Background
Interventional-cardiovascular magnetic resonance (iCMR) is a promising clinical tool for adults and children who need a comprehensive hemodynamic catheterization of the heart. Magnetic resonance (MR) imaging-guided cardiac catheterization offers radiation-free examination with increased soft tissue contrast and unconstrained imaging planes for catheter guidance. The interventional MR technologist plays an important role in the care of patients undergoing such procedures. It is therefore helpful for technologists to under-stand the unique iCMR preprocedural preparation, procedural and imaging workflows, and management of emergencies. The authors report their team’s experience from the National Institutes of Health Clinical Center and a collaborating pediatric site.
Interventional-cardiovascular magnetic resonance (iCMR) was first described in 1996.1 Since then, several groups have reported magnetic resonance (MR) right-sided heart catheterization in humans, using either MR alone or in a combined fluoroscopy-MR approach in adults and children.2–5 In 2013, the authors reported the first comprehensive right-sided heart catheterization (sampling both venae cavae and pulmonary artery branches) performed entirely using MR guidance.6 Laboratory staff at the National Institutes of Health (NIH) Clinical Center have since performed more than 80 MR-guided right-sided heart catheterizations, and the procedure have been reclassified as a standard clinical procedure (not requiring research consent) offered to all eligible patients at our institution.
One advantage of MR over fluoroscopy-guided cardiac catheterization is the combination of invasive cardiac pressures with simultaneous flow CMR (velocity-encoded phase contrast) analysis of blood flow for measurements such as pulmonary vascular resistance and systemic vascular resistance. These hybrid measurements provide a more accurate comprehensive hemodynamic characterization of the heart than do traditional methods.7 In addition, versatile soft tissue contrast and unconstrained imaging plane prescriptions might allow real-time identification of complications related to catheter interaction with surrounding tissue beyond the vascular lumen. Finally, evidence of potential harm from medical radiation to pediatric and adult patients,8–10 as well as to medical staff, encourages radiation-free alternatives to reduce cumulative exposure. This is particularly important in children with complex congenital heart disease who often require multiple catheterizations.
MR-guided cardiac catheterization overcomes the limitations of traditional cardiac catheterization techniques by simultaneously measuring invasive pressures, blood flow, tissue characterization, and cardiac chamber volume in a single, radiation-free, optimized examination. For this reason, MR imaging is emerging as a promising tool for endovascular procedures. This article describes the role of the interventional MR technologist related to preprocedural preparation, procedural workflow, and contingencies for managing emergencies.
Hybrid MR Cardiac Catheterization Lab
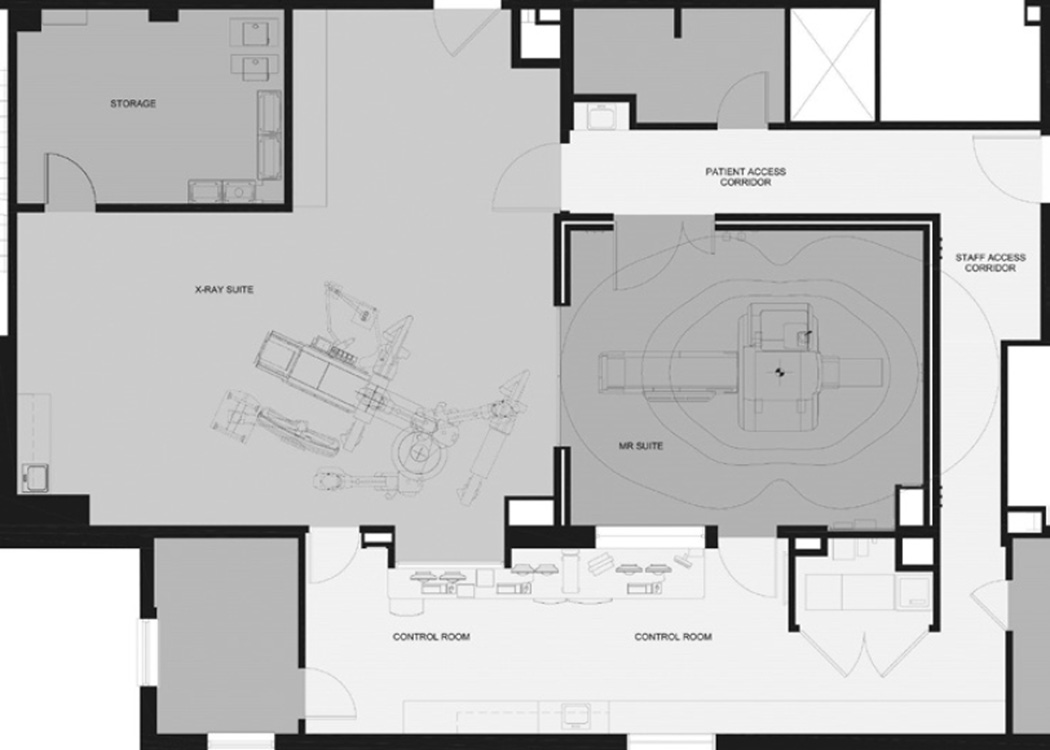
The cardiac catheterization laboratory is part of the Cardiopulmonary Branch of the National Heart, Lung, and Blood Institute and is located in the NIH Clinical Center in Bethesda, Maryland. It is configured with adjacent MR and fluoroscopy suites that include a 1.5T MR system (Aera, Siemens Healthcare), a biplane fluoroscopy interventional system (Axiom Artis Zee, Siemens Healthcare), and an intermodality transport system consisting of a dockable interventional table and transfer board (Combi Table, Siemens Healthcare) for moving the patient between the x-ray and MR sections of the cardiac catheterization suite (see Figure 1). Bay doors maintain the radiofrequency shield of the MR laboratory and contain lead to provide radiation protection when fluoroscopy is in use. With the bay doors closed, these imaging systems can be used independently. The laboratory also has a common control room for the adjoining MR and x-ray laboratories. Greater detail regarding the specific hybrid laboratory set-up and support equipment for MR-guided procedures can be found in related published literature.11 A hybrid imaging suite is not required to perform MR-guided right-sided heart catheterization, provided a nearby room (not necessarily equipped with x-ray) is available for managing emergencies.
Figure 1.
Floor plan of the National Institutes of Health hybrid magnetic resonance (MR) cardiac catheterization lab. Image courtesy of Christopher Dail, project architect, National Heart, Lung, and Blood Institute, Bethesda, MD.
Hemodynamic Recording
Cardiac catheterization requires high-fidelity hemodynamic recording systems to provide measurements for diagnosis and treatment with faster sampling rates and additional data channels than are found with commercial low-fidelity patient monitoring equipment. Furthermore, electrocardiographic (ECG) waveforms are markedly altered when the patient is in the magnet, especially during scanning. This ECG noise is caused by magnetic gradients and the ECG repolarization patterns that result from the magnetohydrodynamic effects of cardiac and aortic blood flow. No commercially available clinical high-fidelity hemodynamic recording systems for MR exist; thus, an NIH physicist developed a 9-lead (7-electrode) surface ECG that interfaces with the standard hemodynamic recording system used in the x-ray laboratory (Sensis, Siemens Healthcare). Using the same disposable pressure transducers for both x-ray and MR, the technologist can record 2 or more invasive blood pressure channels. In addition, the ECG, invasive blood pressure, or pulse oximetry signals can serve as a source for cardiac gating or triggering for MR imaging. Commercial solutions are in development by PinMed Inc.
Audio and Visual Communication
Real-time MR imaging generates excessive acoustic noise that interferes with voice communication, even when using newer shielded gradient systems. For this reason, staff at NIH use a commercial noise-cancelling wireless headset system that permits open-microphone and hands-free communication among operators. It also includes multiple channels for parallel staff-to-patient, staff-to-staff, and staff-to-control room communication (IMROC, Opto-acoustics). Because of the number of open microphones within the MR suite and control room, the physicians advocate strict audio discipline to ease communication. This involves minimizing background conversation, as well as using standardized procedural dialogue (see Table 1).
Table 1.
Dialogue for Audio Disciplinea
| Instructions | Purpose |
|---|---|
| “SVC view” or “RV view” or “PA view” | Aid in tracking catheter tip |
| “Slice 1 ON” or “Slice 2 OFF” | Aid in tracking catheter tip |
| “Sat ON” or “Sat OFF” | Activate or deactivate image saturation |
| “Pause scanning” or “Resume scanning” | Activate or deactivate real-time imaging |
| “RPA sat for pickup” | Notify RN of need for running oxygen saturation test on blood specimen |
| “RPA pressure is 25 over 10; mean 15” | Record hemodynamic data |
Sample instructions provided by physician operators to support staff.
Abbreviations: PA, posteroanterior; RN, registered nurse; RPA, right pulmonary artery; RV, right ventricular; Sat, saturation; SVC, superior vena cava.
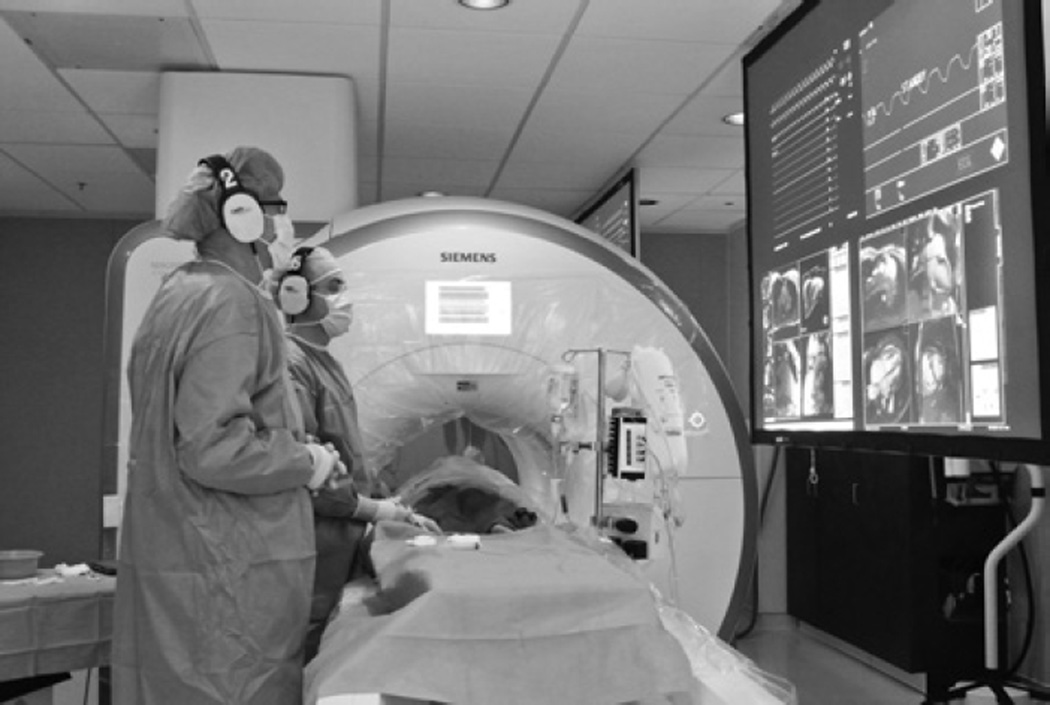
To display hemodynamic data and real-time imaging to in-room staff, the MR suite is equipped with commercial LCD projectors, shielded from radiofrequency interference, directed toward 2 projector screens positioned at the head and foot of the MR scanner. The screens enable visualization of procedural data regardless of the operator’s position within the scanner room. The physicians request prioritization of display inputs so that the most relevant data (invasive blood pressure, ECG, and real-time MR imaging) are displayed in their direct line of sight (see Figure 2). The suite also is equipped with 4 shielded video cameras sending signals to the control room to overcome potential obstructions of the control room window.
Figure 2.
The MR imaging-guided catheterization procedure setup, displaying the draped scanner, noise-suppression headsets, screen showing images from shielded in-room projectors, and ferromagnetic-free procedure table. Image courtesy of the authors.
Real-time MR Display Console
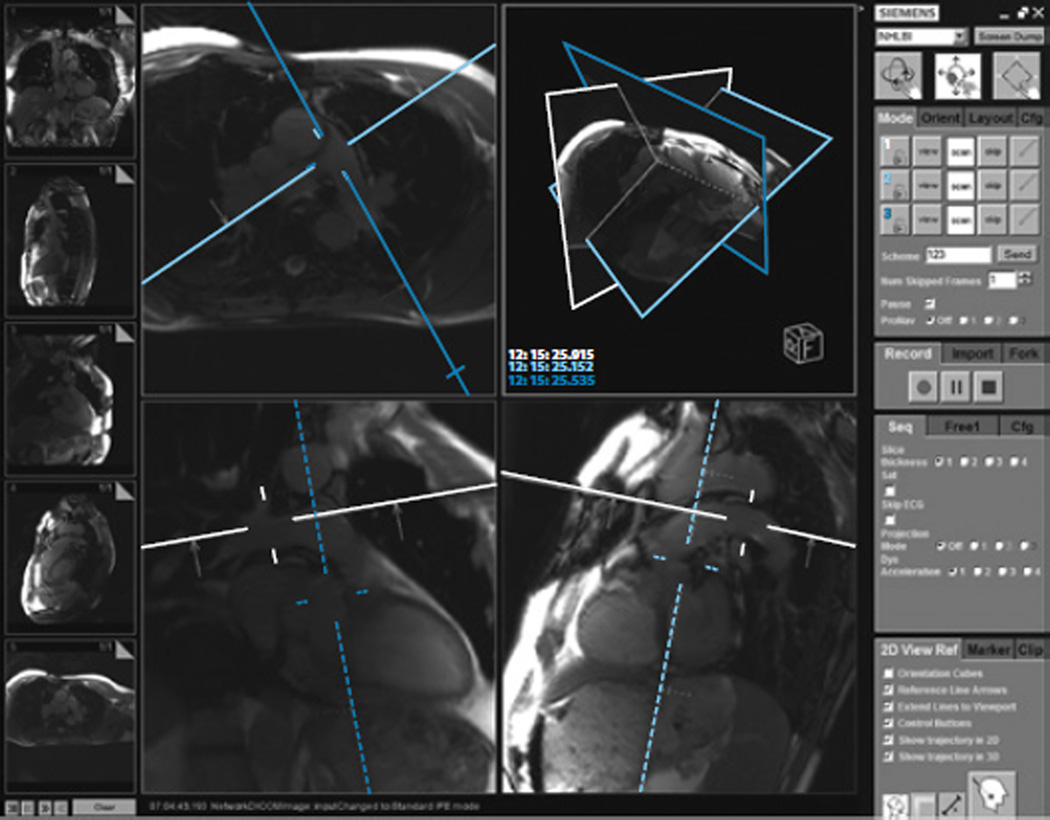
The iCMR system requires real-time visualization of imaging planes to navigate catheters through the heart. The interventional MR technologist uses an investigational real-time MR display console (Interactive Front End [IFE], Siemens Corporate Research) that runs on an independent workstation connected to the scanner host computer (see Figure 3). Similar display consoles are available from other major MR vendors (iDrive, General Electric; iSuite, Philips Healthcare). The IFE application communicates with the real-time imaging sequence, allowing interactive slice manipulation. The main viewing window displays newly acquired slices in real-time, whereas smaller viewing windows within a side panel contain saved slice prescriptions that can be returned to the main viewing/manipulation window as needed.12 NIH physicists have incorporated features, such as interactive slice thickness adjustment, interactive control of parallel imaging acceleration rate,13 and interactive saturation mode, to enhance visualization of gadolinium contrast agents by suppressing tissue signal with a saturation prepulse.
Figure 3.
Interactive Front End (IFE) software for real-time image display. Reference images are stored in the left sidebar. The main panel displays interactive images, which, in this example, are the main, right, and left pulmonary arteries. The right sidebar displays imaging parameters that can be manipulated interactively during real-time imaging. IFE is a work-in-progress by Siemens Corporate Research. Image courtesy of the authors.
The MR Catheterization Team
Traditional cardiac catheterization teams, in their most distilled form, consist of a physician, nurse, and cardiovascular technologist (CVT) or radiologic technologist. The physician serves as the lead operator for the procedure, whereas the nursing and CVT staff provide support by preparing the room, monitoring the patient, documenting vital signs and procedural details, circulating supplies, administering medications, and serving as scrubbed assistants. In the x-ray laboratory, they might be responsible for panning the patient table, adjusting collimation, and providing fluoroscopic image guidance.
In many ways, the MR catheterization team is similar to a traditional catheterization team, apart from additional staff training related to working in an MR environment and the addition of an interventional MR technologist. Furthermore, just as it is common for staff within a traditional catheterization laboratory to cross-cover one another, the interventional MR technologist should be trained to adopt the role of the CVT and be capable of performing those responsibilities in addition to MR-related duties. Optimally, the interventional MR technologist working in the iCMR setting should attain dual certification as a CVT.
Procedure Preparation
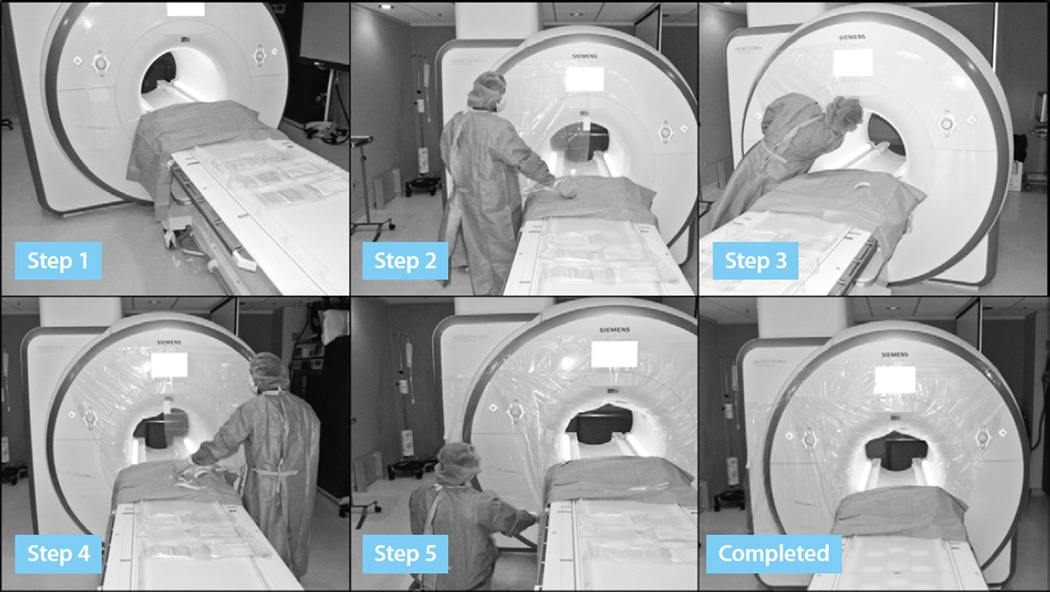
The scanner should be prepared so that gowned staff touching the bore will not contaminate the sterile field. The interventional MR technologist drapes the MR scanner with one medium-sized table drape (102 cm × 145 cm, Cardinal Health) and 3 clear façade drapes with adhesive borders (90 cm × 110 cm, 3M Healthcare), using sterile procedures. The clear drape material allows visualization of the monitor and scanner controls embedded into the scanner façade (see Figure 4). A custom-fitted drape is expected to be commercially available soon.
Figure 4.
Sterile draping of the scanner. Step 1: A table drape covers the MR table abutting the scanner bore, protecting subsequent drapes from contamination during their application. Step 2: The upper-right corner of the first clear drape is adhered to the mid-upper border of the scanner façade, approximately 16 inches above the scanner bore and unfolded as it is adhered laterally to the left. Step 3: The lower-right corner of the same clear drape is passed into the bore and adhered to the upper-inner rim of the scanner bore as far as allowable. The lower adhesive strip is adhered in a diagonal direction toward the head of the scanner table. Step 4: Steps 2 and 3 are repeated for applying the second clear drape to the left side of the scanner, ensuring overlap of the upper portions of the drapes for a continued zone of sterile coverage. Step 5: A third clear drape is applied to the scanner façade, either to the left or right of the scanner bore, depending from which side the physician will be working. The upper border of the third drape should be positioned approximately 8 inches above the scanner bore and extended laterally, providing overlap with the prior drape on the same side. Images courtesy of the authors.
MR Procedure Table
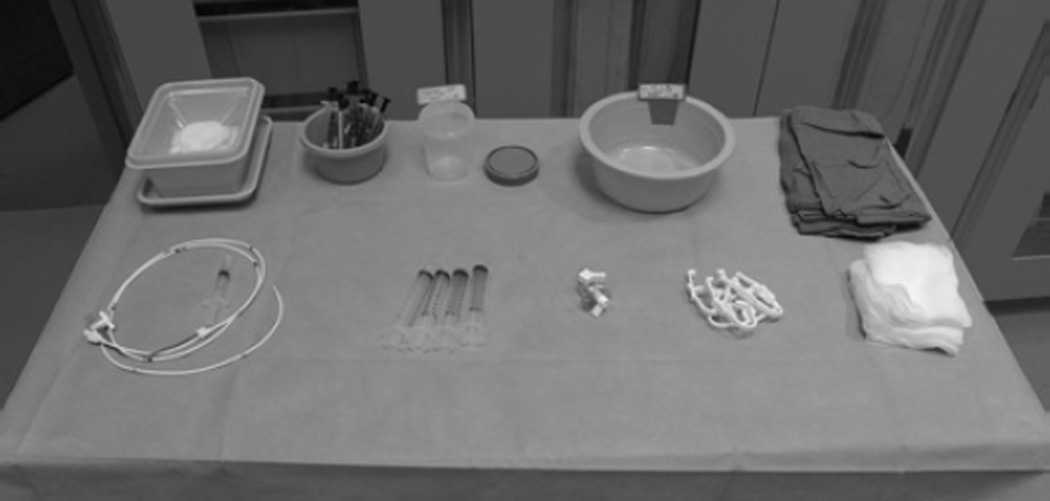
When selecting instruments for an MR right-sided heart catheterization procedure, the interventional MR technologist must consider several factors (see Figure 5). Only nonferromagnetic items should be provided and only on a table tested to be MR safe and clearly marked with the appropriate emblem, as dictated by the American Society for Testing and Materials. Metal towel clips provided in traditional catheterization procedure packs should be replaced with plastic alternatives. A physician performs the vascular access procedure prior to patient transfer to MR, meaning that a scalpel, vascular access needle, and guidewire are not necessary to include on the MR procedure table. Many catheters for imaging-guided procedures incorporate wire braiding to enhance torque control needed to navigate tortuous structures. Although they might not be significantly attracted by magnetic fields, braided catheters create large imaging artifacts.11,14
Figure 5.
MR-safe procedure table. Back row, from left to right: Fluid waste bin, heparinized syringes for blood gases, 1% diluted gadolinium contrast, heparinized saline, and blue towels. Front row, from left to right: balloon wedge end-hole catheter, 10-mL syringes, stopcocks, plastic towel clips, and gauze. Image courtesy of the authors.
Another factor of concern to the interventional MR technologist is the potential for heating that can result from introducing metal devices into a magnetic field. Conductive wires can resonate and heat during radiofrequency excitation related to MR.15–17 In addition, resonating transmission lines can deliver an electric shock to a patient, especially when contacting myocardium, unless adequately insulated. No U.S. Food and Drug Administration (FDA)-approved MR conditional guidewires exist, and for this reason, physicians limit clinical MR-guided catheterizations to the right side of the heart and depend on off-the-shelf, flow-directed, nonwire-braided catheters with inflatable balloon tips for navigational needs within the low-pressure venous system. Multiple small companies have MR-conditional guidewires in the regulatory pipeline. NIH biomedical engineers developed custom MR guidewires, which are undergoing FDA regulatory approval.18,19 MR physicists at NIH also are developing novel pulse sequences to enable safe use of off-the-shelf guidewires.20
Finally, when selecting a catheter for the MR procedure table, the interventional MR technologist should take device conspicuity into account. MR techniques for passive catheter visualization rely on creating an imaging artifact (eg, with ferrous material) or using contrast agents (eg, air-filled or gadolinium-filled balloons). Some groups use CO2-filled balloons to make catheters conspicuous and exploit their bloodstream flotation properties.21 CO2-filled balloons appear as a signal void, as opposed to a bright signal, when filled with gadolinium. NIH physicians prefer a 100:1 mixture of normal saline and gadopentetate dimeglumine (Magnevist, Bayer) because they believe it to be more conspicuous when used in combination with the image saturation mode. Only 10 mL of this mixture provided on the procedure table in a small sterile container is needed to fill the balloon. This strategy, however, does require the device to be confined within the selected imaging slab to be visible, and in the case of balloon-tip catheters, the shaft of the device remains invisible.
Patient Transfer
The patient lies on a thin transfer board for easy transfer between the x-ray and MR sections of the catheterization laboratory. Once vascular access has been obtained, the physician sutures the arterial access sheath in place to prevent accidental dislodgement during the patient’s transfer to the adjacent MR suite. To ensure MR safety, the physician and interventional MR technologist, in tandem, perform a visual check of the patient table for metal items that could become projectiles within the MR environment.
Once the patient is cleared for transfer, the physician—with support from the interventional MR technologist—places 2 additional sterile drapes over the sterile workspace. The first drape is placed lengthwise to protect the vascular access sites during transfer. The second drape is placed crosswise over the patient’s chest and folded over the MR surface coil later, allowing for coil manipulation during the procedure, if needed, without contaminating the sterile field.
During transfer, the technologist pauses midway between the x-ray and MR sections of the laboratory so that various lines connected to the patient can be exchanged for those belonging to MR conditional equipment, ensuring that the patient’s vital signs are monitored at all times (see Box).
Box. Device Lines.
| Accessible port for manual infusion of additional medications |
| Audio communications system |
| Electrocardiographic electrodes, leads/cables for x-ray and MRa |
| End-tidal CO2a |
| Intravenous lines and MR-compatible intravenous pumpsa |
| Invasive blood pressure transducers, 2 channelsa |
| Nitric oxide injector and monitoring device |
| Noninvasive blood pressure cuff |
| Oxygen tubing or anesthesia tubing and gas monitora |
| Pulse oximetrya |
| Surface coil and connectors |
Devices used in the x-ray portion of the catheterization laboratory that are switched to MR-conditional devices during patient transfer at NIH.
One key safety step is placing the peripheral intravenous infusion line used for medicine administration, along with the oxygen tubing, through the MR bore so that they can be monitored at the head-side of the system where the conscious sedation nurse is stationed. Doing so allows expedited removal of the patient from the suite for cardiopulmonary resuscitation, defibrillation, or emergent temporary pacing, if necessary, without concern for lines and tubing becoming dislodged.
Once necessary lines have been positioned, the patient is transferred onto the MR table. The interventional MR technologist then provides the patient with earplugs and a 2-way communication headset. The surface coil is placed after the patient headset is in place so that, in the case of an emergency, the coil can be swiftly removed and chest compressions performed. The patient is positioned at isocenter within the bore and is ready for baseline imaging.
Imaging Workflow
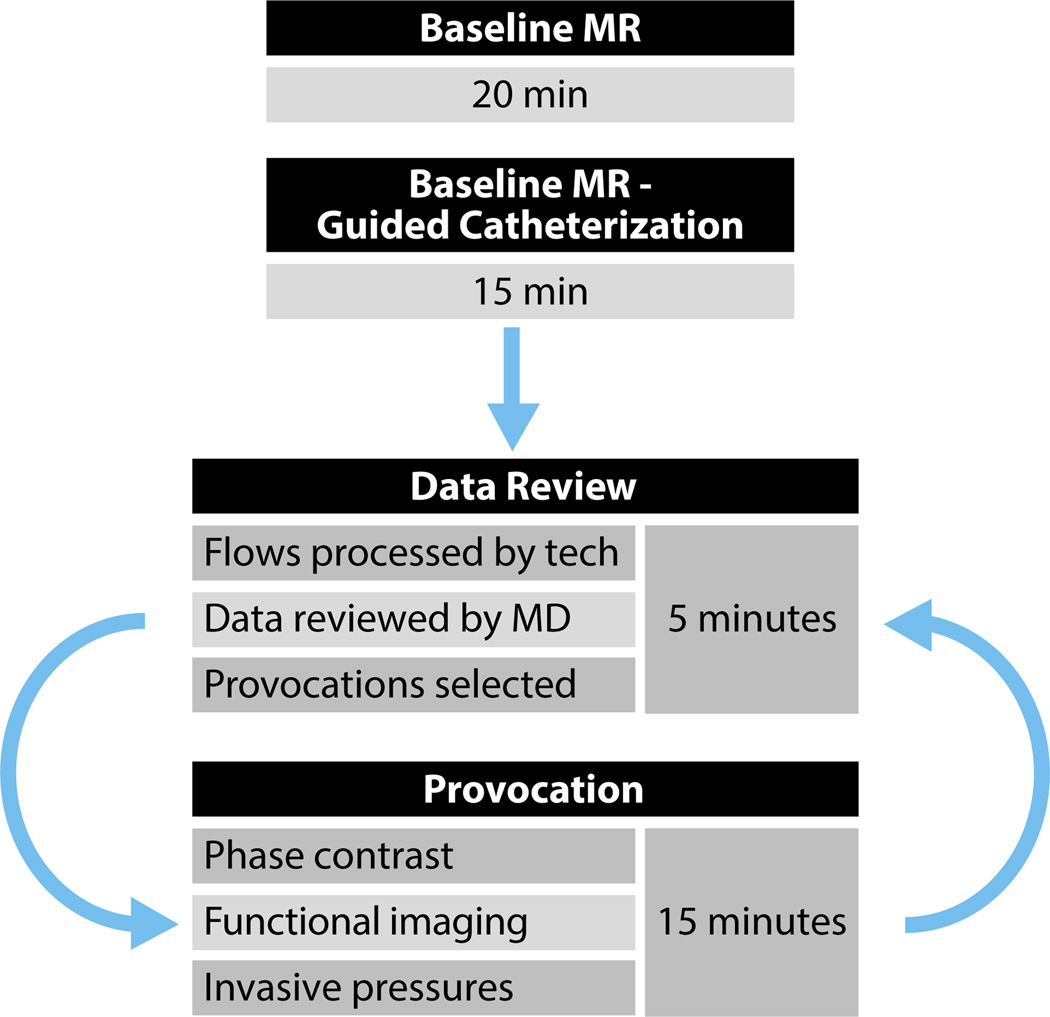
Cardiovascular catheterization often is performed on patients with compromised respiratory function and symptoms of dyspnea yet who require moderate sedation for procedural comfort. For this reason, the interventional MR technologist works with the physician to build a comprehensive, free-breathing imaging protocol that maximizes workflow efficiency. The imaging workflow is broken into 3 key procedural steps (see Figure 6).
Figure 6.
Procedural workflow. The collection of baseline MR imaging and MR-guided catheterization data, followed by a cycle of data review, provocation selection, and administration.
Baseline MR and Catheterization
The purpose of baseline data collection, from a catheterization perspective, is to acquire data related to pulmonary (Qp) and systemic (Qs) blood flow hemodynamics as well as cardiac volumes and ventricular function while the patient is in a resting state. The baseline imaging workflow begins with the interventional MR technologist obtaining traditional cardiac localizers (3-plane, pseudo 2-chamber, and pseudo short-axis localizers). Patient positioning in relation to the scanner isocenter is verified to confirm that the patient is not too deep within the bore to ensure that the femoral vascular access sheaths remain accessible to the physician.
Diagnostic through-plane flow CMR is performed on the ascending aorta and main pulmonary artery to measure Qp and Qs blood flow. To avoid lengthy measurements using erroneous velocity encoding (VENC) factors, the interventional MR technologist first performs brief flow CMR scouts (1 average, ~20 seconds each). Each flow CMR scout contains 3 VENC factors (Qs = 150, 200, and 250; Qp = 100, 150, and 200) to preview the best choice (see Table 2). Baseline diagnostic flow CMR sequences are performed simultaneously with baseline Qp and Qs invasive pressure measurements during MR-guided catheterization.
Table 2.
Common Magnetic Resonance Imaging Catheterization Pulse Sequence Parameters
| Traditional Localizers |
VENC Scout | Diagnostic Flow CMR |
Real-Time LAX Cine |
Real-Time SAX Cine |
Real-Time SSFP (IFE) |
|
|---|---|---|---|---|---|---|
| TR, ms | 2.66 | 4.64 | 4.84 | 2.58 | 2.46 | 2.44 |
| TE, ms | 1.13 | 2.47 | 2.64 | 1.13 | 1.04 | 1.22 |
| Matrix (readout × phase) | 256 × 168 | 192 × 173 | 256 × 256 | 160 × 92 | 160 × 92 | 192 × 144 |
| Slice thickness, mm | 8.0 | 6.0 | 6.0 | 8.0 | 8.0 | 8.0 |
| Flip angle | 80° | 20° | 20° | 45° | 45° | 45° |
| Acceleration factor | 2 | 2 | 2 | 4 | 4 | 4 |
| Scan times | 10 s/9 slices | 23 s | 2–3 min each | 8 s/1 slice | 2 min/11 slices | 3–10 frames/s |
Abbreviations: CMR, cardiac magnetic resonance; IFE, Interactive Front End; LAX, long-axis; SAX, short-axis; SSFP, single-shot fast procession; TR, repetition time; TE, echo time; VENC, velocity-encoded.
Diagnostic long-axis and short-axis views are obtained using a rapid-research real-time myocardial cine imaging sequence with retrospective reconstruction.22 Image acquisition with parallel imaging rate 4 is performed with the patient free breathing over multiple heartbeats (~8 s/slice). An image-based navigator is computed to correct for respiratory motion, and the recorded ECG is used for retrospective cardiac gating. Nonlinear reconstruction is used to produce images with spatial and temporal resolution comparable to conventional breath-hold segmented cine images. Image reconstruction uses open-source software (Gadgetron23), running on multiple nodes hosted on Amazon Cloud, which reduces reconstruction times to approximately 2 minutes for 11 slices.
The interventional MR technologist gives special attention to sequence parameters such as slice prescription, VENC selection, shim adjustments, and cardiac acquisition windows to ensure data accuracy and procedure efficiency. Baseline imaging sequences are set automatically to propagate to specific locations later in the imaging workflow, preventing downtime associated with represcribing similar sequences. Doing so also ensures that imaging acquired at baseline and under physiological provocation differs only in the patient’s hemodynamic state and is not due to differences in imaging parameters.
Baseline MR data collection concludes with prescription of catheterization reference images consisting of orthogonal views of the superior and inferior venae cavae, the right ventricular outflow tract, and the main, right, and left pulmonary arteries to expedite catheter navigation during MR guidance. The interventional MR technologist systematizes the image acquisition and display order of the reference images for efficiency and familiarity of the physician operators, as well as staff. Using the IFE program, up to 3 distinct imaging planes can be displayed simultaneously, allowing orthogonal views of the anatomy of immediate interest on demand. Physicians typically allow 20 minutes for baseline imaging and real-time slice prescription for MR catheterization.
After baseline imaging is completed, MR-guided catheterization begins. Real-time reference images are systematically displayed on the in-room projectors, as requested by the physician operators, to guide navigation through the chambers of the right heart and collect invasive pressure data. Baseline MR-guided catheterization typically requires approximately 15 minutes to complete, although the actual time is dependent on patient anatomy and hemodynamic status. For example, reaching the right ventricle and pulmonary artery can be challenging from groin access in patients with severe tricuspid regurgitation.
Data Review and Physiological Provocation
On completion of baseline MR and MR-guided cardiac catheterization, the physicians conduct an expedited review of the findings. The interventional MR technologist postprocesses the flow CMR data and communicates the results to the physician. The physician might choose to perform additional mea-surements under altered physiological conditions (ie, provocations) including inhaled nitric oxide, rapid infusion of normal saline, or intravenous delivery of vasoconstrictors such as phenylephrine.24 The physicians and support staff at the NIH are exploring the foot pedal exercise as an additional provocation. Postprocessing and review of the data take an average of 3 to 5 minutes to complete. Postprocessing and analysis of the cardiac volume data are performed offline after the procedure, and the findings are incorporated into the final procedural report.
For each provocation, imaging identical to baseline is repeated by the interventional MR technologist once the patient reaches steady state. Following repeat imaging, catheter pressure measurements are repeated under real-time MR guidance. Each provocation administration and measurement takes approximately 15 minutes to perform. Subsequent provocations can be added to the examination with a similar time frame.
From clinical experience with more than 80 patients, the authors have shown the feasibility of performing comprehensive MR-guided right-sided heart catheterizations, including patient transfer, baseline MR and hemodynamic assessment, and repetition with up to 2 provocations in approximately 90 minutes. The interventional MR technologist continues to optimize procedural workflow and the imaging protocol to enhance efficiency.
Emergency Evacuation
The risks of diagnostic cardiac catheterization, regardless of the modality of image guidance, are complete heart block and ventricular fibrillation. Both are life-threatening and require immediate action. Therefore, in this new environment, a process should be established for safe resuscitation and defibrillation. Standard defibrillators can become projectiles within the magnetic field, and commercial MR-safe defibrillators are currently unavailable. In the NIH laboratory, the adjacent x-ray section of the laboratory serves as the evacuation destination for cardiovascular emergencies. The team drills quarterly on evacuating patients from MR to x-ray. By delegating tasks, the team can complete a simulated evacuation and defibrillate in less than one minute, although this has not yet been necessary in practice (see Table 3).
Table 3.
Staff Roles During Emergency Patient Evacuation From MR Suitea
| 1st Operator | 2nd Operator | Interventional CMR Technologist |
Catheter RN | Sedation RN | |
|---|---|---|---|---|---|
| 1 | Provide instructions | ||||
| 2 | Advance table to home position | Open MR bay doors | Secure IV line | ||
| 3 | Remove surface coil | Remove patient headset | Secure airway | ||
| 4 | Start chest compressions | Transfer patient from MR suite | Assistant catheter RN | ||
| 5 | Restrict access to MR suite | Defibrillate/administer medications |
Abbreviations: CMR, cardiac magnetic resonance; IV, intravenous; MR, magnetic resonance; RN, registered nurse.
Each member of the staff has specific duties to facilitate rapid evacuation from the MR suite in case of a life-threatening emergency.
Conclusion
The interventional MR technologist’s role in supporting MR-guided cardiovascular interventions is critical to ensuring procedural goals are accomplished in a safe and systematic manner with minimal downtime. Preprocedure preparation for MR-guided cardiac catheterization is unique, requiring sterile draping of the scanner system, protection of the sterile working field during patient transfer, and exclusion of MR-unsafe devices from the procedure table. In addition, the procedural and imaging workflow should be customized to enable a comprehensive cardiovascular assessment encompassing simultaneous collection of invasive pressures, blood flow, and blood volume measurements. Finally, contingencies for managing emergencies should be considered.
Acknowledgments
The authors would like to thank Alisa Downing, R.T.(R)(MR), for her contribution of proofreading this article.
This article was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (Z01-HL005062). NHLBI and Siemens have a Collaborative Research and Development Agreement on interventional MR. Interactive Front End is a work in progress.
Contributor Information
Jonathan R Mazal, National Institutes of Health in Besthesda, Maryland.
Toby Rogers, National Institutes of Health in Besthesda, Maryland.
William H Schenke, National Institutes of Health in Besthesda, Maryland.
Anthony Z Faranesh, National Institutes of Health in Besthesda, Maryland.
Michael Hansen, Children’s National Medical Center in Washington, DC.
Kendall O’Brien, Children’s National Medical Center in Washington, DC.
Kanishka Ratnayaka, Children’s National Medical Center in Washington, DC.
Robert J Lederman, National Institutes of Health in Besthesda, Maryland.
References
- 1.Atalar E, Bottomley PA, Ocali O, et al. High resolution intravascular MRI and MRS by using a catheter receiver coil. Magn Reson Med. 1996;36(4):596–605. doi: 10.1002/mrm.1910360415. [DOI] [PubMed] [Google Scholar]
- 2.Kuehne T, Yilmaz S, Steendijk P, et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation. 2004;110(14):2010–2016. doi: 10.1161/01.CIR.0000143138.02493.DD. [DOI] [PubMed] [Google Scholar]
- 3.Muthurangu V, Atkinson D, Sermesant M, et al. Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;289(3):H1301–H1306. doi: 10.1152/ajpheart.00957.2004. [DOI] [PubMed] [Google Scholar]
- 4.Razavi R, Hill DL, Keevil SF, et al. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362(9399):1877–1882. doi: 10.1016/S0140-6736(03)14956-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuehne T, Yilmaz S, Schulze-Neick I, et al. Magnetic resonance imaging guided catheterisation for assessment of pulmonary vascular resistance: in vivo validation and clinical application in patients with pulmonary hypertension. Heart. 2005;91(8):1064–1069. doi: 10.1136/hrt.2004.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34(5):380–389. doi: 10.1093/eurheartj/ehs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthurangu V, Taylor A, Andriantsimiavona R, et al. Novel method of quantifying pulmonary vascular resistance by use of simultaneous invasive pressure monitoring and phase-contrast magnetic resonance flow. Circulation. 2004;110(7):826–834. doi: 10.1161/01.CIR.0000138741.72946.84. [DOI] [PubMed] [Google Scholar]
- 8.Ait-Ali L, Andreassi MG, Foffa I, Spadoni I, Vano E, Picano E. Cumulative patient effective dose and acute radiation-induced chromosomal DNA damage in children with congenital heart disease. Heart. 2010;96(4):269–274. doi: 10.1136/hrt.2008.160309. [DOI] [PubMed] [Google Scholar]
- 9.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. gamma-H2AX foci as a biomarker for patient x-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation. 2009;120(19):1903–1909. doi: 10.1161/CIRCULATIONAHA.109.880385. [DOI] [PubMed] [Google Scholar]
- 10.Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175–184. doi: 10.1148/radiol.2511081296. [DOI] [PubMed] [Google Scholar]
- 11.Ratnayaka K, Faranesh AZ, Guttman MA, Kocaturk O, Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance: still tantalizing. J Cardiovasc Magn Reson. 2008;10:62. doi: 10.1186/1532-429X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz CH, Kirchberg KJ, Zuehlsdorff S, et al. Interactive frontend (IFE): a platform for graphical MR scanner control and scan automation. Proc Intl Soc Mag Reson Med. 2005;13.2170 [Google Scholar]
- 13.Hansen MS, Sorensen TS. Gadgetron: an open source framework for medical image reconstruction. Magn Reson Med. 2013;69(6):1768–1776. doi: 10.1002/mrm.24389. [DOI] [PubMed] [Google Scholar]
- 14.White MJ, Thornton JS, Hawkes DJ, et al. Design, operation, and safety of single-room interventional MRI suites: practical experience from two centers. J Magn Reson Imaging. 2015;41(1):34–43. doi: 10.1002/jmri.24577. [DOI] [PubMed] [Google Scholar]
- 15.Konings MK, Bartels LW, Smits HF, Bakker CJ. Heating around intravascular guidewires by resonating RF waves. J Magn Reson Imaging. 2000;12(1):79–85. doi: 10.1002/1522-2586(200007)12:1<79::aid-jmri9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13(1):105–114. doi: 10.1002/1522-2586(200101)13:1<105::aid-jmri1016>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Yeung CJ, Susil RC, Atalar E. RF heating due to conductive wires during MRI depends on the phase distribution of the transmit field. Magn Reson Med. 2002;48(6):1096–1098. doi: 10.1002/mrm.10310. [DOI] [PubMed] [Google Scholar]
- 18.Sonmez M, Saikus CE, Bell JA, et al. MRI active guidewire with an embedded temperature probe and providing a distinct tip signal to enhance clinical safety. J Cardiovasc Magn Reson. 2012;14:38. doi: 10.1186/1532-429X-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basar B, Campbell-Washburn AE, Rogers T, et al. Stiffness-matched segmented metallic guidewire for interventional cardiovascular MRI. J Cardiovasc Magn Reson. 2015;17(suppl 1):P414. doi: 10.1186/s12968-015-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell-Washburn AE, Rogers T, Xue H, Hansen MS, Lederman RJ, Faranesh AZ. Dual echo positive contrast bSSFP for real-time visualization of passive devices during magnetic resonance guided cardiovascular catheterization. J Cardiovasc Magn Reson. 2014;16:88. doi: 10.1186/s12968-014-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miquel ME, Hegde S, Muthurangu V, et al. Visualization and tracking of an inflatable balloon catheter using SSFP in a flow phantom and in the heart and great vessels of patients. Magn Reson Med. 2004;51(5):988–995. doi: 10.1002/mrm.20041. [DOI] [PubMed] [Google Scholar]
- 22.Xue H, Kellman P, LaRocca G, Arai AE, Hansen MS. High spatial and temporal resolution retrospective cine cardiovascular magnetic resonance from shortened free breathing real-time acquisitions. J Cardiovasc Magn Reson. 2013;15:102. doi: 10.1186/1532-429X-15-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue H, Inati S, Sorensen TS, Kellman P, Hansen MS. Distributed MRI reconstruction using Gadgetron-based cloud computing. Magn Reson Med. 2015;73(3):1015–1025. doi: 10.1002/mrm.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers T, Ratnayaka K, Lederman RJ. MRI catheterization in cardiopulmonary disease. Chest. 2014;145(1):30–36. doi: 10.1378/chest.13-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]