable medication.12 Whether those results generalize to other clinics or to non-academic or underserved settings is unclear.
The study purpose was to evaluate the potential scope of implementing multi-drug pharmacogenetic testing in a large safety net health care system. Our aims were to (1) identify the number of patients exposed to at least one of 30 known pharmacogenomically actionable medications and (2) identify the number and types of clinics involved to best direct provider education efforts during implementation. Our guiding questions were as follows: How many patients are exposed to known pharmacogenomically actionable medications? What are the age, race, and gender of those patients? In what clinics do patients receive care?
New contribution
This study extends research on the implementation of pharmacogenomics in several ways. To the best of our knowledge, it is the first to focus on a large, safety net health care system. Prior pharmacogenomic studies have focused on academic health centers12–15 which have greater resources for implementation. It is well documented that poverty is closely aligned with poor health, and that expensive health care complications occur with greater frequency in the underserved, who are at greater risk of emergency room visits, frequent hospital admissions,16 and adverse outcomes from diseases and their treatments.17–20 Thus, data from academic health centers may not generalize to safety net health care systems. Second, measuring the costs and benefit of pharmacogenetics testing is challenging and requires an informatics infrastructure capable of measuring both financial costs and clinical outcomes. As a result, the business case for implementation of genomic and pharmacogenetic testing in large health care systems has not been persuasive.21,22 Data from our study will provide a better understanding of the potential scope of implementation of a multi-drug, multi-gene approach to help build the business case for implementation to help drive change. Third, we interpret our results in the context of health care providers’ educational needs. Gaps in clinician education have been identified as a barrier to implementation of pharmacogenomics15 with one national survey indicating only 10% of nearly 400,000 physicians felt appropriately informed about pharmacogenetic testing.23 No data are available on nurse practitioner knowledge. Identifying the estimated number and type of providers who are likely to encounter pharmacogenomically guided medication orders will also help drive implementation forward.
Conceptual model
This study is based on a three-step model for implementation of pharmacogenetic testing.15 Step 1, obtaining genomic data, requires clear understanding of the patient population, the genomic data storage needs, as well as the genes and variants to be screened. Having addressed the latter two points during planning, the current analysis was undertaken to better understand the patient population. Step 2 of the process relates to interpreting genomic data which will be automated within our implementation system. Step 3, returning genomic data to providers, requires educated clinicians capable of acting upon pharmacogenetic test results. The current analysis of clinic encounters was designed to better understand the number and type of providers likely to be touched by and require education about implementation. Thus, our analysis was designed to provide crucial data for Steps 1 and 3 within the implementation process.
Methods
Design and data source—overview
This was a retrospective analysis of existing electronic medical record data from the Eskenazi Health System in Indianapolis, IN, that was judged to be an exempt study by the institutional review board office. As a result, no informed consent or authorization to use protected health information was required. Three sources of data were used. Some Eskenazi data (including medication-dispensing events) are housed in the Regenstrief Medical Record System (RMRS). There is also a computerized physician order entry system (Gopher).24 Other Eskenazi data (number and locations of health care encounters, diagnoses, dates) are housed in Eskenazi Health’s own clinical data repository. Pharmacogenetic testing has not been previously offered within this health care system.
Identification of medications
As part of the Implementing Genomics in Practice National Institutes of Health/National Human Genome Research Institute (IGNITE NIH/NHGRI)-funded project called INGENIOUS (INdiana GENomics Implementation: an Opportunity for the UnderServed), we identified 30 medications known to be pharmacogenomically actionable. All of the chosen medications had sufficient evidence to guide drug changes or dosing adjustments in practice. The targeted medications were based on extensive literature reviews of peer-reviewed publications, Food and Drug Administration labeling, internal pre-publication data from our groups of scientists, and a strong emphasis on guidelines published by CPIC, the Dutch Pharmacogenetics Working Group, and the Canadian Pharmacogenomics Network for Drug Safety. A working group composed of cross-functional team members representing clinical and scientific specialties reviewed the data, engaged in a series of face-to-face discussions over the period of a year, and reached consensus on the selection of targeted medications and relevant genes and variants to be studied. The working group members included pharmacogeneticists, clinical pharmacologists, the clinical laboratory director, and physicians from nephrology, cardiology, gastroenterology, infectious disease, oncology, pediatrics, obstetrics, geriatrics, and general internal medicine. Our focus on multiple drugs required a multi-gene approach since each drug is affected by specific genetic polymorphisms. The entire process allowed for informed decisions regarding clinical and scientific evidence supporting each targeted medication. Because proton pump inhibitors are used commonly in this health care system, and risk of adverse events can to some extent be stratified based on indications, we limited the cohort with proton pump inhibitors to patients with International Classification of Diseases—Ninth Revision (ICD-9) diagnosis codes that reflected higher risk of adverse events.
Identification of patients
We sought to identify patients who had received a new prescription for any one of the 30 targeted medications. The first inclusion criterion identified patients with at least one new (Gopher) order for any of the 30 medications of interest between 1 July 2013 and 30 June 2014. By “new” order, we meant no previous order for the same medication between April 2011 (when the current version of Gopher went online) and 30 June 2013. We then narrowed this “new” order cohort to those who had received their relevant new medication order at a care encounter site (outpatient, emergency, or inpatient) on the main campus of Eskenazi Health (including Eskenazi Hospital), or at any Eskenazi mental health site in Indianapolis. This step excluded new medication orders at any of Eskenazi Health’s eight off-campus primary care sites in Indianapolis neighborhoods. We adopted this approach because our plan in the prospective trial is to recruit subjects on the main campus. Separately from using order data to define “new,” we also examined medication-dispensing data to define “new.” Medication-dispensing data were not directly linked to the encounter sites. Because medication orders were linked to the encounter sites (Gopher), we used medication orders in our primary analyses. We used medication-dispensing data as a secondary source, to help triangulate the order data to confirm the number of patients.
We then scrutinized how the medication records had actually been stored electronically, for the year 1 July 2013 to 30 June 2014. We reviewed all 3349 orderable medication names in the Gopher system in order to find all possible forms, strengths, and combinations (n = 47) in which the 30 medications of interest could have been ordered. We reviewed all 17,062 medication names stored in the RMRS, in order to find all forms, strengths, and combinations in which relevant dispensing events had been recorded for Eskenazi patients during that year. In this way, we identified many more records than Regenstrief initially provided us. For example, for codeine, Regenstrief initially provided only records containing the full-word “codeine,” but we knew to query for abbreviated forms such as “Acetaminophen-cod #4 tablet.”
Once the patient cohort was identified, demographics contained in the medical record were abstracted. Data included age, gender, and race.
Number and types of clinic encounters
Similarly, for locations of care, we reviewed the way that all unique encounter locations had been stored in the Eskenazi system for patients on at least one of the 30 medications. We then winnowed this set of locations down to those on the main campus inpatient, outpatient, or emergency department, or those within mental health services sites (outpatient or inpatient).
Results
A total of 7039 unique patients had received a new order for one or more of our targeted medications during the 12-month time period meeting our inclusion criteria. Patients represented diverse genders, ages, and races/ethnicities (see Table 1). Races/ethnicities shown in Table 1 were comparable to the adult patients seen within the health care institution during the same time period, the latter being 40% Black, 32% White, 2% Asian, 3% Hispanic, 4% mixed races, and 19% other/unknown. These patients had received a total of 8169 prescriptions, with 20% of the patients receiving prescriptions for two or more of the targeted medications (see Table 1). These numbers suggest that each day on average, about 26 unique patients began a new prescription for one or more of these pharmacogenetically actionable medications.
Table 1.
Demographic characteristics of patients who received a new prescription for one or more known pharmacogenomically actionable medications.
| (n = 7039) | |
|---|---|
| Age (mean, SD) | 51.0 (14.4) |
| Median | 52.5 |
| Range | 21.1 to >89 |
| Gender (n, %) | |
| Female | 3943 (56%) |
| Male | 3096 (44%) |
| Race (n, %) | |
| Black | 2858 (41%) |
| White | 3036 (43%) |
| Asian | 100 (1%) |
| Hispanic | 30 (<1%) |
| Mixed | 99 (1%) |
| Other/unknown | 916 (13%) |
| Number of targeted medications per patient (mean, SD) | 1.2 (0.5) |
| Median (range) | 1.0 (1 to 5) |
| Number of patients taking targeted medications | |
| 1 of the targeted medications | 5648 (80%) |
| 2 | 1173 (17%) |
| 3 | 185 (3%) |
| 4 | 30 (<1%) |
| 5 of the targeted medications | 3 (<1%) |
Table 2 shows the total number of prescriptions by medication. Tramadol, citalopram, and clopidogrel were the three most commonly prescribed overall. No prescriptions were seen for three of the targeted medications: cyclophosphamide, mercaptopurine, or rasburicase. Prescriptions were also relatively rare (<15 Rx) for capecitabine, tacrolimus, and voriconazole.
Table 2.
Number of new prescriptions for each of the targeted medications.
| Targeted medication | No. of Rx | Targeted medication | No. of Rx |
|---|---|---|---|
| 1. Amitriptyline | 521 | 16. Mercaptopurine | 0 |
| 2. Aripiprazole | 215 | 17. Methotrexate | 183 |
| 3. Atazanavir | 111 | 18. Nortriptyline | 216 |
| 4. Atomoxetine | 19 | 19. Phenytoin | 142 |
| 5. Azathioprine | 94 | 20. PPIs | 507 |
| 6. Capecitabine | 14 | 21. Quetiapine | 525 |
| 7. Citalopram | 890 | 22. Rasburicase | 0 |
| 8. Clopidogrel | 820 | 23. Simvastatin | 455 |
| 9. Codeine | 467 | 24. Tacrolimus | 12 |
| 10. Cyclophosphamide | 0 | 25. Tenofovir | 320 |
| 11. Doxepin | 131 | Tenofovir and efavirenz | 188 |
| 12. Efavirenz | 203 | 26. Thioguanine | 0 |
| 13. Escitalopram | 146 | 27. Tramadol | 1388 |
| 14. Fluorouracil (systemic) | 43 | 28. Venlafaxine | 267 |
| 15. Glyburide | 136 | 29. Voriconazole | 10 |
| 30. Warfarin | 557 |
PPI: proton pump inhibitor.
PPI limited indications as noted in the “Methods” section.
Prescriptions originated across the health system in 73 different care sites. Tramadol was among the three most commonly prescribed of the targeted medications across the emergency department, outpatient clinics, and inpatient units (Figures 1–4). Overall, the largest number of prescriptions for each targeted medication originated in the outpatient, non-mental health clinics with these exceptions: (1) clopidogrel, codeine, and phenytoin (emergency department) and (2) aripiprazole and quetiapine (mental health care sites). In total, there were 73 different care sites.
Figure 1.

Medication prescriptions within the emergency department. Figure shows number of prescriptions originating in the emergency department with tramadol, clopidogrel, and codeine being the three most common. No patients were prescribed thioguanine.
Figure 2.
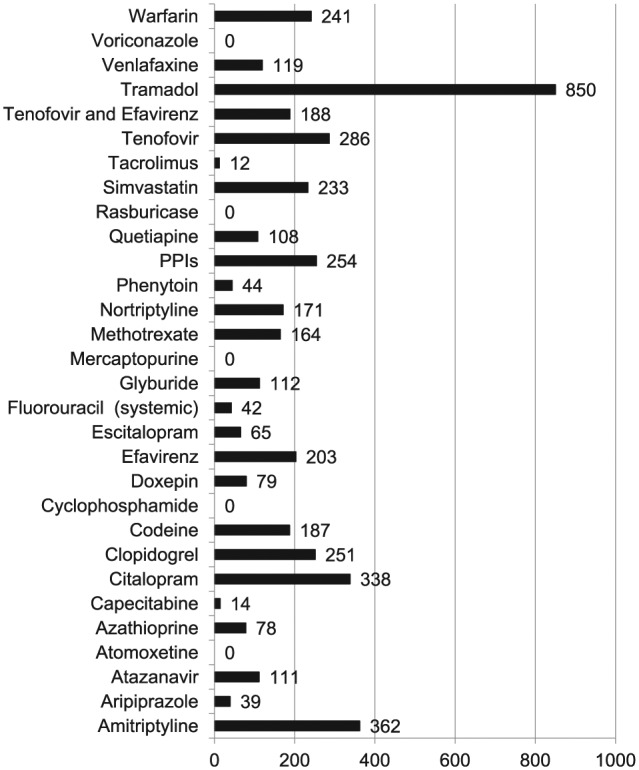
Medication prescriptions within outpatient, non-mental health clinics. Figure shows number of prescriptions originating in non-mental health specific outpatient clinics with tramadol, amitriptyline, and citalopram being the three most common. No patients were prescribed thioguanine.
Figure 3.
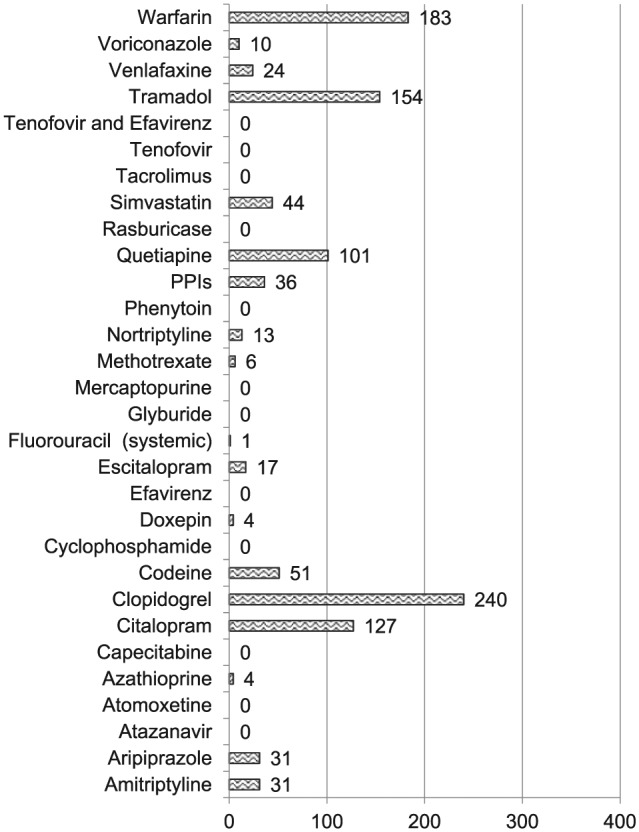
Medication prescriptions within inpatient, non-mental health clinics. Figure shows number of prescriptions originating in non-mental health specific inpatient clinics with clopidogrel, warfarin, and tramadol being the three most common. No patients were prescribed thioguanine.
Figure 4.
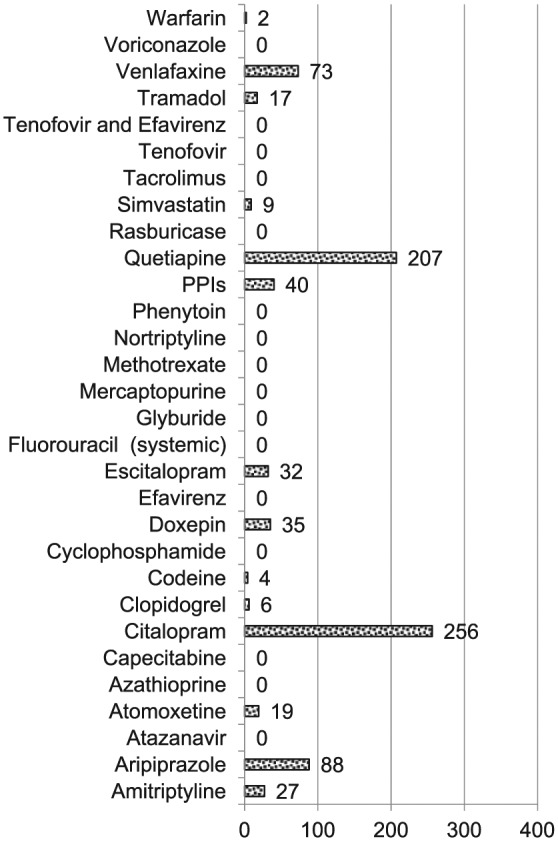
Medication prescriptions within inpatient and outpatient mental health specific settings. Figure shows number of prescriptions originating in mental health specific outpatient clinics and inpatient units with citalopram, quetiapine, and aripiprazole being the three most common. No patients were prescribed thioguanine.
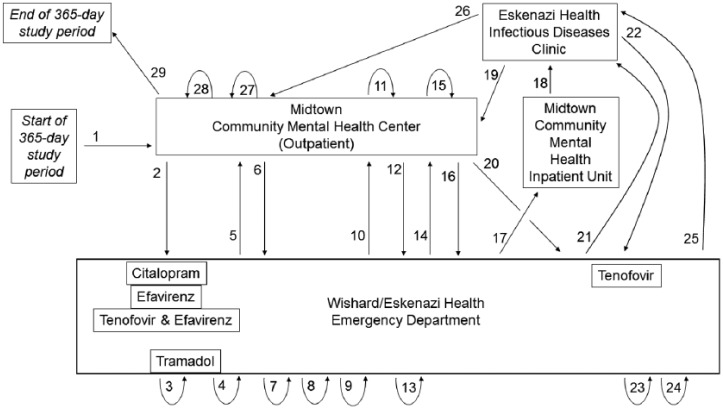
Figure 5 shows the complex trajectory of health care encounters and related prescriptions at different clinical encounter sites for one of the three patients taking five of the targeted medications. During the year, this patient made 28 health care visits to four different care locations, although all prescriptions originated in the emergency department.
Figure 5.
Case study illustration. Figure shows the complex trajectory of health care encounters (numbered arrows) of one patient during a 1-year period. New prescriptions for pharmacogenetically actionable medications (named in boxes) are linked to visits (proximity of boxes to numbered arrows). Tightly curved arrows are used to illustrate repeat visits to the same health care encounter site. Longer straight or curved arrows link visits between one encounter site and another.
Discussion
The goal of personalized medicine is to implement advances in biomarker pharmacology, molecular diagnostics, and genomics to improve public health. For the full benefits of this science to be realized, it is critical that scientific advances made in experimental settings and on a small scale be extended to community practice and that a business case can be made to support such dissemination. Furthermore, it is important that key innovations be extended beyond individual hospital settings to large health care systems, especially those that include underserved populations.
In terms of clinical implementation, our findings showed that a multi-gene, multi-drug pharmacogenetic testing approach would be relevant to a large number of patients who are diverse in terms of age, gender, race, and clinical settings. These data strengthen the case for system-wide rather than select clinic-based implementation, since the latter would be inadequate for capturing the target population and inefficient since patients are seen across many care settings. Our methods for identifying patients using existing electronic medical record data are a cost-efficient means that others can use to evaluate and model subsequent implementation costs. For example, our trial will be based on patients who received an order for a target medication, rather than on pre-emptive genotyping. Our data can be used to model the most cost-efficient frequency for running tests based on the number of samples per day likely to be received and cost variations related to running small, medium, or large batches of samples. Our data could also be useful for those planning pre-emptive genotyping for one or more of the targeted medications.
Implementation requires well-educated prescribers. Our data suggest that educational efforts should be system-wide and ongoing to address any changes or turnover in prescribers. Such large-scale efforts are likely to require asynchronous, cost-efficient educational modules (e.g. web-based) that are either mandatory or incentivized (e.g. continuing education credits). In addition, our case study data can be used during educational efforts to illustrate the potential complexity of incorporating and acting upon pharmacogenetic test results. They also suggest that education include strategies for communicating pharmacogenetic information during handoffs and care transitions. Our data suggest that wide-scale prescriber education efforts that cross different care encounter sites be fully considered during planning so that they do not become a hidden or unexpected cost.
Study findings should be interpreted in light of some limitations. Although the findings provide an important perspective from a safety net rather than tertiary academic health care setting, the data are from a single system and may not be generalizable to other safety net systems. The reason why several of the targeted medications had not been ordered during the previous year is unclear. In addition, because findings are based on the US health care system, they may not generalize to other countries.
Conclusion
Findings document the potential scope of implementing multi-gene, multi-drug pharmacogenetic testing in a large safety net health care system. A large number and diversity of patients and clinics would be directly affected by implementation indicating the need for large-scale education of prescribers and other personnel involved in medication dispensing and handling.
Footnotes
Declaration of Conflicting of Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: The study was deemed to be exempt by the Indiana University Purdue University Institutional Review Board.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Award Number 5U01HG007762 from the National Human Genome Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Human Genome Research Institute or the National Institutes of Health.
Informed consent: Informed consent was not required for this study of existing patient records.
References
- 1. Zhong W, Maradit-Kremers H, St. Sauver J, et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clinic Proc 2013; 88(7): 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaiser Family Foundation. Prescription drug trends, http://www.kff.org/rxdrugs/upload/3057-08.pdf (2010, accessed 10 July 2015).
- 3. Zdanowicz M. Concepts in pharmacogenomics. Bethesda, MD: American Society of Health-System Pharmacists, 2010. [Google Scholar]
- 4. Evans W, McLeod H. Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med 2003; 348(6): 538–549. [DOI] [PubMed] [Google Scholar]
- 5. Ross S, Anand S, Joseph P, et al. Promises and challenges of pharmacogenetics: an overview of study design, methodological and statistical issues. JRSM Cardiovasc Dis 2012; 1(2): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bond CA, Raehl CL. Adverse drug reactions in United States hospitals. Pharmacotherapy 2006; 26(5): 601–608. [DOI] [PubMed] [Google Scholar]
- 7. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42(7): 1017–1025. [DOI] [PubMed] [Google Scholar]
- 8. Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology 2006; 71(1–2): 1–9. [DOI] [PubMed] [Google Scholar]
- 9. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011; 126(2): 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pharmacogenomics Knowledge Base (PharmGKB). CPIC: Clinical Pharmacogenetics Implementation Consortium, http://www.pharmgkb.org/page/cpic (2015, accessed 10 July 2015).
- 11. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labeling, http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm (2015, accessed 10 July 2015).
- 12. Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther 2012; 92(2): 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet 2014; 166C(1): 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Donnell PH, Danahey K, Jacobs M, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care—initial results of the University of Chicago “1,200 Patients Project.” Am J Med Genet C Semin Med Genet 2014; 166C(1): 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol 2015; 55: 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health 2009; 86(2): 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaw LJ, Merz CN, Bittner V, et al. Importance of socioeconomic status as a predictor of cardiovascular outcome and costs of care in women with suspected myocardial ischemia. Results from the National Institutes of Health, National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Womens Health 2008; 17(7): 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazzarino AI, Hamer M, Stamatakis E, et al. Low socioeconomic status and psychological distress as synergistic predictors of mortality from stroke and coronary heart disease. Psychosom Med 2013; 75(3): 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheung MR. Lack of health insurance increases all cause and all cancer mortality in adults: an analysis of National Health and Nutrition Examination Survey (NHANES III) data. Asian Pac J Cancer Prev 2013; 14(4): 2259–2263. [DOI] [PubMed] [Google Scholar]
- 20. Nitzkorski JR, Willis AI, Nick D, et al. Association of race and socioeconomic status and outcomes of patients with rectal cancer. Ann Surg Oncol 2013; 20(4): 1142–1147. [DOI] [PubMed] [Google Scholar]
- 21. Davis JC, Furstenthal L, Desai AA, et al. The microeconomics of personalized medicine: today’s challenge and tomorrow’s promise. Nat Rev Drug Discov 2009; 8(4): 279–286. [DOI] [PubMed] [Google Scholar]
- 22. Trusheim MR, Austin MJ, Rausch C, et al. Uncertain prognosis for high-quality diagnostics: clinical challenges, economic barriers and needed reforms. Pharmacogenomics 2013; 14(3): 325–334. [DOI] [PubMed] [Google Scholar]
- 23. Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther 2012; 91(3): 450–458. [DOI] [PubMed] [Google Scholar]
- 24. Regenstrief Institute. Computerized physician order entry (CPOE), http://www.regenstrief.org/cbmi/areas-excellence/computerized-physician-order-entry-cpoe/ (2015, accessed 10 July 2015).