Abstract
Numerous studies suggest that several long non‐coding RNAs (lncRNAs) play critical roles in bladder cancer development and progression. Long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) is highly expressed in bladder cancer tissues and cells, and it has been shown to play an important role in regulating aggressive phenotypes of bladder cancer cells. However, little is known about the molecular mechanism of lncRNA‐UCA1‐mediated bladder cancer cell migration and invasion. Here, we show that overexpression of lncRNA‐UCA1 could induce EMT and increase the migratory and invasive abilities of bladder cancer cells. Mechanistically, lncRNA‐UCA1 induced EMT of bladder cancer cells by upregulating the expression levels of zinc finger E‐box binding homeobox 1 and 2 (ZEB1 and ZEB2), and regulated bladder cancer cell migration and invasion by tumor suppressive hsa‐miR‐145 and its target gene the actin‐binding protein fascin homologue 1 (FSCN1). Furthermore, we also observed a positive correlation between lncRNA‐UCA1 and ZEB1/2 expression, and a negative correlation between lncRNA‐UCA1 and hsa‐miR‐145 expression in bladder cancer specimens. Importantly, we found that lncRNA‐UCA1 repressed hsa‐miR‐145 expression to upregulate ZEB1/2, whereas the suppression of hsa‐miR‐145 could upregulate lncRNA‐UCA1 expression in bladder cancer cells. Moreover, the binding site for hsa‐miR‐145 within exons 2 and 3 of lncRNA‐UCA1 contributed to the reciprocal negative regulation of lncRNA‐UCA1 and hsa‐miR‐145. Taken together, our results identified that lncRNA‐UCA1 enhances bladder cancer cell migration and invasion in part through the hsa‐miR‐145/ZEB1/2/FSCN1 pathway. Therefore, lncRNA‐UCA1 might act as a promising therapeutic target for the invasion and metastasis of bladder cancer.
Keywords: Bladder cancer, epithelial to mesenchymal transition, Hsa‐miR‐145, long non‐coding RNA, UCA1
Human bladder cancer is one of the most common malignancies in the world and high rates of recurrence and metastasis are the primary causes of mortality.1 The molecular mechanism of cancer metastasis is quite complex and several pathways or factors have been implicated in regulating the metastatic potential of cancer cells.2 Epithelial–mesenchymal transition is commonly regarded as a mechanism for metastatic dissemination that endows cancer cells with more aggressive potential.3 Accumulating evidence indicates that multiple regulators, including protein coding genes and non‐coding RNAs, can trigger EMT to support cancer cell migration and invasion.4
Long non‐coding RNAs (lncRNAs) are defined as a class of transcripts with a low protein‐coding capacity that modulate several signaling pathways to serve oncogenic or tumor suppressive roles during tumorigenesis.5, 6 Specifically, lncRNAs have been shown to promote EMT and metastasis in various types of cancer by acting as a competing endogenous RNA (ceRNA) to sponge microRNAs (miRNAs), for example, lincRNA‐RoR induces EMT to promote breast cancer progression and metastasis by acting as a ceRNA to miR‐205,7 and lncRNA‐activated by transforming growth factor‐β (lncRNA‐ATB) can also promote EMT and hepatocellular carcinoma (HCC) cell invasion by competitively binding the miR‐200 family.8 Additionally, miRNAs can directly target not only mRNAs but also lncRNAs. For example, hsa‐miR‐125b suppresses bladder cancer progression by negatively regulating lncRNA metastasis‐associated lung adenocarcinoma transcript 1.9 Long non‐coding RNA growth arrest‐specific 5 (GAS5) serves as a miR‐21 target to regulate cell growth, apoptosis and cell invasion. Furthermore, lncRNA‐GAS5 can also repress miR‐21 expression, thus forming a reciprocal repression regulatory loop.6 Therefore, the interaction between lncRNAs and miRNAs has been reported to play an important role in cancer development and progression.
Urothelial cancer‐associated 1 (UCA1) is first identified as an oncogenic lncRNA in bladder cancer, which has been reported to regulate bladder cancer cell proliferation, migration, invasion chemoresistance, and metabolism.10, 11, 12, 13 Subsequent studies revealed that the upregulation of lncRNA‐UCA1 in several types of tumor tissues, including tongue squamous cell carcinoma, melanoma, and esophageal squamous cell carcinoma, is statistically correlated with lymph node metastasis.14, 15, 16 In gastric cancer, high lncRNA‐UCA1 expression correlated with tumor invasion depth.17 Notably, lncRNA‐UCA1 depletion can attenuate the migratory ability of tongue squamous cell carcinoma, melanoma, and esophageal squamous cell carcinoma cells, whereas overexpression of lncRNA‐UCA1 can enhance ovarian cancer cell migration and invasion, suggesting that lncRNA‐UCA1 functions as a pivotal regulator of cellular migration and invasion.18 Recently, it has been reported that lncRNA‐UCA1 promotes HCC cell migration and invasion by inhibiting miR‐216b and activating the fibroblast growth factor receptor 1/ERK pathway.19 However, the mechanism by which lncRNA‐UCA1 modulates bladder cancer invasion and metastasis remains poorly understood.
In this study, we discovered that overexpression of lncRNA‐UCA1 induces EMT, migration, and invasion of bladder cancer cells. Furthermore, lncRNA‐UCA1 promotes bladder cancer cell migration and invasion through the hsa‐miR‐145–zinc finger E‐box binding homeobox 1/2 (ZEB1/2)–fascin homologue 1 (FSCN1) pathway. In addition, we identified that there is a reciprocal repression regulatory loop between lncRNA‐UCA1 and hsa‐miR‐145. Therefore, our studies elucidated the mechanism that lncRNA‐UCA1 promotes bladder cancer cell migration and invasion and provided a novel therapeutic target for bladder cancer metastasis.
Materials and Methods
Cell culture and tissue samples
Human bladder cancer cells 5637, T24, and UMUC2 were grown in RPMI‐1640 (Gibco, Grand Island, NY, USA) with 10% bovine calf serum and maintained at 37°C under a humidified 5% CO2 atmosphere. For depletion of lncRNA‐UCA1, 5637 and T24 cells were transfected with pRNAT‐U6.1/shUCA1 and pRNAT‐U6.1/negative control plasmid. For overexpression of lncRNA‐UCA1, UMUC2 cells were transfected with pcDNA3.1‐UCA1 and pcDNA3.1 plasmid. Human bladder cancer tissues were obtained from the First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China). The protocols used in the study were approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University. Twenty‐five specimens of pathologically diagnosed biopsy specimens were obtained from patients with bladder tumors (Table 1).
Table 1.
Clinical characteristics of patients with bladder cancer (n = 25)
| Patient no. | Gender | Age, years | T stage | Lymph node status |
|---|---|---|---|---|
| 1 | Male | 72 | T1 | N0 |
| 2 | Male | 76 | T3 | N0 |
| 3 | Male | 56 | T1 | N0 |
| 4 | Male | 48 | T3 | N2 |
| 5 | Male | 62 | T1 | N0 |
| 6 | Male | 78 | T2 | N0 |
| 7 | Male | 63 | T2 | N0 |
| 8 | Female | 58 | T1 | N0 |
| 9 | Male | 52 | T4 | N0 |
| 10 | Female | 53 | T1 | N0 |
| 11 | Female | 68 | T1 | N0 |
| 12 | Male | 68 | T1 | N0 |
| 13 | Male | 74 | T2 | N0 |
| 14 | Male | 61 | T2 | N0 |
| 15 | Male | 69 | T3 | N1 |
| 16 | Male | 67 | T2 | N0 |
| 17 | Male | 62 | T3 | N2 |
| 18 | Male | 75 | T3 | N0 |
| 19 | Male | 67 | T3 | N0 |
| 20 | Male | 63 | T2 | N0 |
| 21 | Female | 70 | T1 | N0 |
| 22 | Male | 67 | T2 | N0 |
| 23 | Male | 58 | T1 | N0 |
| 24 | Male | 75 | T2 | N0 |
| 25 | Male | 67 | T2 | N0 |
Vector construction
The full‐length lncRNA‐UCA1 was amplified by PCR, and the PCR products were digested and ligated into pMIR‐REPORT luciferase vector (Ambion, Life Technologies, Carlsbad, CA, USA). The hsa‐miR‐145 binding site mutations were generated using a QuikChange Multi Site‐Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). Mutant primers are listed in Table 2.
Table 2.
Primers and shRNAs used in this study of bladder cancer cell migration and invasion
| Gene | Sequence (5′–3′) | Experimental use |
|---|---|---|
| UCA1 | CTCTCCATTGGGTTCACCATTC | Real‐time PCR |
| GCGGCAGGTCTTAAGAGATGAG | ||
| E‐cadherin | GCTGCTCTTGCTGTTTCTTCG | Real‐time PCR |
| CCGCCTCCTTCTTCATCATAG | ||
| Vimentin | AAGTTTGCTGACCTCTCTGAGGCT | Real‐time PCR |
| CTTCCATTTCACGCATCTGGCGTT | ||
| N‐cadherin | GCCCCTCAAGTGTTACCTCAA | Real‐time PCR |
| AGCCGAGTGATGGTCCAATTT | ||
| ZEB1 | TTCAAACCCATAGTGGTTGCT | Real‐time PCR |
| TGGGAGATACCAAACCAACTG | ||
| ZEB2 | CGCTTGACATCACTGAAGGA | Real‐time PCR |
| CTTGCCACACTCTGTCCATT | ||
| Snail1 | TCCAGAGTTTACCTTCCAGCA | Real‐time PCR |
| CTTTCCCACTGTCCTCATCTG | ||
| Snail2 | CTACAGCGAACTGGACACACA | Real‐time PCR |
| GCCCCAAAGATGAGGAGTATC | ||
| Twist1 | GTCCGCAGTCTTACGAGGAG | Real‐time PCR |
| GTCTGAATCTTGCTCAGCTTGT | ||
| Twist2 | ACAAGCTGAGCAAGATCCAGAC | Real‐time PCR |
| GCTGGTCATCTTATTGTCCATC | ||
| FSCN1 | GGCAAGTTTGTGACCTCCAAGAA | Real‐time PCR |
| AGCCGATGAAGCCATGCTC | ||
| ACTB (β‐actin) | TCCCTGGAGAAGAGCTACGA | Real‐time PCR |
| AGCACTGTGTTGGCGTACAG | ||
| miR‐145 binding site | GCCATATGAAGACACCCTCGATCAGTCC | Site‐directed mutagenesis |
| GGACTGATCGAGGGTGTCTTCATATGGC | ||
| UCA1 shRNA control | gatccTTCTCCGAACGTGTCACGTttcaagaga | RNA interference |
| ACGTGACACGTTCGGAGAAttttttggaaa | ||
| UCA1 shRNA | gatccGTTAATCCAGGAGACAAAGAttcaaga | RNA interference |
| gaTCTTTGTCTCCTGGATTAACttttttggaaa |
Bioinformatic analysis
The putative miRNA binding sites on lncRNA‐UCA1 sequences were predicted by an RNAhybrid software program (http://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid) with the minimum free energy cutoff set at −22 kcal/mol.
Quantitative real‐time PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA). First‐strand cDNA was synthesized with random primers using a RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, Waltham, MA, USA) or commercial miRNA reverse transcription PCR kit (RiboBio, Guangzhou, China). Quantitative real‐time PCR was carried out using a SYBR Premix Ex Taq II (Takara, Dalian, China) on a CFX96 real‐time PCR System (Bio‐Rad, Hercules, CA, USA), and the results were normalized with U6 or β‐actin as an internal control. Primers are listed in Table 2.
Western blot analysis
Cells were lysed in RIPA buffer containing protease inhibitor (Roche, Nutley, NJ, USA). Protein samples were separated by SDS‐PAGE and transferred to nitrocellulose membranes. The membranes were incubated with E‐cadherin (Abcam, Hong Kong, China), N‐cadherin (Cell Signaling Technology, Danvers, MA, USA), Vimentin, Snail1, ZEB1 (all Cell Signaling Technology), ZEB2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), FSCN1 (Abcam), and β‐actin (Cell Signaling Technology) primary antibodies. Protein expression was assessed by ECL chemiluminescent regents (Pierce, Rockford, IL, USA) and the intensity of the protein bands was quantified by densitometry (Image Lab software, Bio‐Rad, Hercules, CA, USA) and normalized to the corresponding β‐actin bands.
Wound healing assay
Cells were seeded at a density of 1 × 106 cells/well onto six‐well plates and incubated overnight. Wounds were created by scratching cell monolayers with a sterile 200‐μL plastic pipette tip. Cells were further incubated in serum‐free medium for 24 or 36 h and images were monitored at different time points by phase contrast microscopy (Nikon, Tokyo, Japan) (original magnification, ×100).
Migration and invasion assays
The invasion assay was carried out using a 24‐well Millicell chamber containing a Matrigel‐coated membrane. The migration assay was carried out in a similar fashion without the Matrigel coating. Cells (5 × 105 cells in serum‐free medium) were seeded in the top chamber. The bottom wells were filled with complete medium. Cells on the upper membrane surface were wiped off using a cotton swab and the lower membrane surface was fixed with methanol, stained with 0.1% crystal violet, and counted in five random fields (original magnification, ×200).
Immunofluorescence
Bladder cancer cells were fixed, permeabilized, and incubated with E‐cadherin or vimentin antibodies, and then incubated with the Cy3‐conjugated IgG (Invitrogen). Cellular nuclei were counterstained with DAPI (Roche). Cells were detected with a fluorescence microscope (Nikon).
Luciferase reporter assay
Bladder cancer cells were cultured overnight until 70% confluence. Transient transfection of the lncRNA‐UCA1 luciferase reporter plasmid and internal control Renilla luciferase plasmid were carried out with the X‐treme GENE HP DNA transfection reagent (Roche). After 48 h of transfection, luciferase activity was measured using a dual‐luciferase reporter gene assay system (Promega, Madison, WI, USA).
Statistical analysis
All statistical analyses were carried out using GraphPad Prism Software (GraphPad Software, Inc., San Diego, CA, USA). Statistical evaluations were determined using Student's t‐test (two‐tailed) and Pearson's correlation analysis. P‐value < 0.05 was considered significant.
Results
Ectopic overexpression of lncRNA‐UCA1 induces EMT of bladder cancer cells
To investigate whether lncRNA‐UCA1 regulates bladder cancer cell migration and invasion through inducing EMT, we first established the stable expression of lncRNA‐UCA1 in the bladder cancer UMUC2 cell line transfected with pcDNA3.1 or pcDNA3.1‐UCA1 plasmid, and the efficiency of ectopic overexpressed lncRNA‐UCA1 was confirmed by quantitative real‐time PCR (Fig. 1a). We then observed the phenotype changes of UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1 plasmid. As shown in Figure 1(b), UMUC2 cells overexpressing lncRNA‐UCA1 showed a mesenchymal‐like phenotype, whereas control cells retained the epithelial phenotype. Moreover, we detected the expression levels of epithelial marker E‐cadherin and mesenchymal markers N‐cadherin and vimentin in bladder cancer cells. Overexpression of lncRNA‐UCA1 could decrease the E‐cadherin mRNA and protein levels and increase the N‐cadherin and vimentin mRNA and protein levels (Fig. 1c,d). Using the immunofluorescence assay, we found that overexpression of lncRNA‐UCA1 could decrease E‐cadherin expression in the cell membrane and increase vimentin expression (Fig. 1e). Taken together, our data suggested that lncRNA‐UCA1 acts as an important EMT inducer of bladder cancer cells.
Figure 1.
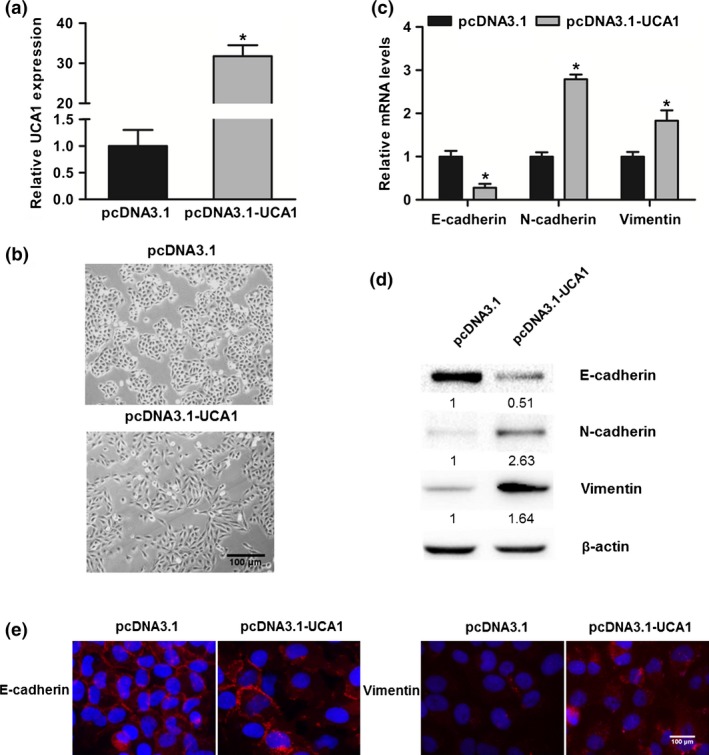
Overexpression of long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) induces EMT in bladder cancer cells. (a) Overexpression efficiency of lncRNA‐UCA1 in UMUC2 cells was detected by quantitative PCR. (b) The cell morphology of UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1 was observed by phase contrast microscopy. (c) Quantitative PCR analysis of E‐cadherin, N‐cadherin, and vimentin mRNA levels in UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (d) Western blot analysis of E‐cadherin, N‐cadherin, and vimentin protein levels in UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (e) Immunofluorescence analysis of the expression of E‐cadherin or vimentin in 5637 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. All data are the averages of at least three independent experiments and data are presented as mean ± SD. *P < 0.05.
Long non‐coding RNA UCA1 induces EMT of bladder cancer cells by ZEB1/2
To determine whether lncRNA‐UCA1 functions as an EMT inducer through modulating EMT‐related regulators, we detected the expression levels of EMT‐related regulators in three bladder cancer cell lines with different expression levels of lncRNA‐UCA1 (Fig. 2a). Among these EMT‐related regulators, we found that overexpression of lncRNA‐UCA1 in UMUC2 cells significantly increased Snail1, ZEB1, and ZEB2 mRNA and protein expression levels (Fig. 2b,c). Furthermore, knockdown of lncRNA‐UCA1 in 5637 cells also decreased ZEB1/2 mRNA and protein expression levels (Fig. 2d,e). We detected the expression levels of lncRNA‐UCA1 and ZEB1/2 in 25 bladder cancer specimens and found that lncRNA‐UCA1 RNA levels were positively correlated with ZEB1/2 mRNA levels in bladder cancer specimens (Fig. 2f,g). These results suggested that lncRNA‐UCA1 may be involved in regulating EMT of bladder cancer cells, at least in part, and be mediated through ZEB1/2.
Figure 2.
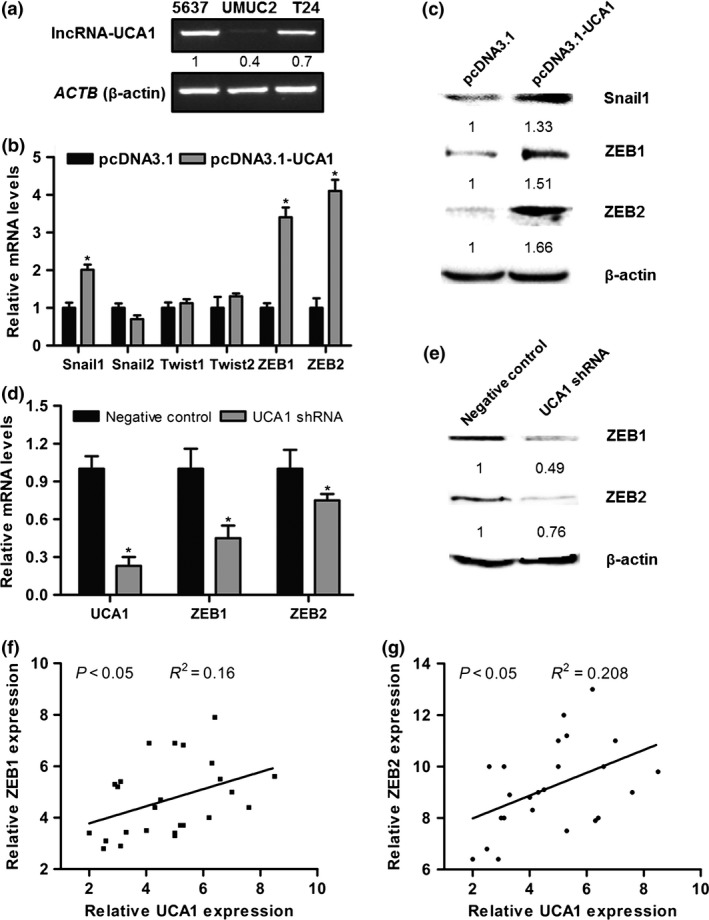
Long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) induces EMT of bladder cancer cells through zinc finger E‐box binding homeobox 1 and 2 (ZEB1/2). (a) LncRNA‐UCA1 expression levels in 5637, UMUC2, and T24 cells were analyzed by PCR. ACTB (β‐actin) was used as the internal control. (b) Quantitative PCR analysis of Snail1/2, Twist1/2, and ZEB1/2 mRNA levels in UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (c) Western blot analysis of Snail1 and ZEB1/2 protein levels in UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (d, e) Quantitative PCR and Western blotting were carried out to detect the expression levels of ZEB1/2 in 5637 cells transfected with shRNA control and lncRNA‐UCA1 shRNA. (f, g) Correlation analysis of lncRNA‐UCA1 and ZEB1/2 expression levels in human bladder cancer specimens (n = 25). All data are the averages of at least three independent experiments and data are presented as mean ± SD. *P < 0.05.
Long non‐coding RNA UCA1 regulates bladder cancer cell migration and invasion
To explore the roles of lncRNA‐UCA1 in bladder cancer invasion and metastasis, we examined the effects of lncRNA‐UCA1 in bladder cancer cell migration and invasion. As shown in Figure 3(a–c), overexpression of lncRNA‐UCA1 in UMUC2 cells increased cell mobility compared with control cells, and its overexpression also downregulated the invasive potential of UMUC2 cells compared with control cells. In addition, depletion of lncRNA‐UCA1 in 5637 cells decreased cell mobility compared with control cells, and its depletion also downregulated the invasive potential of 5637 cells compared with control cells (Fig. 3d–g). Therefore, these results indicated that lncRNA‐UCA1 plays a crucial role in regulating the migration and invasion of bladder cancer cells.
Figure 3.
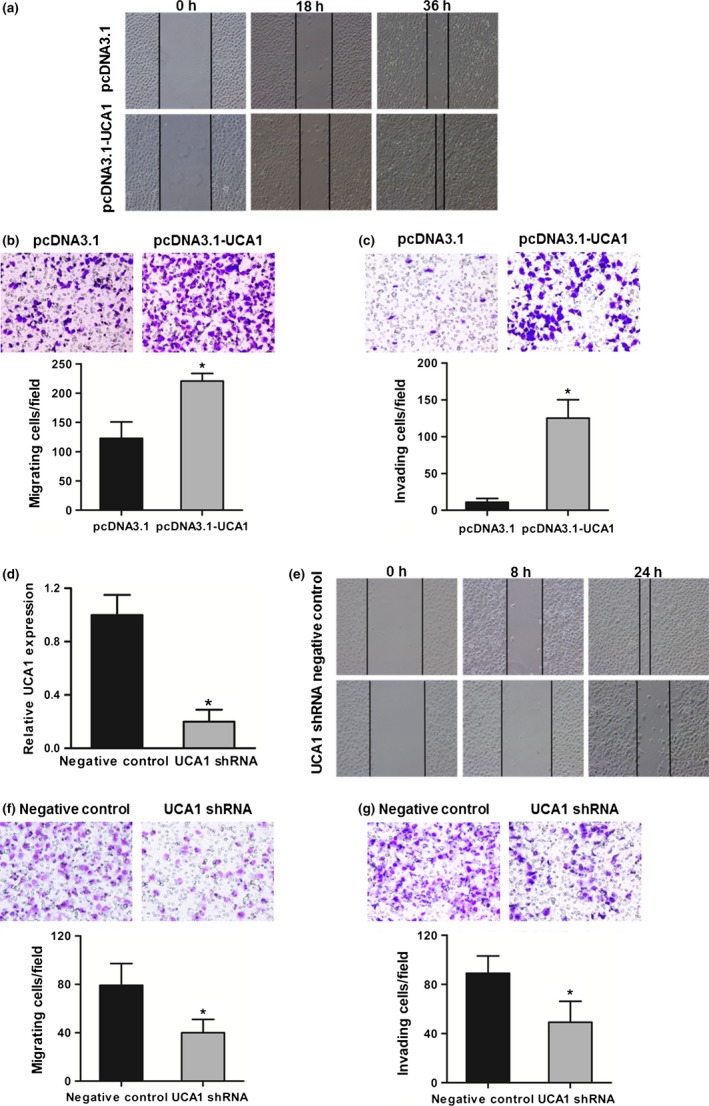
Long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) promotes bladder cancer cell migration and invasion. (a) Wound healing assay was carried out to evaluate the motility of UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (b) Migration assay was carried out to assess the migratory ability of UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (c) Invasion assay was used to examine the invasive ability of UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1. (d) Suppression efficiency of lncRNA‐UCA1 shRNA in 5637 cells was detected by quantitative PCR. (e) The motility of 5637 cells transfected with shRNA control and lncRNA‐UCA1 shRNA was analyzed by wound healing assay. (f, g) The migratory and invasive abilities of 5637 cells transfected with shRNA control or lncRNA‐UCA1 shRNA was assessed by migration and invasion assays. All data are the averages of at least three independent experiments and data are presented as mean ± SD. *P < 0.05.
Long non‐coding RNA UCA1 regulates bladder cancer cell migration and invasion by the hsa‐miR‐145‐FSCN1 pathway
To address the regulatory mechanism of lncRNA‐UCA1 in bladder cancer cell migration and invasion, we measured the expression levels of several miRNAs that are involved in regulating EMT or cellular migration and invasion. Among these miRNAs, overexpression of lncRNA‐UCA1 clearly inhibited hsa‐miR‐145 expression in UMUC2 cells (Fig. 4a). We also found that overexpression of lncRNA‐UCA1 in UMUC2 cells markedly repressed hsa‐miR‐145 expression in a dose‐dependent manner (Fig. 4b). Conversely, when we depleted lncRNA‐UCA1 expression in 5637 and T24 cells by lncRNA‐UCA1 shRNA, the expression level of hsa‐miR‐145 was significantly upregulated (Fig. 4c,d). Recent studies have shown that hsa‐miR‐145 inhibits bladder cancer cell migration and invasion through FSCN1.20 Thus, we also investigated whether lncRNA‐UCA1 could control the expression of the hsa‐miR‐145 target gene FSCN1. We found that overexpression of lncRNA‐UCA1 in UMUC2 cells significantly increased FSCN1 mRNA and protein levels, whereas depletion of lncRNA‐UCA1 in 5637 cells markedly repressed the FSCN1 mRNA and protein levels (Fig. 4e,f). In addition, we detected the expression levels of lncRNA‐UCA1 and hsa‐miR‐145 in 25 bladder cancer specimens. We found that lncRNA‐UCA1 RNA levels were negatively correlated with hsa‐miR‐145 RNA levels in bladder cancer specimens (Fig. 4g). Altogether, our data indicated that lncRNA‐UCA1 regulates bladder cancer cell migration and invasion, in part by the hsa‐miR‐145–FSCN1 pathway.
Figure 4.
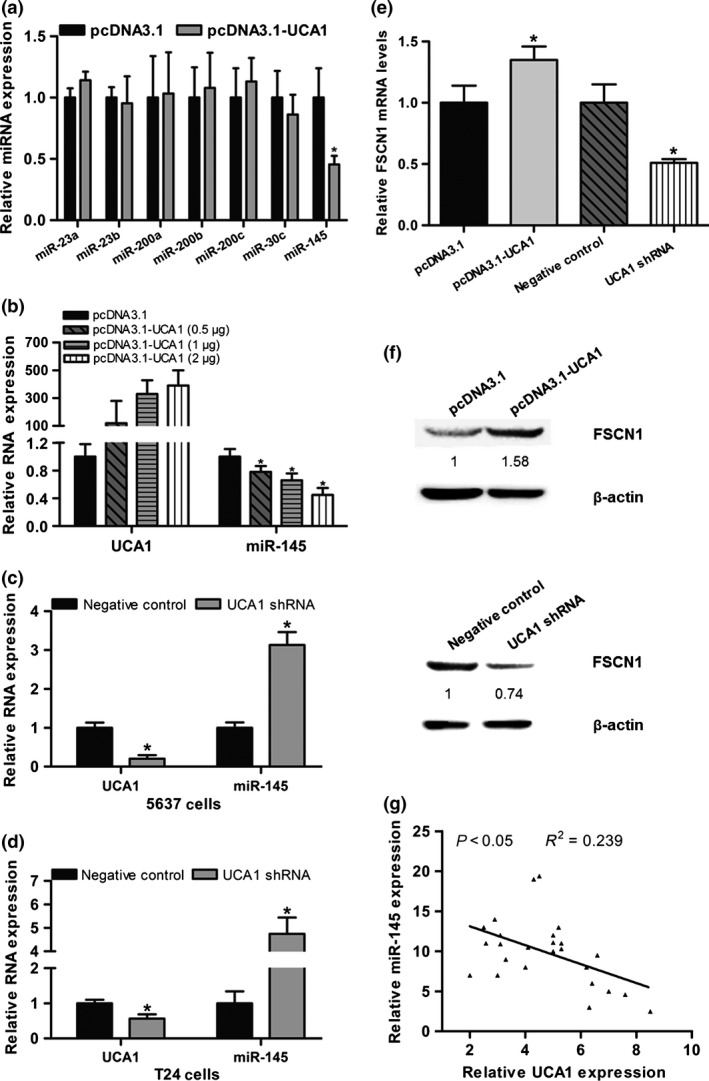
Long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) promotes bladder cancer cell migration and invasion through the hsa‐miR‐145–fascin homologue 1 (FSCN1) pathway. (a) Quantitative PCR analysis of miRNA expression levels in UMUC2 cells transfected with pcDNA3.1 or pcDNA3.1‐UCA1. (b) Quantitative PCR analysis of lncRNA‐UCA1 and hsa‐miR‐145 RNA levels in UMUC2 cells transfected with pcDNA3.1 and pcDNA3.1‐UCA1 at a final concentration of 0.5, 1, and 2 μg. (c, d) Quantitative PCR analysis of lncRNA‐UCA1 and hsa‐miR‐145 RNA levels in 5637 and T24 cells transfected with shRNA control or lncRNA‐UCA1 shRNA. (e, f) Quantitative PCR and Western blot analyses of lncRNA‐UCA1 and FSCN1 expression levels in UMUC2 cells transfected with pcDNA3.1 or pcDNA3.1‐UCA1 and 5637 cells transfected with shRNA control or lncRNA‐UCA1 shRNA. (g) Correlation analysis of lncRNA‐UCA1 and hsa‐miR‐145 expression levels in human bladder cancer specimens (n = 25). All data are the averages of at least three independent experiments and data are presented as mean ± SD. *P < 0.05.
Reciprocal repression of lncRNA‐UCA1 and hsa‐miR‐145 in bladder cancer cells
Recently, lncRNAs have been identified as ceRNAs by interacting with miRNAs. Furthermore, miRNAs also negatively regulate lncRNAs expression by binding with lncRNAs. To further investigate the relationship between lncRNA‐UCA1 and hsa‐miR‐145, we used an hsa‐miR‐145 mimic and inhibitor to observe the alteration of lncRNA‐UCA1 expression in UMUC2 cells. Hsa‐miR‐145 mimic could significantly decrease lncRNA‐UCA1 expression (Fig. 5a), whereas hsa‐miR‐145 inhibitor significantly increased lncRNA‐UCA1 expression (Fig. 5b).
Figure 5.
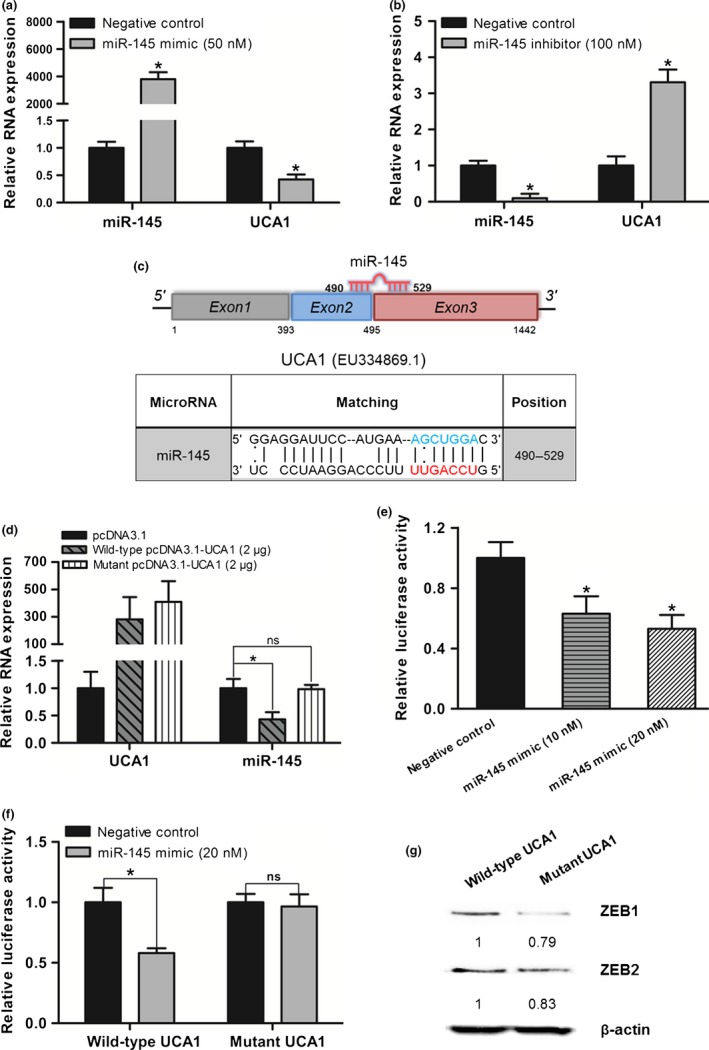
Reciprocal repression of long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1) and hsa‐miR‐145 in bladder cancer cells. (a, b) Quantitative PCR analysis of lncRNA‐UCA1 and hsa‐miR‐145 RNA levels in UMUC2 cells transfected with hsa‐miR‐145 mimic and negative control or hsa‐miR‐145 inhibitor and negative control. (c) Schematic representation of the putative binding site of hsa‐miR‐145 on lncRNA‐UCA1. The red nucleotides were the seed sequences of hsa‐miR‐145 and the blue nucleotides (target sites) were deleted in the mutant lncRNA‐UCA1 constructs. (d) Quantitative PCR analysis of lncRNA‐UCA1 and hsa‐miR‐145 RNA levels in UMUC2 cells transfected with wild‐type pcDNA3.1‐UCA1 or mutant pcDNA3.1‐UCA1 at a final concentration of 2 μg. (e) LncRNA‐UCA1 luciferase reporter constructs transfected into UMUC2 cells together with negative control or hsa‐miR‐145 mimic at a final concentration of 10 or 20 nM. (f) Wild‐type lncRNA‐UCA1 luciferase reporter constructs or mutant lncRNA‐UCA1 luciferase reporter constructs transfected into UMUC2 cells together with negative control or hsa‐miR‐145 mimic at a final concentration of 20 nM. (g) Western blot analysis of zinc finger E‐box binding homeobox 1 and 2 (ZEB1/2) protein levels in UMUC2 cells transfected with wild‐type pcDNA3.1‐UCA1 or mutant pcDNA3.1‐UCA1. All data are the averages of at least three independent experiments and are presented as mean ± SD. *P < 0.05. ns, non‐significant, P > 0.05.
To further confirm the reciprocal repression of lncRNA‐UCA1 and hsa‐miR‐145 by interacting with the putative hsa‐miR‐145 binding site within lncRNA‐UCA1, we used the RNAhybrid program to predict the putative complementary sequences for hsa‐miR‐145 in lncRNA‐UCA1. The putative binding site for hsa‐miR‐145 was predicted at exons 2 and 3 of lncRNA‐UCA1, and the minimum free energy of hsa‐miR‐145 : lncRNA‐UCA1 duplex was −26.2 kcal/mol (Fig. 5c). Furthermore, we constructed the lncRNA‐UCA1 overexpressing plasmid with mutations at the hsa‐miR‐145 binding site (Fig. 5c). We observed that overexpression of mutant lncRNA‐UCA1 in UMUC2 cells did not repress hsa‐miR‐145 expression compared with wild‐type lncRNA‐UCA1 (Fig. 5d). Additionally, we also cloned the full‐length of lncRNA‐UCA1 into luciferase reporter constructs (pMIR‐REPORT) and transfected it into UMUC2 cells with hsa‐miR‐145 mimic or negative control. Luciferase assay confirmed that transient transfection hsa‐miR‐145 mimic into UMUC2 cells reduced the activity of pMIR‐REPORT‐UCA1 (Fig. 5e). The mutations of the hsa‐miR‐145 binding site in pMIR‐REPORT‐UCA1 could abolish the downregulation of luciferase activity mediated by hsa‐miR‐145 mimic (Fig. 5f). Importantly, recent studies have shown that hsa‐miR‐145 represses EMT of cancer cells by directly targeting ZEB2 and indirectly targeting ZEB1.21, 22, 23 We then observed the expression of ZEB1/2 in UMUC2 cells transfected with lncRNA‐UCA1 overexpressing plasmid, which contains the wild‐type or mutated hsa‐miR‐145 binding site. Overexpression of mutant lncRNA‐UCA1 in UMUC2 cells could downregulate the expression levels of ZEB1/2 (Fig. 5g). These data indicated that lncRNA‐UCA1 is a target of hsa‐miR‐145, and lncRNA‐UCA1 can also inhibit hsa‐miR‐145 expression to upregulate ZEB1/2. Therefore, we concluded that lncRNA‐UCA1 and hsa‐miR‐145 form a reciprocal repression regulatory loop to regulate bladder cancer progression.
Discussion
Growing evidence indicates that a large number of lncRNAs have critical roles in cancer progression and metastasis, and the mechanisms by which lncRNAs contribute to cancer invasion and dissemination have gained remarkable attention.24, 25 Epithelial–mesenchymal transition is a well‐characterized transcriptional process that has been shown to facilitate invasion and metastatic dissemination of cancer cells. Recent observations have reported that lncRNAs can server as the key inducers of EMT in various cancer cells.26, 27 Previously, we have shown that lncRNA‐UCA1 is aberrantly upregulated in bladder cancer tissues and implicated in promoting migration and invasion of bladder cancer cells. Other recent studies have also shown that lncRNA‐UCA1 is overexpressed in a wide range of tumor types, including breast cancer, colorectal cancer, gastric cancer, and HCC.14, 15, 16, 17, 18, 19, 28 Remarkably, these studies have revealed that the upregulation of lncRNA‐UCA1 in cancer cells is sufficient to enhance migration and invasion in vitro. Although the roles of lncRNA‐UCA1 in multiple tumor types have been intensively investigated, the molecular mechanism of lncRNA‐UCA1‐mediated bladder cancer cell migration and invasion remains unclear. In this study, we found that ectopic overexpression of lncRNA‐UCA1 in bladder cancer cell induces EMT as well as cell migration and invasion. Therefore, our data strongly support that lncRNA‐UCA1 promotes bladder cancer cell migration and invasion by acting as an inducer of EMT.
Both ZEB1 and ZEB2 are known to trigger the initiation of EMT by binding to E‐boxes on the E‐cadherin promoter, thus repressing its promoter activity and expression.29 In the present study, we investigated the expression of ZEB1 and ZEB2 in lncRNA‐UCA1‐overexpressed bladder cancer cells. Indeed, we showed that overexpression of lncRNA‐UCA1 markedly increased ZEB1 and ZEB2 mRNA and protein expression levels in UMUC2 cells. In addition, our previous studies have shown that lncRNA‐UCA1 enhances glycolysis of bladder cancer cells though repressing hsa‐miR‐143 to increase hexokinase 2 expression. Accumulating evidence indicates that hsa‐miR‐143 and hsa‐miR‐145 form a cluster to colocalize at the chromosome 5q32 locus, and both hsa‐miR‐143 and hsa‐miR‐145 as tumor suppressors are frequently downregulated in various epithelial tumors, including bladder cancer.30, 31, 32, 33 Importantly, hsa‐miR‐145 has been shown to inhibit proliferation, migration, and invasion in a broad range of cancers by controlling numerous target genes.34, 35, 36, 37 In bladder cancer cells, hsa‐miR‐145 inhibits cell proliferation, migration, and invasion through FSCN1.20 Therefore, we hypothesized whether lncRNA‐UCA1 promotes bladder cancer progression through hsa‐miR‐145 and its target genes. We observed that overexpression of lncRNA‐UCA1 significantly suppresses hsa‐miR‐145 expression and increases hsa‐miR‐145 target gene FSCN1 expression, which is associated with cancer cell migration and invasion. Furthermore, hsa‐miR‐145 can also repress EMT in human cancer cells by directly targeting EMT‐related factors ZEB2 and Oct4, and indirectly targeting the Oct4 target gene ZEB1.21, 22, 23 Similarly, our study found that overexpression of lncRNA‐UCA1 with mutations at the hsa‐miR‐145 binding site can decrease the expression levels of ZEB1/2 in bladder cancer cells. These data indicated that lncRNA‐UCA1 upregulates the expression levels of ZEB1/2 in bladder cancer cells by suppressing hsa‐miR‐145; the other molecular mechanisms of lncRNA‐UCA1‐mediated upregulation of ZEB1/2 remain to be further investigated. Conclusively, our results showed that lncRNA‐UCA1 promotes bladder cancer cell migration and invasion, in part by hsa‐miR‐145 and its downstream factors ZEB1/2 and FSCN1 (Fig. 6).
Figure 6.
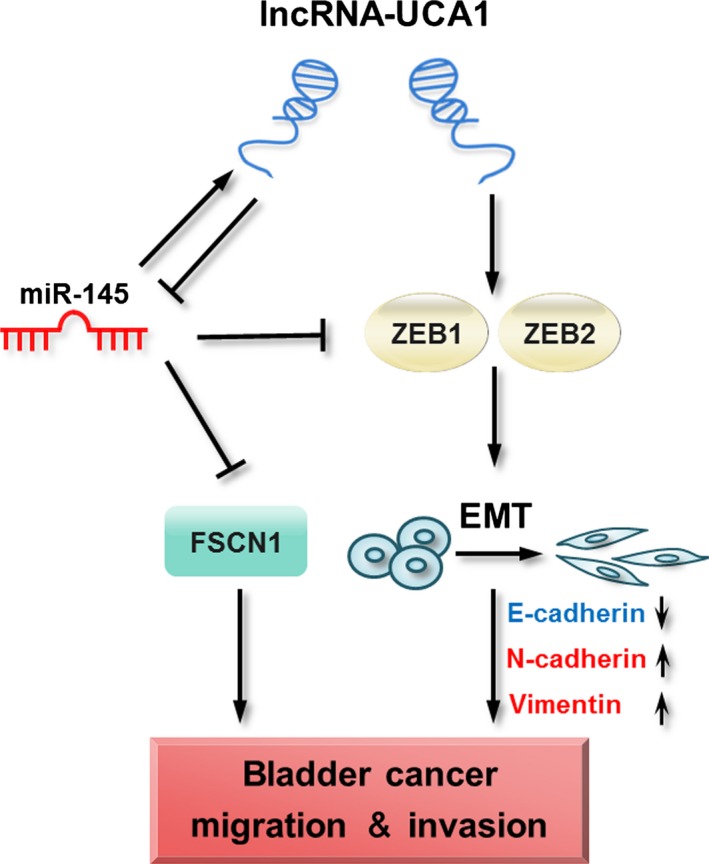
Schematic representation of the proposed mechanism of long non‐coding RNA urothelial cancer‐associated 1 (lncRNA‐UCA1)‐mediated bladder cancer progression through the hsa‐miR‐145–zinc finger E‐box binding homeobox 1 and 2 (ZEB1/2)–fascin homologue 1 (FSCN1) pathway.
Additionally, it is well documented that lncRNAs impact cellular functions by acting as molecular sponges or ceRNA to affect the miRNAs and their targets.38, 39, 40 LincRNA‐RoR has been identified as a ceRNA for hsa‐miR‐145 to regulate its target genes Oct4, Sox2, and Nanog in human embryonic stem cells and endometrial cancer stem cells.41, 42 Notably, lincRNA‐RoR is a hypoxia‐responsive lncRNA that modulates hypoxia signaling pathways through regulating hsa‐miR‐145 in HCC cells.43 Similarly, our previous study showed that lncRNA‐UCA1 is a hypoxia‐responsive lncRNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion.44 In our current work, we found that overexpression of lncRNA‐UCA1 represses hsa‐miR‐145 expression, whereas depletion of lncRNA‐UCA1 can restore the expression level of hsa‐miR‐145 in bladder cancer cells, and the hsa‐miR‐145 binding site at exons 2 and 3 of lncRNA‐UCA1 contributes to the repression of hsa‐miR‐145 mediated by lncRNA‐UCA1. In particular, several lncRNAs including lncRNA‐UCA1 are novel targets for miRNAs. In bladder cancer, a recent study indicated that lncRNA‐UCA1 is a direct target of hsa‐miR‐1 by interacting with the hsa‐miR‐1 binding site within lncRNA‐UCA1.45 Our study also confirmed that lncRNA‐UCA1 is a direct target of hsa‐miR‐145 through interacting with the hsa‐miR‐145 binding site at exons 2 and 3 of lncRNA‐UCA1. Taken together, our results uncovered that oncogenic lncRNA‐UCA1 and tumor suppressive hsa‐miR‐145 forms a reciprocal repression regulatory loop to promote bladder cancer cell migration and invasion (Fig. 6).
In conclusion, our study showed that lncRNA‐UCA1 functions as an inducer of EMT to promote bladder cancer invasion and metastasis through the hsa‐miR‐145–ZEB1/2–FSCN1 pathway, and propose that the lncRNA‐UCA1‐hsa‐miR‐145 interaction plays an important regulatory role in bladder cancer progression. Further studies are needed to investigate the diagnostic value and therapeutic significance of lncRNA‐UCA1 in metastatic progression of bladder cancer.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81372151, 81572735, and 81502529) and the First Affiliated Hospital Foundation of Xi'an Jiaotong University of China (Grant No. 2014YK9).
Cancer Sci 107 (2016) 18–27
Funding Information National Natural Science Foundation of China; First Affiliated Hospital Foundation of Xi'an Jiaotong University of China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011; 147: 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 4. Guo F, Parker KB, Yang D et al Post‐transcriptional regulatory network of epithelial‐to‐mesenchymal and mesenchymal‐to‐epithelial transitions. J Hematol Oncol 2014; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu X, Feng Y, Zhang D et al A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell 2014; 26: 344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Z, Zhu Z, Watabe K et al Negative regulation of lncRNA GAS5 by miR‐21. Cell Death Differ 2013; 20: 1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hou P, Zhao Y, Li Z et al LincRNA‐ROR induces epithelial‐to‐mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis 2014; 5: e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan JH, Yang F, Wang F et al A long noncoding RNA activated by TGF‐beta promotes the invasion‐metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014; 25: 666–81. [DOI] [PubMed] [Google Scholar]
- 9. Han Y, Liu Y, Zhang H et al Hsa‐miR‐125b suppresses bladder cancer development by down‐regulating oncogene SIRT7 and oncogenic long noncoding RNA MALAT1. FEBS Lett 2013; 587: 3875–82. [PubMed] [Google Scholar]
- 10. Wang XS, Zhang Z, Wang HC et al Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res 2006; 12: 4851–8. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non‐protein‐coding RNA up‐regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett 2008; 582: 1919–27. [DOI] [PubMed] [Google Scholar]
- 12. Fan Y, Shen B, Tan M et al Long non‐coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J 2014; 281: 1750–8. [DOI] [PubMed] [Google Scholar]
- 13. Li Z, Li X, Wu S, Xue M, Chen W. Long non‐coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR‐STAT3/microRNA143 pathway. Cancer Sci 2014; 105: 951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Z, Wu L, Wang L, Yang Y, Meng Y, Yang H. Increased expression of the long non‐coding RNA UCA1 in tongue squamous cell carcinomas: a possible correlation with cancer metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 89–95. [DOI] [PubMed] [Google Scholar]
- 15. Tian Y, Zhang X, Hao Y, Fang Z, He Y. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat‐1 in metastasis of melanoma. Melanoma Res 2014; 24: 335–41. [DOI] [PubMed] [Google Scholar]
- 16. Li JY, Ma X, Zhang CB. Overexpression of long non‐coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol 2014; 7: 7938–44. [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng Q, Wu F, Dai WY et al Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol 2015; 17: 640–6. [DOI] [PubMed] [Google Scholar]
- 18. Wang F, Zhou J, Xie X et al Involvement of SRPK1 in cisplatin resistance related to long non‐coding RNA UCA1 in human ovarian cancer cells. Neoplasma 2015; 62: 432–8. [PubMed] [Google Scholar]
- 19. Wang F, Ying HQ, He BS et al Upregulated lncRNA‐UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR‐216b and activation of FGFR1/ERK signaling pathway. Oncotarget 2015; 6: 7899–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiyomaru T, Enokida H, Tatarano S et al miR‐145 and miR‐133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer 2010; 102: 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cioce M, Ganci F, Canu V et al Protumorigenic effects of mir‐145 loss in malignant pleural mesothelioma. Oncogene 2014; 33: 5319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu J, Qiu M, Jiang F et al MiR‐145 regulates cancer stem‐like properties and epithelial‐to‐mesenchymal transition in lung adenocarcinoma‐initiating cells. Tumour Biol 2014; 35: 8953–61. [DOI] [PubMed] [Google Scholar]
- 23. Ren D, Wang M, Guo W et al Double‐negative feedback loop between ZEB2 and miR‐145 regulates epithelial‐mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res 2014; 358: 763–78. [DOI] [PubMed] [Google Scholar]
- 24. Gupta RA, Shah N, Wang KC et al Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prensner JR, Iyer MK, Sahu A et al The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet 2013; 45: 1392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fan Y, Shen B, Tan M et al TGF‐beta‐induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res 2014; 20: 1531–41. [DOI] [PubMed] [Google Scholar]
- 27. Han Y, Ye J, Wu D et al LEIGC long non‐coding RNA acts as a tumor suppressor in gastric carcinoma by inhibiting the epithelial‐to‐mesenchymal transition. BMC Cancer 2014; 14: 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang J, Zhou N, Watabe K et al Long non‐coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis 2014; 5: e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial‐mesenchymal transition. Sci Signal 2014; 7: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chivukula RR, Shi G, Acharya A et al An essential mesenchymal function for miR‐143/145 in intestinal epithelial regeneration. Cell 2014; 157: 1104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshino H, Enokida H, Itesako T et al Tumor‐suppressive microRNA‐143/145 cluster targets hexokinase‐2 in renal cell carcinoma. Cancer Sci 2013; 104: 1567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villadsen SB, Bramsen JB, Ostenfeld MS et al The miR‐143/‐145 cluster regulates plasminogen activator inhibitor‐1 in bladder cancer. Br J Cancer 2012; 106: 366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol 2013; 10: 396–404. [DOI] [PubMed] [Google Scholar]
- 34. Sachdeva M, Zhu S, Wu F et al p53 represses c‐Myc through induction of the tumor suppressor miR‐145. Proc Natl Acad Sci USA 2009; 106: 3207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaman MS, Chen Y, Deng G et al The functional significance of microRNA‐145 in prostate cancer. Br J Cancer 2010; 103: 256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kano M, Seki N, Kikkawa N et al miR‐145, miR‐133a and miR‐133b: tumor‐suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer 2010; 127: 2804–14. [DOI] [PubMed] [Google Scholar]
- 37. Gao P, Xing AY, Zhou GY et al The molecular mechanism of microRNA‐145 to suppress invasion‐metastasis cascade in gastric cancer. Oncogene 2013; 32: 491–501. [DOI] [PubMed] [Google Scholar]
- 38. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell 2011; 146: 353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cesana M, Cacchiarelli D, Legnini I et al A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011; 147: 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kallen AN, Zhou XB, Xu J et al The imprinted H19 lncRNA antagonizes let‐7 microRNAs. Mol Cell 2013; 52: 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Xu Z, Jiang J et al Endogenous miRNA sponge lincRNA‐RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self‐renewal. Dev Cell 2013; 25: 69–80. [DOI] [PubMed] [Google Scholar]
- 42. Zhou X, Gao Q, Wang J, Zhang X, Liu K, Duan Z. Linc‐RNA‐RoR acts as a “sponge” against mediation of the differentiation of endometrial cancer stem cells by microRNA‐145. Gynecol Oncol 2014; 133: 333–9. [DOI] [PubMed] [Google Scholar]
- 43. Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia‐signaling pathways by extracellular linc‐RoR. J Cell Sci 2014; 127: 1585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xue M, Li X, Li Z, Chen W. Urothelial carcinoma associated 1 is a hypoxia‐inducible factor‐1alpha‐targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol 2014; 35: 6901–12. [DOI] [PubMed] [Google Scholar]
- 45. Wang T, Yuan J, Feng N et al Hsa‐miR‐1 downregulates long non‐coding RNA urothelial cancer associated 1 in bladder cancer. Tumour Biol 2014; 35: 10075–84. [DOI] [PubMed] [Google Scholar]


