Abstract
The effects of propranolol in the treatment of anxiety disorders have not been systematically evaluated previously. The aim was to conduct a systematic review and meta-analysis of randomised controlled trials, addressing the efficacy of oral propranolol versus placebo or other medication as a treatment for alleviating either state or trait anxiety in patients suffering from anxiety disorders. Eight studies met the inclusion criteria. These studies concerned panic disorder with or without agoraphobia (four studies, total n = 130), specific phobia (two studies, total n = 37), social phobia (one study, n = 16), and posttraumatic stress disorder (PTSD) (one study, n = 19). Three out of four panic disorder trials qualified for pooled analyses. These meta-analyses found no statistically significant differences between the efficacy of propranolol and benzodiazepines regarding the short-term treatment of panic disorder with or without agoraphobia. Also, no evidence was found for effects of propranolol on PTSD symptom severity through inhibition of memory reconsolidation. In conclusion, the quality of evidence for the efficacy of propranolol at present is insufficient to support the routine use of propranolol in the treatment of any of the anxiety disorders.
Keywords: Propranolol, anxiety disorders, panic disorder, meta-analysis
Introduction
Propranolol, a ß1,2-adrenoreceptor antagonist, competes at receptor level with catecholamines, thereby blocking their orthosympathetic effects (Black et al., 1964). Clinically, propranolol is used widely to target peripheral sites of the noradrenergic system to treat hypertension, coronary artery disease and tachyarrhythmias (Freemantle et al., 1999; Fuster et al., 2006; Webb et al., 2010). Furthermore, propranolol can be deployed to block ß-adrenoreceptors in the central nervous system, as the lipophilic compound readily enters the blood–brain barrier.
Soon after the discovery of propranolol in the early 1960s, Turner and Granville-Grossman fortuitously noted its anxiolytic effects in an attempt to reduce tachycardia caused by hyperthyroidism (Turner and Granville-Grossman, 1965). Ever since, propranolol has gained increasing interest in psychiatry. Several trials studying off-label use of propranolol would follow, such as its use in the treatment of high trait anxiety (Becker, 1976; Kathol et al., 1980; Meibach et al., 1987; Wheatley, 1969), substance disorder and withdrawal symptoms (Grosz, 1972), schizophrenia (Yorkston et al., 1974), autism (Ratey et al., 1987), and aggression (Fleminger et al., 2006). In addition, propranolol has been shown to mitigate milder distressing states such as exam nerves (Brewer, 1972; Drew et al., 1985; Stone et al., 1973), stage fright (Brantigan et al., 1982), performance anxiety in musicians (Clark and Agras, 1991), performance anxiety in surgeons (Elman et al., 1998), and fear of undergoing surgery (Dyck and Chung, 1991; Jakobsson et al., 1995; Mealy et al., 1996).
Propranolol’s generic status, and the fact that selective serotonin reuptake inhibitors (SSRIs) have become first-line pharmacological treatment across the range of anxiety disorders (Baldwin et al., 2014), have probably contributed to a gradually declining attention for the agent as a potential treatment of anxiety-related conditions. More recently however, with advanced insights into the way the brain processes emotional experiences and their pivotal role in the development and persistence of several mental disorders (McGaugh, 2000), the psychopharmacological properties of propranolol have regained research attention (Johansen et al., 2011; Kindt et al., 2009; Soeter and Kindt, 2010).
Whereas propranolol was first studied as a general anxiolytic in the treatment of anxiety disorders, today it is mainly the amnesic effect on retrieved fear memory that is the subject of interest. To this end, there is evidence to suggest that after a fear memory is recollected, and followed specifically by a prediction error (a discrepancy between actual and expected negative events), propranolol selectively blocks protein synthesis, thereby prohibiting the ‘reconsolidation’ of the fear memory while sparing declarative memory (Debiec and Ledoux, 2004; Finnie and Nader, 2012; Kindt et al., 2009; Merlo et al., 2014, 2015; Nader et al., 2000; Sevenster et al., 2013). A recent meta-analysis of eight experiments with healthy human volunteers (total n = 308) supported this line of reasoning as it was found that compared with placebo, propranolol administered before memory reactivation is capable of reducing the expression of cue-elicited fear responses (Lonergan et al., 2013). The latter findings have tempted several authors to suggest that propranolol has potential for the treatment of anxiety disorders that are rooted in the presence of disturbing memories, particularly posttraumatic stress disorder, or PTSD (e.g. Gardner, 2010; Giles, 2005; Lehrer, 2012). Yet, it should be noted that the treatment approach in which propranolol is employed as an ‘amnesic agent’ to reduce traumatic memory differs from the use of propranolol as a general anxiolytic agent in the treatment of anxiety disorders.
Clinical evidence for the effects of propranolol in the treatment of anxiety disorders has never before been systematically reviewed. Accordingly, in an effort to determine the current place of propranolol within the therapeutic armamentarium of treatments for anxiety disorders, the aim of this study was to review both published and unpublished reports of randomised controlled trials (RCTs) that evaluated the effects of oral propranolol versus placebo or other medication as a treatment for alleviating state and/or trait anxiety in patients suffering from anxiety disorders. In addition, meta-analyses of pooled summary statistics were undertaken where possible.
Method
Systematic review
A systematic review was performed, which is reported in accordance with the PRISMA Statement (Moher et al., 2009).
Eligibility criteria
Only placebo-controlled, comparative parallel group and crossover RCTs were eligible when they included human subjects with any of the anxiety disorders as included in the current version (American Psychiatric Association, 2013) or previous versions of the Diagnostic and Statistical Manual (DSM) for an evaluation of the therapeutic effects of propranolol. Unpublished abstracts and reports were also considered. The comparator was either a placebo or other medication. The search excluded experimental fear conditioning trials and secondary prevention trials. There was no restriction on the basis of sample size, duration of follow-up, primary or secondary outcomes, duration or severity of symptoms, presence of comorbid disorders, or demographic variables of subjects. The search was not restricted to any language.
Information sources and search
An electronic systematic literature search, updated until 18 March 2014, was performed in the online databases: PubMED (all indexed years), Ovid Embase (Embase Classic and Embase 1947 to present), PsycINFO (1806 to present), Web of Science SCI-EXPANDED 1975–present [v.5.13.1], and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) that includes unpublished reports, using the following strategy (see Table 1). When necessary, authors of included articles were contacted in order to retrieve summary continuous data that were not provided in their trial report.
Table 1.
Search terms.
| PubMED (all indexed years) |
| (Propranolol OR Propanolol OR Avlocardyl OR AY-20694 OR AY 20694 OR AY20694 OR Betadren OR Dexpropranolol OR Inderal OR Obsidan OR Obzidan OR Propranolol Hydrochloride OR Hydrochloride, Propranolol OR Rexigen OR Anaprilin OR Anapriline OR Dociton) AND (anxiety disorder OR anxieties OR fear OR anxiolytic OR anxiolytics OR dread OR worry OR antianxiety OR emotional trauma OR angst OR anxious OR panic OR terror OR terrors OR horror OR horrors) |
| Ovid Embase (Embase Classic and Embase 1947 to present) |
|
| PsycINFO (1806 to present) |
|
| Web of Science SCI-EXPANDED 1975 - present [v.5.13.1] |
| Propranolol (Category Term: PSYCHIATRY) |
| WHO ICTRP |
| Propranolol [Recruitment status: ALL] |
Trial selection
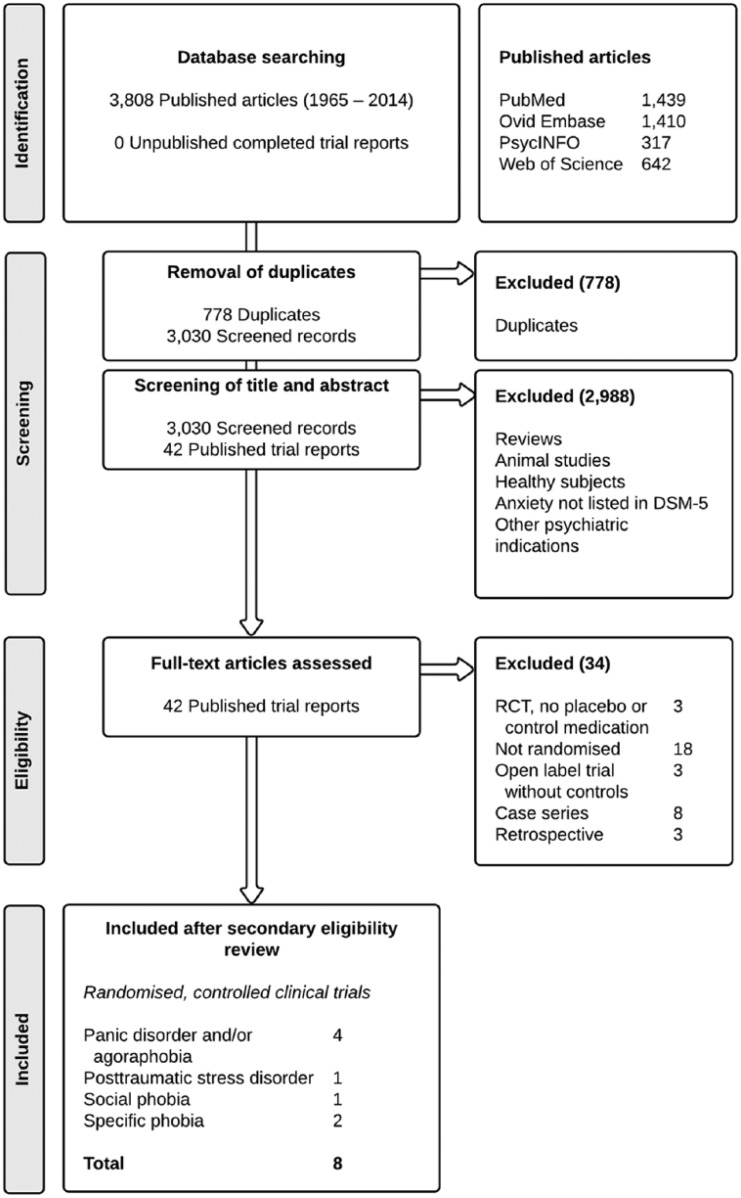
During the primary screening process, two raters (SAS and RvW) independently assessed the information in the title and abstract of retrieved articles on their eligibility in a standardised but non-blinded manner. Thereafter, full text articles were retrieved and screened for eligibility. RCTs were included when both raters regarded all inclusion criteria (i.e. RCT, comparator agent, anxiety disorders) as fulfilled. Unpublished reports were also considered in order to rule out publication bias. There were no disagreements in assessment and inclusion. Figure 1 shows a flow diagram of the inclusion process.
Figure 1.
Flow of information through the different phases of the systematic search.
Data extraction and collection
One reviewer (SAS) extracted the data, and a second reviewer (RvW) checked the extracted items. The following information was obtained from each trial: (a) description of the trials, including the primary researcher, the year of publication, and the source of funding; (b) characteristics of the interventions, including the number of participants randomised to treatment and control groups, the number of total dropouts per group as well as the number that dropped out due to adverse effects, the dose of the medication and the period over which it was administered; (c) characteristics of trial methodology, including the diagnostic criteria employed; (d) characteristics of participants, including gender distribution and mean and range of ages; and (e) outcome measures employed (both primary and secondary), and summary statistics of reported continuous (means and standard deviations; SD) and dichotomous outcome measures (categorical treatment outcome). Summary outcome data were entered into Review Manager (RevMan software, version 5.2; Cochrane Collaboration, 2012). Eight authors were contacted for further information. Seven responded but none provided requested information or data.
Risk of bias
To ascertain the validity of eligible RCTs, two of the reviewers (SAS and AJvW) independently assessed the quality of the trials by means of the Cochrane Collaboration’s tool for assessing risk of bias (Higgins et al., 2011a). Disagreement in assessment was solved by discussion with a third person (AdJ).
Meta-analysis
Meta-analyses of pooled summary statistics were undertaken only if trials were combinable; i.e. if these included participants with the same anxiety disorder and if treatment effects in these trials were expressed in the same variable.
Data analysis and synthesis
For dichotomous treatment outcomes of interest, relative risks with 95% confidence intervals (95%-CIs) were used as the summary statistic. Results from continuous data were expressed as weighted mean differences (WMDs) with 95%-CIs. These data were pooled over studies using invariance weighting. Results were combined using the random effects model, in order to prevent substantially overstated precision of final estimates of effects even when statistical heterogeneity was low (I2 < 60% and p > 0.10; Schmidt et al., 2009). Data from only the first treatment period of crossover trials was included in the calculation of summary statistics if there was insufficient data available to include a paired analysis (Higgins et al., 2011b). Data from both periods were included only when the correlation between participants’ responses to the interventions in the different phases was provided in the trial report.
Sensitivity analysis
To explore the degree to which the findings of the meta-analysis could be affected by bias, sensitivity analyses were performed, when considered appropriate.
Results
Study selection
The initial search yielded a total of 3030 citations after adjusting for duplicates (see Figure 1). After a primary screening process, 42 published trial reports (all in English language) were read full text for detailed examination. It appeared that 34 trials needed to be excluded after secondary review due to their study design (i.e. the full text article revealed that these trials were not randomised or that there was no comparator medication). Our eligibility criteria were met by a total of eight published trials of propranolol for the treatment of panic disorder with or without agoraphobia (four studies, n = 130), specific phobia (two studies, n = 37), social phobia (one study, n = 16), and PTSD (one study, n = 19).
Study characteristics
The characteristics and results of included trials are summarised in Table 2.
Table 2.
Randomised, controlled clinical trials evaluating the effects of oral propranolol in the treatment of anxiety disorders (1981–2008).
| Author | Design | Participants | Intervention | Outcome measures | Results |
|---|---|---|---|---|---|
| Panic disorder with or without agoraphobia | |||||
| Noyes et al., 1984 | Crossover trial, double-blind |
n = 21 Panic disorder and agoraphobia (DSM-III) Mean age of completers: 34.8y (range 20–61), 62% females |
4 wk Treatment period
diazepam 5–40 mg/day (median 30) vs. propranolol 80–320 mg/day (median 240) for 2 wk, followed by a 1-wk washout period after which treatment allocation was reversed for 2 wks |
No clear primary outcome
Trait anxiety SCL-90 HAM-A Trait anxiety Severity of symptom scale Relief of symptoms scale Social impairment scale Panic attack frequency |
• Patients with illnesses of moderate severity responded better to both drugs than did those with severe illnesses, t = 4.00, p = 0.06 for relief, t = 6.31, p = 0.02 for improvement • Loss to follow-up: 6/21 patients (29%) dropped out during the first 2 wks • No record of reasons for withdrawal per group |
| Munjack et al., 1985 | Crossover trial, single-blind |
n = 38 Panic disorder or agoraphobia with panic attacks (DSM-III) Mean age of completers: 36y (range 23–61), 61% females. No record of number excluded |
6 wk Treatment period
Imipramine up to 300 mg/day vs. propranolol up to 160 mg/day Followed by 1 wk tapering and 1 wk drug-free period Second arm, 6 wk vice-versa drug allocation |
Primary outcomes: Number of panic attacks Avoidance behaviour (ordinal subjective ratings) Secondary outcomes: State and trait anxiety SCL-90 Fear Questionnaire Wolpe-Lang Fear Inventory MMPI (non-self-report measure) |
• There were no significant differences between imipramine and propranolol on questionnaires, on clinical ratings of effectiveness, on panics, or level of functioning • 15/38 (39%) dropouts (groups not specified), reasons reported included: refusing treatment allocation and switching to other group (1 in propranolol group, 2 in imipramine) side effects (6), unknown (6) |
| Munjack et al., 1989 | Parallel groups, double-blind |
n = 64 Panic disorder or agoraphobia with panic attacks (DSM-III) Mean age of completers: 31y (range 18–62), 69% females. No record of number excluded |
5 wk Treatment period
Alprazolam up to 6 mg/day vs. propranolol up to 240 mg/day vs. up to 12 capsules of placebo per day |
No clear primary outcome
Trait anxiety HAM-A Sheehan’s Panic and Anxiety Scales Marks-Sheehan Phobia Scale Depression HAM-D Side Effects Checklist |
• The alprazolam group improved more than the placebo group on the Assessor Phobia Fear Scale, t = 3.03; df = 34; p < 0.004, and the Assessor Phobia Avoidance Scale, t = 3.16; df = 34; p < 0.003, but no superiority over propranolol was found • The alprazolam group improved more than the propranolol group (t = 3.16; df = 34; p < 0.003) and the placebo group (t = 2.36; df = 37; p < 0.02) on the Assessor Phobia Avoidance Scale • 4/19 (21%) Dropouts in propranolol group, 5/16 (31%) dropouts in placebo group, and 0 in the alprazolam group; of which 4 (group unspecified) indicated to drop out due to side effects |
| Ravaris et al., 1991 | Parallel groups, double-blind (study psychiatrists were “objective” assessors and aware of allocation) |
n = 33 Agoraphobia with panic disorder or panic disorder ± phobic avoidance Alprazolam completers: mean age 37.5y ± 9.0, 93% female; Propranolol completers: mean age 33.2y ± 9.0, 93% female |
6 wk Treatment period
Alprazolam 5.0 ± 2.3 mg/day (range 1–30) vs. propranolol 182.0 ± 60.5 mg (range 30–300) |
No clear primary outcome; only data from ‘most clinically pertinent findings’ presented
Trait anxiety HAM-A; Marks-Sheehan Phobia Scale; Sheehan’s Patient Rated Anxiety Scale, Clinician Rated Anxiety Scale, and Panic and Anxiety Attack Scale; Physician’s Global Improvement Scale; SCL Scale Panic attack frequency |
• There were no significant between-drug differences at any time, with both drugs significantly suppressing panic attacks • At the end of 6 weeks, compared with baseline, both drugs produced reductions in HAM-A generalised anxiety (alprazolam t[13] = 3.90, p < 0.002, propranolol t[14] = 6.04, p < 0.001), but no between-drug differences at any point • 3/33 (9%) Early-phase dropouts (groups not specified), one drug related in propranolol group (insufficient effect and depressive symptoms after 14 days). 1 subject retrospectively did not meet panic disorder entry criteria, leaving a final sample of 29 patients |
| Posttraumatic stress disorder | |||||
| Brunet et al., 2008 | Parallel groups, double-blind |
n = 19 Chronic (>10 years duration) PTSD patients (DSM-IV) Propranolol completers: mean age 34.8y ± 10.1; Placebo completers: mean age 35.1y ± 10.5; no data on gender No record of number excluded |
Two consecutive doses
After 20-min script-driven imaginary exposure to traumatic event: 40 mg short-acting propranolol vs. placebo; 2 h later 60 mg long-acting propranolol vs. placebo |
Primary outcomes: physiologic responding during mental imagery of traumatic event (1 wk later) HR, SC, left corrugator EMG |
• Overall physiologic responding during mental imagery of trauma after 1 wk was smaller in propranolol group, F(3,15) = 5.1; p = 0.007, η2 = 0.49 • HR and SC, but not EMG, responses were significantly smaller in propranolol group • Number of dropouts not reported, nor reasons for withdrawal per group |
| Specific phobia | |||||
| Fagerström et al., 1985 | Crossover trial, double-blind |
n = 14 Phobic females (snake or spider phobia) No data on gender and age No record of exclusion criteria or number of refusals |
Repeated measurements (one dose of each 3 conditions each) Condition 1: propranolol 80 mg/day vs. atenolol 50 mg/day vs. placebo 90 min before exposure to pictures of phobic stimulus Followed by 1 wk washout period Condition 2 (re-allocated) Followed by 1 wk washout period Condition 3 (re-allocated) |
No clear primary outcome
State anxiety VAS Physiological responding HR, BP, finger tip temperature |
• No general effect of the β-blocking drugs on subjective anxiety • For the high cardiovascular reactors compared with the low group, propranolol was associated with a trend towards higher anxiety ratings, F(2.24) = 3.42, p < 0.10, MSe = 1147.06 • Number of dropouts not reported, nor reasons for withdrawal per group |
| Liu et al., 1991 | Parallel groups, double-blind |
n = 23 Dental phobics (DSM-III R) No data on gender and age No record of number excluded |
Single dose
propranolol 80–120 mg (12) vs. placebo 80–120 mg (11) 1 h before exposure to actual dental treatment |
No clear primary outcome
State anxiety VAS-scores (anxiety, aversiveness, pain); videotape observer-rated anxious behaviour |
• Lower self-reported anxiety during injection phase vs. placebo; 5.5 (2.75) vs. 7.45 (2.0), p = 0.033 (one-tailed) • Less overall pain intensity and aversiveness in propranolol group; 20.8 (23.5) vs. 45.5 (29.1), p = 0.015 • No significant difference for observer-rated fear behaviour • No dropouts because no follow-up |
| Social phobia | |||||
| Falloon et al., 1981 | Parallel groups, double-blind |
n = 16 Social phobia (DSM-III) ± panic attacks (4) or alcoholism (3) Mean age of completers: 27y (range 18–52), 38% females. No record of exclusion criteria or number of refusals |
4 wk Treatment period
Propranolol 160–320 mg/day vs. placebo (Treatment period followed a 4-wk social skills training without medication) Follow-up assessment 6 months after treatment (81% response) |
No clear primary outcome
Trait anxiety Social Anxiety Questionnaire (6 months follow-up) State anxiety Global tension/anxiety scale; observer (therapist)-rated anxiety behaviour |
• A repeated measures analysis of variance revealed no significant differences or trends between the drug and placebo on any outcome measures (p = NS) • 3/9 (33%) Dropouts in propranolol group, 1/7 placebo (14%) in placebo group, reasons reported were unspecified side effects |
Panic disorder with or without agoraphobia
Four trials found mixed effects of propranolol versus other medication in the treatment of panic disorder with or without agoraphobia (Munjack et al., 1985, 1989; Noyes et al., 1984; Ravaris et al., 1991). Three of these four studies (Munjack et al., 1989; Noyes et al., 1984; Ravaris et al., 1991) qualified for formal meta-analyses.
Posttraumatic stress disorder
Only one trial with chronic PTSD patients showed that 40 mg of short-acting oral propranolol given prior to imaginary exposure, followed by 60 mg of long-acting oral propranolol, statistically reduced physiological responding (reduced heart rate and skin conductance) during imaginary exposure 1 week later (F(3,15) = 5.1; p = 0.007, η2 = 0.49) (Brunet et al., 2008). No data on PTSD symptom severity endpoints were provided in the study report.
Specific phobia
One trial with specific (dental) phobia subjects was found (Liu et al., 1991). It showed that, as compared with placebo, 80–120 mg of oral propranolol significantly diminished self-reported state anxiety during injection of local dental anaesthesia (self-reported anxiety at injection: 5.5 (2.75) versus 7.45 (2.0), p = 0.033, one-tailed). One 3-week crossover trial with specific (animal type: snake or spider) phobia subjects by reported no significant effects of propranolol on self-reported trait anxiety (Fagerström et al., 1985).
Social phobia
One trial with social phobia subjects was found. It reported absence of statistically significant effects of propranolol on state and trait anxiety 6 months follow-up after a social skills training (Falloon et al., 1981).
Risk of bias
Study quality
Risk of bias judgements are summarised in Figure 2. The majority of trials (9/12; 75%) failed to provide complete outcome data. On average, 19 percent (37 out of 191) of the participants in the six trials that provided dropout data did not reach study endpoint; hence the risk of attrition bias is considerable. The authors of one trial reported that “data from only the most clinically pertinent findings” were presented in the paper, although they specified all outcome measures that were initially recorded (Ravaris et al., 1991). Therefore, this trial was judged to have a high risk of selective outcome reporting.
Figure 2.
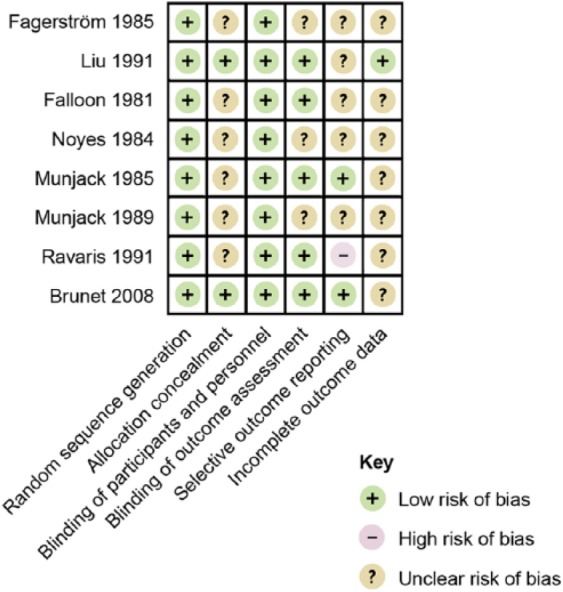
Risk of bias summary figure.
Publication bias
No unpublished reports of completed RCTs were obtained. Three RCTs were found (ClinicalTrials.gov Identifiers: NCT00645450, NCT00648375 and NCT01239173) that had been terminated because of inadequate subject recruitment. The authors of these three discontinued trials were contacted. All responded, but none provided data.
Meta-analyses
Propranolol versus benzodiazepines in panic disorder with or without agoraphobia
Meta-analysis I. Outcome: number of panic attacks after 2 weeks of treatment
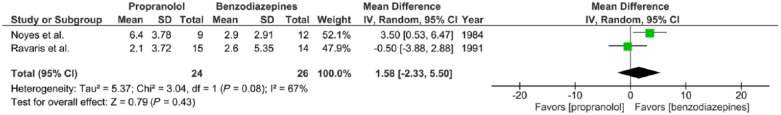
Two trials report the effects of propranolol (30–320 mg/day) versus benzodiazepines (1–30 mg/day alprazolam or 5–40 mg/day diazepam) prescribed to adults with panic disorder with or without agoraphobia for a 2-week period, expressed in mean number of panic attacks after 2 weeks of treatment (Noyes et al., 1984; Ravaris et al., 1991). Effect size for each study was expressed as a WMD. There was substantial statistical heterogeneity of studies (two trials, 50 participants in total; χ2 = 3.04, df = 1, p = 0.08, I2 = 67%). No significant difference was found between propranolol and benzodiazepines (mean difference = 1.58, 95%-CI [–2.33; 5.50], Z = 0.79, p = 0.43) (Figure 3(a)).
Figure 3(a).
Meta-analysis I. Propranolol versus benzodiazepines in panic disorder with or without agoraphobia (outcome: number of panic attacks after 2 weeks of treatment).
Meta-analysis II. Outcome: HAM-A after 2 weeks treatment
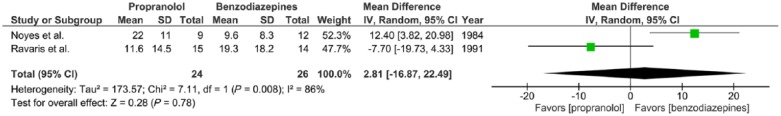
Two trials report the effects of propranolol (30–320 mg/day) versus benzodiazepines (1–30 mg/day alprazolam or 5–40 mg/day diazepam) given to adults with panic disorder with or without agoraphobia for a 2-week period, expressed in mean HAM-A after 2 weeks of treatment (Noyes et al., 1984; Ravaris et al., 1991). WMDs were calculated for continuous summary data obtained from the Hamilton Anxiety Rating Scale (HAM-A), a widely used scale to assess the severity of symptoms of anxiety (Hamilton, 1959). There was substantial statistical heterogeneity of studies (two trials, 50 participants in total; χ2 = 7.11, df = 1, p = 0.008, I2 = 86%). No significant difference was found between propranolol and benzodiazepines (mean difference = 2.81, 95%-CI [–16.87; 22.49], Z = 0.28, p = 0.78) (Figure 3(b)).
Figure 3(b).
Meta-analysis II. Propranolol versus benzodiazepines in panic disorder with or without agoraphobia (outcome: HAM-A after 2 weeks treatment).
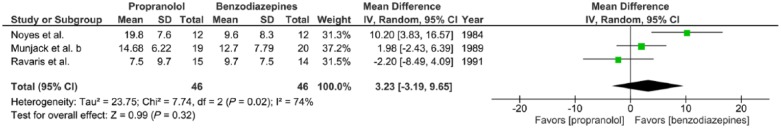
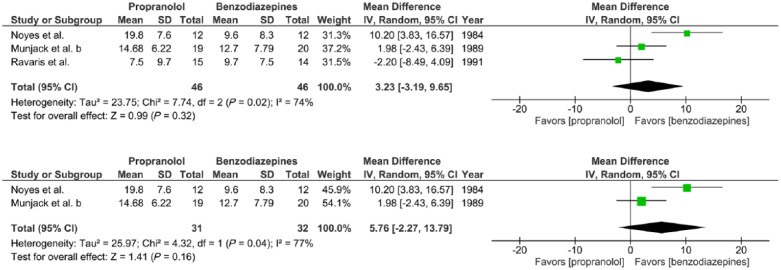
Meta-analysis III. Outcome: HAM-A after 2–6 weeks treatment
Three trials report the effects of propranolol (20–320 mg/day) versus benzodiazepines (0.5–30 mg/day alprazolam or 5–40 mg/day diazepam) given to adults with panic disorder with or without agoraphobia for a 2–6-week period, expressed in mean HAM-A scores at completion of treatment (Munjack et al., 1989; Noyes et al., 1984; Ravaris et al., 1991). There was substantial statistical heterogeneity of studies (three trials, 92 participants in total; χ2 = 7.74, df = 2, p = 0.02, I2 = 74%). No significant overall effect was found (mean difference = −2.20, 95%-CI [–8.49; 4.09], Z = 0.99, p = 0.32) (Figure 3(c)).
Figure 3(c).
Meta-analysis III. Propranolol versus benzodiazepines in panic disorder with or without agoraphobia (outcome: HAM-A after 2–6 weeks treatment).
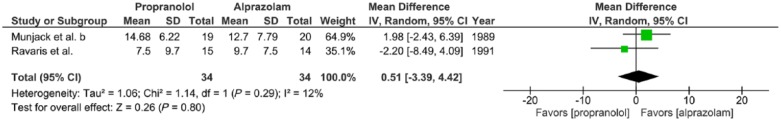
Meta-analysis IV. Outcome: HAM-A after 6 weeks treatment
Two trials examined the effects of propranolol (30–300 mg/day) versus alprazolam (1–30 mg/day) given to adults with panic disorder with or without agoraphobia for a 2-week period, expressed in mean HAM-A after 6 weeks of treatment (Munjack et al., 1989; Ravaris et al., 1991). There was little statistical heterogeneity of studies (two trials, 68 participants in total; χ2 = 1.14, df = 1, p = 0.29, I2 = 12%). No significant difference was found between propranolol and alprazolam (mean difference = 0.51, 95%-CI [–3.39; 4.42], Z = 0.26, p = 0.80) (Figure 3(d)).
Figure 3(d).
Meta-analysis IV. Propranolol versus alprazolam in panic disorder with or without agoraphobia (outcome: HAM-A after 6 weeks treatment).
Sensitivity analysis
For one study, included in all four meta-analyses, a high risk of selective outcome reporting was found (Ravaris et al., 1991). The impact of this bias on the findings of our meta-analyses was assessed. After excluding this study only one study remained for meta-analyses I, number of panic attacks after 2 weeks of treatment and meta-analyses II, HAM-A after 2 weeks treatment (Noyes et al., 1984), and one study remained for the meta-analysis IV, HAM-A after 6 weeks treatment (Munjack et al., 1989). For the meta-analysis III, HAM-A after 2–6 weeks treatment, two studies remained (Munjack et al., 1989; Noyes et al., 1984) of which the effects of compared treatment (benzodiazepines and propranolol) no longer reached statistical significance (two trials, 63 participants in total; MD = 5.76 [–2.27 to 13.79], Z = 1.41, p = 0.16) (Figure 3(e)).
Figure 3(e).
Sensitivity analysis. Meta-analysis III with one study with high risk of selective outcome reporting included and excluded (Ravaris et al., 1991).
Discussion
This systematic review and meta-analysis shows a lack of well-designed clinical studies. This limits the scientific evidence and allows neither firm conclusions in favour or against the use of propranolol in the treatment of anxiety disorders, nor recommendations for informed decision-making in clinical practice. More specifically, our meta-analyses found no statistical difference between the effects of propranolol and benzodiazepines on anxiety and panic attack frequency (Munjack et al., 1989; Noyes et al., 1984; Ravaris et al., 1991). In addition, four moderate risk of bias trials failed to show solid evidence on the therapeutic effect of propranolol in patients with dental phobia (Liu et al., 1991), animal-type specific phobia (Fagerström et al., 1985), and social phobia (Falloon et al., 1981). No RCTs were available on the effects of propranolol in the treatment of any of other anxiety disorders (e.g. generalised anxiety disorder, obsessive–compulsive disorder (OCD), separation anxiety disorder, or selective mutism; note that PTSD and OCD have been relocated to separate chapters in the DSM-5 (American Psychiatric Association, 2013). Moreover, despite widespread suggestions (Gardner, 2010; Giles, 2005; Lehrer, 2012), no evidence was found for the effects of propranolol on PTSD symptom severity, through inhibition of memory reconsolidation (Brunet et al., 2008) or any other mechanism.
To date, statistical equivalence of the efficacy of propranolol versus benzodiazepines regarding the treatment of individuals with panic disorder with or panic disorder without agoraphobia has not been shown. Because the evidence converges to suggest that propranolol and benzodiazepines prescribed in clinical settings have similar effectiveness in the short-term treatment of these conditions (Munjack et al., 1989; Noyes et al., 1984; Ravaris et al., 1991), other factors also need to be considered. First, it takes time for an effect to become prominent upon administration. SSRIs generally require a period of 2–4 weeks, while in some patients with panic disorder the onset of action may take up to 12 weeks (Michelson et al., 2001; Oehrberg et al., 1995). Therefore, early adjuvant therapy with propranolol may be taken into consideration. Second, the side effects profile should be taken into account. Whereas the clinical effects of benzodiazepines are considered equivalent to the first-line pharmacotherapy of panic disorder (SSRIs; Mitte, 2005; Roy-Byrne et al., 2013; Wilkinson et al., 1991), they carry a high risk of unwanted sedative effects, cognitive impairment and dependence, and tolerance will develop over time (Baldwin et al., 2014). Conversely as compared with benzodiazepines, the side effects of SSRIs are temporary, reversible and relatively benign, albeit still considerably frequent (among others, >10% gastrointestinal complaints, fatigue, insomnia, and headache; Sandoz, 2012). Although side effects of propranolol occur less frequently (among others, 1–10% sleeping disturbances, nightmares, transient fatigue, and cold extremities; Roy-Byrne et al., 2013; Sandoz, 2012), the extent of the supporting evidence base is currently broader and more consistent for SSRIs than for propranolol (Otto et al., 2001). Therefore, it would seem most reasonable not to divert from current treatment guidelines recommending SSRIs as the first-line medication for panic disorder (Baldwin et al., 2014) until robust data regarding the comparative efficacy and tolerability of propranolol versus SSRIs become available.
With regard to the therapeutic effects of propranolol, it has been proposed that propranolol’s anxiolytic properties may result from its peripheral (autonomic) rather than its central activity (Balon et al., 1990; Clark, 1986; Roy-Byrne et al., 2006). This may explain the lack of evidence for propranolol’s efficacy in the long-term treatment of anxiety disorders other than panic disorder. To this end, it seems most likely that propranolol aids in breaking a vicious cycle of anxiety in which catastrophic misappraisal of bodily sensations of orthosympathetic origin, such as palpitations or increased ventilation, fuel the occurrence of panic attacks. This explanation is supported by research that found that subjects suffering from high levels of general trait anxiety improved little on propranolol (Becker, 1976; Kathol et al., 1980; Meibach et al., 1987; Wheatley, 1969), whereas more favourable effects were found in the treatment of performance anxieties, in which enhanced sensitivity for adrenergic hyperactivation may similarly initiate the fear response (Brantigan et al., 1982; Brewer, 1972; Clark and Agras, 1991; Drew et al., 1985; Elman et al., 1998; Stone et al., 1973).
The present systematic review was limited by the moderate number of small studies examining the effects of propranolol on anxiety disorders, and by the risk of bias these trials presented. Notably, the average loss to follow-up was nearly one-fifth of all participants. As withdrawal reasons were seldom reported, the possibility of selective loss to follow-up in some studies could not be ruled out.
In conclusion, the quality of evidence for the efficacy of propranolol at present is insufficient to support the routine use of propranolol in the treatment of any of the anxiety disorders.
Acknowledgments
The authors would like to thanks Joost G Daams, MA, clinical librarian at the Academic Medical Centre of the University of Amsterdam, for helping constitute adequate search terms for the electronic database search.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th Edition Washington, DC: American Psychiatric Association. [Google Scholar]
- Baldwin DS, Anderson IM, Nutt DJ, et al. (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 28: 403–439. [DOI] [PubMed] [Google Scholar]
- Balon R, Yeragani VK, Pohl R, et al. (1990) Somatic and psychological symptoms during isoproterenol-induced panic attacks. Psychiatry Res 32: 103–112. [DOI] [PubMed] [Google Scholar]
- Becker AL. (1976) Oxprenolol and propranolol in anxiety states. A double-blind comparative study. S Afr Med J 50: 627–629. [PubMed] [Google Scholar]
- Black JW, Crowther AF, Shanks RG, et al. (1964) A new adrenergic betareceptor antagonist. Lancet 1: 1080–1081. [DOI] [PubMed] [Google Scholar]
- Brantigan CO, Brantigan TA, Joseph N. (1982) Effect of beta blockade and beta stimulation on stage fright. Am J Med 72: 88–94. [DOI] [PubMed] [Google Scholar]
- Brewer C. (1972) Beneficial effect of beta-adrenergic blockade on ‘exam nerves’. Lancet 2: 435. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, et al. (2008) Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42: 503–506. [DOI] [PubMed] [Google Scholar]
- Clark DB, Agras WS. (1991) The assessment and treatment of performance anxiety in musicians. Am J Psychiatry 148: 598–605. [DOI] [PubMed] [Google Scholar]
- Clark DM. (1986) A cognitive approach to panic. Behav Res Ther 24: 461–470. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. (2004) Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129: 267–272. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Barnes JN, Evans SJ. (1985) The effect of acute beta-adrenoceptor blockade on examination performance. Br J Clin Pharmacol 19: 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JB, Chung F. (1991) A comparison of propranolol and diazepam for preoperative anxiolysis. Can J Anaesth 38: 704–709. [DOI] [PubMed] [Google Scholar]
- Elman MJ, Sugar J, Fiscella R, et al. (1998) The effect of propranolol versus placebo on resident surgical performance. Trans Am Ophthalmol Soc 96: 283–291; discussion 291–294. [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO, Hugdahl K, Lundström N. (1985) Effect of beta-receptor blockade on anxiety with reference to the three-systems model of phobic behavior. Neuropsychobiology 13: 187–193. [DOI] [PubMed] [Google Scholar]
- Falloon IR, Lloyd GG, Harpin RE. (1981) The treatment of social phobia. Real-life rehearsal with nonprofessional therapists. J Nerv Mental Dis 169: 180–184. [DOI] [PubMed] [Google Scholar]
- Finnie PSB, Nader K. (2012) The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev 36: 1667–1707. [DOI] [PubMed] [Google Scholar]
- Fleminger S, Greenwood RJ, Oliver DL. (2006) Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst Rev 4: CD003299. [DOI] [PubMed] [Google Scholar]
- Freemantle N, Cleland J, Young P, et al. (1999) beta Blockade after myocardial infarction: Systematic review and meta regression analysis. BMJ 318: 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Rydén LE, Cannom DS, et al. (2006) ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice. Circulation 114: e257–e354. [DOI] [PubMed] [Google Scholar]
- Gardner D. (2010) A pill to block out the bad memories: Experts make breakthrough to help people forget traumatic past events. Daily Mail, 23rd November Available at: http://www.dailymail.co.uk/health/article-1332181/Experts-make-breakthrough-help-people-forget-traumatic-past-events.html#ixzz2yqLLsBQ7 (accessed 8 July 2015).
- Giles J. (2005) Beta-blockers tackle memories of horror. Nature 436: 448–449. [DOI] [PubMed] [Google Scholar]
- Grosz HJ. (1972) Narcotic withdrawal symptoms in heroin users treated with propranolol. Lancet 2: 564–566. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1959) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. [DOI] [PubMed] [Google Scholar]
- Higgins J, Altman D, Sterne J. (2011a) Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S. (eds), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0., The Cochrane Collaboration. Available at: http://www.cochrane-handbook.org/ (accessed 9 July 2015).
- Higgins J, Deeks J, Altman D. (2011b) Chapter 16: Special topics in statistics. In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Available at: http://www.cochrane-handbook.org/ (accessed 9 July 2015).
- Jakobsson J, Rane K, Ryberg G. (1995) Oral premedication one hour before minor gynaecological surgery – does it have any effect? A comparison between ketobemidone, lorazepam, propranolol and placebo. Acta Anaesthesiol Scand 39: 359–363. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, et al. (2011) Molecular mechanisms of fear learning and memory. Cell 147: 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathol RG, Noyes R, Slymen DJ, et al. (1980) Propranolol in chronic anxiety disorders. A controlled study. Arch Gen Psychiatry 37: 1361–1365. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. (2009) Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci 12: 256–258. [DOI] [PubMed] [Google Scholar]
- Lehrer J. (2012) The forgetting pill erases painful memories forever. Wired, 17th February Available at: http://www.wired.com/2012/02/ff_forgettingpill/all/ (accessed 8 July 2015).
- Liu HH, Milgrom P, Fiset L. (1991) Effect of a beta-adrenergic blocking agent on dental anxiety. J Dent Res 70: 1306–1308. [DOI] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-Figueroa LA, Pitman RK, et al. (2013) Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J Psychiatry Neurosci 38: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. (2000) Memory – a century of consolidation. Science 287: 248–251. [DOI] [PubMed] [Google Scholar]
- Mealy K, Ngeh N, Gillen P, et al. (1996) Propranolol reduces the anxiety associated with day case surgery. Eur J Surg 162: 11–14. [PubMed] [Google Scholar]
- Meibach RC, Dunner D, Wilson LG, et al. (1987) Comparative efficacy of propranolol, chlordiazepoxide, and placebo in the treatment of anxiety: A double-blind trial. J Clin Psychiatry 48: 355–358. [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozee ZY, et al. (2014) Reconsolidation and extinction are dissociable and mutually exclusive processes: Behavioral and molecular evidence. J Neurosci 34: 2422–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Everitt BJ. (2015) Enhancing cognition by affecting memory reconsolidation. Curr Opin Behav Sci 4: 41–47. [Google Scholar]
- Michelson D, Allgulander C, Dantendorfer K, et al. (2001) Efficacy of usual antidepressant dosing regimens of fluoxetine in panic disorder: Randomised, placebo-controlled trial. Br J Psychiatry 179: 514–518. [DOI] [PubMed] [Google Scholar]
- Mitte K. (2005) A meta-analysis of the efficacy of psycho- and pharmacotherapy in panic disorder with and without agoraphobia. J Affect Disord 88: 27–45. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjack DJ, Crocker B, Cabe D, et al. (1989) Alprazolam, propranolol, and placebo in the treatment of panic disorder and agoraphobia with panic attacks. J Clin Psychopharmacol 9: 22–27. [PubMed] [Google Scholar]
- Munjack DJ, Rebal R, Shaner R, et al. (1985) Imipramine versus propranolol for the treatment of panic attacks: A pilot study. Compr Psychiatry 26: 80–89. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726. [DOI] [PubMed] [Google Scholar]
- Noyes R, Anderson DJ, Clancy J, et al. (1984) Diazepam and propranolol in panic disorder and agoraphobia. Arch Gen Psychiatry 41: 287–292. [DOI] [PubMed] [Google Scholar]
- Oehrberg S, Christiansen PE, Behnke K, et al. (1995) Paroxetine in the treatment of panic disorder. A randomised, double-blind, placebo-controlled study. Br J Psychiatry 167: 374–379. [DOI] [PubMed] [Google Scholar]
- Otto MW, Tuby KS, Gould RA, et al. (2001) An effect-size analysis of the relative efficacy and tolerability of serotonin selective reuptake inhibitors for panic disorder. Am J Psychiatry 158: 1989–1992. [DOI] [PubMed] [Google Scholar]
- Ratey JJ, Bemporad J, Sorgi P, et al. (1987) Open trial effects of beta-blockers on speech and social behaviors in 8 autistic adults. J Autism Dev Disord 17: 439–446. [DOI] [PubMed] [Google Scholar]
- Ravaris CL, Friedman MJ, Hauri PJ, et al. (1991) A controlled study of alprazolam and propranolol in panic-disordered and agoraphobic outpatients. J Clin Psychopharmacol 11: 344–350. [PubMed] [Google Scholar]
- Roy-Byrne P, Stein M, Hermann R. (2013) Pharmacotherapy for panic disorder.In: Post T. (ed.) UpToDate®. Waltham, MA: UpToDate®; Available at: http://www.uptodate.com/contents/pharmacotherapy-for-panic-disorder (accessed 9 July 2015). [Google Scholar]
- Roy-Byrne PP, Craske MG, Stein MB. (2006) Panic disorder. Lancet 368: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Sandoz (2012) 1.3.1.1. Summary of product characteristics: Paroxetine Sandoz® 10/20/30 tablets RVG 33722–3–26613. Almere. [Google Scholar]
- Schmidt FL, Oh I-S, Hayes TL. (2009) Fixed- versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. Br J Math Stat Psychol 62: 97–128. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. (2013) Prediction error governs pharmacologically induced amnesia for learned fear. Science 339: 830–833. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. (2010) Dissociating response systems: Erasing fear from memory. Neurobiol Learn Mem 94: 30–41. [DOI] [PubMed] [Google Scholar]
- Stone W, Gleser G, Gottschalk L. (1973) Anxiety and beta-adrenergic blockade. Arch Gen Psychiatry 29: 620–622. [DOI] [PubMed] [Google Scholar]
- Turner P, Granville-Grossman KL. (1965) Effect of adrenergic receptor blockade of the tachycardia of thyrotoxicosis and anxiety state. Lancet 2: 1316–1318. [DOI] [PubMed] [Google Scholar]
- Webb AJS, Fischer U, Mehta Z, et al. (2010) Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 375: 906–915. [DOI] [PubMed] [Google Scholar]
- Wheatley D. (1969) Comparative effects of propranolol and chlordiazepoxide in anxiety states. Br J Psychiatry 115: 1411–1412. [DOI] [PubMed] [Google Scholar]
- Wilkinson G, Balestrieri M, Ruggeri M, et al. (1991) Meta-analysis of double-blind placebo-controlled trials of antidepressants and benzodiazepines for patients with panic disorders. Psychol Med 21: 991–998. [DOI] [PubMed] [Google Scholar]
- Yorkston NJ, Zaki SA, Malik MK, et al. (1974) Propranolol in the control of schizophrenic symptoms. BMJ 4: 633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]