Abstract
Changes in synaptic connections are considered essential for learning and memory formation1–6. However, it is unknown how neural circuits undergo continuous synaptic changes during learning while maintaining lifelong memories. Here we show, by following postsynaptic dendritic spines over time in the mouse cortex7–8, that learning and novel sensory experience lead to spine formation and elimination by a protracted process. The extent of spine remodelling correlates with behavioural improvement after learning, suggesting a crucial role of synaptic structural plasticity in memory formation and storage. Importantly, a small fraction of new spines induced by novel experience, together with most spines formed early during development and surviving experience-dependent elimination, are preserved throughout the entire life of an animal. These studies indicate that learning and daily sensory experience leave minute but permanent marks on cortical connections and suggest that lifelong memories are stored in largely stably connected synaptic networks.
One remarkable feature of the mammalian brain is its capacity to integrate new information throughout life while stably maintaining memories. Coincident with these two seemingly mutually exclusive attributes of the brain are plasticity and stability of synaptic connections1–11. It is well-established that the strength and number of synaptic connections can undergo rapid and extensive changes after sensory alterations and learning throughout life1,2,4,6,9,12–19. On the other hand, recent studies have shown that dendritic spines, the postsynaptic sites of excitatory synapses, are remarkably stable in adult life7–9. Therefore, synaptic connections are not only capable of undergoing rapid changes in response to new experience but also can serve as substrates for long-term information storage. However, it remains unknown how and to what degree synapses reorganize during learning and how such reorganization is transformed into lifelong memories.
To address these questions, we used transcranial two-photon microscopy to examine how fluorescently labelled dendritic spines of layer V pyramidal neurons in the mouse cortex are altered and maintained in response to skill learning or novel sensory experience7,20–22. We first examined spine dynamics in the primary motor cortex after motor skill learning on an accelerated rotarod20,23 (see Methods). In this rotarod learning task, animals changed their gait pattern and learned specific movement strategies beyond simply running quickly23. In the forelimb area of the motor cortex, rotarod training over 2 days leads to a significant increase (~5–7%) in spine formation in both young (1 month of age) and adult (>4 months) mice (P < 0.001; Fig. 1a–f and Supplementary Table). The increased spine formation was not observed in mice subjected to running similar distances on a slowly rotating rotarod and was region specific, occurring in the forelimb motor cortex but not in the barrel cortex (Fig. 1f). Notably, after being trained for 2 days, spine formation over the next 2 days remained significantly higher if mice were trained with a different type of motor task (reverse running) than if mice were subjected to the same type of training or no training (P < 0.005; Fig. 1a, g). Thus, motor learning experience, not just physical exercise, induces rapid spine formation within 2 days in the primary motor cortex.
Figure 1. Motor learning and novel sensory experience promote rapid dendritic spine formation.
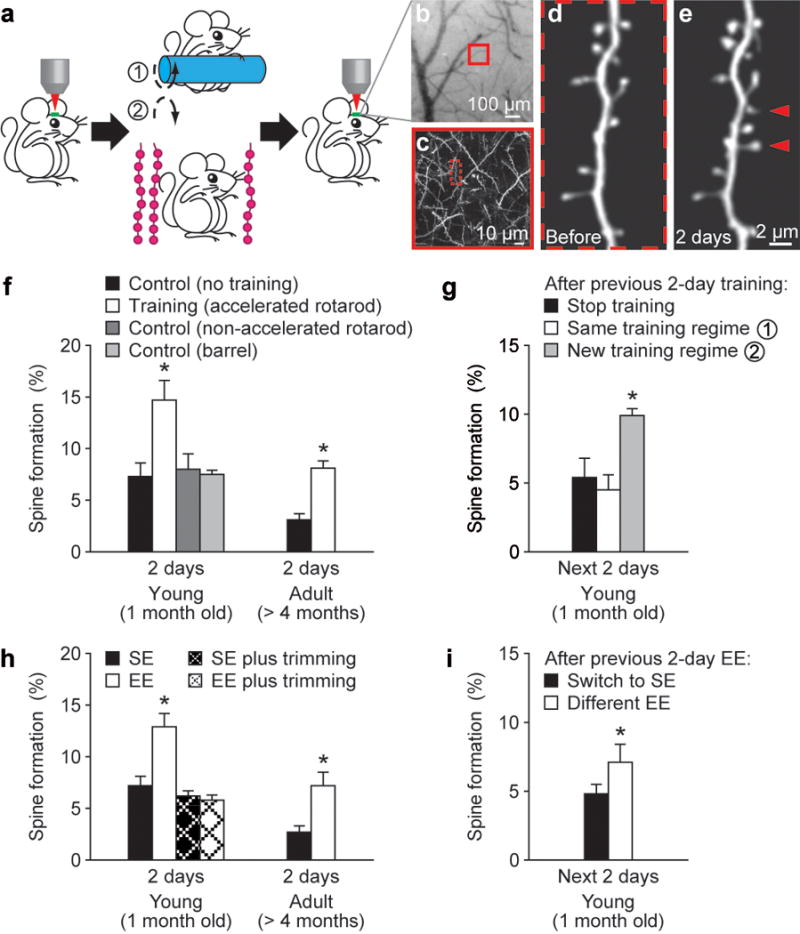
a, Transcranial two-photon imaging of spines before and after rotarod training or sensory enrichment. b, CCD camera view of the vasculature of the motor cortex. c, Two-photon image of apical dendrites from the boxed region in b. A higher-magnification view of a dendritic segment in c is shown in d. d–e, Repeated imaging of a dendritic branch before (d) and after rotarod training (e). Arrowheads indicate new spines formed over 2 days. f, Percentage of new spines formed within 2 days in the motor cortex was significantly higher in young or adult mice after training as compared with controls with no training or running on a non-accelerated rotarod. No increase in spine formation was found in the barrel cortex after training. g, After previous 2-day training, only a new training regime (reverse running) caused a significant increase in spine formation. h, EE increased spine formation over 2 days in the barrel cortex in both young and adult animals. No significant increase in spine formation was found under EE when the whiskers were trimmed. i. After previous 2-day EE, animals switched to a different EE showed a higher rate of spine formation than those returned to SE. Data are presented as mean ± s.d. *P < 0.005. See Supplementary Table for the number of animals in each group.
To further understand experience-dependent spine plasticity, we examined the impact of novel sensory experience on spine formation in the barrel cortex, the primary somatosensory area for whisker sensation, by switching animals from a standard housing environment (SE) to an enriched environment (EE) (see Methods). When either young or adult mice were switched from SE to EE, spine formation over 1–2 days was significantly (~5%) higher than that under SE (Fig. 1a, h; P < 0.001; Supplementary Fig. 1). After being housed in an EE for 2 days, spine formation over the next 2 days remained significantly higher if mice were housed in a different EE than if mice were switched from EE to SE (P < 0.005; Fig. 1i). Notably, sensory deprivation by whisker trimming prevented the increase in spine formation associated with EE over 2 days (P > 0.2; Fig. 1h). Thus, novel sensory whisker experience, not simply exploratory activity of the animals under EE, induces new spine formation in the barrel cortex. It is worth mentioning that regardless of animals’ ages, neither EE nor motor learning increased the number of new dendritic filopodia, spine precursors7,8,24, over 2 days (Supplementary Fig. 2). Together, these findings indicate that at different stages of animals’ lives, learning and novel sensory experience induce rapid and extensive spine formation in functionally relevant cortical regions.
To gain insights into the functional significance of new spines, we examined the maintenance of new spines under various conditions (with or without skill learning, housed under EE or SE). We found that regardless of the animals’ ages or conditions, a small fraction of new spines formed over 2 days remained over next 2 weeks while most new spines (>75%) were eliminated (Fig. 2a–c and Supplementary Fig. 3). Interestingly, a significantly larger fraction of new spines lasted over 2 weeks when the mice were trained for 4–14 consecutive days than when they were trained for only 2 days (P < 0.05; Fig. 2b). Similarly, a larger fraction of new spines remained if mice continued to stay in the EE than if they were switched from EE to SE or stayed under EE but with their whiskers trimmed (Fig. 2c). Thus, although new spines are rapidly induced by novel experience (Fig. 1f, h), only a small fraction of them are maintained over weeks by a protracted process facilitated by persistent experience.
Figure 2. A fraction of newly formed spines persists over weeks and correlates with performance after learning.
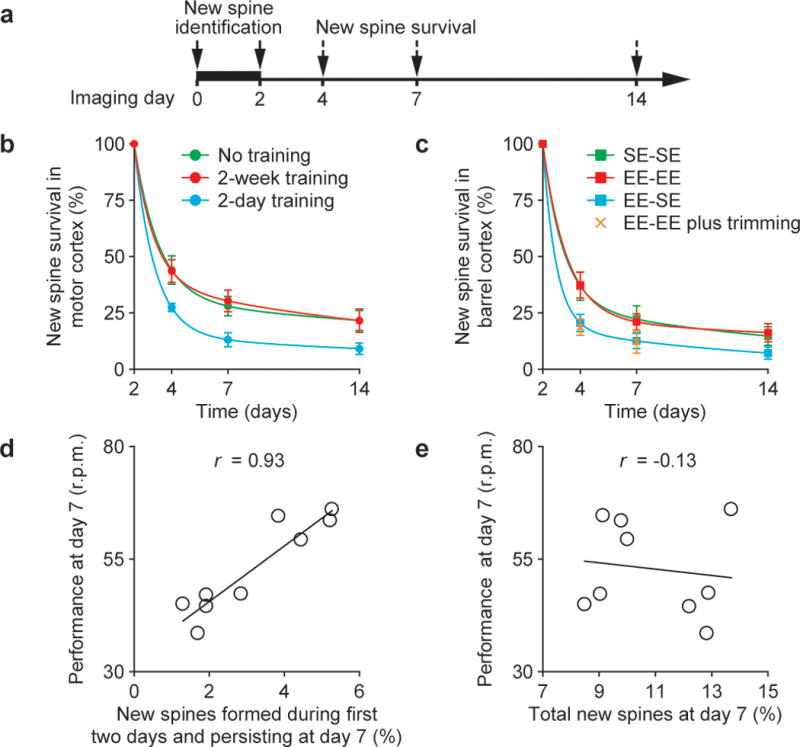
a, New spines induced by novel experience were identified in the first 2 days and followed over time. b, c, The survival of new spines (mean ± s.d.) over time under various conditions. A significantly larger fraction of new spines remained in mice trained repeatedly or housed under EE continuously. The lines represent two exponential fittings (r2 = 1). d, e, An animal’s performance at day 7 strongly correlated with new spines formed during the first 2-day training and persisting at day 7 (d), but did not correlate with the total new spines accumulated from day 0 to 7 (e). Each circle represents an individual animal. The linear regression lines and correlation coefficients (r) are shown.
Many lines of evidence suggest that functional reorganization of mammalian cortex associated with motor and sensory training consists of a fast phase (within an individual training session) and a slow phase (between training sessions)22,25. The improvement of performance between sessions reaches a plateau over days to weeks and can persist for months to years20,22,23,25. The survival of a fraction of new spines for weeks suggests that they may be important for slow-phase learning and memory retention. Indeed, we found that the proportion of new spines that were formed within the first 2 days and remained at day 7 highly correlated with the retention of learned motor skills, as quantified by the average running speed that mice mastered on an accelerated rotarod (r = 0.93; Fig. 2d and Supplementary Fig. 4). In contrast, the extent of new spines accumulated from the beginning of training until day 7 did not correlate with motor skill performance (r = −0.13; Fig. 2e), underscoring the importance of experience-specific spine formation rather than increased spine turnover in general. The strong correlation between maintained new spines and slow-phase learning suggests that new spines are important for the reorganization of cortical circuits that underlie new motor skills. Furthermore, because a fraction of new spines induced by novel sensory experience are maintained, they may be important for receptive field reorganization in barrel cortex and contribute to whisker-based decision making2,26.
In addition to promoting synapse formation, experience plays an important role in eliminating excessive and imprecise synaptic connections formed early during development3–6,9. To understand experience-dependent synaptic remodelling better, we examined the elimination of early formed spines in young mice subjected to motor training or exposed to EE. We found that in 1-month-old mice, neither motor training nor novel sensory experience increased the elimination of existing spines or filopodia over 2 days in motor or barrel cortex (P > 0.4; Fig. 3a, b and Supplementary Fig. 2). However, a significant increase in spine elimination (~4.5%) was observed in motor cortex when mice were subjected to training for 7–14 days (P < 0.05; Fig. 3a). Similarly, more spines were eliminated in barrel cortex if mice continued to stay in EE for 7–30 days than if they were switched from EE to SE or stayed under EE but with their whiskers trimmed (P < 0.05; Fig. 3b). Furthermore, we found that the elimination of spines that have existed for at least 2 days was increased by new experience over 4–5 days (P < 0.05; Fig. 3c). Because the spines in this pool have likely all made synaptic contacts with axonal terminals15,24, these results suggest that new experience leads to the pruning of existing synapses and could cause significant functional changes in cortical circuits. Indeed, we found that 1 week after motor training, motor performance strongly correlated with the degree of spine elimination (r = 0.94; Fig. 3d). Thus, motor learning and novel sensory experience involve not only new spine formation but also permanent removal of connections established early in life (Supplementary Fig. 5).
Figure 3. Novel experience promotes spine elimination.
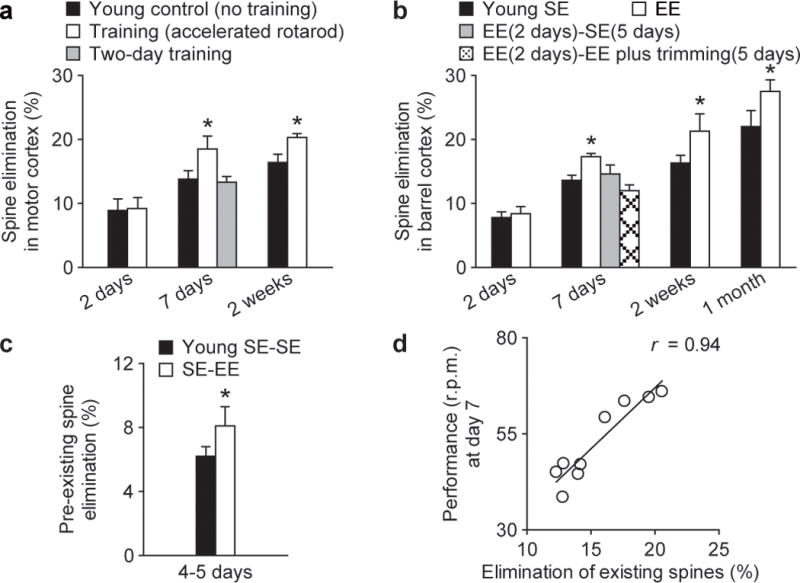
a, b, Percentage of spines eliminated (mean ± s.d.) in young animals under various conditions. Rotarod training (a) or EE (b) for at least 7 days increased the elimination of existing spines (P < 0.05). c, EE increased the elimination of spines that existed for more than 2 days before EE exposure (P < 0.05). d, The elimination of existing spines over 7 days strongly correlated with an animal’s performance on day 7 (r = 0.94). Each circle represents an individual animal.
Although the above findings are consistent with the general notion that structural synaptic plasticity is critical for learning and memory, they raise a fundamental issue about how ongoing experience-induced synaptic reorganization can be reconciled with the stability needed to support lifelong memories. To address this issue, we first examined whether new spines could be maintained over a lifetime. If a significant number of new spines could last throughout an animal’s lifespan, they could directly contribute to permanent memory storage. Otherwise, lifelong memory storage cannot rely on these new spines and may involve continuous rewiring of synaptic networks.
To distinguish between these possibilities, we examined the survival of new spines over many months in motor and barrel cortices. We found that ~4–5% of new spines formed over 2 days persisted for at least 3 months in motor cortex (2 of 42 spines formed after 2-day training) and for at least 5 months in barrel cortex (2 of 50 new spines). Thus, a tiny fraction of daily formed new spines (~0.2% of the total spines) could persist for 3–5 months. Because it is difficult to measure directly and accurately a small fraction of new spines surviving over many months, we estimated long-term survival of new spines based on the fact that the accumulation of new spines depends on the formation rate of new spines and their survival fraction (Fig. 4a and Supplementary Information 1). Because the rate of spine formation is relatively constant throughout adult life (Supplementary Fig. 6) and the survival fraction of new spines is comparable under constant environment (Figs 2b, c and 5), we found that our direct measurement of new spine accumulation over time in barrel cortex can be best fitted by three exponential components with time constants of ~1.5 days, ~1–2 months and ~73–80 months, respectively (Fig. 4a and Supplementary Information 1). The first two exponential components suggest that most daily formed spines have an average lifetime of ~1.5 days and a small fraction have an average lifetime of ~1–2 months. Importantly, the third component suggests that ~0.8% of daily formed new spines have an average lifetime of ~80 months under SE and ~73 months under EE (Fig. 4a and Supplementary Information 1). Because the degree of spine formation and the survival of new spines are comparable between motor and barrel cortices, a similar degree of daily generated new spines in motor cortex are also expected to last over the entire life of an animal.
Figure 4. Maintenance of daily formed new spines and spines formed during early development throughout life.
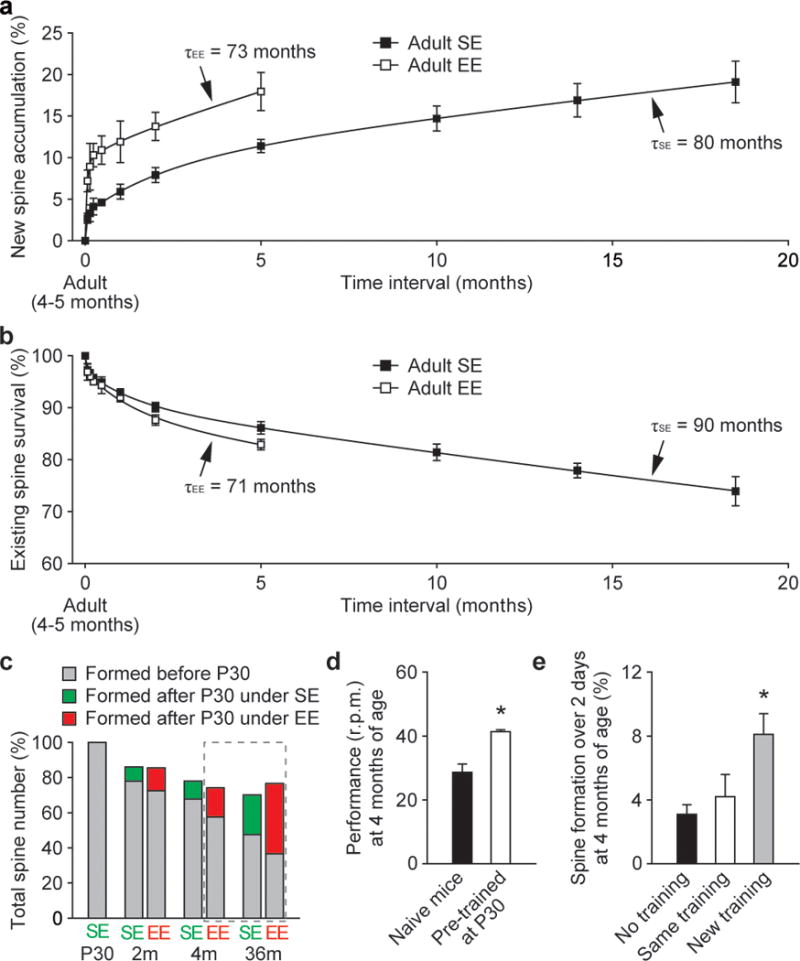
a, New spine accumulation over time under SE and EE. Three exponential fits show that ~0.8% of daily formed new spines decay with a time constant of 80 months under SE and 73 months under EE. b, The percentage of adult spines remaining over time under SE and EE. Three exponential fits show that ~90% of adult spines have an average lifetime of 90 months under SE and 71 months under EE. c, A large fraction of spines formed before P30 persisted throughout life under SE or EE. The projections based on a and b are shown in the dashed frame. d, Mice previously trained at P30 for 7 days showed better performance (mean ± s.e.m.) when assessed at 4 months of age than naive mice (P < 0.01). e, Only a new training regime (reverse running) caused an increase in spine formation in previously trained animals. Spine data are presented as mean ± s.d.
Based on the survival function of new spines and ~5–7% spine formation over 2 days under EE or motor learning conditions (Fig. 1), we estimated that the number of new spines formed over 2 days and persisting at the end of life would be ~0.04% of the total spines in motor or barrel cortex (assuming the mouse lifespan is ~36 months; Supplementary Information 2). Given the large quantity of spines in the mouse cortex, the number of learning-induced and subsequently maintained new spines could be ~2 × 106, sufficiently many to have a significant and lifelong impact on neural network functions and an animal’s behaviour27,28 (Supplementary Information 2).
Although a fraction of daily generated spines persist and could directly contribute to lifelong memory storage, it is important to note that they represent a minute portion (~0.04%) of the total spine population at the end of an animal’s life and likely have their impact on the animal’s behaviour in the context of existing circuitry rather than acting alone. Because the pruning of existing spines is an important aspect of learning (Fig. 3), this raises the question of whether early formed spines would persist throughout adult life. If a fraction of early formed spines were maintained over a lifetime, they may serve as substrates for preserving basic cortical functions and early memories. Otherwise, the physical substrates of early memories would have to be re-established in synaptic networks that are formed later in adulthood.
To address this question, we measured the survival of existing spines over many months in barrel cortex under SE and EE. We found that in 4-month-old adult mice, ~86% and ~83% of existing spines are maintained over a period of 5 months under SE and EE, respectively (Fig. 4b). Based on the survival of existing spines over 5–18.5 months, we estimated that ~90% adult spines have an average lifetime of ~90 months under SE and ~71 months under EE (Fig. 4b and Supplementary Information 3). Furthermore, we found that ~78% and ~73% of existing spines are maintained from postnatal day 30 (P30) to 2 months of age under SE and EE, respectively (Fig. 3b). Assuming a lifespan of 36 months, ~48% (under SE) and ~37% (under EE) of spines existing at P30 would remain at the end of life (Fig. 4c). Thus, regardless of housing environments, a large fraction of spines that are formed before P30 in barrel cortex would persist throughout life. Because motor learning and novel sensory experience lead to a similar degree of spine remodelling in either young or adult mice (Figs 1–3 and Supplementary Table), a large fraction of early formed spines are also expected to be stably maintained in the motor cortex. Together, these results suggest that spines formed early during development and surviving experience-dependent elimination could provide a scaffold for basic cortical function and lifelong memory storage.
By examining how spines reorganize and maintain in response to novel experiences (Figs 1–4), our studies have revealed the existence of two populations of stable spines in synaptic circuits. One population constitutes new spines specifically induced by novel experience and maintained later in life. The other population comes from a large spine pool formed during early postnatal development, pruned by developmental experiences and surviving throughout adulthood. Because spines in both populations have an average lifetime between 70 and 90 months (Fig. 4a, b), ~60–70% of them could persist over an animal’s life and directly support lifelong memories in synaptic circuits.
One prediction from such a synaptic model of memory storage is that information should still be maintained even though ~30–40% of synapses in the circuitry are lost. To test this experimentally, we trained animals on the rotarod task from P30 to P37 and tested their performance at 4 months of age, when ~30% of spines that existed at P30 were eliminated in barrel and motor cortices (Fig. 4c and Supplementary Table). We found that animals previously trained at P30 could still maintain their learned motor skills when tested again at 4 months of age (Fig. 4d). Notably, the same training regime did not result in a significant increase in spine formation over 2 days in these previously trained mice (P > 0.2), whereas a different training regime did (P < 0.02) (Fig. 4e). These findings are consistent with the above synaptic model of memory storage, suggesting that dynamic (~30% spine loss) but largely stable circuits could maintain previously acquired skills.
By studying spine dynamics of layer V pyramidal cell apical dendrites, our results suggest that spine maintenance is a fundamental feature of neural circuits important for memory storage. However, it remains unclear whether the same rule regulating spine dynamics on layer V apical dendrites applies to spines in other cell types or cortical layers or regions. As shown below, by analysing age-dependent developmental profiles of spine number, we found evidence that stably maintaining a fraction of new spines and spines formed early in life is likely a general rule for lifelong information storage in the cortex.
Many lines of evidence indicate that developmental change in synapse number is remarkably similar across different cortical layers and regions in a variety of species7,8,29,30. We found that in the dendrites of layer V and VI pyramidal neurons in mouse barrel cortex, the number of spines rose rapidly after birth, underwent a substantial net loss during late postnatal life and declined slowly throughout adulthood (Fig. 5a–c). Importantly, in the apical dendrites of layer V pyramidal cells, we found that the substantial net loss of spines during postnatal development was due to a combination of two factors: (1) a tremendous burst in spine formation early in life was followed by a rapid decline in spine formation from P19 to P30 (Fig. 5d); and (2) regardless of developmental stages, only a small fraction of newly formed spines were maintained by a similar prolonged process (Fig. 5e and Supplementary Fig. 7). Specifically, a substantial net loss of spines occurred during the late postnatal period because early formed spines (before P19) continued to be eliminated from P19 to P60 at a rate higher than that of new spine addition. The remarkably similar patterns of developmental spine loss in different cortical layers and species suggest that both a rapid decline in spine formation and maintenance of a fraction of new spines by a prolonged process are general rules in the development of the mammalian cortex (Supplementary Information 4).
Figure 5. Spine maintenance in different cell types and cortical layers.
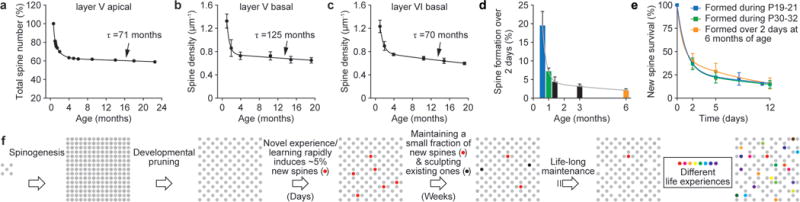
a–c, Age-dependent change in spine number is remarkably similar across different cell types/cortical layers in barrel cortex and contains information on spine dynamics. Total spine number (percentage of P19) of layer V pyramidal cell apical dendrites (a) was measured through in vivo imaging. Spine densities (mean ± s.e.m.) of layer V and layer VI pyramidal cell basal dendrites (b, c) were measured on dendritic segments located 50–100 μm from the soma in fixed brain slices. d, Spine formation rate declined rapidly from P19 to P30 and remained low thereafter. e, Regardless of animals’ ages (P19, P30, 6 months), a fraction of new spines formed over 2 days were maintained over a similar protracted process. f, Schematic summary of spine remodelling and maintenance throughout life. Spines are rapidly formed after birth, undergo experience-dependent pruning during postnatal development and remain largely stable in adulthood. Learning or novel sensory experience induces rapid formation of new spines (~5% of total spines) within 1–2 days. Only a tiny fraction of new spines (~0.04% of total spines) survive the first few weeks in synaptic circuits and are stably maintained later in life. Novel experience also results in the pruning of a small fraction of existing spines formed early during development. New stable spines induced by novel experience, together with existing spines formed during early development and surviving experience-dependent pruning, provide an integrated and stable structural basis for lifelong memory storage, despite ongoing plasticity in synaptic networks.
If stably maintaining a fraction of new spines by a prolonged process is a common rule, do most adult spines in other cells and layers persist as those of layer V pyramidal cell apical dendrites? Assuming that spine formation is constant throughout adulthood and all spines that survive a prolonged process have the same average lifetime, τ, the total number of adult spines would change according to the equation A + Be−t/τ (Supplementary Information 5). Based on the gradual decline in spine number of apical dendrites of layer V pyramidal cells (Fig. 5a), we estimated that the average lifetime of adult spines is ~71 months. This number is highly comparable to the average lifetime of new stable spines (Fig. 4a; 73–80 months) or existing spines (Fig. 4b; 71–90 months) that we measured with the in vivo imaging approach. Furthermore, based on the age-dependent decline in spine density of basal dendrites of layer V and VI pyramidal cells (Fig. 5b, c), we estimated that the average lifetime of adult spines on these dendrites is ~70–125 months. Together, these projections suggest that (1) developmental profiles of spine number contain important information on spine dynamics, and (2) most adult spines in other cell types and cortical layers could be stably maintained and serve as substrates for long-term information storage.
Determining how long-lasting memories are stored in neuronal circuits remains a great challenge. Because synapses undergo rapid changes in response to environmental perturbations, it is unknown how dynamic synaptic circuits maintain indelible memories. Here we show that, despite ongoing circuit plasticity, two populations of stable spines are important for maintaining lifelong memories. Specifically, our findings suggest that a minute fraction of new spines (~0.04% of total spines) induced by novel experience, together with spines formed early during development and remaining after experience-dependent pruning, represent a unique and stable physical entity for lifelong memory storage (Fig. 5f and Supplementary Discussion). The fact that most spines in such an entity persist underscores the fundamental importance of stably connected synaptic circuits in lifelong memory storage.
METHODS SUMMARY
Mice expressing YFP (H-line) were used in all the experiments. Sensory enrichment was conducted by placing mice in standard mouse cages containing strings of beads whose positions were changed daily. Motor training was performed by placing mice on an accelerated motorized rod. The rotation speed was recorded when the animal could not keep up with the rotating-rod and fell. The performance was measured as the average speed animals achieved during the 20-trial training session per day. The procedure of in vivo transcranial two-photon imaging, spine density measurement and data quantification was described previously7,8. P values were calculated using Student’s t-test.
METHODS
Experimental animals
Mice expressing YFP in Layer V pyramidal neurons (H-line) were purchased from the Jackson Laboratory and group-housed in the Skirball animal facilities. All experiments were done in accordance with institutional guidelines.
Sensory enrichment
Sensory enrichment was conducted in standard mouse cages containing strings of beads hanging from the top of the cages (Supplementary Movies 1 and 2). The positions of bead strings were changed daily. Mice could move freely in these cages and had to navigate through the strings of beads to obtain food and water.
Rotarod training procedure
An EZRod system with a test chamber (44.5 cm × 14 cm × 51 cm, Accuscan Instruments) was used in this study. Animals were placed on the motorized rod (30 mm in diameter) in the chamber. The rotation speed gradually increased from 0 to 100 r.p.m. over the course of 3 min. The time latency and rotation speed were recorded when the animal was unable to keep up with the increasing speed and fell. Rotorod training/testing was performed in one 30-min session per day (20 trials in total). Performance was measured as the average speed animals achieved during the 20 trials. For control experiments, animals were either trialled 20 times by placing them on the still rod for 2 min, then dropped to the bottom of the chamber (no-training control), or by forcing them to run on the rod rotating at a constant speed of 15 r.p.m. (non-accelerated rotarod control, 60 min in total with a 20-s break every 5 min). A reverse running regime was introduced to provide pre-trained mice with a new motor learning experience. In this regime, animals were forced to run backwards on the rotating rod (speed increased gradually from 0 to 50 r.p.m. over 3 min) for 20 trials.
Identification of the forelimb region of the motor cortex and the barrel cortex
The location of imaging in the motor cortex is 1.3 mm anterior to the bregma and 1.2 mm lateral from the midline. In a previously published study31, this region has been identified through microstimulation as the location of forelimb representations in the same mouse strain as we used in our study. We confirmed this region of forelimb representations by microstimulation in our own hands. In addition, the location of imaging in the barrel cortex is 1.1 mm posterior to the bregma and 3.4 mm lateral from the midline. We have previously confirmed this location is within the barrel cortex using cytochrome oxidase staining9. Because our imaging window was rather small (200 μm × 200 μm), we chose to use stereotaxic coordinates of previously mapped forelimb and barrel regions as the guide to study spine dynamics in motor and barrel cortices.
In vivo transcranial two-photon imaging
The degree of spine formation and elimination was obtained from longitudinal studies by imaging the mouse cortex through a thinned-skull window. Because thinning the skull to ~20 μm at each imaging session without damaging the cortex becomes difficult after several chronic imaging sessions, we designed our experiments such that the same animals were imaged no more than four times. For the measurement of new spine survival in Fig. 2b, c, most but not all of the data came from chronic imaging of the same mice. For the measurement of new spine accumulation and existing spine survival in Fig. 4a, b, a total of 57 animals were used (most of them were imaged twice, eight of them were imaged three or four times).
The surgery and imaging procedures are described below.
Anesthetize the mouse with an intraperitoneal injection of ketamine/xylazine mix (20 mg ml−1 ketamine, 3 mg ml−1 xylazine in saline, 5–6 μl g−1 body weight).
Carefully shave the hair of the scalp with a double-edged razor blade. Make a midline incision of the scalp with sterile surgical scissors. The incision should extend from the middle of the ears to the frontal area.
Remove the periosteum tissue with a microsurgical blade. The brain area to be imaged was localized based on the stereotactic coordinates and marked with a fine marker.
Place a small amount of glue around the edges of the internal opening of the skull holding plate and press it against the skull for a few seconds. Make sure that the area to be imaged is exposed in the centre of the internal opening of the skull holding plate.
Wait approximately 5 min until the plate is stably glued to the skull and then place the mouse on a cotton pad on top of an optional heating pad. Attach the skull holding plate to the skull immobilization device. Wash away unpolymerized glue with artificial cerebrospinal fluid.
Use a high-speed micro-drill to thin a circular area of skull (typically ~0.5–1 mm in diameter) over the region of interest under a dissection microscope. Drilling should be done intermittently to avoid overheating. Replace the artificial cerebrospinal fluid periodically and wash away the bone debris.
The mouse skull consists of two thin layers of compact bone, sandwiching a thick layer of spongy bone. The spongy bone contains tiny cavities arranged in concentric circles and multiple canaliculi that carry blood vessels. Remove the external layer of the compact bone and most of the spongy bone with the drill. Some bleeding from the blood vessels running through the spongy bone may occur during the thinning process. This bleeding will usually stop spontaneously within a few minutes.
After removing most of the spongy bone, use a microsurgical blade to continue the thinning process until a very thin (~20 μm) and smooth preparation (~200 μm in diameter) is achieved.
Use a conventional epifluorescence microscope to check if dendrites and spines in the area of interest can be clearly visualized at this stage. The thickness of the skull can also be directly determined by visualization of the skull with a two-photon microscope.
A CCD (charge-coupled device) camera can be used to acquire a high-quality picture of the brain vasculature, which is used as a landmark for future relocation.
Carefully move the mouse to the two-photon microscope and select an area for two-photon imaging. The selected area is then carefully identified and marked in the CCD vasculature map.
Tune the two-photon microscope to the appropriate wavelength (920 nm for yellow fluorescent protein). Imaging is achieved by using 1.1 numerical aperture ×60 water-immersion objectives.
Obtain a low-magnification stack of fluorescently labelled neuronal processes at ×1 zoom, which serves as a more precise map for relocation of the same area at later time points in addition to the CCD image of brain vasculature. The stack is typically taken within ~200 μm below the pial surface. Additional higher magnification (×3 digital zoom) images can be taken by electronically moving the imaged area.
For re-imaging the same region, find the thinned region based on the brain vasculature map. Carefully remove the connective tissue that has re-grown on top of the thinned region using a microsurgical blade, and check the image quality with the two-photon microscope. The skull may need to be re-thinned.
Use a microsurgical blade to shave the skull carefully until a clear image can be obtained.
Find the imaged region under a fluorescence microscope. Align the region according to a ×1 zoom map under the two-photon microscope, then zoom in to ×3 to align it further.
After the image is precisely aligned with the first view, take images as previously described.
Data analysis
National Institutes of Health ImageJ software was used to analyse image stacks. The same dendritic segments were identified from three-dimensional stacks taken from different time points with high image quality (ratio of signal to background noise >4:1). The number and location of dendritic protrusions (protrusion length was more than one-third the dendritic shaft diameter) were identified in each view without previous knowledge of the animal’s age, the interval between views or the order of the views. The total number of spines (n) was pooled from dendritic segments of different animals. Filopodia were identified as long, thin structures (generally larger than twice the average spine length, ratio of head diameter to neck diameter <1.2:1 and ratio of length to neck diameter >3:1). The remaining protrusions were classified as spines. No subtypes of spines were separated. Three-dimensional stacks were used to ensure that tissue movements and rotation between imaging intervals did not influence spine identification. Spines or filopodia were considered the same between views if their positions remained the same distance from relative adjacent landmarks. Spines were considered different if they were more than 0.7 μm away from their expected positions based on the first view.
Changes in cortical volume associated with motor skill learning and EE have been well documented previously32–35. It is important to note that because we measured spine dynamics on the same dendrites in the same animals over time, our measurements of spine elimination and formation were not sensitive to changes in cortical volume.
Supplementary Material
This movie file shows mice in Cage 1 which is a bead-string hanging enriched cage in which 200 strings of plastic beads were evenly hung from the top of the cage grid. The mice need to navigate through the bead-strings to get access to food and water.
This movie file shows mice in Cage 2 which is a bead-string hanging enriched cage in which 200 strings of plastic beads were evenly hung from the top of the cage grid. The mice need to navigate through the bead-strings to get access to food and water.
Acknowledgments
This work was supported by National Institutes of Health R01 NS047325 and a Dart Foundation Fellowship to W.-B.G. and by an Ellison/AFAR Postdoctoral Fellowship to G.Y. We thank members of the Gan laboratory for their comments.
Footnotes
Author Contributions G.Y. and W.-B.G. conceived the experiments. G.Y. performed and analysed most experiments on motor cortex and all the experiments on barrel cortex. F.P. conducted and analysed some of the experiments on motor cortex. G.Y. and W.-B.G. performed the data fitting. W.-B.G. wrote the manuscript.
References
- 1.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 2.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 4.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Phil Trans R Soc Lond B. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 5.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 6.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol (Lond) 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 8.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 10.Purves D, Hadley RD. Changes in the dendritic branching of adult mammalian neurones revealed by repeated imaging in situ. Nature. 1985;315:404–406. doi: 10.1038/315404a0. [DOI] [PubMed] [Google Scholar]
- 11.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 12.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 13.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 14.Kleim JA, Vij K, Ballard DH, Greenough WT. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–721. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 17.Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai HM. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 20.Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 22.Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. 2004;81:211–216. doi: 10.1016/j.nlm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 25.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 26.Celikel T, Sakmann B. Sensory integration across space and in time for decision making in the somatosensory system of rodents. Proc Natl Acad Sci USA. 2007;104:1395–1400. doi: 10.1073/pnas.0610267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arenz A, Silver RA, Schaefer AT, Margrie TW. The contribution of single synapses to sensory representation in vivo. Science. 2008;321:977–980. doi: 10.1126/science.1158391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- 29.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 31.Li CX, Waters RS. Organization of the mouse motor cortex studied by retrograde tracing and intracortical microstimulation (ICMS) mapping. Can J Neurol Sci. 1991;18:28–38. doi: 10.1017/s0317167100031267. [DOI] [PubMed] [Google Scholar]
- 32.Anderson BJ, Eckburg PB, Relucio KI. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem. 2002;9:1–9. doi: 10.1101/lm.43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond MC, et al. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J Comp Neurol. 1966;128:117–26. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- 34.Grossman AW, Churchill JD, Bates KE, Kleim JA, Greenough WT. A brain adaptation view of plasticity: is synaptic plasticity an overly limited concept? Prog Brain Res. 2002;138:91–108. doi: 10.1016/S0079-6123(02)38073-7. [DOI] [PubMed] [Google Scholar]
- 35.Kleim JA, Pipitone MA, Czerlanis C, Greenough WT. Structural stability within the lateral cerebellar nucleus of the rat following complex motor learning. Neurobiol Learn Mem. 1998;69:290–306. doi: 10.1006/nlme.1998.3828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This movie file shows mice in Cage 1 which is a bead-string hanging enriched cage in which 200 strings of plastic beads were evenly hung from the top of the cage grid. The mice need to navigate through the bead-strings to get access to food and water.
This movie file shows mice in Cage 2 which is a bead-string hanging enriched cage in which 200 strings of plastic beads were evenly hung from the top of the cage grid. The mice need to navigate through the bead-strings to get access to food and water.


