The use of various animal models for cardiovascular diseases has significantly advanced our understanding of the pathophysiology of these diseases. In addition, these animal models have been quite useful in testing novel treatment strategies. However, there are inter-species differences in manifestation of certain diseases that precludes modeling them in animals (1). Treatment strategies that work in animal models do not always translate into effective therapies in humans. For this reason, there is a critical need for using human-based cellular/tissue platforms to understand the pathological mechanisms involved and to evaluate efficient treatments. The development of technology that induces human adult fibroblasts to revert back to pluripotent stem cells by Yamanaka et al. has opened new avenues in disease modeling (2). The induced pluripotent stem cells (iPSC) can be then differentiated into a variety of cell types including cardiovascular ones, as shown in Figure 1. Despite the fact that the derived cardiovascular cells are physiologically closer to fetal and neonatal cells, a number of genetic disorders with cardiovascular manifestations have been reproduced using this technology, including hypertrophic cardiomyopathy (3), LEOPARD syndrome (4), long QT syndrome (LQTS) (5), cathecholaminergic polymorphic ventricular tachycardia (6), arrhythmogenic right ventricular dysplasia (7), and familial dilated cardiomyopathies (8). Most of these studies have focused on deriving cardiomyocytes from patient-specific iPSCs and studying the resulting phenotype. The usual approach is to obtain fibroblasts (usually skin biopsy) from specific patients with genetic diseases, to derive stem cells, and then to differentiate them into cardiomyocytes. Following a number of weeks in culture, the cardiomyocytes will develop disease features such as abnormal contractile function, dys-regulated calcium movements, altered electrical, and an increase in hypertrophic markersand can serve as a platform to model the disease. In this issue of JACC, Wang et al. (9) propose a different approach where genetic mutations are directly introduced by genome engineering in in generic embryonic stem cells or non-diseased iPSCs. The engineered pluripotent cells can then be differentiated into cardiomyocytes (as shown in Figure 2) with the hope that they will display disease features as compared to the un-edited cell lines (so called isogenic controls). This approach is supported by recent developments in site-specific genome engineering using designer nucleases (molecular scissors) that cut DNA sequences within the chromosome and then can be replaced by another sequence template. Three different types of molecular scissors have been developed which can be designed to cut at specific sites: 1) zinc finger nucleases (ZFN) (10), 2) transcription activator-like effector nuclease (11), and 3) clustered regularly inter-spaced short palindromic repeats (CRISPR) (12). These designer nucleases each have unique features, but the capability of each one to target specific DNA sequences make them a very useful tool for manipulating pluripotent cells. To support this approach, Wang et al. introduced the ion channel genes KCNQ1 and KCNH2 with dominant negative mutations causing LQTS type-1 and -2, respectively, into both ES and iPSCs (9). LQTS is a genetic-based disease disorder typified by prolonged cardiac repolarization that leads to lethal ventricular arrhythmias (13). More than 12 different types of LQTS have been defined and each due to mutations in a distinctive cardiac ion channel (13). LQTS type 1 (LQTS1) is based on a mutation in the KCNQ1 gene, which encodes the slow component of the delayed rectifier potassium current (IKs) channel, and it is characterized by a prolonged action potential duration and trafficking defect of the KCNQ1 channel. LQTS type 2 (LQTS2) is based on a mutation in the hERG gene (the human Ether-à-go-go-Related Gene) that codes for the alpha subunit of the repolarizing IKr current in the cardiac action potential. The investigators used ZFNs to place these mutated genes in a safe site within the human chromosome 19, the AAVS1 locus. AAVS1 is a well-validated “safe harbor” to host the DNA fragment with expected function (14). It has an open chromatin structure and is transcription-competent. Most importantly, there are no known adverse effects on the cell resulting from the inserted DNA fragment of interest in the AAVS1 locus (14). The investigators then derived cardiomyocytes, which displayed characteristics of LQTS phenotype including prolongation of the action-potential duration, compared to the un-edited control cells. The authors also tested pharmacological agents, such as a calcium channel blocker, Nifedipine, and a KATP channel opener Pinacidil on the derived cells. In doing so, the authors have nicely modeled the physiological features of LQTS types 1 and 2, and the developed cell lines can be used to differentiate cardiomycoytes and become test beds for novel therapeutics for LQTS.
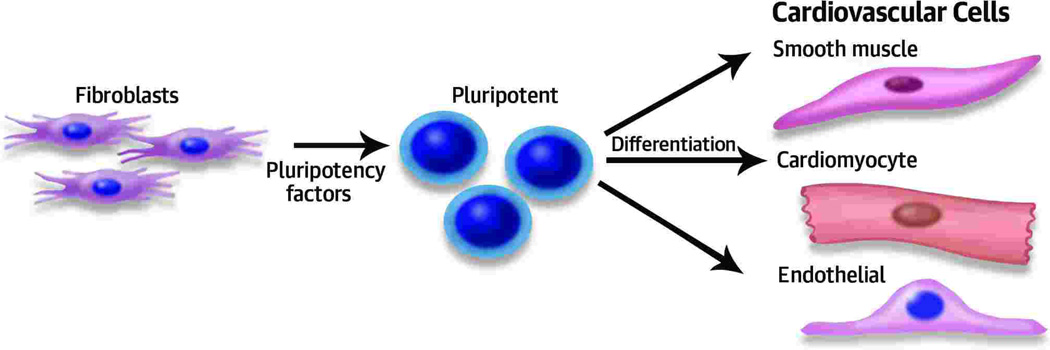
Figure 1.
Derivation of pluripotent stem cells from fibroblasts using pluripotency factors (Oct3/4, Sox2, Klf4, and c-Myc). The pluripotent cells can be differentiated into specific cardiovascular cells in specialized media.
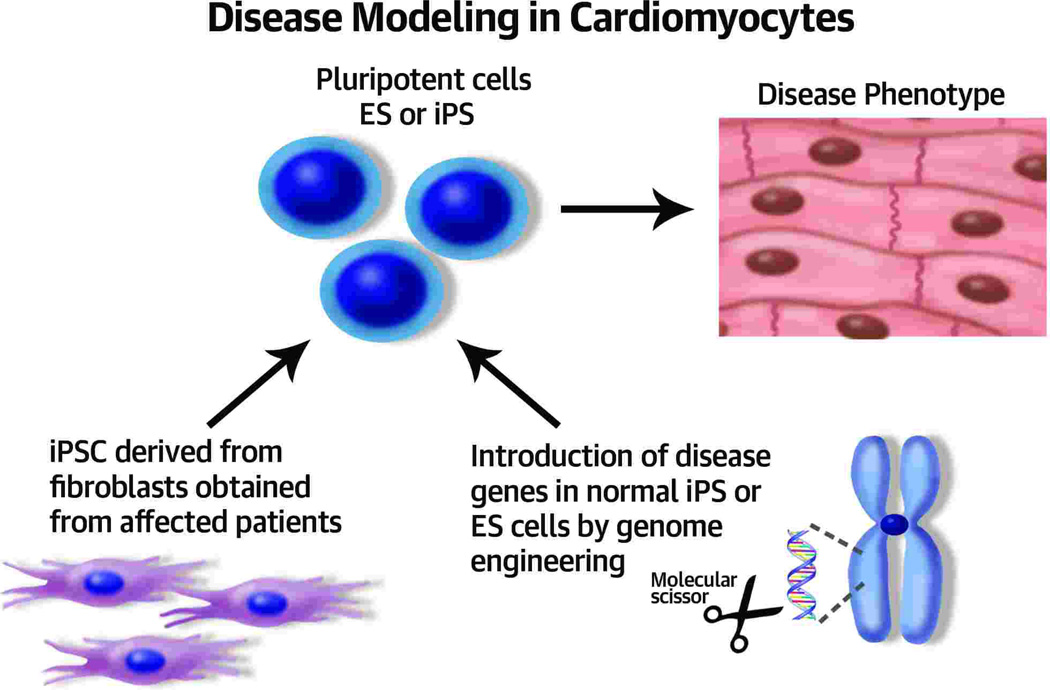
Figure 2. Disease modeling.
Fibroblasts from affected patients can be used to induce pluripotent cells which can be differentiated to cardiomyocytes which will in turn display pathological features of the disease. Sequence-specific altering tools such as zinc finger nucleases (ZFNs), Transcription Activator-Like effector Nucleases (TALENs), and the Clustered Regularly Interspaced Short Palindromic rRepeats (CRISPRs) can be used to insert mutated genes or correct them in pluripotent and stem cells.
This study offers hope that genome engineering will allow us to study the molecular mechanisms underlying the pathology of genetically based cardiovascular diseases in the dish. There are still a number of improvements that are required for this technology to be more effective in modeling cardiac disorders. In the current study, Wang et al. used a safe harbor from where to express the mutated gene while the normal genes were being expressed concurrently. Molecular scissors, such as TALENs and CRISPRs, can be used to excise the KCNQ1 and hERG genes and then through homologous recombination, the mutated genes can be replaced in the same site. These tools can also be used to make genomic replacement of a DNA fragment at a single nucleotide position. This would circumvent the effects of expressing the mutated gene in a remote site (e.g., AAVS1). The cardiomyocytes derived using iPSCs are in general immature and may not represent the physiological characteristics of adult cardiomyocytes (15). Methods to mature these cardiomyocytes both physically and by genetic editing are being actively studied. In addition, tissue engineering permits the reconstruction of three-dimensional (3D) muscles, allowing a large number of physiological parameters to be measured in these tissues (15). Finally, genetic background can strongly influence the clinical phenotype caused by diseased genes. Re-introducing specific mutations in genes of interest in a generic genome is a wonderful approach to speed up the development of functional genomic studies but will provide limited information on the role of modifying and/or interacting genes.
The study by Wang et al. represents an exciting first step in producing cardiac cells that recapitulate LQTS by inserting the mutated genes in safe harbor sites. It will be important to develop new methods for reconstructing the 3D design of adult vascular and myocardial tissue to allow the measurements of meaningful physiological parameters. The future of drug discovery in cardiology will depend not on these human models of genetically based cardiovascular diseases but also on acquired forms of cardiac disorders.
Acknowledgments
This work is supported by NIH R01 HL117505, HL 119046 and P50 HL112324 (to RJH) and R01 HL113497 (to JSH).
Footnotes
Disclosures: Both authors declare no conflicts of interest.
References
- 1.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. P Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Lan F, Lee AS, Liang P, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvajal-Vergara X, Sevilla A, D'Souza SL, et al. Patient-specific induced pluripotent stem-cell-derived models of leopard syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itzhaki I, Maizels L, Huber I, et al. Modelling the long qt syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 6.Novak A, Barad L, Zeevi-Levin N, et al. Cardiomyocytes generated from cpvtd307h patients are arrhythmogenic in response to beta-adrenergic stimulation. J Cell Mol Med. 2012;16:468–482. doi: 10.1111/j.1582-4934.2011.01476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C, Wong J, Wen J, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific ipscs. Nature. 2013;494:105–110. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun N, Yazawa M, Liu J, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Liang P, Lan F, et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long qt phenotype for drug testing. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.04.057. Current Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urnov FD, Rebar EJ, Holmes MC, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 11.Juillerat A, Dubois G, Valton J, et al. Comprehensive analysis of the specificity of transcription activator-like effector nucleases. Nucleic Acids Res. 2014;42:5390–5402. doi: 10.1093/nar/gku155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10:957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz PJ, Ackerman MJ, George AL, Jr, et al. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiyaboonchai A, Mac H, Shamsedeen R, et al. Utilization of the aavs1 safe harbor locus for hematopoietic specific transgene expression and gene knockdown in human es cells. Stem Cell Res. 2014;12:630–637. doi: 10.1016/j.scr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull IC, Karakikes I, Serrao GW, et al. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28:644–654. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]