Abstract
Bauhinia purprea agglutinin (BPA) is a well‐known lectin that recognizes galactosyl glycoproteins and glycolipids. In the present study, we firstly found that BPA bound to human prostate cancer specimens but not to normal prostate ones. Therefore, we sought to develop BPA‐PEG‐modified liposomes (BPA‐PEG‐LP) encapsulating anticancer drugs for the treatment of prostate cancer. We examined the tumor targetability of BPA‐PEG‐LP with human prostate cancer DU145 cells, and observed that fluorescently labeled BPA‐PEG‐LP dominantly associated with the cells via the interaction between liposome‐surface BPA and cell‐surface galactosyl molecules. We also observed that BPA‐PEG‐LP accumulated in the prostate cancer tissue after the i.v. injection to DU145 solid cancer‐bearing mice, and strongly bound to the cancer cells. In a therapeutic study, DU145 solid cancer‐bearing mice were i.v. injected thrice with BPA‐PEG‐LP encapsulating doxorubicin (BPA‐PEG‐LPDOX, 2 mg/kg/day as the DOX dosage) or PEG‐modified liposomes encapsulating DOX (PEG‐LPDOX). As a result, BPA‐PEG‐LPDOX significantly suppressed the growth of the DU145 cancer cells, whereas PEG‐LPDOX at the same dosage as DOX showed little anti‐cancer effect. The present study suggested that BPA‐PEG‐LP could be a useful drug carrier for the treatment of human prostate cancers.
Keywords: Bauhinia purprea agglutinin, drug delivery system, liposome, prostate cancer, targeting
Lectins are generally defined as proteins that possess the potential to selectively recognize and strongly bind to sugar chains of glycoproteins and glycolipids, and the expression of lectins is widely observed in animals, microorganisms and plants. Because interactions between sugar chains and proteins play important roles in not only cell differentiation and proliferation,1 but also memory formation,2 the immune system3 and tumorigenesis,4 lectins are thought to be one kind of the key molecules that control both physiological and pathological cell signals. Therefore, lectins are often used as detection tools for determining blood type5 and diagnosing a viral infection6 or cancer development.7 In addition, scientific researchers routinely use lectins as biological molecular tools for specific purification, staining and detection of certain proteins.8, 9 As lectins possess the ability to bind to specific sugar chains, they have become available as probes for targeting drugs or nanoparticle drug carriers to diseased organs. In fact, tomato lectin can be used as a probe targeting the lumen of the small intestines;10 and the application of lectins for use in drug delivery systems (DDS) is ongoing to improve the efficiency of drug delivery to the lungs, brain and other organs for the treatment of various diseases such as cancer.11, 12
Liposomes are lipid‐based nanoparticles that are widely used for drug encapsulation and as a DDS, because they have characteristics suitable for clinical use. In particular, liposomal anti‐cancer agents are expected to achieve efficient cancer therapy, because liposomes having long blood‐circulation potential owing to modification by polyethylene glycol (PEG) effectively accumulate in cancer tissue.13, 14 However, there are considerable problems with the PEG‐based modification of liposomes and nanoparticles: for example, decreased interaction of those drug nanocarriers with target cells and impaired intracellular uptake of encapsulated materials into the cells. This PEG dilemma sometimes impairs the therapeutic efficiency of drugs encapsulated in PEGylated nanocarriers. To overcome these disadvantages and to enhance the specific accumulation of nanocarriers, many researchers have applied an active targeting strategy by attaching molecular targeting probes to the top of the PEG chains to improve the efficiency of drug delivery into the target cells for obtaining enhanced therapeutic efficacy.15 For this reason, identification of targeting molecules that can specifically and strongly recognize the target is desirable to resolve this problem. Boland et al.16 and Kawa et al.17 reported that Bauhinia purprea agglutinin (BPA) binds dominantly to cancerous cells in the colon and pancreas, respectively. BPA is a well‐known lectin that recognizes sugar chains that terminate in galactose. In the present study, we firstly observed that BPA bound to prostate cells in specimens from patients with prostate cancer but not to the regions of normal prostate tissues from those individuals. Furthermore, we used BPA as a probe for active targeting of liposomes to human prostate cancers in order to achieve the effective delivery of anti‐cancer drugs to cancer cells for prostate cancer chemotherapy.
Materials and Methods
Agents
Dipalmitoylphosphatidylcholine (DPPC), cholesterol and methoxy‐polyethyleneglycol (2000)‐conjugated distearoylphosphatidylethanolamine (DSPE‐MPEG) were gifts from Nippon Fine Chemical (Takasago, Hyogo, Japan). DSPE‐PEG‐NHS (N‐hydroxysuccinimide) was purchased from NOF (Tokyo, Japan). 1,1′‐Dioctadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine perchlorate (DiIC18) was obtained from Life Technologies Japan (Tokyo, Japan). BPA, FITC‐conjugated BPA (BPA‐FITC) and biotin‐conjugated BPA were kindly donated by J‐OIL MILLS (Tokyo, Japan).
Animals
BALB/c nu/nu male mice were purchased from Japan SLC (Shizuoka, Japan). All animal experiments were approved by the Animal and Ethics Committee of the University of Shizuoka. The animals were cared for according to the Animal Facility Guidelines of the University of Shizuoka.
Cell culture
Human prostate cancer cells lines DU145 and LNCaP were cultured in RPMI‐1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% heat‐inactivated FBS (AusGeneX Pty Ltd, Brisbane, Australia), 100 μg/mL streptomycin (MP Biomedicals, Irvine, CA, USA) and 100 units/mL penicillin (MP Biomedicals) at 37°C in a humidified atmosphere with 5% CO2.
Histological analysis
A deparaffinized tissue array slide (human normal prostate and cancer tissue; SuperBioChips Laboratories, Seoul, Korea) was blocked with 1% BSA‐PBS for 30 min. Then, biotin‐conjugated BPA (50 μg/mL) was added to the array slide; and incubation was carried out for 1 h at room temperature. After a wash, the slide was next incubated with streptavidin‐conjugated HRP (5 μg/mL; Vector Laboratories, Burlingame, CA, USA) for 30 min. To visualize the bound BPA molecules, we incubated the array slide for approximately 5 min in the solution provided in a DAB liquid substrate system (Sigma‐Aldrich Co. LLC, St. Louis, MO, USA). The tissues on the array slide were then observed with a light microscope.
Preparation of Bauhinia purprea agglutinin‐PEG‐LP
Dipalmitoylphosphatidylcholine and cholesterol (2:1 as a molar ratio) dissolved in chloroform were evaporated and dried for at least 1 h under reduced pressure to form a thin lipid film in a round‐bottomed flask. The lipids were then hydrated with PBS (pH 7.4), after which the liposome solution was subsequently frozen in liquid nitrogen and thawed repeatedly for three cycles, and then incubated in a sonic bath for 15 min at 55°C. Then, the sonicated liposomes were passed through 100‐nm pore‐size polycarbonate filters (Nuclepore, Cambridge, MA, USA) with a Lipe extruder (Northern Lipids, Burnaby, BC, Canada). For radiolabeling of liposomes, [3H]cholesteryl hexadecyl ether (PerkinElmer Japan, Tokyo, Japan) was mixed in the lipid/chloroform solution before the evaporation. For fluorescence labeling of liposomes, DiIC18 was added in the same manner.
For the modification of the liposomal surface with BPA, DSPE‐PEG‐NHS was used as a linker molecule. DSPE‐PEG‐NHS or DSPE‐PEG was mixed with the solution of plain liposomes, and the mixture was then incubated at 55°C for 15 min to obtain PEG‐NHS‐modified liposomes or PEG‐modified liposomes (PEG‐LP), respectively. Then, BPA dissolved in borate buffer (pH 8.4) was added to the PEG‐NHS‐modified liposomes; and the coupling reaction of NHS with BPA was carried out at room temperature overnight. Uncoupled BPA was separated from the BPA‐PEG‐modified liposomes (BPA‐PEG‐LP) by three cycles of ultracentrifugation (550 000 g, 15 min, 4°C, CS120GXL; Hitachi, Tokyo, Japan). The amount of BPA was measured by HPLC. Particle size and ζ‐potential of liposomes were determined with a ZetaSizer Nano ZS (Malvern Instruments, Worcestershire, UK).
To prepare the heat‐inactivated BPA‐PEG‐modified liposomes, BPA was firstly incubated in a water bath at 80°C for 30 min, and then the modification of it to liposomes was performed in a similar manner as described above.
DOX encapsulation into liposomes
A remote‐loading method was used for encapsulation of DOX into the liposomes.18 In brief, the thin lipid film was hydrated with 0.3 M citric acid solution (pH 4.0). After the sizing of liposomes, the liposomes were neutralized with 0.5 M sodium carbonate and buffered with 20 mM HEPES solution (pH 7.4) to generate a pH gradient. Then, DOX (1 mg/mL) solution was added to the liposome solution; and incubation was carried out at 65°C for 1 h. Free DOX was removed by ultracentrifugation, and the pelleted liposomes were resuspended in PBS. Modification of the liposomes with BPA was then done as described above.
Liposome binding assay
DU145 cells (8.0 × 104 cells/well) or LNCaP cells (4.0 × 104 cells/well) were seeded on a 24‐well plate and incubated at 37°C overnight. After the removal of medium, the cells were incubated in fresh medium containing DiI‐labeled PEG‐LP, BPA‐PEG‐LP, or heat‐inactivated BPA‐PEG‐LP for 3, 12 or 24 h. Then the cells were washed with PBS and lysed with 0.1% SDS/Tris buffer solution (pH 7.4). To determine the binding and uptake of liposomes into the cells, the fluorescence intensity of DiI (Ex. 549 nm; Em. 592 nm) was measured by using an Infinite M200 microplate reader (Tecan Japan, Kawasaki, Japan). The detection gains for the measurement of fluorescence intensity were automatically set by Tecan i‐Control software.
For the binding inhibition assay, DU145 cells were incubated with the liposomes in the presence of glucose or galactose at the concentration of 0.1, 1 or 10 mM; and the fluorescence in the cells was then measured in a similar manner.
Biodistribution analysis
DU145 cells in a Matrigel‐containing medium were implanted s.c. (5 × 106 cells/0.2 mL/mouse) into 5‐week‐old BALB/c nu/nu male mice to prepare solid cancer‐bearing mice. [3H]‐labeled PEG‐LP or BPA‐PEG‐LP was i.v. injected into the mice via a tail vein; and after distribution of the liposomes for 3 or 24 h, the mice were killed under anesthesia. Blood and organs were then harvested and solubilized with Solvable (PerkinElmer). Sample solutions were subsequently mixed with Hionic‐Fluor (PerkinElmer), and the radioactivity was measured by using a liquid scintillation counter (LSC‐7400; Hitachi Aloka Medical, Tokyo, Japan). The distribution data were presented as the percentage of injected dose per whole tissue. The total weight of plasma was assumed to be 4.38% of the body weight.
Intratumoral distribution
DiI‐labeled PEG‐LP or BPA‐PEG‐LP was i.v. injected into DU145‐bearing mice via a tail vein; and after 24 h, the mice were killed under anesthesia. Their solid cancers were perfused with an excess amount of PBS in order to remove the circulating liposomes in the blood and then dissected. The cancer tissues were embedded in optimal cutting temperature compound (Sakura, Torrance, CA, USA), frozen at −80°C and sectioned in a 10‐μm thickness. Then, the sections were mounted on MAS‐coated slides (Matsunami Glass Ind., Ltd., Kishiwada, Japan). The cancer sections were washed with PBS, and incubated with protein‐blocking solution containing 3% BSA in PBS for 15 min at room temperature. Next, they were incubated with FITC‐conjugated anti‐CD31 rat IgG antibody (eBioscience) in 1% BSA‐PBS (5 μg/mL) for 1 h at room temperature. These sections were washed with PBS and then fixed with 4% paraformaldehyde‐PBS. After washing with PBS, the sections were incubated with DAPI for nuclear staining. Finally, the sections were mounted with PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific, Cheshire, UK). The localization of liposomes and blood vessel cells in the cancer was observed by use of a fluorescence microscope (IX71; Olympus Corporation, Tokyo, Japan).
In vitro anti‐proliferative assay
DU145 cells (1.0 × 104 cells/well) were seeded onto a 96‐well plate and cultured overnight. DOX‐encapsulated PEG‐modified liposomes (PEG‐LPDOX) or DOX‐encapsulated BPA‐PEG‐LP (BPA‐PEG‐LPDOX) with the DOX dose at 10 μg/mL was added to the cells; and 3 h later the cells were washed thrice with PBS. Then, they were cultured in fresh medium without liposomes for 48 h. The viable cells were determined by performing a WST‐8 assay with a Cell Counting Kit‐8 (Dojindo Laboratory, Kumamoto, Japan).
Therapeutic experiment
DU145 cells were implanted s.c. (5 × 106 cells/0.2 mL/mouse) into 5‐week‐old BALB/c nu/nu male mice, and PEG‐LPDOX or BPA‐PEG‐LPDOX solution (0.2 mL) with a DOX dosage of 2 mg/kg/day was i.v. injected via a tail vein once a week for 3 weeks starting from day 29 after the implantation. The tumor volume and the body weight changes were monitored daily. The tumor volume was calculated according to the following formula: Tumor volume = 0.4 × a × b 2 (a, largest diameter; b, smallest diameter).
Statistical analysis
One‐way anova followed by Tukey honestly significant difference (HSD) post hoc pairwise comparison test was used for multiple group comparisons. In the case of two‐group comparisons, Student's t‐test was used.
Results
Selective binding of Bauhinia purprea agglutinin to human prostate cancer specimens
To demonstrate the potential of BPA to target human prostate cancer, we performed histological analysis by using a tissue array of human prostate cancer specimens (Gleason score = 7–9). As the result, BPA specifically bound to the region of the tissue containing prostate cancer cells, whereas the binding was rarely observed in the normal prostate region (Fig. 1). This result suggests that BPA possessed the potential to bind selectively to human prostate cancer cells.
Figure 1.
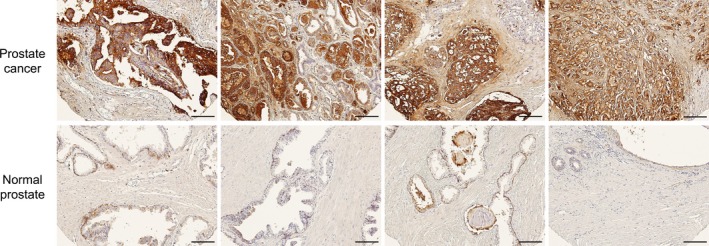
Histological analysis of Bauhinia purprea agglutinin (BPA) binding in human prostate cancer specimens. Biotin‐conjugated BPA was added to tissue array slides (n = 4) bearing regions of prostate cancer tissue (upper images, Gleason score = 7–9) or normal prostate tissue (lower images); and the tissues were secondly reacted with streptavidin‐HRP conjugate. After DAB staining, the bright‐light images were obtained with a light microscope. Scale bars: 200 μm.
Characterization of Bauhinia purprea agglutinin‐PEG‐LP and Bauhinia purprea agglutinin‐PEG‐LPDOX
Bauhinia purprea agglutinin‐PEG‐LP and BPA‐PEG‐LPDOX showed similar particle sizes and ζ‐potentials in comparison with those of PEG‐LP and PEG‐LPDOX: The average particle sizes of PEG‐LP, BPA‐PEG‐LP, PEG‐LPDOX and BPA‐PEG‐LPDOX were 132, 139, 153 and 159 nm, respectively; and the ζ‐potentials, −2.1, −4.3, −4.6 and −6.0 mV, respectively. When the stabilities of PEG‐LP, BPA‐PEG‐LP PEG‐LPDOX and BPA‐PEG‐LPDOX after 24 h incubation with FBS were examined, the particle sizes of liposomes were not changed and the DOX encapsulation in both PEG‐LPDOX and BPA‐PEG‐LPDOX was kept in over 90% efficiency (data not shown).
Association of Bauhinia purprea agglutinin‐PEG‐LP to human prostate cancer cells
To evaluate the specificity of binding of BPA‐PEG‐LP to prostate cancer cells, we incubated DU145 cells with fluorescently labeled BPA‐PEG‐LP and then observed the fluorescence in these cells. The results indicated that the association of BPA‐PEG‐LP to DU145 cells occurred in a time‐dependent manner and was significantly higher than that of BPA‐free PEG‐LP (Fig. 2a). Similar results were obtained in the experiment using LNCaP cells (Fig. 2b). When heat‐inactivated BPA was used for the modification of the liposomes, the association of the liposomes to DU145 cells was not enhanced but was at a level similar to that obtained with PEG‐LP (Fig. 2c). Furthermore, to confirm the interaction of BPA with the sugar chain on the surface of the prostate cancer cells, a binding inhibition assay was performed in the presence of the monosaccharide galactose. The binding of BPA‐PEG‐LP to DU145 cells was dose‐dependently inhibited by the galactose (Fig. 2d). In addition, glucose failed to inhibit the binding (Fig. 2d). These data indicate that BPA‐PEG‐LP dominantly binds to prostate cancer cells through the interaction between the BPA on the liposome surface and galactocyl molecules on the cell surface.
Figure 2.
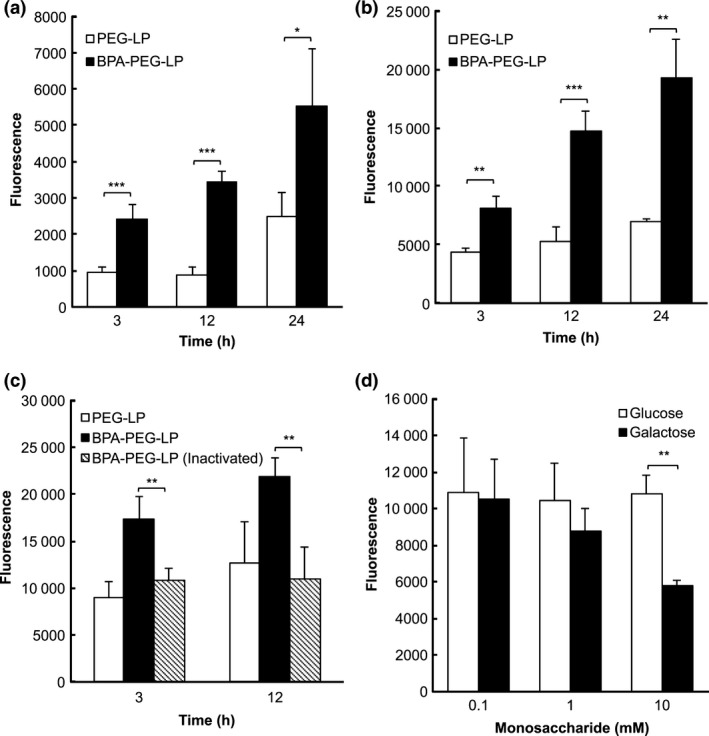
Bauhinia purprea agglutinin (BPA)‐mediated association of BPA‐PEG‐LP with human prostate cancer cells. DiI‐labeled PEG‐LP or BPA‐PEG‐LP was added to DU145 cells (a) or LNCaP cells (b); and incubation was carried out for 3, 12 or 24 h at 37°C. For inactivation of BPA‐PEG‐LP, BPA‐PEG‐LP was heated at 80°C for 30 min before the experiment and incubated with DU145 cells for 3 or 12 h (c). For the competitive inhibition assay (d), BPA‐PEG‐LP was incubated for 6 h with DU145 cells in the presence of galactose or glucose at the indicated concentrations. The fluorescence of DiI in the cells was determined by measuring the fluorescence intensity at the excitation and emission wavelengths of 549 and 592 nm, respectively. Data are shown as the mean ± SD. Significant differences are shown with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001, Student's t‐test).
Biodistribution of Bauhinia purprea agglutinin‐PEG‐LP in DU145 cancer‐bearing mice
Next, the accumulation of BPA‐PEG‐LP in the organs after i.v. injection into DU145‐bearing mice was examined. As a result, the retention of BPA‐PEG‐LP in the bloodstream at 3 h after the injection was a little lower than that of PEG‐LP; and the other distribution profile was quite similar between them (Fig. 3a). It is notable that BPA‐PEG‐LP showed a biodistribution similar to that of PEG‐LP, suggesting that BPA‐PEG‐LP had accumulated in the tumor tissue by the EPR effect, which was a prerequisite for their specific binding to the cancer cells. At 24 h after the injection, whereas the retention of BPA‐PEG‐LP in the bloodstream was significantly low and the accumulation of BPA‐PEG‐LP in the liver was higher than that of PEG‐LP, the accumulation in the tumor was not much different between BPA‐PEG‐LP and PEG‐LP (Fig. 3b). These results are consistent with the distribution of other cancer‐active targeting liposomes: PEG‐modification is sufficient to endow liposomes with accumulation in tumors, but intratumoral distribution and the association of liposomes with cancer cells are different between PEG‐LP and active targeting probe‐conjugated PEG‐LP.19
Figure 3.
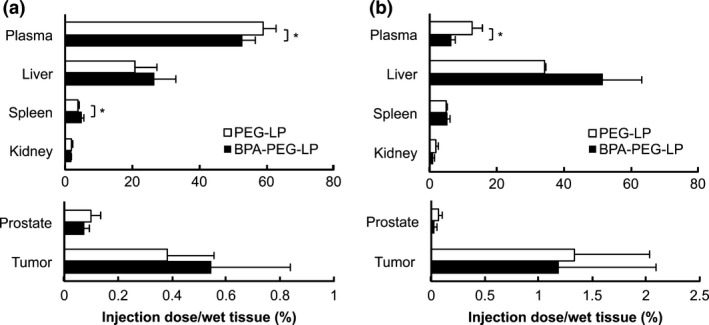
Biodistribution of Bauhinia purprea agglutinin (BPA)‐PEG‐LP in DU145 cancer‐bearing mice. [3H]‐labeled PEG‐LP or BPA‐PEG‐LP was i.v. injected into DU145 cancer‐bearing mice via a tail vein and allowed to circulate for (a) 3 or (b) 24 h. After harvesting the organs, the radioactivity at each time was measured by a liquid scintillation counter. Data are presented as the percentage of the injected dose per whole tissue. Data are shown as the mean ± SD. Significant difference is shown with an asterisk (*P < 0.05, Student's t‐test).
Distribution of Bauhinia purprea agglutinin‐PEG‐LP in DU145 solid cancers
In the response to the results of quantitative analysis of liposome accumulation in tumor tissue, we next examined intratumoral distribution of BPA‐PEG‐LP in the DU145 solid cancers. Fluorescently labeled PEG‐LP or BPA‐PEG‐LP was i.v. injected into the DU145‐bearing mice and the distribution of liposomes in the tumor tissues was compared. Immunohistological analysis revealed that higher DiI fluorescence was detected in the tumor sections treated with BPA‐PEG‐LP than in those treated with PEG‐LP (Fig. 4). Moreover, the images which liposomes were associated with the cells were obtained in BPA‐PEG‐LP‐treated mice. These results indicate that the intratumoral distribution of BPA‐PEG‐LP was obviously different from that of PEG‐LP, suggesting that BPA‐PEG‐LP strongly bound to the prostate cancer cells via the interaction of BPA with target molecules in the tumor tissue.
Figure 4.
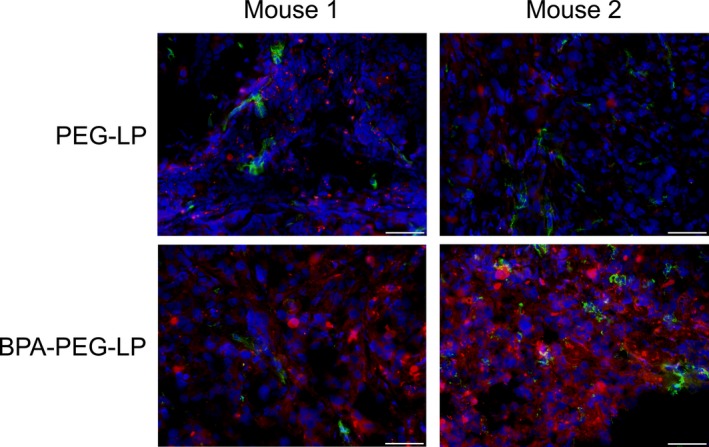
Intratumoral distribution of DiI‐labeled Bauhinia purprea agglutinin (BPA)‐PEG‐LP in DU145 solid cancer. DiI‐labeled PEG‐LP or BPA‐PEG‐LP (red) was i.v. injected via a tail vein into DU145‐bearing mice and allowed to circulate for 24 h. After tumor perfusion with PBS, the solid cancers were dissected and 10‐μm frozen cancer sections were prepared. Then, immunostaining for both blood vessels (green) and cell nuclei (blue) was carried out by using FITC‐conjugated anti‐CD31 antibody and DAPI, respectively. The fluorescence in the sections was observed under a fluorescence microscope. Scale bar: 50 μm.
Cytotoxicity of Bauhinia purprea agglutinin‐PEG‐LPDOX against DU145 cells
Next, we examined the in vitro cytotoxic effect of BPA‐PEG‐LPDOX against human prostate cancer cells. As a result, BPA‐PEG‐LPDOX inhibited the proliferation of DU145 cells at the DOX doses of both 3 and 10 μg/mL, and the inhibition was significantly higher than that obtained with PEG‐LPDOX at 3 μg/mL of DOX dose (Fig. 5). The result indicates that BPA modification enhanced the anti‐cancer effect of PEG‐modified liposomal DOX on human prostate cancer cells.
Figure 5.
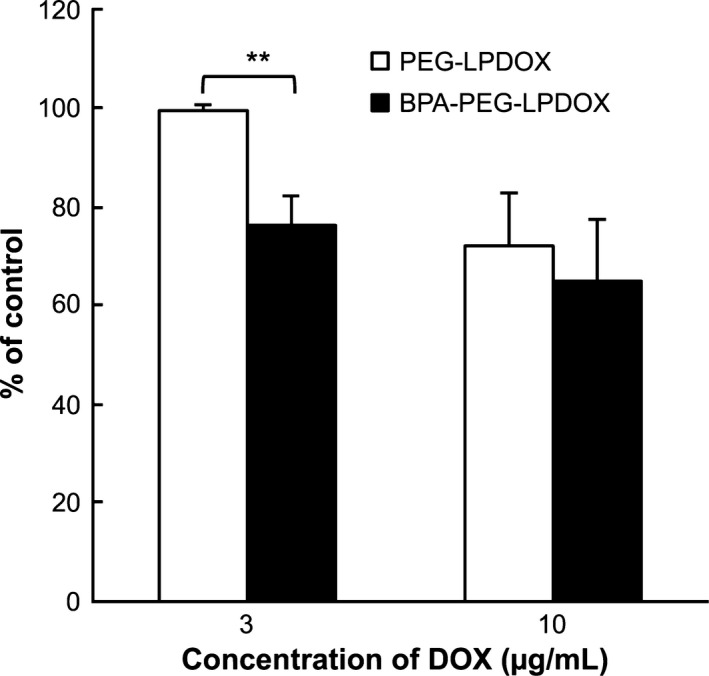
Anti‐proliferative effect of Bauhinia purprea agglutinin (BPA)‐PEG‐LPDOX on DU145 cells. PEG‐LPDOX and BPA‐PEG‐LPDOX at 3 or 10 μg/mL as the dose of DOX or PBS (control) was added to DU145 cells, and incubation was carried out for 3 h at 37°C. After washing the cells with PBS, the cells were cultured in fresh medium without liposomes for 48 h. Then the viable cells were determined by performing a WST‐8 assay. Data are shown as the mean of percent of control ± SD. Significant differences are shown with asterisks (**P < 0.01, Student's t‐test).
Prostate cancer therapy with Bauhinia purprea agglutinin‐PEG‐LPDOX
To elucidate the cancer therapeutic effect of BPA‐PEG‐LPDOX, we i.v. injected such liposomes into DU145 solid cancer‐bearing mice at the DOX dosage of 2 mg/kg/day via a tail vein once a week three times, and measured the tumor size every day. The result indicated that BPA‐PEG‐LPDOX strongly suppressed the cancer growth, as the tumor volume in the BPA‐PEG‐LPDOX‐treated mice was significantly smaller than that in the PEG‐LPDOX‐treated mice (Fig. 5b). When the body weight changes of the mice were monitored as an indicator of a side effect of DOX, no remarkable decrease in body weight was observed in any of the mice (data not shown). These results indicate that BPA‐PEG‐LPDOX possessed a potent therapeutic effect on human prostate cancer cells.
Discussion
Nowadays, the rate of prostate cancer is rising, especially in the developed countries. Corresponding to this situation, the importance of prostate cancer diagnosis has become a matter of focus, because the treatment of prostate cancer in its early stages can significantly improve the survival rate. PSA, a serine proteinase glycoprotein, is a suitable marker for detecting prostate cancer, because the amount of PSA in the bloodstream of the patient begins to increase with the development of prostate cancer.20 In addition, it is known that some tumor‐associated carbohydrate antigens such as sialyl LewisX (sLeX) can be indicators of tumor malignancy in some types of cancer, including prostate cancer.21, 22 These findings point to the importance of cell‐surface sugar chains in tumor development and the possibility of using them as targets for drug delivery and molecular imaging. In fact, some researchers have used lectins for whole‐body imaging of cancer and as an active targeting probe of nanoparticles for drug delivery.23
Our present research aimed at developing an effective prostate cancer therapy by utilizing the interaction between a lectin on the liposomal surface and sugar chains on prostate cancer cells. BPA is a well‐known lectin that binds to sugar chains terminating in galactose, such as Gal‐GalNAc; and of glycoproteins having this terminal sugar, Thomsen–Friedenreich antigen is the most famous.24 This antigen is a tumor‐associated molecule involved in prostate carcinogenesis; and its expression in prostate cancer cells increases with the development of tumor malignancy.25 Pacis et al.26 also studied the relationship between the expression of carbohydrates on the surface of prostate cancer cells and tumor malignancy, and reported that the potential of human prostate cancer cells to metastasize to bone increases with an increase in the expression of carbohydrates terminating in galactose. In the present study, we found that BPA strongly bound to human prostate cancer tissue, whereas it did not bind to normal prostate tissue in the same specimen (Fig. 1). Moreover, we observed that BPA specifically bound to cells of the LNCaP human prostate cancer line and showed that this binding was inhibited in the presence of an excess amount of galactose (Fig. S1). These results suggest that BPA bound to human prostate cancer cells via cell‐surface antigens such as the Thomsen–Friedenreich antigen, which would explain why BPA bound to cancerous, but not to normal, prostate tissue. In contrast, we have examined the targetability of BPA to the other human cancers by similarly comparing the binding potential between the cancer and the normal tissues. The results indicated that BPA strongly bound to lung, breast, colon and bladder cancer tissues compared with their normal tissues (Table S1). These results suggest that BPA could be applicable as a targetable probe for wide spectrum of human cancers.
To utilize the binding potential of BPA for the treatment of prostate cancer, we modified the liposomal surface with BPA for delivering anti‐cancer drugs to prostate cancer tissue. As the results showed, BPA‐PEG‐LP of approximately 140 nm in size could be successfully prepared; and the association of BPA‐PEG‐LP with human prostate cancer cells was significantly higher than that of PEG‐LP (Fig. 2a,b). Furthermore, the binding of BPA‐PEG‐LP to DU145 cells was clearly cancelled by heat‐inactivation of BPA or by competitive inhibition by an excessive amount of galactose (Fig. 2c,d), suggesting that the binding of BPA‐PEG‐LP to prostate cancer cells was dependent on the preferential interaction between BPA on the liposomal surface and the cell‐surface galactosyl sugar chains.
The biodistribution analysis indicated that BPA‐PEG‐LP showed a long circulation‐time potential, when they were i.v. injected into DU145 solid cancer‐bearing mice. Subsequently, BPA‐PEG‐LP highly accumulated in the solid cancer; and this accumulation was quite similar to that of PEG‐LP (Fig. 3): this result was dominantly due to the EPR effect of long‐circulating nanoparticles, which was a prerequisite for the specific binding of BPA‐PEG‐LP to the cancer cells. In fact, our previous study demonstrated that active targeting peptide‐conjugated PEG‐modified liposomes show a biodistribution pattern similar to that of PEG‐modified liposomes in tumor‐bearing mice, although the intratumoral distribution of these liposomes is much different.19 Thus, to elucidate the difference of liposome distribution in prostate cancer tissue between BPA‐PEG‐LP and PEG‐LP, the liposome localization in DU145 cancer tissues was fluorometrically observed. BPA‐PEG‐LP was widely distributed in parenchyma of the cancer tissues; and most of the liposomes seemed to bind to or be taken up into the cells (Fig. 4). In contrast, PEG‐LP was rarely observed in the cancer tissues. We assumed that this difference was caused by the ligand‐mediated targeting potential of BPA‐PEG‐LP: BPA‐PEG‐LP strongly bound to prostate cancer cells via the ligand‐receptor interaction and was preferentially incorporated into the cells, whereas PEG‐LP was poorly incorporated into the cells because of the shielding effect of PEG on the recognition of these cells by certain proteins and immune cells.
In light of the targetability of BPA‐PEG‐LP to human prostate cancer cells, we then examined the in vitro cytotoxic effect of BPA‐PEG‐LPDOX against DU145 cells. BPA‐PEG‐LPDOX inhibited the proliferation of DU145 cells and this effect of BPA‐PEG‐LPDOX was higher than that obtained with PEG‐LPDOX (Fig. 5). This result suggests that the enhanced cytotoxicity was brought by ligand‐mediated targeting of BPA‐PEG‐LPDOX to prostate cancer cells. There is much evidence that active targeting liposomes such as antibody‐modified liposomes are taken up into cells by a receptor‐mediated endocytotic pathway and that the encapsulated drugs are then released into the cytosol following lysosomal degradation.27 There are also some reports about lectin‐mediated drug delivery into cells. Qaddoumi et al.28 reported that some lectins were taken up into cells by endocytotic pathway after interaction with membrane receptors and Gabor et al.29 further demonstrated that a kind of lectin, wheat germ agglutinin (WGA), was subject to the proteolytic degradation in lysosome following endocytosis. Thus, we assume that the mechanism by which BPA‐PEG‐LPDOX was effectively taken up into the cells was endocytosis, which occurred after binding of these modified liposomes to the cell‐surface sugar chains. Following endocytosis, the intraliposomal DOX would have been released to exert its cytotoxic action.
Finally, we performed a cancer therapeutic experiment using BPA‐PEG‐LPDOX. Because liposomal DOX has already been commercialized for the treatment of several cancers all over the world and some candidates of liposomal DOX are now in clinical studies for prostate cancer,30 we choose DOX as a cytotoxic agent. Three treatments of DU145‐bearing mice with BPA‐PEG‐LPDOX strongly suppressed the tumor growth in these mice; and the tumor volume of the BPA‐PEG‐LPDOX‐treated mice was significantly smaller than that of the PEG‐LPDOX‐treated mice (Fig. 6). Furthermore, the body‐weight decrease as an indicator of side effects was not observed in BPA‐PEG‐LPDOX‐treated mice (data not shown). We strongly believe that this enhanced therapeutic effect was due to the potential of BPA to target prostate cancer and efficient delivery of DOX to the cells.
Figure 6.
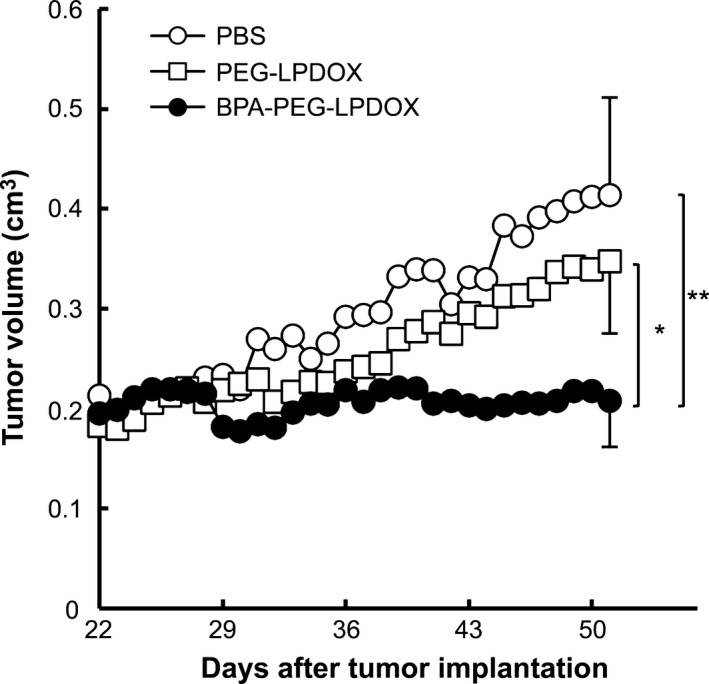
Suppression of prostate cancer growth by the treatment with Bauhinia purprea agglutinin (BPA)‐PEG‐LPDOX. Therapeutic effect of BPA‐PEG‐LPDOX on DU145 cancer‐bearing mice. PEG‐LPDOX or BPA‐PEG‐LPDOX (2 mg/kg/day as DOX dosage) was i.v. injected via a tail vein into DU145 cancer‐bearing mice (n = 4–6) once a week for a total of three treatments (day 29, 36 and 43). The tumor volume was monitored daily starting after day 22. Data are shown as the mean. SD are shown with bars at day 51. Significant differences are shown with asterisks (*P < 0.05; **P < 0.01, Tukey HSD).
In the present study, we succeeded in preparing active targeting liposomes bearing the lectin BPA; the liposomes enabled delivery of DOX, an anticancer drug, to prostate cancer cells and suppressed their proliferation. Because BPA could specifically recognize cancer cells in the human prostate but not normal cells in this tissue, BPA could be a potent active targeting probe of nanocarriers for delivering anti‐cancer drugs to prostate cancer cells.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Binding of Bauhinia purprea agglutinin (BPA) to human prostate LNCaP cancer cells. BPA‐FITC or BSA‐FITC was added to LNCaP cells in the absence or presence of 200 mM galactose on a slide glass, and the cells were then incubated at 37°C for 1 h. The fluorescence was observed under a fluorescence microscope. The green image shows the distribution of the lectin, and blue images show the nuclei stained by DAPI. Scale bars: 100 μm.
Table S1. Binding potential of Bauhinia purprea agglutinin (BPA) to human cancer tissues. Binding potential of BPA to cancer and normal tissues in each organ are summarized and shown in stages from weak (+) to strong (++++).
Acknowledgments
This work was supported by a research grant from Sugiyama Chemical and Industrial Laboratory and by the University of Shizuoka.
Cancer Sci 107 (2016) 53–59
Funding Information Sugiyama Chemical and Industrial Laboratory.
References
- 1. Mizuguchi S, Uyama T, Kitagawa H et al Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans . Nature 2003; 423: 443–48. [DOI] [PubMed] [Google Scholar]
- 2. Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, Hsieh‐Wilson LC. Dynamic O‐GlcNAc modification regulates CREB‐mediated gene expression and memory formation. Nat Chem Biol 2012; 8: 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geijtenbeek TB, Gringhuis SI. Signalling through C‐type lectin receptors: shaping immune responses. Nat Rev Immunol 2009; 9: 465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kobata A, Amano J. Altered glycosylation of proteins produced by malignant cells, and application for the diagnosis and immunotherapy of tumours. Immunol Cell Biol 2005; 83: 429–39. [DOI] [PubMed] [Google Scholar]
- 5. Judd WJ. The role of lectins in blood group serology. Crit Rev Clin Lab Sci 1980; 12: 171–214. [DOI] [PubMed] [Google Scholar]
- 6. Hideshima S, Hinou H, Ebihara D et al Attomolar detection of influenza A virus hemagglutinin human H1 and avian H5 using glycan‐blotted field effect transistor biosensor. Anal Chem 2013; 85: 5641–44. [DOI] [PubMed] [Google Scholar]
- 7. Kawai K, Kojima T, Miyanaga N et al Lectin‐reactive alpha‐fetoprotein as a marker for testicular tumor activity. Int J Urol 2005; 12: 284–89. [DOI] [PubMed] [Google Scholar]
- 8. Roth J. Lectins for histochemical demonstration of glycans. Histochem Cell Biol 2011; 136: 117–30. [DOI] [PubMed] [Google Scholar]
- 9. Madera M, Mechref Y, Novotny MV. Combining lectin microcolumns with high‐resolution separation techniques for enrichment of glycoproteins and glycopeptides. Anal Chem 2005; 77: 4081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bies C, Lehr CM, Woodley JF. Lectin‐mediated drug targeting: history and applications. Adv Drug Deliv Rev 2004; 56: 425–35. [DOI] [PubMed] [Google Scholar]
- 11. Wen Z, Yan Z, Hu K et al Odorranalectin‐conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson's disease following intranasal administration. J Control Release 2011; 151: 131–38. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell MJ, Chen CS, Ponmudi V, Hughes AD, King MR. E‐selectin liposomal and nanotube‐targeted delivery of doxorubicin to circulating tumor cells. J Control Release 2012; 160: 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature 1996; 380: 561–62. [DOI] [PubMed] [Google Scholar]
- 14. Maeda H, Matsumura Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv Drug Deliv Rev 2011; 63: 129–30. [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa K, Asai T, Shigematsu H et al Development of anti‐HB‐EGF immunoliposomes for the treatment of breast cancer. J Control Release 2012; 160: 274–80. [DOI] [PubMed] [Google Scholar]
- 16. Boland CR, Montgomery CK, Kim YS. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A 1982; 79: 2051–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawa S, Kato M, Oguchi H, Kobayashi T, Furuta S, Kanai M. Preparation of pancreatic cancer‐associated mucin expressing CA19‐9, CA50, Span‐1, sialyl SSEA‐1, and Dupan‐2. Scand J Gastroenterol 1991; 26: 981–92. [DOI] [PubMed] [Google Scholar]
- 18. Ichikawa K, Asai T, Shimizu K et al Suppression of immune response by antigen‐modified liposomes encapsulating model agents: a novel strategy for the treatment of allergy. J Control Release 2013; 167: 284–89. [DOI] [PubMed] [Google Scholar]
- 19. Sugiyama T, Asai T, Nedachi YM et al Enhanced active targeting via cooperative binding of ligands on liposomes to target receptors. PLoS One 2013; 8: e67550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Angelis G, Rittenhouse HG, Mikolajczyk SD, Blair Shamel L, Semjonow A. Twenty years of PSA: from prostate antigen to tumor marker. Rev Urol 2007; 9: 113–23. [PMC free article] [PubMed] [Google Scholar]
- 21. Martensson S, Bigler SA, Brown M, Lange PH, Brawer MK, Hakomori S. Sialyl‐Lewis(x) and related carbohydrate antigens in the prostate. Hum Pathol 1995; 26: 735–39. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa T, Endo Y, Watanabe M et al Adhesional function of canine mammary gland tumor cells expressing sialyl Lewis X. J Vet Med Sci 2009; 71: 1225–28. [DOI] [PubMed] [Google Scholar]
- 23. Sakuma S, Yano T, Masaoka Y et al In vitro/in vivo biorecognition of lectin‐immobilized fluorescent nanospheres for human colorectal cancer cells. J Control Release 2009; 134: 2–10. [DOI] [PubMed] [Google Scholar]
- 24. Yu LG. The oncofetal Thomsen–Friedenreich carbohydrate antigen in cancer progression. Glycoconj J 2007; 24: 411–20. [DOI] [PubMed] [Google Scholar]
- 25. Janssen T, Petein M, Van Velthoven R et al Differential histochemical peanut agglutinin stain in benign and malignant human prostate tumors: relationship with prostatic specific antigen immunostain and nuclear DNA content. Hum Pathol 1996; 27: 1341–47. [DOI] [PubMed] [Google Scholar]
- 26. Pacis R, Pilat M, Yamazaki K, Pienta K. Differential carbohydrate expression in tumorigenic vs nontumorigenic prostate cell‐lines. Int J Oncol 1995; 7: 1349–54. [DOI] [PubMed] [Google Scholar]
- 27. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 2013; 65: 36–48. [DOI] [PubMed] [Google Scholar]
- 28. Qaddoumi M, Lee VH. Lectins as endocytic ligands: an assessment of lectin binding and uptake to rabbit conjunctival epithelial cells. Pharm Res 2004; 21: 1160–66. [DOI] [PubMed] [Google Scholar]
- 29. Gabor F, Schwarzbauer A, Wirth M. Lectin‐mediated drug delivery: binding and uptake of BSA‐WGA conjugates using the Caco‐2 model. Int J Pharm 2002; 237: 227–39. [DOI] [PubMed] [Google Scholar]
- 30. Kroon J, Metselaar JM, Storm G, van der Pluijm G. Liposomal nanomedicines in the treatment of prostate cancer. Cancer Treat Rev 2014; 40: 578–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Binding of Bauhinia purprea agglutinin (BPA) to human prostate LNCaP cancer cells. BPA‐FITC or BSA‐FITC was added to LNCaP cells in the absence or presence of 200 mM galactose on a slide glass, and the cells were then incubated at 37°C for 1 h. The fluorescence was observed under a fluorescence microscope. The green image shows the distribution of the lectin, and blue images show the nuclei stained by DAPI. Scale bars: 100 μm.
Table S1. Binding potential of Bauhinia purprea agglutinin (BPA) to human cancer tissues. Binding potential of BPA to cancer and normal tissues in each organ are summarized and shown in stages from weak (+) to strong (++++).


