Abstract
Numerous studies have explored whether the antibody response to influenza vaccination in elderly adults is as strong as it is in young adults. Results vary, but tend to indicate lower post-vaccination titers (antibody levels) in the elderly, supporting the concept of immunosenescence – the weakening of the immunological response related to age. Because the elderly in such studies typically have been vaccinated against influenza before enrollment, a confounding of effects occurs between age, and previous exposures, as a potential extrinsic reason for immunosenescence.
We conducted a four-year study of serial annual immunizations with inactivated trivalent influenza vaccines in 136 young adults (16 to 39 years) and 122 elderly adults (62 to 92 years). Compared to data sets of previously published studies, which were designed to investigate the effect of age, this detailed longitudinal study with multiple vaccinations allowed us to also study the effect of prior vaccination history on the response to a vaccine.
In response to the first vaccination, young adults produced higher post-vaccination titers, accounting for pre-vaccination titers, than elderly adults. However, upon subsequent vaccinations the difference in response to vaccination between the young and elderly age groups declined rapidly. Although age is an important factor when modeling the outcome of the first vaccination, this term lost its relevance with successive vaccinations. In fact, when we examined the data with the assumption that the elderly group had received (on average) as few as two vaccinations prior to our study, the difference due to age disappeared.
Our analyses therefore show that the initial difference between the two age groups in their response to vaccination may not be uniquely explained by immunosenescence due to ageing of the immune system, but could equally be the result of the different pre-study vaccination and infection histories in the elderly.
Keywords: influenza vaccine, elderly, vaccine efficacy, immunosenescence, repeated vaccination
1. Introduction
The World Health Organization and many national health authorities recommend yearly influenza vaccination for people at risk of developing serious complications, including elderly persons over a defined age limit (typically 60 or 65 years). Various studies have described lower serological responses to vaccination in elderly than in young human adults [1–4]. For example, Beyer et al. [5] described how ten studies revealed a better immune response in young subjects than in elderly, 16 could not detect a significant difference, and four found an increased response in the elderly. Another quantitative meta-analysis of 31 studies consistently found lower seroprotection and seroconversion rates in the elderly compared to younger adults [6], findings that are in agreement with results from a database of 48 serological trials performed for regulatory purposes [6, 7]. Thus most but not all published studies of serological comparisons report a lower antibody response to influenza vaccination in the elderly than in the young adults. A weakened immunological response related to age is known as immunosenescence, and this explanation is commonly used to explain the lower antibody response in elderly cohorts to vaccination.
Here we consider two different mechanistic drivers for immunosenescence. One mechanism concerns intrinsic drivers towards immunosenescence based on the ageing of the immune system, a complex process that is not yet fully understood, and may involve the age-dependent functioning of T-cells and a decreased output of naïve T-cells as a result of involution of the thymus [8–11]. Such an intrinsic immunosenescent process has been observed in studies of influenza-naïve rhesus macaques, where ageing results in declined antibody response to influenza vaccination [12, 13].
The effects of such intrinsic immunological drivers may be compounded by extrinsic, or environmental, drivers of immunosenescence. An example of such an environmental contribution towards immunosenescence in reactions to influenza vaccine is previous infection with cytomegalovirus (CMV). CMV antibodies have been reported to increase pro-inflammatory potential, which contributes to unresponsiveness of the immune system. Because the presence of CMV antibody strongly correlates with age, this would also explain lower serological responses to vaccination against influenza in the elderly [14–16]. Similarly, studies on the effect of repeated vaccination in the elderly have proposed the explanation that prior vaccination may attenuate subsequent immune responses upon re-exposure to influenza [17, 18].
Because humans partaking in vaccination studies are not naïve to influenza infection and their history of vaccination prior to enrollment is typically unknown, it is difficult to establish the relative contribution and possible interdependence of age and exposure history on immunosenescence. We designed a four-year cohort vaccination study to delineate the intertwined effect of age and repeated exposures on the response to influenza vaccination.
2. Materials and methods
2.1. Subjects and study design
The study was performed from 1996 to 1999 in healthy community-dwelling young and elderly adults living in Hampton Roads, Virginia, United States. The young adults had never received influenza vaccine, and older adults may have been vaccinated previously, but not for at least two years prior to their enrollment in the study. Subjects consented upon enrollment to participation for the duration of the study. The Institutional Review Board of Eastern Virginia Medical School, Norfolk, VA, approved the study protocol and informed consent form. All study participants received an intramuscular injection of the standard dose of trivalent seasonal influenza vaccine (Fluzone®, Sanofi) in each of the study years in which they were enrolled. The health status of all participants of both age groups was very good. All subjects were contacted in the fall of each year to schedule a vaccination visit. Post-vaccination follow-up visits were scheduled in October of each year. Blood samples included 5 cc of serum collected just prior to vaccination, and four weeks post-vaccination.
142 healthy young adults (20–40 years) and 122 healthy older adults (≥ 65 years) completed the study, i.e., their sequence of vaccinations was uninterrupted during the years, and their pre- and post-vaccination antibody titers were available for all vaccination events and influenza strains involved. The two age groups consisted of four cohorts each, as each year a new cohort of young and elderly adults entered the study. Table 1A shows the numbers of vaccinees per year and cohort. The vaccine strains changed once for each of the three (sub)types in the course of the study, as shown in Table 1B.
Table 1.
| A. Numbers of volunteers, according to age, year of entering the study, and vaccinations within the study. The table also shows the compliance of participants during the study. For example, the cohort of young adults started with 55 young individuals in year 1 (1996), of whom 30 also participated in the second year, 22 in the third year, and 18 in the final year (diagonal). | ||||||
|---|---|---|---|---|---|---|
| Age group | Number of vaccinations (Nv) |
Number of vaccination events | ||||
| 1996 | 1997 | 1998 | 1999 | All | ||
| Young adults | 1 | 55 | 25 | 32 | 24 | 136 |
| 2 | 30 | 14 | 15 | 59 | ||
| 3 | 22 | 8 | 30 | |||
| 4 | 18 | 18 | ||||
| All | 55 | 55 | 68 | 65 | 243 | |
| Elderly adults | 1 | 33 | 42 | 32 | 15 | 122 |
| 2 | 27 | 38 | 25 | 90 | ||
| 3 | 24 | 32 | 56 | |||
| 4 | 21 | 21 | ||||
| All | 33 | 69 | 94 | 93 | 289 | |
| B. Vaccine strain for each year and subtype for both age groups. Vaccine and titration strains were taxonomically identical, except for the A-H1N1 subtype in 1997 (vaccine: A/Beijing/262/95, titration: A/Johannesburg/33/94). | |||
|---|---|---|---|
| Year | A-H3N2 | A-H1N1 | B |
| 1996 | Nanchang/933/95 | Texas/36/91 | Harbin/7/94 |
| 1997 | Nanchang/933/95 | Beijing/262/95 | Harbin/7/94 |
| 1998 | Sydney/5/97 | Beijing/262/95 | Harbin/7/94 |
| 1999 | Sydney/5/97 | Beijing/262/95 | Yamanashi/166/98 |
2.2 Serum Antibody Titres
Hemagglutination inhibition assays (HIA) were performed using a single stock source for each of the hemagglutinin antigens (supplied by Centers for Disease Control) and representing the strains of virus contained in the vaccine. HIA was performed as previously described [19] using two-fold dilutions of serum from 1/10 to 1/1024. Titers of <1/10 were calculated as 1/5. Geometric mean titers were calculated using log conversion for each dilution.
2.3. Linear regression models
Heteroscedasticity robust ordinary least squares, a type of linear regression model, was used to determine the effects of age and vaccination history on individual post-vaccination titers, Tpost, using the heteroskedasticity robust regression (option r) in Stata 12 software. In all calculations pre- and post-vaccination HI titers (Tpre- and Tpost-values) were log2-transformed logarithms of measured titer levels. For an undetectable HI titer (<10, indicating a ‘seronegative’ person), a value of 5 was imputed. Group log titer means were re-exponentiated and presented as geometric mean titers GMTs throughout the text.
The initial regression model was Tpost = A + Bpre * Tpre, where A is the y-axis intercept, Tpre the pre-vaccination titer, and Bpre the regression coefficient (additional increase in Tpost per unit increase of Tpre). Subsequently, age group (G: young adults = 0, elderly adults = 1) and number of vaccinations within the study (NV: values from 1 to 4) were then added to the regression models as independent variables: Tpost = A + Bpre * Tpre + Bagegroup * G and Tpost = A + Bpre * Tpre + Bagegroup * G + Bnv * NV. The respective regression coefficients were designated Bagegroup and Bnv. All analyses were run for the three virus (sub)types separately, and for all (sub)types combined.
3. Results
The effects of previous vaccinations and age on response to vaccination
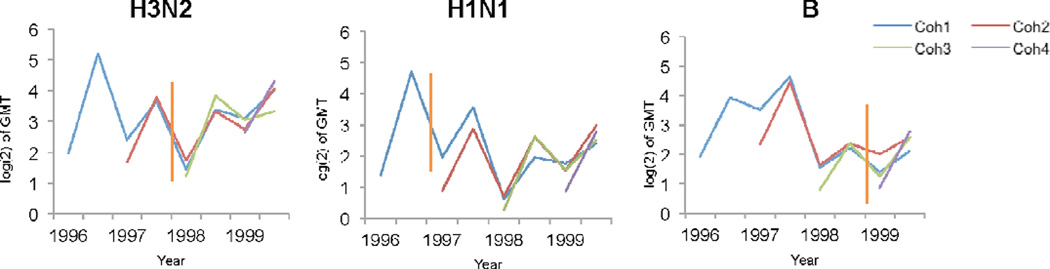
We studied the antibody levels from individuals vaccinated as part of the four year cohort, based on their hemagglutination inhibition (HI) assay titers. Figure 1 shows the geometric mean titer (GMT) values by vaccine (sub)type, year and cohort. When all titers were grouped without distinction by virus (sub)type, cohort, or trial year, the pre-vaccination GMTs were virtually identical in both age groups (16.4 in young adults versus 15.5 in elderly adults), but the post-vaccination GMTs were a little over twice as high in the young adults (80.3) than in the older adults (38.8), indicating an age effect. The A/H1N1 subtype was associated with a much larger age group ratio (3.1 ratio) than the other two vaccine components (1.6 ratio for A-H3N2 and 1.8 ratio for B).
Figure 1.
Titers as log(2) of GMT by cohort are shown for the pre-vaccination and post-vaccination data per year. There are two data points for each year in the plots, the first for pre-vaccination titer, and the second for the post-vaccination titer. Vertical lines in orange represent changes in vaccine strain.
A first look at the effect of age as an explanatory variable in regression models of the response to vaccination suggests that age is an important determinant of the titer increase induced by vaccination. In the regression model including only pre-vaccination titers as an independent variable to predict the post-vaccination titers, the predictive power of the model (R2) is 23.7%, but increases by about 7% when age is included (Table 2).
Table 2.
Regression analysis. Predicting post vaccination titer, by pre vaccination titer (left), including age as an independent variable (right).*
| Independent variables |
Estimates of regression model | Independent variables |
Estimates of regression model | ||
|---|---|---|---|---|---|
| Pre-titre | A (y-intercept) | 2.433*** | Pre-titre | A (y-intercept) | 3.535*** |
| Bpre | 0.600*** | Bpre | 0.594*** | ||
| Age | BAge | −0.021*** | |||
| R2 | 0.257 | R2 | 0.328 | ||
Notice that the inclusion of age as an independent variable contributes a highly significant effect and increases the R2.
p<0.001
As also reported in other studies [1, 4, 20], we find that the number of previous vaccinations received also has an effect on the titer increase in response to vaccination. Table 3 shows in its top section that number of vaccinations alone can explain 4% of the variance in post-vaccination titers. In a regression model with pre-vaccination titer as a predictor of post-vaccination titer, the number of previous vaccinations adds 7% to the percentage of variance explained by the model, and in a regression model with pre-vaccination titer and age group as predictors of post-vaccination titer, number of previous vaccinations adds a further 6% to the percentage of variance explained.
Table 3.
Results of regression models with post vaccination titer Tpost as the dependent variable in every case.^
| Variables | Estimates of regression model | Variables | Estimates of regression model | ||
|---|---|---|---|---|---|
| Nr vaccinations | A (y-intercept) | 3.738* | |||
| BNV | −0.369* | ||||
| R2 | 0.039 | ||||
| Pre-titre | A (y-intercept) | 2.433* | Pre-titre | A (y-intercept) | 2.777* |
| Bpre | 0.600* | Bpre | 0.642* | ||
| Nr vaccinations | BNV | −0.502* | |||
| R2 | 0.257 | R2 | 0.327 | ||
| Pre-titre Age group |
A (y-intercept) | 2.993 | Pre-titre Age group |
A (y-intercept) | 3.248* |
| Bpre | 0.592* | Bpre | 0.692* | ||
| Bagegroup | −1.006* | Bagegroup | −0.911* | ||
| Nr vaccinations | BNV | −0.448* | |||
| R2 | 0.335 | R2 | 0.390 | ||
The regression models on the right include number of previous vaccinations BNV. Notice that number of previous vaccinations has a statistically significant negative effect and contributes to the R2 in every case.
p<0.001
The age difference is limited to a difference between age groups
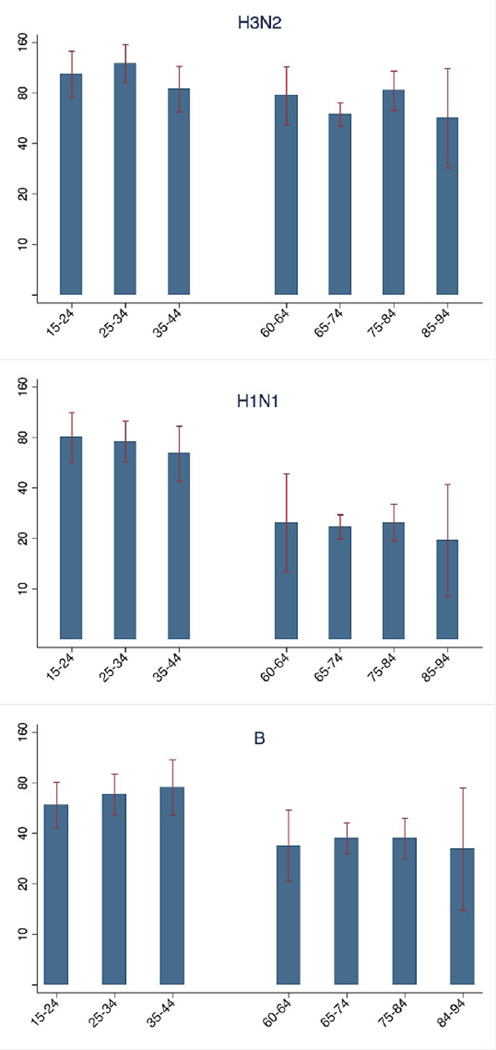
Age appears to be an important factor determining the post-vaccination titer, based on the pre-vaccination titer and number of vaccinations. We subsequently wanted to test if this age effect was also observed within an age group. Figure 2 shows the post-vaccination GMTs, per (sub)type and decade of age. While there is a clear difference between the two age groups, there is little variation in the response to vaccination within each of the two age groups. In particular, the very old persons (84–94 years old) reacted similarly to vaccination as the other elderly persons (60 – 83 years old). It is worth noting however that there are only 13 individuals in the 84–94 grouping, and that these elderly may be fitter than average as this study deliberately only includes ambulatory elderly.
Figure 2.
Post-vaccination antibody levels (GMT) for different age classes are shown.
The difference in the effect of age within age group versus between age groups is also seen in the linear regression analyses shown in Table 4. Age is an insignificant factor when regressing post-vaccination titer on pre-vaccination titer and age within either of the two age groups: it explains none of the variation in post-vaccination titer data. However, in the dataset comprising all individuals from both age groups, age can explain 7.6% of the observed post-vaccination titer variation. In summary, we find no effect of age on post-vaccination titer within the age groups, but a marked effect between the two age groups.
Table 4.
Summarized results of the regression post-vaccination titer = A + BAge* Age for each of the two age groups, and for the combined dataset.*
| Independent variables |
Estimates of regression model | |||
|---|---|---|---|---|
| Old adults | Young adults | All | ||
| Age | A (y-intercept) | 3.330* | 3.909* | 4.565* |
| BAge | 0.006 (P=0.490) | 0.003 (P=0.739) | −0.022 (P=0.000) | |
| R2 | 0.001 | 0.000 | 0.077 | |
Notice that age only has a predictive effect in the combined dataset and not within either age group.
The effect of the number of previous vaccinations on the response to vaccination
The previous analyses suggest that age is an important determinant of the response to vaccination, but the difference in response to vaccination is only present between age groups. Since young and elderly groups also differ in their vaccination history and because the results in Table 3 indicated that the number of previous vaccinations influences the response to vaccination, we further investigated the individual responses in relation to the number of administered vaccinations for each person.
Table 5A lists, for each number of previous vaccinations, the results of regressions where the post-vaccination titer was predicted from pre-vaccination titer and an “age group” term (young or old). In this analysis the first regression uses the first vaccination event recorded for all individuals, the second regression uses the second vaccination event for all those individuals that have a second vaccination, and so on until the fourth regression which only includes the first cohort, because this is the only cohort that is vaccinated four times. It can be seen in Table 5A that as subjects in the elderly adult group enter the study in any of the four years and receive their first vaccination, their post-vaccination titer will be significantly lower than the average post-vaccination titer for young adults and elderly combined (Bagegroup equals −1.46 2-fold HI units). As the vaccination history of the elderly and young groups converges over 2nd, 3rd and 4th vaccinations, the difference caused by age status declines monotonically from −1.46 to −0.43 to −0.16 to 0.075, and age group as a predictor of post-vaccination titer gradually becomes insignificant.
Table 5.
|
A Regression analysis predicting post-titre, by pre-titre and including age group, by number of vaccinations.^ | ||||||
|---|---|---|---|---|---|---|
| Independent variables |
Estimates of regression model |
Number of vaccinations (NV) | ||||
| Total | 1 | 2 | 3 | 4 | ||
| Pre-titre Age group |
A (y intercept) | 2.43*** | 3.54*** | 2.36*** | 1.79*** | 1.04*** |
| Bpre | 0.60** | 0.65*** | 0.60*** | 0.68*** | 0.84*** | |
| Bagegroup | −1.46*** | −0.43** | −0.16 | 0.075 | ||
| R2 | 0.29 | 0.42 | 0.46 | 0.73 | ||
|
B Regression analysis predicting post-titre, by pre-titre and excluding age group, by number of vaccinations. | ||||||
|---|---|---|---|---|---|---|
| Independent variables |
Estimates of regression model |
Number of vaccinations (NV) | ||||
| Total | 1 | 2 | 3 | 4 | ||
| Pre-titre | A (y intercept) | 2.43*** | 3.00*** | 2.03*** | 1.69*** | 1.09*** |
| Bpre | 0.60** | 0.56*** | 0.64*** | 0.69*** | 0.84*** | |
| R2 | 0.257 | 0.16 | 0.40 | 0.46 | 0.73 | |
p<0.01
p<0.001
Notice that the significance for age-group, and the difference in R2 between Table 4A and Table 4B decline with number of vaccinations.
Comparing the regression results of Table 5B with those of Table 5A similarly shows the vanishing age effect: this comparison reveals that age group adds to the explanatory power of the model in the first vaccination event, because the R2 increases from 0.16 to 0.29 when we include age group in the model predicting the post-vaccination titer from the pre-vaccination titer for the first vaccination. In the second vaccination event the difference only increases from 0.40 to 0.42, and for the third and fourth events the effect of age on the regression model has completely disappeared.
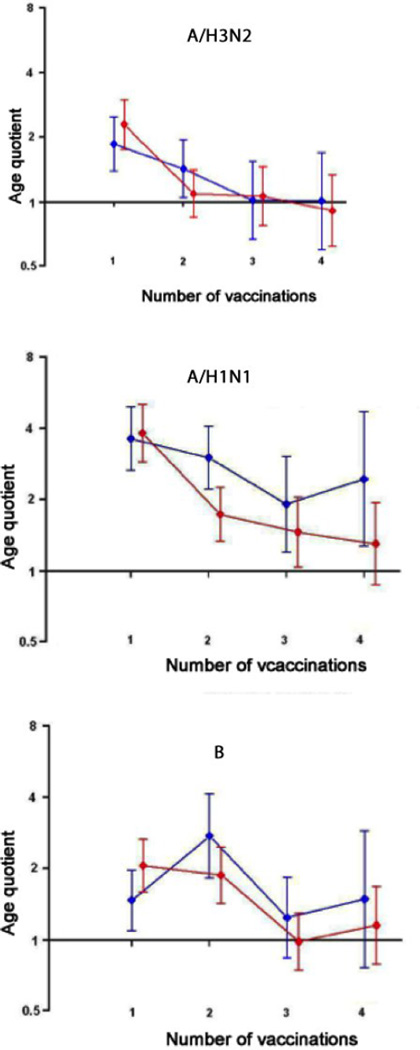
Figure 3 shows the age group ratios of young adult post-vaccination titers to elderly adult post-vaccination titers by number of vaccinations, for all three virus (sub)types. Because these age-group titer ratios may be biased by pre-vaccination level, as mean pre-vaccination titers generally rise with increasing number of vaccinations, we adjusted post-vaccination titers to correct for pre-vaccination titer levels using the Bpre estimates of the regression models [21]. The resulting adjusted age group titer ratios shown in Figure 3 (red) become more precise, as seen by narrower confidence intervals, than the unadjusted age group post-vaccination titer ratios. The age effect is observed for the first vaccination, yet declines after further vaccinations, though not monotonically, for all (sub)types. For A/H3N2, it takes only two vaccinations for the two age groups’ serological responses to converge, and for A/H1N1, four vaccinations. The same pattern of declining difference between age groups with successive vaccinations is evident in a graph of the difference between the intercepts of the regression model post vaccination titer = A + B* pre-vaccination titer, per age group and per cohort for the two age groups (Supplementary Figure S1).
Figure 3.
Young adults’ post-vaccination titers as multiples of elderly adults’ post-vaccination titers according to virus (sub)type and number of vaccinations, raw (blue) and adjusted for pre-vaccination log titer (red).
The two variables age and number of previous vaccinations are confounded
The younger adults in our study had never been vaccinated against influenza; the elderly adults may have received multiple influenza vaccinations up to two years before the study. The two age groups start with a clear difference in post-vaccination titer level after the first vaccination within the study, which may be attributable to this difference in vaccination history, rather than age: the age term will proxy some of the negative effect attributable to previous vaccination history. Since there is no parameter that independently captures history, the exposure history difference will be attributed to the only parameter that distinguishes the two groups, namely age. Thus, we next investigated whether the observed age difference is likely to be caused by age per se (intrinsic immunosenescence), or by the extrinsic effect of these previous vaccinations.
As we have seen in Table 5A and Figure 3, once individuals received two or more vaccinations within the study, the age effect disappeared. We therefore tested if the regression model could be used to estimate the unknown number of previous vaccinations in the elderly group. To this end, we varied the regression model as shown in Table 2 (right side) by replacing the number of vaccinations (NV) with a new variable, the augmented number of vaccinations (NVaug). For young adults, NVaug was the same as NV because these people had not been vaccinated prior to the study; for elderly adults however, NVaug was set to NV+2 to account for vaccinations received prior to the study.
Table 6 shows the regression results using the augmented number of previous vaccinations, and can be compared with Table 2 (right side), the same regression model using the documented, non-augmented number of previous vaccinations. The estimates for the y-intercept, the slopes for pre-vaccination titers and (augmented) number of vaccinations, and R2 were exactly the same in Table 6 and Table 2. However, the age group coefficient changed dramatically: it was highly negative in the non-augmented model (−0.911, P<0.001), but close to zero and insignificant in the augmented model (−0.014, P=0.893).
Table 6.
Regression analysis on post-vaccination log titre, by pre-vaccination log titre, augmented number of vaccinations, and age group.^
| Variables included | Estimates of regression model | |
|---|---|---|
| A (y-intercept) | 3.696* | |
| Pre-titre | Bpre | 0.630* |
| Augmented number | BNVaug | −0.448* |
| of vaccinations | Bage | −0.014 |
| Age group | (P=0.893) | |
| R2 | 0.390 | |
Notice that the coefficient on age group becomes insignificant when the augmented number of previous vaccinations is included.
P<0.001.
Comparison of these tables therefore demonstrates that age loses significance as a predictor of the response to vaccination, whilst the R2 increases when using the augmented number of previous vaccinations. A number of previous vaccinations augmented by anywhere between 1.3 and 2.3 has the same explanatory effect as a combination of the documented number of previous vaccinations and age group (Supplementary Table S1 and Supplementary Figure S2). These results show that the influence of age per se disappears when supposing two previous vaccination events in the elderly before entering the study, and that age as a predictor of the antibody titer in response to vaccination is thus equivalent to vaccination history.
4. Discussion
When antibody response after a single vaccination is studied in groups of young and elderly adults, usually a clear difference with a larger response to vaccination in young adults is observed. Importantly, in our study as well as many others, elderly participants had already been vaccinated against influenza prior to the study, to various degrees, and young participants usually had not. At least since the 1980s, vaccination of the entire elderly population is a common target in many developed and developing countries [22], where such vaccination studies are performed. Thus, it is difficult to enroll representative, previously unvaccinated, groups of elderly persons. As a result, any effect of age on the immune response is intrinsically correlated and necessarily closely linked to vaccination history.
The present cohort study where individuals were repeatedly vaccinated allowed us to analyze the effect of repeated vaccination separately from the effect of age in the same cohort study. Using these data, we inferred the effect of repeated exposure on the response to vaccination, and showed that only two vaccinations prior to the study can account for the entire observed difference between the young and elderly age groups.
A limitation of this study is the lack of reliable infection history preceding this experiment, which is expected to differ between the age groups, and the lack of data on post-vaccination infection. Serum antibody titres are a standard accepted [23] though indirect and necessarily imperfect measure of vaccine efficacy.
When an analysis leaves out an important explanatory factor, an omitted variable bias is created. Regression models compensate for the omitted variable by over- or underestimating included explanatory variables that are correlated with the omitted variable. In the present case, the lack of a variable for number of previous vaccinations is compensated for by a distortion in the correlated age variable. As a result, being elderly appears to be a determinant of the antibody response to vaccination, whereas at least part of actual determinant could be vaccination history. It should also be noted that any source of difference in exposure history, including prior infections, may lead to a similar situation, in which age and exposure history are confounded.
Our results are in line with previous studies, describing a weakened serological response to vaccination in elderly adults, i.e. an immunosenescent effect. However, we put forward the explanation that, in addition or instead of resulting from intrinsic ageing of the immune system, this immunosenescence effect could be enhanced, or more parsimoniously explained by an extrinsic driver: the previous vaccination and infection history of the elderly group, related to the yearly influenza vaccination campaigns. Of course, we do not deny the general existence of immunosenescence. It is well established that ageing modulates many immune functions. However, given the well-documented influence of repeated vaccination [20, 24, 25], it seems that comparative serological data sets containing an elderly group with an undocumented number of previous vaccinations prior to enrollment, and a vaccination-free young group, is an inaccurate way to explore immunosenescence. Indeed, a simple age effect would not explain how the titer difference between age groups changes for subsequent vaccinations. In our view, carefully controlled studies are needed to establish the effect of immunosenescence in this context, studies where either vaccination and infection history among the two age groups is comparable, or sufficient information on the history is known such that differences can be controlled for.
Supplementary Material
Acknowledgments
Supported by the NIH First Award R29AG11876 (PI: D. Powers), NIH Director’s Pioneer Award, program grant P0050/2008 from the Human Frontier Science Program, European Union FP7 program EMPERIE (22349). This work was supported by the award of a Fellowship in Biomedical Informatics from the Medical Research Council (UK) and a Junior Research Fellowship from Homerton College Cambridge to JMF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Bruijn IA, Remarque EJ, Beyer WE, le Cessie S, Masurel N, Ligthart GJ. Annually repeated influenza vaccination improves humoral responses to several influenza virus strains in healthy elderly. Vaccine. 1997;15(12–13):1323–1329. doi: 10.1016/s0264-410x(97)00019-4. Epub 1997/08/01. [DOI] [PubMed] [Google Scholar]
- 2.Glathe H, Bigl S, Grosche A. Comparison of humoral immune responses to trivalent influenza split vaccine in young, middle-aged and elderly people. Vaccine. 1993;11(7):702–705. doi: 10.1016/0264-410x(93)90252-s. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. The Journal of clinical investigation. 2011;121(8):3109–3119. doi: 10.1172/JCI57834. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology. 2007;53(6):411–418. doi: 10.1159/000110579. Epub 2007/11/03. [DOI] [PubMed] [Google Scholar]
- 5.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7(5):385–394. doi: 10.1016/0264-410x(89)90150-3. Epub 1989/10/01. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 7.Voordouw AC, Beyer WE, Smith DJ, Sturkenboom MC, Stricker BH. Evaluation of serological trials submitted for annual re-licensure of influenza vaccines to regulatory authorities between 1992 and 2002. Vaccine. 2009;28(2):392–397. doi: 10.1016/j.vaccine.2009.09.138. Epub 2009/11/03. [DOI] [PubMed] [Google Scholar]
- 8.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. The Journal of pathology. 2007;211(2):144–156. doi: 10.1002/path.2104. Epub 2007/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appay V, Sauce D, Prelog M. The role of the thymus in immunosenescence: lessons from the study of thymectomized individuals. Aging. 2010;2(2):78–81. doi: 10.18632/aging.100122. Epub 2010/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fülöp Ts. Handbook on immunosenescence : basic understanding and clinical applications. New York: Dordrecht: Springer; 2009. [Google Scholar]
- 11.Aspinall R, Andrew D. Thymic involution in aging. Journal of clinical immunology. 2000;20(4):250–256. doi: 10.1023/a:1006611518223. Epub 2000/08/12. [DOI] [PubMed] [Google Scholar]
- 12.Carroll TD, Matzinger SR, Barry PA, McChesney MB, Fairman J, Miller CJ. Efficacy of influenza vaccination of elderly rhesus macaques is dramatically improved by addition of a cationic lipid/DNA adjuvant. The Journal of infectious diseases. 2014;209(1):24–33. doi: 10.1093/infdis/jit540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coe CL, Lubach GR, Kinnard J. Immune senescence in old and very old rhesus monkeys: reduced antibody response to influenza vaccination. Age. 2012;34(5):1169–1177. doi: 10.1007/s11357-011-9356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29(29–30):4869–4874. doi: 10.1016/j.vaccine.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 15.Wald A, Selke S, Magaret A, Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. Journal of medical virology. 2013;85(9):1557–1560. doi: 10.1002/jmv.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21(25–26):3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 17.Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging and disease. 2012;3(1):68–90. Epub 2012/04/14. [PMC free article] [PubMed] [Google Scholar]
- 18.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25(16):3066–3069. doi: 10.1016/j.vaccine.2007.01.025. Epub 2007/02/06. [DOI] [PubMed] [Google Scholar]
- 19.BPD WCCfI. The hemagglutination inhibition test for influenza viruses. DHEW, PHS, CDC, Center for Infectious Disease. 1981:1–21. 1981([revised]) [Google Scholar]
- 20.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Archives of internal medicine. 1999;159(2):182–188. doi: 10.1001/archinte.159.2.182. Epub 1999/02/02. [DOI] [PubMed] [Google Scholar]
- 21.Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–6033. doi: 10.1016/j.vaccine.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 22.van Essen GA, Palache AM, Forleo E, Fedson DS. Influenza vaccination in 2000: recommendations and vaccine use in 50 developed and rapidly developing countries. Vaccine. 2003;21(16):1780–1785. doi: 10.1016/s0264-410x(03)00072-0. Epub 2003/04/11. [DOI] [PubMed] [Google Scholar]
- 23.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC medical research methodology. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(10):1375–1385. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):14001–14006. doi: 10.1073/pnas.96.24.14001. Epub 1999/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.