Abstract
Cyanine dyes are well known for their bright fluorescence and utility in biological imaging. Yet, cyanines also readily photoisomerize to produce non-emissive dark states. Co-illumination with a secondary, red-shifted light source on-resonance with the longer wavelength absorbing dark state reverses the photoisomerization and returns the cyanine dye to the fluorescent manifold, increasing steady-state fluorescence intensity. Modulation of this secondary light source dynamically alters emission intensity, drastically improving detection sensitivity and facilitating fluorescence signals to be recovered from otherwise overwhelming background. Red and near-IR emitting cyanine derivatives have been synthesized with varying alkyl chain lengths and halogen substituents to alter dual-laser fluorescence enhancement. Photophysical properties and enhancement with dual laser modulation were coupled with density functional calculations to characterize substituent effects on dark state photophysics, potentially improving detection in high background biological environments.
Introduction
A valuable tool in biological imaging, near infrared (NIR) fluorescence benefits from low tissue autofluorescence and relatively deep tissue penetration.1–4 Stable, targetable, red and near IR probes are thus highly desirable for in vitro and in vivo imaging applications. Organic fluorophores have the additional advantage that their optical properties can be tailored to maximize sensitivity and compatibility through directed chemical synthesis.5 Since a usable fluorescence contrast agent needs to be hydrophilic, photostable, and strongly emissive, squaraine dyes, porphyrin derivatives, BODIPY (borondipyrromethane) analogs,6 and cyanines continue to find the most applications.7, 8
Cyanines, in particular, have high extinction coefficients, moderate fluorescence quantum yields, and generally good biocompatibility coupled with straightforward labeling strategies.9 Further, structural modification enables tailoring of their photophysics for various biomedical applications. Varying alkyl chain length, for example, has been shown to affect molar absorptivity and fluorescence quantum yield10 and halogenation on the polymethine chain has been used to improve cyanine targeting to G-quadruplexes.11 Also, halogenated pentamethine cyanines with quaternary ammonium side chains have proven useful as labels in biodegradable scaffolds.12 Importantly, cyanine dyes can be reliably switched between fluorescent and dark states, which has proven them useful in optical switching applications such as data storage and super-resolution microscopy.13, 14
The photophysics of Cy5 have been thoroughly studied using fluorescence correlation spectroscopy (FCS)15 and transient absorption,16 leading to the observation of multiple dark states, including photoisomers and triplet levels. Naturally in the all-trans ground state, cyanine π-electrons are well-modeled by a simple particle in a box or 1-D metal model.17 For Cy5, the cis-photoisomer absorption is red-shifted by ~45 nm, yielding a transient absorption at 690 nm with a lifetime of 150 μs.16 The relatively long cis-Cy5 lifetime enables significant buildup of this dark state under even low steady-state excitation. Excitation of cis-Cy5 in its absorption band, however, photoreverts the Cy5 to the trans state and recovers fluorescence with enhancements of up to 50%.18 Although Cy5 excitation also shows triplet state dynamics for both trans- and cis-isomers, the T1 levels have much shorter lifetimes of ~35 μs and 6 μs for the trans and cis isomers, respectively.16 Thus, our approach of fluorescence excitation combined with secondary laser co-illumination that is on-resonance with the transient absorption dynamically modulates the Cy5 fluorescence intensity via optical depopulation of primarily the photoisomerized dark state.
Using Synchronously Amplified Fluorescence Intensity Recovery (SAFIRe)19 we can modulate the long-wavelength secondary laser to modulate the cis vs. trans-Cy5 ground state populations, thereby modulating collected fluorescence and enhancing signal to noise. Such fluorescence recovery from high background was demonstrated for the commercially available parent Cy5 in solution18 and buried within tissue mimicking phantoms.20 To understand and improve detection sensitivity, we synthesized cyanine structural variants and utilized optical modulation methods as a screening tool to assess how variations in cyanine structure affect modulatability and, therefore, signal recovery in high background fluorescence experiments. Our specific emphasis is on understanding the bright state and dark state photophysics to enhance and tailor optically recovered ground state populations.
Experimental
Sulfo-Cy5, Sulfo-Cy7, and Cy5.5 all in NHS ester form (Lumiprobe), and Merocyanine 540 (Sigma-Aldrich) were used as received. Cy5 derivatives were synthesized by reacting two equivalents of individual heterocyclic salts with either the commercially obtained malondialdehyde bisphenylimine salt or using our halogenated reagent (Figure S1, see supplementary information for synthetic details) in the presence of acetic anhydride with sodium acetate. This general cyanine structure is shown in Figure 1. Cyanine solutions were prepared by dissolution in DMSO followed by dilution to experimental concentrations. All dual laser experiments were performed on an inverted microscope (Olympus IX71) using a 60×, 1.2 NA water immersion objective. All solution data were acquired by focusing ~30 microns into solution. Signal was collected in a confocal arrangement with a 100-μm multimode fiber serving as the pinhole and directing the emission to a photon-counting avalanche photodiode (APD, Perkin–Elmer). Intensity trajectories were recorded using a Becker-Hickl SPC-630 single photon counting board to time-stamp individual photon arrival times. Primary excitation was performed with a 514.5-nm Argon-ion laser (Coherent Innova 90C), 594 or 633 nm He-Ne lasers (JDS Uniphase and Melles-Griot), or a 730 nm diode (Thorlabs), and secondary co-illumination was done with a Ti:sapphire laser (Coherent Mira 900) with wavelengths ranging from 710 to 830 nm, operating in continuous wave mode. Primary and secondary excitation beams were spatially overlapped in the microscope after combining on a dichroic mirror. Appropriate band pass filters blocked the primary and secondary excitation wavelengths to only let the desired fluorescence signals reach the detector. When necessary to distinguish timescales comparable to those of diffusion, a defocusing lens was used to increase the laser spot size, shifting diffusion-based fluctuations to much longer timescales. An electro-optic modulator (Conoptics) was used to modulate the amplitude of the long-wavelength secondary laser beam, with recorded time traces being binned at least 2.2 times faster than the highest modulation frequency. Data were processed by Fourier transformation of each time correlated single photon counting fluorescence intensity time trace. The corresponding FFT peak amplitude at each modulation frequency was divided by the DC peak amplitude to calculate modulation depth.
Figure 1.
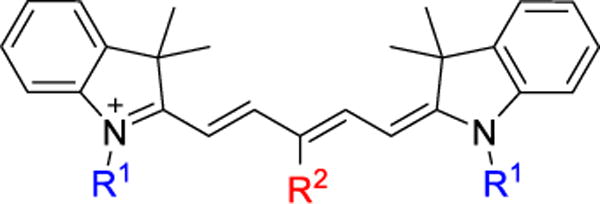
General structure of Cy5 analogs. Modifications were incorporated as alkyl substitutions at the heterocyclic nitrogens (R1) and halogen incorporation on the meso position of the polymethine bridge (R2).
DFT calculations were performed using Gaussian 0921 with Becke’s three-parameter hybrid density function in combination with the Lee-Yang-Parr correlation functional (B3LYP) and the effective core potential (ECP) basis set Los Alamos ECP plus double zeta (LanL2DZ). Ground state geometries of Cy5 and its synthesized derivatives were optimized and the energy levels calculated, and then time-dependent density functional theory (TDDFT) was used to calculate transition energies for the geometry optimized ground states. The polarizable continuum model (PCM) was applied for ground state geometry optimizations and excited state absorption spectra calculations to determine the solvation effects of DMSO, as applied in experimental conditions.22–26
Results
Cyanine photophysics and dark state recovery kinetics were investigated under single and dual laser excitation. Compounds (dissolved in DMSO) were co-excited with a 594 nm primary and 710 nm square wave-modulated secondary laser for Synchronously Amplified Fluorescence Intensity Recovery (SAFIRe).19 This dual laser method uses the primary excitation to produce fluorescence and populate the dark state, while the much lower energy secondary laser depopulates the non-fluorescent dark state, shifting population to the bright state faster than the dark state would naturally decay. By modulating this secondary laser, we directly modulate Cy5 fluorescence, shifting its signal to a unique, very-low-background detection frequency. At sufficiently high modulation frequencies, the system has insufficient time to establish steady-state populations, meaning that measurements of modulation depth, m, vs. modulation frequency, ν, report on the time to establish dark and bright manifold steady state populations, and is given by:27–29
| (1) |
When a specific timescale exists, fitting frequency-dependent modulation depth curves to equation 1 enables extraction of the characteristic time, τc. When a good fit to a single characteristic time, τc, is not possible, one can specify the time at which the modulation depth drops by half to extract a characteristic time.18 Changing with primary laser intensity, the inverse of τc is the characteristic frequency, or rate kc = kon + kooff, where (σabs is the absorption cross section, Ipri is the primary laser intensity, and ΦD is the dark state quantum yield) and kooff is the natural dark state decay rate constant.30 The parameters kon and kooff (or their inverses τon and τooff) can be used to determine the rates at which molecules enter and exit the dark state, allowing one to determine fluorescence enhancement,31
| (2) |
in which τoff is the dark state lifetime with the secondary laser on. The data in Figure 2A demonstrate this process, showing a diminishing modulation depth with increasing modulation frequency, and the fitted characteristic frequencies at varying primary power are used to extract on and off times (Figure 2B).
Figure 2.
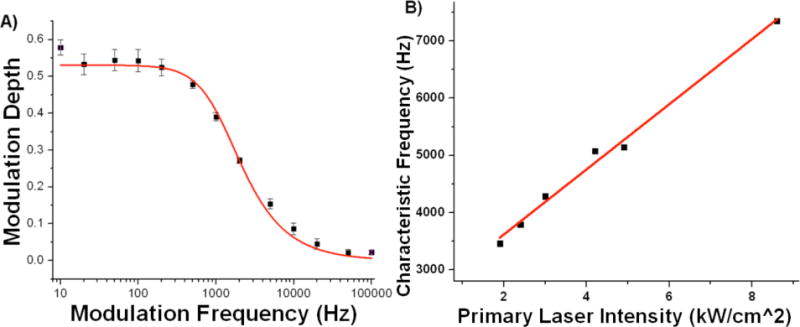
A) Cy5 derivative MHI97 (See Table 1) modulation depth as a function of modulation frequency and fit to equation 1. Data were collected for 1 second at each modulation frequency. B) MHI97 characteristic frequency as a function of primary intensity and the line to which it fits.
At low modulation depths (<15%), extracting accurate photophysical parameters can be difficult from modulation depth vs. frequency curves. Instead, by achieving steady state dark state population through extended primary illumination,32 thermal recovery from the dark state occurs upon rapidly turning the primary laser off. By varying the primary illumination off period, Toff, the fraction of molecules in the bright state, nB/ntot will recover to a degree that depends on the natural off time, τoffo according to equation 3:
| (3) |
By fitting the experimentally determined fraction of molecules in the bright state as a function of the laser off period we can determine the natural dark state decay rate. This process is illustrated in Figure 3 where Figure 3A shows a large intensity drop off with 300 μs between laser pulses, Figure 3B demonstrates less drop off with 100 μs between pulses, in Figure 3C there is even less decay with 50 μs between pulses, and finally in Figure 3D the intensity drop is the least significant. This shows that the longer the laser off period, the greater the recovery from the dark state, which yields a higher initial fluorescence intensity when the primary laser is turned back on.
Figure 3.
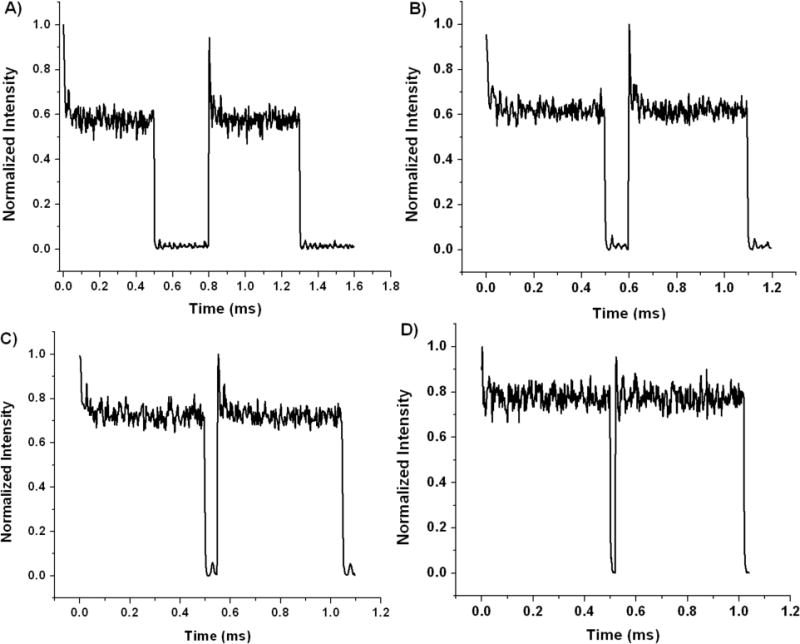
Fluorescence traces showing ground state recovery for MHI106 using different primary laser off periods, Toff. Using an electro-optic modulator, the primary laser is turned on for 500 μs and off for A) 300 μs, B) 100 μs, C) 50 μs, and D) 20 μs. Laser spot size was expanded 300 μm2, to increase diffusion timescales to ~10ms, such that it does not interfere with the modulation timescales. For each panel, data were collected for 20 seconds and modulation cycle averages are taken and ratios of initial to final intensities within the average primary illumination period, Ton, as a function of Toff are fit to equation 3.
Even with the seemingly minor structural changes to Cy5 indicated in Figure 1, modulation depth and photophysical parameters are strongly affected by slight substituent changes. Using modulation frequency dependence and bright state recovery methods (Figures 2 & 3), photophysical on and off times were extracted (Table 1). Overall, with the Cy5 derivatives, modulation depth increases with alkyl chain length and decreases with increasing halogen mass.
Table 1.
Summary of experimental results including enhancement and on and off times for Cy5 derivatives. R1 and R2 positions are as shown in Figure 1. For τon and τoff measurements, the primary and secondary laser intensities were 350 and 640 W/cm2, respectively.
| Dye | R1 | R2 | Mod depth | τoffo (μs) | τoff (μs) | τon(μs) | ϕD |
|---|---|---|---|---|---|---|---|
| Cy5 | 50% (±4) | 73(±7) | 20(±3) | 88(±9) | .011(±.002) | ||
| MHI84 | CH3 | H | 40% (±2) | 87(±4) | 25(±2) | 130(±8) | .0077(±.001) |
| LO4 | CH2CH3 | H | 50% (±4) | 130(±3) | 40(±3) | 140(±7) | .0072(±.001) |
| MHI97 | (CH2)3CH3 | H | 58% (±2) | 210(±8) | 40(±5) | 250(±11) | .004(±5*10−4) |
| MHI106 | CH3 | Cl | 28% (±3) | 60(±8) | 45(±7) | 10(±3) | .10(±.007) |
| E27 | CH3 | Br | 13% (±1) | 25(±3) | 20(±3) | 7(±2) | .14(±.01) |
| E63 | CH2CH2OAc | Cl | 20% (±1) | 50(±5) | 37(±4) | 25(±3) | .040(±.003) |
| E65 | CH2CH2OAc | Br | 7% (±2) | 12(±3) | 10(±2) | 7(±2) | .14(±.009) |
| Cy5.5 | 12% (±2) | 140(±11) | 118(±10) | 100(±3) | .012(±.002) | ||
| Cy7 | 15% (±3) | 16(±3) | 13(±3) | 6(±2) | .21(±.01) | ||
| Merocyanine 540 | 40% (±3) | 216(±15) | 123(±10) | 110(±11) | .016(±.001) |
The ~100 μs natural dark state lifetimes suggest that a ground state process such as photoisomerization leads to the modulatable metastable dark state, as suggested previously for commercially available Cy5.18 To probe the ground state energy differences between modulatable dark and bright states, thermally equilibrated samples were either kept in the dark or pre-illuminated with the secondary laser, followed by primary-only illumination to determine initial ground state population with and without prior secondary illumination (Figure 4). The relative initial fluorescence intensities with and without secondary pre-illumination then report on the energy difference between trans and photoreversible cis isomers involved in modulation. Assuming the dark state is completely depleted upon secondary laser pre-illumination, a Boltzmann distribution enables experimental trans-cis energy differences for all cyanine derivatives to be determined (Table 2). The relatively low energy difference between bright and dark states further supports the idea that a photoisomer, and not the much higher energy triplet level, is the experimentally modulatable dark state.
Figure 4.
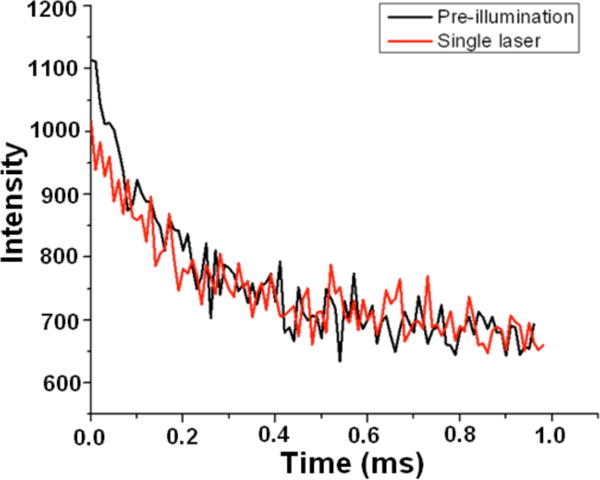
Optical recovery of thermally populated dark states. Primary-only induced fluorescence from MHI97 with (black) and without (red) secondary laser pre-illumination. The increased fluorescence with secondary-only pre-illumination relative to no secondary pre-illumination generates higher initial fluorescence intensity by optically recovering molecules from the thermally populated dark state.
Table 2.
Summary of ground state DFT computational results: Cis23-Trans and Cis12-Trans refer to the energy differences between the ground states of the different cis-photoisomers and the all-trans ground states. (Note: Due to short lifetimes, E65 and Cy7 exhibited insufficient dark state buildup to determine experimental cis-trans values.) Ea is the activation energy obtained by Arrhenius calculations using the experimental kooff and setting A = 1012 s−1.33
| Cis23-Trans (eV) | Cis12-Trans (eV) | Exp Cis-Trans (eV) | Ea (eV) | |
|---|---|---|---|---|
| Cy5 | .1345 | .3091 | .0757(±.01) | .465(±.04) |
| MHI84 | .1366 | .3087 | .0592(±.008) | .470(±.03) |
| LO4 | .1366 | .3157 | .0505(±.007) | .480(±.01) |
| MHI97 | .09528 | .2758 | .0419(±.007) | .492(±.04) |
| MHI106 | .1608 | .7520 | .0905(±.02) | .460(±.05) |
| E27 | .1621 | .8439 | .0732(±.008) | .438(±.05) |
| E63 | .1593 | .9554 | .0762(±.009) | .455(±.05) |
| E65 | .1488 | 1.212 | .419(±.06) | |
| Cy5.5 | .1349 | .3033 | .110(±.012) | .482(±.04) |
| Cy7 | .2086 | .7661 | .426(±.07) | |
| Merocyanine 540 | .2985 | .1628 | .0540(±.008) | .493(±.04) |
Although the timescales and pre-illumination experiments suggest that photoisomerization and back isomerization give rise to optical modulation, photoisomerization can potentially occur about any of the bonds along the cyanine polymethine chain (Figure 5A–D for Cy5, Cy5.5, Cy7 and merocyanine 540, respectively). To gain further insight into the isomerization mechanism, density functional calculations were employed to compare ground and excited state energies of the different possible isomers. The B3LYP functional and LanL2DZ basis set were used, taking into account DMSO solvation with the PCM, analogous to previous Cy5 calculations.22 Geometry optimizations and ground state energy calculations were performed on the all-trans and each of the various cis-photoisomers, and TDDFT was used to calculate vertical excitation energies (see SI). Experimental and calculated cis-trans energy differences are compared in Table 1. Arrhenius-extracted activation energies for cis to trans regeneration of the bright manifold are also given as calculated from the experimentally derived rate constants for dark state decay, koffo.
Figure 5.
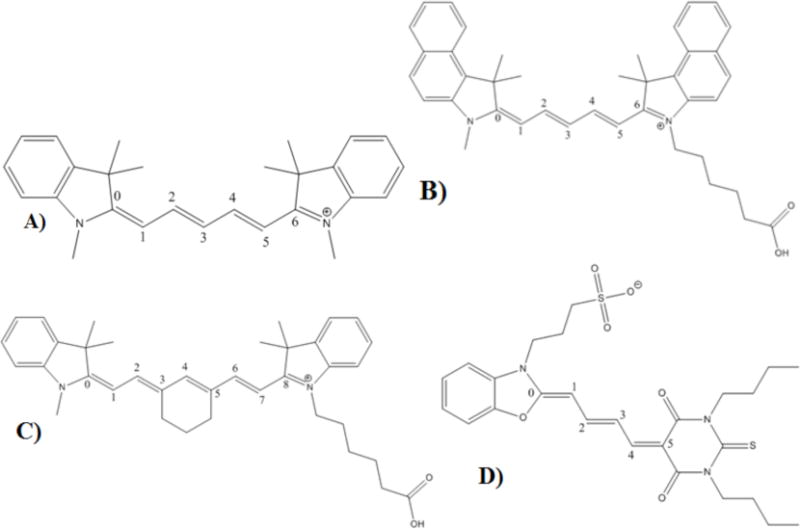
All-trans isomers of A) Cy5, B) Cy5.5, C) Cy7, D) Merocyanine 540 numbered to show isomerization possibilities.
Discussion
The fluorescence enhancement of Cy5 is due to optical depopulation of a dark state, and derivatives’ modulation depth generally increases with increasing alkyl chain length and decreases with increasing halogen atomic mass at the central carbon (Table 2). Despite the added bulk to the polymethine chain, halogens seem to shorten the on and off times, while long alkyl chains appear to slow the forward and back-isomerization processes.
Comparing the DFT and experimental energy differences between cis and trans, the modulatable dark states of all cyanines other than Merocyanine 540 appear to result from isomerization around the 2–3 carbon-carbon bond. For Merocyanine, isomerization appears to occur about the 1–2 bond. The 2–3 cis isomer (and 1–2 cis for Merocyanine 540) and trans isomer energy difference is in fair agreement (~factor of 2) with the experimentally obtained values, while the 1–2 isomer (and 2–3 for Merocyanine) is higher in energy, and much higher in the halogenated compounds. For calculated energies of the other photoisomers, please see the SI.
The ratio of off to on times is the most important criterion governing modulation depth. A fluorescent dye with high modulation depth quickly feeds into a long-lived dark state and is efficiently depopulated by a secondary laser. Experimental on and off times are shorter with halogens, and it appears that although the quantum yield of trans to 2–3 cis photoisomerization increases, the 2–3 cis ground state stability decreases with increased halogen size, or possibly that the triplet level may be longer lived and becomes the more populated dark state level in these molecules. Although these factors may combine to decrease modulation depth, the low energy difference between ground and modulatable dark state measured by secondary laser pre-illumination argues against the involvement of a triplet level, as this would presumably be significantly higher in energy than the ground S0 level for either isomer. This argues in favor of steric effects that make dark state population increasingly difficult with increased halogen mass.
As mentioned above, the photophysical rates into and out of the dark state control the steady state dark state population. The larger the achievable steady-state dark state population, the larger the possible enhancement upon complete depopulation through secondary illumination. MHI97, for example, exhibits the highest enhancement and is the slowest to photoisomerize, but because the dark state is long-lived, a high modulation depth is recorded. On the other hand, halogenated E27 and E65 rapidly photoisomerize, but also back-photoisomerize very quickly, leading to low dark state buildup and low overall modulation depths. All of the Cy5 variants have similar secondary-induced τoff values, minimizing its impact with these derivatives. Thus, within this family of Cy5-like compounds, structural modifications that increase the ratio of τooff to τon for a given primary excitation intensity have the greatest effect on overall enhancement. Through such optical control of emission with tailorable on and off times, applications in superresolution imaging may also become possible at faster timescales,13, 14, 34–37 possibly coupled with the improved sensitivity afforded by modulation-based SAFIRe.27
Interestingly, Cy5.5 has photophysical on and off times similar to the best Cy5 derivatives, but shows much lower enhancement. While R1 and R2 substitutions showed the same trend as with Cy5, the lower modulation depths precluded full photophysical characterization, except for commercial Cy5.5. In contrast to Cy5, the low enhancement appears to arise from inefficient dark state depletion – an interpretation corroborated by FCS data which show a higher dark state population for Cy5.5 than for Cy5 when both primary and secondary lasers are on (Figure S2).
Considering the range of cyanines studied, increased conjugation length appears negatively correlated with dark state buildup. Merocyanine 540, for example, exhibits a relatively high modulation depth due to its long-lived dark state and fast forward photoisomerization rate, but its dark state is less efficiently depleted by the secondary laser, causing the modulation depth to not be as high as Cy5 and some of its related analogues. Cy7 exhibits a short-lived dark state, similar to the brominated Cy5 derivatives, and therefore does not offer as much fluorescence recovery as the Cy5 compounds or Merocyanine 540. When Cy7 isomerization is chemically prevented around the central double bond as in Fig. 5, however, isomerization is forced to occur around other bonds, likely limiting accessible photoisomers to increase photoreversible dark state populations and modulation depth.
The combination of ground vs. dark state thermal populations, natural dark state lifetimes, and density functional theory calculations enable improved understandings of cis and trans photoisomer energy differences. DFT was used to calculate optimized ground state energies for the various isomers and vertical transition energies. Relative calculated energy differences are compared with experimental values to lend insight into which isomers are likely to give rise to the modulatable dark states for each cyanine derivative. It appears that the halogenated compounds have a larger energy difference between cis and trans isomers, a fact that could explain lower the activation barrier and observed higher reverse photoisomerization rates. On the contrary, DFT suggests that compounds with longer alkyl chains have smaller energy differences separating isomers, corroborating the observed larger activation energies and longer τooff values. Cy5.5 does have a higher than expected experimental cis-trans energy difference, but thermal reversion is also low, suggesting a higher barrier to ground state photoisomerization. For Cy5, its derivatives, and Cy5.5, the experimental values are closer in magnitude to the DFT energy differences between the Cis23 rather than the Cis12 isomer, suggesting that Cis23 is the photoisomer responsible for the dark state. In Merocyanine 540, however, the Cis12, rather than the Cis23, appears to be responsible for the photo-induced dark state, based on similarity of calculated isomer energy differences with experimental values. Time-dependent DFT excited state calculations further corroborate this interpretation as only the calculated Cis23 (and Cis12 for Merocyanine) vertical transition energies are slightly and appropriately38 red-shifted from that of the all-trans isomer absorption. This data is included in the supporting information.
Conclusion
The enhancement and optical modulation of Cy5 fluorescence using red-shifted secondary co-illumination to depopulate photoaccessible dark states18 can be modified with conjugation length, and alkyl and halogen substitutions. A pentamethine chain is optimal for fluorescence enhancement; Cy3 has no enhancement and Cy7 has less than Cy5 and its related compounds, presumably in both cases related to shortened dark state residence time. Adding an extra phenyl ring to the indole structure also decreases modulation depth, as in the case of Cy5.5. Our experiments suggest this is due to inefficient dark state depletion. Longer alkyl chains on the nitrogen atoms in Cy5 derivatives increase modulation depth because they lengthen the dark state lifetime, while halogenation of the polymethine chain causes a reduction in modulation depth because it decreases the dark state lifetime.
Dark state depopulation relating to modulatable fluorescence is observable in fluorescence correlation spectroscopy (Fig. S2), but is convolved with the diffusion time giving rise to fluctuations. Thus, bulk experiments employing modulation depth vs. modulation frequency in larger spots have enabled us to probe the dark states giving rise to the enhanced sensitivity possible through optically modulated fluorescence. Common to many cyanines, modulatability through long-wavelength dark state illumination enables photophysics to be elucidated and correlated with structural differences.28 Combining these studies with DFT calculations generates mechanistic insight into the nature of the dark states giving rise to enhancement. Through further studies, such understandings are likely to ultimately improve fluorescence modulation depth through molecular design, facilitating the tailoring of modulation timescales to study specific molecular or intermolecular interactions. While substituents affect modulation depth, it is really the ratio between dark and bright state residence times that determines overall modulation depth. The magnitudes of the rates into and out of these states determine the characteristic frequencies and it is these rates that are individually altered through substituent effects – most likely arising from steric interactions destabilizing certain isomeric forms. Correlating DFT calculations and experimental rates and thermal populations lends further insight into the nature of the optically modulatable dark state for each cyanine dye.
Supplementary Material
Acknowledgments
RMD gratefully acknowledges financial support from NIH R01AI107116. We thank Rebecca Gieseking, Khanh Do, and Sean Ryno for theoretical discussions and assistance with density functional calculations.
Footnotes
Supporting Information. Cyanine synthesis, additional ground state DFT calculations, excited state DFT calculations, SAFIRe experimental data for Cy5.5 compounds, FCS data for cyanine compounds. “This material is available free of charge via the Internet at http://pubs.acs.org.”
References
- 1.Weissleder R. A Clearer Vision for in Vivo Imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 2.Frangioni JV. In Vivo near-Infrared Fluorescence Imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Weissleder R, Tung C-H, Mahmood U, Bogdanov A. In Vivo Imaging of Tumors with Protease-Activated near-Infrared Fluorescent Probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder R. Scaling Down Imaging: Molecular Mapping of Cancer in Mice. Nat Rev Cancer. 2002;2:11–18. doi: 10.1038/nrc701. [DOI] [PubMed] [Google Scholar]
- 5.Escobedo JO, Rusin O, Lim S, Strongin RM. Nir Dyes for Bioimaging Applications. Curr Opin Chem Biol. 2010;14:64–70. doi: 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umezawa K, Nakamura Y, Makino H, Citterio D, Suzuki K. Bright, Color-Tunable Fluorescent Dyes in the Visible–near-Infrared Region. J Am Chem Soc. 2008;130:1550–1551. doi: 10.1021/ja077756j. [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Zhang E, Su Y, Cheng T, Shi C. A Review of Nir Dyes in Cancer Targeting and Imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey GT, Vaughan JC, Chen KH, Bates M, Zhuang X. Evaluation of Fluorophores for Optimal Performance in Localization-Based Super-Resolution Imaging. Nat Methods. 2011;8:1027–1036. doi: 10.1038/nmeth.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitus M, Ranjit S. Cyanine Dyes in Biophysical Research: The Photophysics of Polymethine Fluorescent Dyes in Biomolecular Environments. Q Rev Biophys. 2011;44:123–151. doi: 10.1017/S0033583510000247. [DOI] [PubMed] [Google Scholar]
- 10.Chapman G, Henary M, Patonay G. The Effect of Varying Short-Chain Alkyl Substitution on the Molar Absorptivity and Quantum Yield of Cyanine Dyes. Anal Chem Insights. 2011;6:29. doi: 10.4137/ACI.S6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanjunda R, Owens EA, Mickelson L, Alyabyev S, Kilpatrick N, Wang S, Henary M, Wilson WD. Halogenated Pentamethine Cyanine Dyes Exhibiting High Fidelity for G-Quadruplex DNA. Bioorg Med Chem. 2012;20:7002–7011. doi: 10.1016/j.bmc.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens EA, Hyun H, Kim SH, Lee JH, Park G, Ashitate Y, Choi J, Hong GH, Alyabyev S, Lee SJ, et al. Highly Charged Cyanine Fluorophores for Trafficking Scaffold Degradation. Biomed Mater. 2013;8:014109. doi: 10.1088/1748-6041/8/1/014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates M, Blosser TR, Zhuang X. Short-Range Spectroscopic Ruler Based on a Single-Molecule Optical Switch. Phys Rev Lett. 2005;94:108101. doi: 10.1103/PhysRevLett.94.108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilemann M, Margeat E, Kasper R, Sauer M, Tinnefeld P. Carbocyanine Dyes as Efficient Reversible Single-Molecule Optical Switch. J Am Chem Soc. 2005;127:3801–3806. doi: 10.1021/ja044686x. [DOI] [PubMed] [Google Scholar]
- 15.Widengren J, Schwille P. Characterization of Photoinduced Isomerization and Back-Isomerization of the Cyanine Dye Cy5 by Fluorescence Correlation Spectroscopy. J Phys Chem A. 2000;104:6416–6428. [Google Scholar]
- 16.Huang Z, Ji D, Wang S, Xia A, Koberling F, Patting M, Erdmann R. Spectral Identification of Specific Photophysics of Cy5 by Means of Ensemble and Single Molecule Measurements. J Phys Chem A. 2006;110:45–50. doi: 10.1021/jp0562936. [DOI] [PubMed] [Google Scholar]
- 17.Baker TA, Gellene GI. A Hybrid Ab Initio/Free Electron Computational Model for Conjugated Dye Molecules: Simple Cyanines and Oxonols. J Comput Chem. 2000;21:943–953. [Google Scholar]
- 18.Fan C, Hsiang J-C, Dickson RM. Optical Modulation and Selective Recovery of Cy5 Fluorescence. ChemPhysChem. 2012;13:1023–1029. doi: 10.1002/cphc.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards CI, Hsiang J-C, Dickson RM. Synchronously Amplified Fluorescence Image Recovery (SAFIRe) J Phys Chem B. 2009;114:660–665. doi: 10.1021/jp909167j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar S, Fan C, Hsiang J-C, Dickson RM. Modulated Fluorophore Signal Recovery Buried within Tissue Mimicking Phantoms. J Phys Chem A. 2013;117:9501–9509. doi: 10.1021/jp312071n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al. Gaussian 09, Revision B.01. Gaussian, Inc; Wallingford, CT: 2009. [Google Scholar]
- 22.Bamgbelu A, Wang J, Leszczynski J. Tddft Study of the Optical Properties of Cy5 and Its Derivatives. J Phys Chem A. 2010;114:3551–3555. doi: 10.1021/jp908485z. [DOI] [PubMed] [Google Scholar]
- 23.Bertolino CA, Ferrari AM, Barolo C, Viscardi G, Caputo G, Coluccia S. Solvent Effect on Indocyanine Dyes: A Computational Approach. Chem Phys. 2006;330:52–59. [Google Scholar]
- 24.Champagne B, Guillaume M, Zutterman F. Tddft Investigation of the Optical Properties of Cyanine Dyes. Chem Phys Lett. 2006;425:105–109. [Google Scholar]
- 25.Baraldi I, Carnevali A, Caselli M, Momicchioli F, Ponterini G, Berthier G. Theoretical and Photophysical Study of Photoisomerism of Cyanine Dyes: Bisphenylaminopentamethine Cyanine (Bppc) J Mol Struct: THEOCHEM. 1995;330:403–410. [Google Scholar]
- 26.Momicchioli F, Baraldi I, Berthier G. Theoretical Study of Trans-Cis Photoisomerism in Polymethine Cyanines. Chem Phys. 1988;123:103–112. [Google Scholar]
- 27.Hsiang J-C, Jablonski AE, Dickson RM. Optically Modulated Fluorescence Bioimaging: Visualizing Obscured Fluorophores in High Background. Acc Chem Res. 2014;47:1545–1554. doi: 10.1021/ar400325y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatzogiannis E, Zhu X, Kao Y-T, Min W. Observation of Frequency-Domain Fluorescence Anomalous Phase Advance Due to Dark-State Hysteresis. J Phys Chem Lett. 2011;2:461–466. [Google Scholar]
- 29.Zhu X, Min W. Frequency-Domain Phase Fluorometry in the Presence of Dark States: A Numerical Study. Chem Phys Lett. 2011;516:40–44. [Google Scholar]
- 30.Jablonski AE, Vegh RB, Hsiang J-C, Bommarius B, Chen Y-C, Solntsev KM, Bommarius AS, Tolbert LM, Dickson RM. Optically Modulatable Blue Fluorescent Proteins. J Am Chem Soc. 2013;135:16410–16417. doi: 10.1021/ja405459b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petty JT, Fan C, Story SP, Sengupta B, Sartin M, Hsiang J-C, Perry JW, Dickson RM. Optically Enhanced, near-Ir, Silver Cluster Emission Altered by Single Base Changes in the DNA Template. J Phys Chem B. 2011;115:7996–8003. doi: 10.1021/jp202024x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widengren J. Fluorescence-Based Transient State Monitoring for Biomolecular Spectroscopy and Imaging. J R Soc, Interface. 2010 doi: 10.1098/rsif.2010.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chibisov AK, Zakharova GV, Gorner H. Effects of Substituents in the Polymethine Chain on the Photoprocesses in Indodicarbocyanine Dyes. J Chem Soc, Faraday Trans. 1996;92:4917–4925. [Google Scholar]
- 34.van der Velde JHM, Ploetz E, Hiermaier M, Oelerich J, de Vries JW, Roelfes G, Cordes T. Mechanism of Intramolecular Photostabilization in Self-Healing Cyanine Fluorophores. ChemPhysChem. 2013;14:4084–4093. doi: 10.1002/cphc.201300785. [DOI] [PubMed] [Google Scholar]
- 35.Bates M, Huang B, Dempsey GT, Zhuang X. Multicolor Super-Resolution Imaging with Photo-Switchable Fluorescent Probes. Science. 2007;317:1749–1753. doi: 10.1126/science.1146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang B, Wang W, Bates M, Zhuang X. Three-Dimensional Super-Resolution Imaging by Stochastic Optical Reconstruction Microscopy. Science. 2008;319:810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flors C. Photoswitching of Monomeric and Dimeric DNA-Intercalating Cyanine Dyes for Super-Resolution Microscopy Applications. Photochem Photobiol Sci. 2010;9:643–648. doi: 10.1039/b9pp00119k. [DOI] [PubMed] [Google Scholar]
- 38.Alarcon E, Aspee A, Gonzalez-Bejar M, Edwards AM, Lissi E, Scaiano JC. Photobehavior of Merocyanine 540 Bound to Human Serum Albumin. Photochem Photobiol Sci. 2010;9:861–869. doi: 10.1039/c0pp00079e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


