Correction of the article
In the results section of the abstract the two sentences
For 79 % of the radiopharmaceuticals, the new calculations gave a lower effective dose per unit administered activity than earlier estimated.” Should be “For 63 % of the radiopharmaceuticals, the new calculations gave a lower effective dose per unit administered activity than earlier estimated.”
“As a mean for all radiopharmaceuticals, the effective dose was 25 % lower.” Should be“As a mean for all radiopharmaceuticals, the effective dose was 11 % lower.”
In the results section in the main article four sentences should be changed
“The calculated values are lower than earlier presented values for 79 % of the radiopharmaceuticals.” Should be “The calculated values are lower than earlier presented values for 63 % of the radiopharmaceuticals.”
“As a mean for all 338 radiopharmaceuticals, the values are 25 % lower.” Should be “As a mean for all 338 radiopharmaceuticals, the values are 11 % lower.”
“The effective doses are larger for females than for males in 62 % of all 338 radiopharmaceuticals.” Should be “The effective doses are larger for females than for males in 99 % of all 338 radiopharmaceuticals.”
“Only for 125I Iodine Hippuran with unilateral renal blockage and an abnormal kidney function there is a difference of more than 100 % between the new and the old E/A0 values.” Should be “Only for 99mTc Apcitide and 99mTc labelled colloids, small colloids and normal liver condition there is a difference of more than 100 % between the new and the old E/A0 values.”
In the Discussion section in the main article eight sentences should be changed
“For radiopharmaceuticals with a significant uptake in adipose tissue as for 14C- and 3H-labelled neutral fat and free fatty acids or in the male gonads, the effective dose will be higher for males than for females.” Should be “For radiopharmaceuticals with a significant uptake in adipose tissue as for 14C- and 3H-labelled neutral fat and free fatty acids, the effective dose will be higher for males than for females.”
“For 18 F-labelled substances, E/A0 varies between 0.013 and 0.019 mSv/MBq (less than a factor of 1.5).” Should be “For 18 F-labelled substances, E/A0 varies between 0.013 and 0.021 mSv/MBq (a factor of 1.6).”
“For 11C-substances, E/A0 varies between 0.0025 and 0.0055 mSv/MBq (around a factor of 2.2).” Should be “For 11C-substances, E/A0 varies between 0.0011 and 0.0087 mSv/MBq (around a factor of 8.0). “
“Also for 99mTc-labelled substances, the range of E/A0 values is limited to 0.0017 to 0.016 mSv/MBq (a factor of 9.6).” Should be “Also for 99mTc-labelled substances, the range of E/A0 values is limited to 0.0022 to 0.020 mSv/MBq (a factor of 8.8).”
“For all the 18 F substances, there is a reduction in effective dose estimation by 29 % in average.” Should be “For all the 18 F substances, there is a reduction in effective dose estimation by 26 % in average.”
“For 11C-substances, two radiopharmaceuticals show a higher effective dose and 11 have a lower effective dose than previously published values.” Should be “For 11C-substances, nine radiopharmaceuticals show a higher effective dose and four have a lower effective dose than previously published values.”
“In 50 of the 62 99mTc-substances, the effective dose estimations give lower values than previous estimations.” Should be “In 38 of the 62 99mTc-substances, the effective dose estimations give lower values than previous estimations.”
“Using the new estimations, the collective effective dose is estimated at 292 manSv, i.e. 13 % lower value than earlier estimated.” Should be “Using the new estimations, the collective effective dose is estimated at 295 manSv, i.e. 12 % lower value than earlier estimated.”
In the Conclusions there are two sentences that should be changed
“For 268 radiopharmaceuticals out of 338, the new calculations show lower effective dose values than previous estimates.” Should be “For 212 radiopharmaceuticals out of 338, the new calculations show lower effective dose values than previous estimates.”
“For 68 radiopharmaceuticals, the new calculations results in an increased value of the estimated effective dose.” Should be “For 120 radiopharmaceuticals, the new calculations results in an increased value of the estimated effective dose.”
Figure 1 should be changed to (only the figure not the text)
Fig. 1.
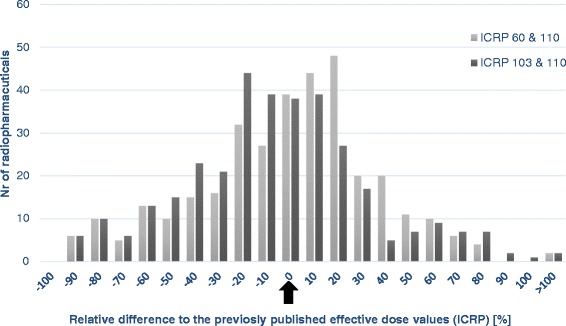
A histogram of the relative difference between different dose values. The relative difference between the old published effective dose per unit administered activity and the effective dose values calculated with the new phantom (ICRP 110) and with (1) the new (ICRP 103) and (2) the previous (ICRP 60) tissue weighting factors. The arrow indicates identical results between old and new estimations
Almost all numbers have been changed in Table 1 and a new corrected Table 1 is presented below (table text to Table 1 does not need to be changed).
Table 1.
Effective dose from the 55 radiopharmaceuticals in ICRP publication 106, determined using three different methods
| Radiopharmaceuticals | (E/A0)1 [mSv/MBq] | (E/A0)2 [mSv/MBq] | ((E/A0)2-(E/A0)1)/(E/A0)1 [%] | (E/A0)3 [mSv/MBq] | ((E/A0)3-(E/A0)1)/(E/A0)1 [%] | (E/A0)3 male [mSv/MBq] | (E/A0)3 female [mSv/MBq] |
|---|---|---|---|---|---|---|---|
| Phantom | MIRD | ICRP/ICRU | ICRP/ICRU | ICRP/ICRU | ICRP/ICRU | ||
| wT | ICRP 60 | ICRP 60 | ICRP 103 | ICRP 103 | ICRP 103 | ||
| 3H Tritium labelled neutral fat & free fatty acids | 2.2E-01 | 9.81E-02 | −55 | 1.80E-01 | −18 | 2.44E-01 | 1.16E-01 |
| 11C Carbon acetate | 3.5E-03 | 4.26E-03 | 22 | 3.65E-03 | 4 | 3.33E-03 | 3.97E-03 |
| 11C Carbon amino acids | 5.6E-03 | 5.76E-03 | 3 | 5.26E-03 | −6 | 4.91E-03 | 5.61E-03 |
| 11C Carbon brain receptor substances | 4.3E-03 | 3.70E-03 | −14 | 3.62E-03 | −16 | 3.23E-03 | 4.00E-03 |
| 11C Carbon methionine | 8.4E-03 | 5.88E-03 | −30 | 5.11E-03 | −39 | 4.50E-03 | 5.72E-03 |
| 11C Carbon (2-11C)thymidine | 2.7E-03 | 3.01E-03 | 11 | 3.04E-03 | 13 | 2.77E-03 | 3.32E-03 |
| 11C Carbon, realistic maximum | 1.1E-02 | 6.04E-03 | −45 | 5.08E-03 | −54 | 4.36E-03 | 5.80E-03 |
| 14C Carbon labelled neutral fat & free fatty acids | 2.1E + 00 | 1.88E + 00 | −10 | 2.65E + 00 | 26 | 3.14E + 00 | 2.15E + 00 |
| 14C Carbon labelled urea, normal case, orally administered | 3.1E-02 | 2.67E-02 | −14 | 2.72E-02 | −12 | 2.46E-02 | 2.98E-02 |
| 15O Oxygen water | 1.1E-03 | 9.63E-04 | −12 | 9.33E-04 | −15 | 8.72E-04 | 9.93E-04 |
| 18 F Fluoride labelled amino acids | 2.3E-02 | 2.27E-02 | −1 | 2.07E-02 | −10 | 1.92E-02 | 2.21E-02 |
| 18 F Fluoride labelled brain receptor substances | 2.8E-02 | 2.01E-02 | −28 | 2.02E-02 | −28 | 1.82E-02 | 2.22E-02 |
| 18 F Fluoride FDG | 1.9E-02 | 1.69E-02 | −11 | 1.71E-02 | −10 | 1.53E-02 | 1.88E-02 |
| 18 F Fluoride L-dopa | 2.5E-02 | 1.75E-02 | −30 | 1.57E-02 | −37 | 1.37E-02 | 1.76E-02 |
| 51Cr Chromium EDTA | 2.0E-03 | 1.65E-03 | −18 | 1.43E-03 | −29 | 1.23E-03 | 1.62E-03 |
| 67Ga Gallium citrate | 1.0E-01 | 9.29E-02 | −7 | 9.08E-02 | −9 | 8.14E-02 | 1.00E-01 |
| 68Ga Gallium labelled EDTA | 4.0E-02 | 2.41E-02 | −40 | 2.14E-02 | −47 | 1.89E-02 | 2.40E-02 |
| 75Se Selenium labelled amino acids | 2.2E + 00 | 2.39E + 00 | 8 | 2.27E + 00 | 3 | 2.14E + 00 | 2.39E + 00 |
| 75Se Selenium labelled bile acid SeHCAT | 6.9E-01 | 3.01E-01 | −56 | 3.48E-01 | −50 | 3.16E-01 | 3.79E-01 |
| 99mTc Technetium apcitide | 4.7E-03 | 1.13E-02 | 140 | 1.19E-02 | 153 | 1.10E-02 | 1.29E-02 |
| 99mTc Technetium labelled small colloids, intratumoural adm. time to removal 18 h | 2.0E-03 | 3.14E-03 | 57 | 3.96E-03 | 98 | 3.49E-03 | 4.43E-03 |
| 99mTc Technetium labelled small colloids, intratumoural adm time to removal 6 h | 1.2E-03 | 1.78E-03 | 48 | 2.24E-03 | 86 | 1.98E-03 | 2.50E-03 |
| 99mTc Technetium EC, normal renal function | 6.3E-03 | 4.63E-03 | −27 | 3.66E-03 | −42 | 3.04E-03 | 4.29E-03 |
| 99mTc Technetium ECD | 7.7E-03 | 5.97E-03 | −23 | 5.64E-03 | −27 | 5.01E-03 | 6.27E-03 |
| 99mTc Technetium furifosmin, exercise | 8.9E-03 | 6.57E-03 | −26 | 6.78E-03 | −24 | 6.16E-03 | 7.40E-03 |
| 99mTc Technetium furifosmin, resting subject | 1.0E-02 | 6.99E-03 | −30 | 7.19E-03 | −28 | 6.53E-03 | 7.85E-03 |
| 99mTc Technetium labelled HIG | 7.0E-03 | 9.83E-03 | 40 | 9.42E-03 | 35 | 8.93E-03 | 9.92E-03 |
| 99mTc Technetium labelled HM-PAO | 9.3E-03 | 1.05E-02 | 13 | 9.78E-03 | 5 | 8.95E-03 | 1.06E-02 |
| Tc-99 m Technetium labelled IDA derivatives, normal hepato-biliary conditions | 1.7E-02 | 9.39E-03 | −45 | 9.73E-03 | −43 | 8.93E-03 | 1.05E-02 |
| 99mTc Technetium labelled MAA | 1.1E-02 | 1.34E-02 | 22 | 1.40E-02 | 27 | 1.27E-02 | 1.53E-02 |
| 99mTc Technetium labelled MAG3, normal renal function | 7.0E-03 | 5.12E-03 | −27 | 4.00E-03 | −43 | 3.29E-03 | 4.70E-03 |
| 99mTc Technetium labelled non-absorbable markers, orally administered fluids | 1.9E-02 | 1.06E-02 | −44 | 1.07E-02 | −44 | 9.93E-03 | 1.14E-02 |
| 99mTc Technetium labelled non-absorbable markers, orally administered solids | 2.4E-02 | 1.13E-02 | −53 | 1.15E-02 | −52 | 1.07E-02 | 1.24E-02 |
| 99mTc Technetium labelled MIBI, exercise | 7.9E-03 | 6.55E-03 | −17 | 6.29E-03 | −20 | 5.80E-03 | 6.78E-03 |
| 99mTc Technetium labelled MIBI, resting subject | 9.0E-03 | 6.81E-03 | −24 | 6.61E-03 | −27 | 6.14E-03 | 7.07E-03 |
| 99mTc Technetium labelled monoclonal antibodies, intact antibody | 1.2E-02 | 1.17E-02 | −3 | 1.08E-02 | −10 | 9.95E-03 | 1.16E-02 |
| 99mTc Technetium pertechnegas | 1.2E-02 | 1.52E-02 | 26 | 1.50E-02 | 25 | 1.39E-02 | 1.61E-02 |
| 99mTc Technetium pertechnetate, intravenous blocking agent given | 4.2E-03 | 4.34E-03 | 3 | 4.02E-03 | −4 | 3.58E-03 | 4.46E-03 |
| 99mTc Technetium pertechnetate, intravenous no blocking agent given | 1.3E-02 | 1.60E-02 | 23 | 1.58E-02 | 22 | 1.48E-02 | 1.68E-02 |
| 99mTc Technetium pertechnetate orally, no blocking agent | 1.4E-02 | 6.48E-03 | −54 | 6.36E-03 | −55 | 5.83E-03 | 6.89E-03 |
| 99mTc Technetium labelled phosphates and phosphonates, normal uptake and excretion | 5.7E-03 | 4.55E-03 | −20 | 3.99E-03 | −30 | 3.38E-03 | 4.59E-03 |
| 99mTc Technetium labelled erythrocytes | 7.0E-03 | 1.06E-02 | 51 | 1.11E-02 | 59 | 1.02E-02 | 1.20E-02 |
| 99mTc Technetium technegas | 1.5E-02 | 1.79E-02 | 19 | 1.90E-02 | 27 | 1.71E-02 | 2.08E-02 |
| 99mTc Technetium tetrofosmin, exercise | 6.9E-03 | 5.54E-03 | −20 | 5.67E-03 | −18 | 5.15E-03 | 6.20E-03 |
| 99mTc Technetium tetrofosmin, resting subject | 8.0E-03 | 5.92E-03 | −26 | 6.15E-03 | −23 | 5.57E-03 | 6.72E-03 |
| 99mTc Technetium labelled white blood cells (leukocytes) | 1.1E-02 | 1.28E-02 | 16 | 1.02E-02 | −7 | 9.24E-03 | 1.12E-02 |
| 111In Indium labelled HIG | 1.7E-01 | 2.23E-01 | 31 | 2.15E-01 | 26 | 1.99E-01 | 2.31E-01 |
| 111In Indium labelled monoclonal antibodies, intact antibody | 3.3E-01 | 2.88E-01 | −13 | 2.74E-01 | −17 | 2.49E-01 | 2.99E-01 |
| 111In Indium octreotide | 5.4E-02 | 6.74E-02 | 25 | 5.93E-02 | 10 | 5.34E-02 | 6.51E-02 |
| 123I Iodide, thyroid uptake 35 % | 2.2E-01 | 2.59E-01 | 18 | 2.13E-01 | −3 | 1.95E-01 | 2.30E-01 |
| 123I Iodine BMIPP | 1.6E-02 | 1.70E-02 | 6 | 1.71E-02 | 7 | 1.56E-02 | 1.87E-02 |
| 123I Iodine IPPA | 1.6E-02 | 1.72E-02 | 7 | 1.72E-02 | 8 | 1.56E-02 | 1.87E-02 |
| 123I Iodine labelled brain receptor substances | 5.0E-02 | 3.60E-02 | −28 | 3.65E-02 | −27 | 3.30E-02 | 4.00E-02 |
| 123I Iodine Hippuran, normal renal function | 1.2E-02 | 8.88E-03 | −26 | 7.06E-03 | −41 | 5.98E-03 | 8.15E-03 |
| 123I Iodine MIBG | 1.3E-02 | 1.67E-02 | 28 | 1.67E-02 | 28 | 1.51E-02 | 1.82E-02 |
| 123I Iodine labelled monoclonal antibodies, intact antibody | 2.9E-02 | 3.29E-02 | 13 | 2.94E-02 | 1 | 2.68E-02 | 3.21E-02 |
| 124I Iodide, thyroid uptake 35 % | 1.5E + 01 | 1.41E + 01 | −6 | 1.15E + 01 | −23 | 1.05E + 01 | 1.25E + 01 |
| 125I Iodide, thyroid uptake 35 % | 1.4E + 01 | 1.85E + 01 | 32 | 1.50E + 01 | 7 | 1.38E + 01 | 1.62E + 01 |
| 131I Iodide, thyroid uptake 35 % | 2.4E + 01 | 2.68E + 01 | 11 | 2.15E + 01 | −10 | 1.98E + 01 | 2.33E + 01 |
| 131I Iodine, Hippuran, normal renal function | 5.2E-02 | 1.89E-02 | −64 | 1.53E-02 | −71 | 1.29E-02 | 1.78E-02 |
| 131I Iodine, labelled monoclonal antibodies, intact antibody | 4.7E-01 | 4.40E-01 | −6 | 3.59E-01 | −24 | 3.26E-01 | 3.94E-01 |
| 131I Iodine NP59 | 1.8E + 00 | 2.02E + 00 | 12 | 1.74E + 00 | −3 | 1.60E + 00 | 1.89E + 00 |
| 201Tl Thallium ion | 1.4E-01 | 1.27E-01 | −10 | 1.02E-01 | −27 | 9.90E-02 | 1.05E-01 |
A new Supplemental file is given in a separate file named “Additional file 1: Table S1”.
Additional file 1
Effective dose from all the radiopharmaceuticals published by the ICRP, determined using three different methods. (E/A0) 1 is the previously published effective dose per unit administered activity (E/A0) by ICRP, (E/A0)2 is (E/A0) dose calculated with the new phantoms and old tissue weighting factors while (E/A0) 3 is with the new phantoms and new weighting factors. (E/A0)2-(E/A0) 1))/ (E/A0)1 and ((E/A0) 3-(E/A0)1)/ (E/A0)1 is the difference in % of the new values compared to the old. (E/A0)3 male and (E/A0)3 are the effective dose estimations generated from the equivalent dose of each gender separately using the new phantoms and new weighting factors. (DOCX 65 kb)


