Abstract
The importance of measuring the quality of cancer care has been well recognized in many developed countries, but has never been successfully implemented on a national level in Japan. We sought to establish a wide‐scale quality monitoring and evaluation program for cancer by measuring 13 process‐of‐care quality indicators (QI) using a registry‐linked claims database. We measured two QI on pre‐treatment testing, nine on adherence to clinical guidelines on therapeutic treatments, and two on supportive care, for breast, prostate, colorectal, stomach, lung, liver and cervical cancer patients who were diagnosed in 2011 from 178 hospitals. We further assessed the reasons for non‐adherence for patients who did not receive the indicated care in 26 hospitals. QI for pretreatment testing were high in most hospitals (above 80%), but scores on adjuvant radiation and chemoradiation therapies were low (20–37%), except for breast cancer (74%). QI for adjuvant chemotherapy and supportive care were more widely distributed across hospitals (45–68%). Further analysis in 26 hospitals showed that most of the patients who did not receive adjuvant chemotherapy had clinically valid reasons for not receiving the specified care (above 70%), but the majority of the patients did not have sufficient reasons for not receiving adjuvant radiotherapy (52–69%) and supportive care (above 80%). We present here the first wide‐scale quality measurement initiative of cancer patients in Japan. Patients without clinically valid reasons for non‐adherence should be examined further in future to improve care.
Keywords: Quality indicators, Quality of health care, Management and analysis of medical data, Oncology Service, Insurance claim reporting
Measuring the quality of cancer care has become widely accepted as an integral part of cancer care in many developed countries with descriptive investigations showing that not all patients are receiving optimal care and maximum survival benefit from the most up‐to‐date cancer treatments.1 Establishing a system of quality surveillance that can detect the gaps and disparities in the delivery of care can not only help health‐care providers to improve the services they provide, but also allow policy‐makers to target areas for improvement in its cancer control plans.
Establishing such a system is often restricted by the health system's capacity to gather information from multiple sources for accurate and comprehensive measurement, as was the challenge in Japan. Increased awareness for the need to establish a systematic monitoring and evaluation program prompted the Ministry of Health, Labour and Welfare to fund a working group of nationally‐renowned experts to develop indicators of cancer care quality in 2007,2, 3 and to use them in a series of pilot programs4, 5, 6, 7 to test the feasibility of conducting quality measurements in a number of cancer treating hospitals. However, the pilot programs faced great challenges. Thoroughness and clarity of clinical documentation varied greatly with physicians, which resulted in high inter‐rater variability despite the labor‐intensive work of conducting medical chart reviews. This led investigators to conclude that without appropriate investments in human resources to conduct chart reviews and improve clinical documentation by physicians, the method was unsuitable for wide‐scale implementation.
Building on the experiences of these pilot studies, we launched a quality‐of‐care monitoring program using administrative claims data and the cancer registry as data sources for large‐scale measurement without investing in new data system infrastructure. We also assessed the limitations of using administrative data for systematic quality measurement, by reviewing the reasons for non‐adherence within the medical charts of patients who did not receive standard care as specified in the quality indicators. In this report, we present the results of the first large‐scale cancer care quality measurement initiative in Japan.
Methods
Participating hospitals
We invited all 397 Designated Cancer Care Hospitals (DCCH) to participate in the quality measurement program. DCCH are large cancer‐treating hospitals that are accredited by the Ministry of Health, Labour, and Welfare to serve as major providers of cancer care to patients in Japan.8, 9 The invitation was also left open to non‐designated cancer care hospitals that had submitted cancer registry data to the National Cancer Center (NCC) in 2011. A total of 178 hospitals, including 6 non‐designated hospitals, from 44 prefectures participated in the program. This study was approved by the NCC's institutional review board.
Data collection
We used Hospital‐based Cancer Registry (HBCR) data to identify colorectal, lung, stomach, liver, breast, prostate and cervical cancer patients who were diagnosed with cancer in 2011 from the participating hospitals. HBCR10 is a compulsory cancer incidence reporting system for all DCCH (optional for non‐designated hospitals), and follows the same national coding rules to record tumor morphology and topography codes, stage and other variables, such as the dates, methods and routes of cancer diagnosis, and information on first‐course treatments (i.e. presence/absence of curative surgery, endoscopic treatment, chemotherapy, radiotherapy and other treatments). We excluded non‐carcinoma (i.e. sarcoma and lymphoma) patients and patients who did not receive their first cancer treatment at the participating hospitals from our analysis.
Once we created a list of patients who were eligible for quality assessment, we distributed the list of patients’ HBCR IDs to the participating hospitals for collection of health claims data. The health claims data have a standardized data format nationwide that allows researchers, insurers and administrators to use the database to analyze hospital activity, patterns of care and adequacy of hospital reimbursements. It contains information on dates, costs, quantities and doses of both hospital and out‐of‐hospital prescriptions, procedures, and materials from both inpatient and outpatient settings of all patients of all insurance types.11 We developed a software that allowed hospitals to abstract the health claims data of only the cancer patients who were listed on the HBCR ID list, while replacing all personal identifiers with HBCR IDs and encrypting the data for secure data submission. We collected health claims data from September 2010 to December 2012, and linked them to the HBCR data for analysis.
To assess the generalizability of the hospitals and patients that participated in the study, we calculated the differences in hospital and patient characteristics of those who were included in the study compared to those who were not included in the study using a two‐sample Student's t‐test and Pearson's χ2‐test.
Quality indicators
Among the 206 QIs initially developed by the expert panel in 2007 (revised in 2010), which encompassed various aspects of care quality such as the adequacy of psychosocial support, patient‐centeredness and care coordination,12 we selected process‐of‐care QIs that measured the delivery of standard cancer care as indicated in clinical guidelines. In contrast to outcome measures, these QIs did not require risk adjustments to be made when comparing their results,13 and were measurable from administrative data. We chose eight QIs for five cancers (stomach, colorectal, lung, breast and liver cancer), two QIs on supportive care, and added three more QIs on cervical and prostate cancers that we developed with specialists using clinical guidelines. In total, we selected 13 QIs for seven cancers for measurement (Table 3).
Table 3.
List of quality indicators (QI) and scores
| Cancer types | Target patients | Specified care | Number of patients (N) | Proportion of patients receiving specified care (% [95% confidence interval]) |
|---|---|---|---|---|
| Stomach | ||||
| QI 1 | pStage II or III (excluding pT1, pT3N0) gastric cancer patients who were discharged within 6 weeks of receiving curative surgery | Patients who received S‐1 adjuvant chemotherapy within 8 weeks of surgery | 2258 | 62.8 [60.8, 64.8] |
| Colorectal | ||||
| QI 2 | pStage III colorectal cancer patients who received curative surgery | Patients who received one of the following adjuvant chemotherapy within 8 weeks of surgery: 5FU + LV, UFT + LV, FOLFOX, Cape, CapeOX† | 5127 | 44.8 [43.4, 46.2] |
| Lung | ||||
| QI 3 | Stage I–II non‐small cell lung cancer patients | Patients who received resection surgery or stereotactic body radiotherapy | 10 026 | 85.4 [84.7, 86.1] |
| QI 4 | pStage II–IIIA non‐small cell lung cancer patients who received curative surgery | Patients who received adjuvant platinum‐based chemotherapy within 6 months of surgery | 1691 | 49.0 [46.6, 51.4] |
| Breast | ||||
| QI 5 | Breast cancer patients under the age of 70, who received breast‐conserving surgery | Patients who received postoperative radiation within 20 weeks of surgery | 8258 | 73.6 [72.7, 74.6] |
| QI 6 | Patients with invasive breast cancer | Patients tested for HER2‡ | 16 332 | 83.3 [82.7, 83.8] |
| QI 7 | pT3–4 and not pN0, or pN2‐3 breast cancer patients | Patients who received postoperative radiation within 20 weeks | 966 | 26.5 [23.7, 29.4] |
| Liver | ||||
| QI 8 | Liver cancer patients who underwent liver resection surgery for the first time | Patients who received indocyanine green testing prior to the date of surgery | 2094 | 91.4 [90.1, 92.6] |
| Prostate | ||||
| QI 9 | pT3N0M0 prostate cancer patients who received surgical resection | Patients who received postoperative radiation or hormone therapy within a year of surgery | 772 | 19.6 [16.8, 22.5] |
| QI 10 | Locally advanced prostate cancer patients (cT3‐4cN1‐3 and M0) | Patients receiving combined androgen blockade therapy | 1973 | 80.9 [79.1, 82.7] |
| Cervical | ||||
| QI 11 | Cervical cancer patients clinically staged cT1‐2, but pathologically pN1‐3, M0 | Patients who received postoperative chemoradiation within 6 months | 197 | 37.1 [30.3, 44.2] |
| All (Supportive care) | ||||
| QI 12 | Patient who received highly‐emetic chemotherapy | Patients who received prophylaxtic anti‐emetics with aprepitant (NK1 antagonist§), serotonin antagonist (5HT3 antagonist¶), and corticosteroids at the same time as chemotherapy treatment | 10 104 | 68.1 [67.2, 69.0] |
| QI 13 | Patients who were started on narcotics in an outpatient settings for the first time | Patients who received laxatives to prevent constipation before or at the same time a narcotic is started | 2610 | 64.9 [63.1, 66.8] |
†Cape, capecitabine; CapeOX, capecitabine and oxaliplatin; FOLFOX, 5‐fluorouracil, leucovorin and oxaliplatin; 5FU + LV, 5‐fluorouracil and leucovorin; UFT + LV, uracil/ftorafur and leucovorin; ‡HER2: human epidermal growth receptor; §NK1, neurokinin 1; ¶5HT3: 5‐hydroxytryptamine.
Among these were two indicators on pre‐treatment testing (human epidermal growth receptor 2 [HER2] testing for invasive breast cancer patients and indocyanine green [ICG] testing before liver resection), two QIs on standard treatment (resection or stereotactic body radiotherapy [SBRT] for stage I–II non‐small cell lung cancer [NSCLC] patients, and combined androgen blockade [CAB] therapy for locally advanced prostate cancer patients), three QIs on adjuvant chemotherapy (postoperative chemotherapies for stage III colorectal cancer patients, stage II–III gastric cancer patients and stage II–IIIA NSCLC patients), four QIs on adjuvant radiotherapy (postoperative radiotherapy for breast cancer patients who had breast‐conserving surgery, p [pathological] T3‐4 [and not pN0] or pN2‐3 breast cancer patients, pT3N0M0 prostate cancer patients and pN1‐3M0 cervical cancer patients), and two indicators on supportive treatments (antiemetic drug prescription for patients receiving highly‐emetic chemotherapy and laxative prescription for patients starting narcotics).
Statistical analyses
For each of the 13 QIs, we calculated the percentage and confidence intervals (CIs) of patients who received the indicated care among those who met the specified characteristics of the QI for each hospital. The original wording of the QI that were initially developed12 were that if clinically valid rationale was given in medical charts for not having provided the indicated care, these patients are to be considered as having met the QI. As we could not assess this using claims data, we did not account for clinical reasons for not fulfilling the indicated care in the analyses.
For indicators on adjuvant treatments, we excluded from the analyses patients who had preoperative chemotherapy or radiotherapy. We also excluded all chemotherapy drugs administered on the day of surgery to exclude drugs used for intraoperative chemoperfusions. For all QIs, we created centipede plots depicting the distribution of QI scores from the lowest scoring hospital to the highest scoring hospital along with their 95% CIs. All analyses were conducted independently by two investigators to double‐check for accuracy using Stata 13.1 (Stata Corporation, College station, TX, USA).
Reasons for non‐adherence to quality indicators
We returned the list of HBCR IDs of patients who did not receive the specified care indicated in the QI, and asked the participating hospitals to voluntarily report back the reasons why patients did not receive the specified care. Twenty‐six hospitals participated in the reporting. We examined the frequencies of the reasons for non‐adherence among patients who did not fulfill the QIs.
Results
Among 136 245 colorectal, stomach, lung, breast, prostate, cervical and liver cancer patients who were registered in the HBCR in 2011 from 178 DCCHs, we were able to link health claims data for 94.2% (128 353) of the patients. The differences in the characteristics of hospitals and patients in the study compared to those who were not in the study are demonstrated in Table 1. Hospitals in the study had similar hospital volume compared to hospitals that were not in the study (765 vs 719 respectively, P = 0.29), but the proportion of academic institutions was smaller among hospitals in the study (15.7 vs 26.7% respectively, P = 0.01). The mean age of patients among hospitals in the study was similar to those who were not in the study (67.4 vs 67.4 respectively, P = 0.23). The distribution of sex, cancer type and stages of patients were similar, although the difference was statistically significant due to large patient size.
Table 1.
Hospital and patient characteristics of patients in the study compared to those not included in the study using 2011 hospital‐based cancer registry data
| In the study | Not in the study | P‐values | |
|---|---|---|---|
| n (% or SD) | n (% or SD) | ||
| Hospital characteristics | |||
| Number of hospitals | 178 | 225 | |
| Academic hospitals (%)† | 28 (15.7) | 60 (26.7) | P = 0.01* |
| Hospital volume, mean (SD)‡ | 765.4 (439.1) | 719.0 (426.0) | P = 0.29 |
| Patient characteristics | |||
| Number of patients, (row %) | 136 245 (45.7) | 161 785 (54.3) | |
| Age, mean (SD)‡ | 67.4 (13.2) | 67.4 (13.1) | P = 0.23 |
| Sex, male (%)‡ | 76 771 (56.3) | 93 357 (57.7) | P < 0.01* |
| Cancer type† | |||
| Colorectal (%) | 30 954 (22.7) | 37 265 (23.0) | |
| Stomach (%) | 26 755 (19.6) | 33 585 (20.8) | |
| Lung (%) | 24 363 (17.9) | 29 444 (18.2) | |
| Breast (%) | 20 863 (15.3) | 21 805 (13.5) | |
| Prostate (%) | 17 253 (12.7) | 20 229 (12.5) | |
| Cervical (%) | 8286 (6.1) | 10 374 (6.4) | |
| Liver (%) | 7771 (5.7) | 9083 (5.6) | P < 0.01* |
| Stage† | |||
| 0 (%) | 15 285 (11.2) | 18 239 (11.3) | |
| I (%) | 43 351 (31.8) | 52 700 (32.6) | |
| II (%) | 31 889 (23.4) | 37 334 (23.1) | |
| III (%) | 20 721 (15.2) | 24 524 (15.2) | |
| IV (%) | 23 227 (17.1) | 26 629 (16.5) | |
| Unknown (%) | 1772 (1.3) | 2359 (1.5) | P < 0.01* |
*Statistical significance at alpha = 0.05. †Pearson's χ2‐test. ‡Two sample Student's t‐test.
The characteristics of 128 353 patients whose health claims data were linked to HBCR data and were included in the analysis, are shown in Table 2. The mean age was 67.2 (standard deviation 13.3) and 56.0% were male. Cervical and breast cancer patients were younger than other cancer patients (46.2 and 59.1 years old vs 69–72 years old). Cancer stages were identified in 99% of the patients.
Table 2.
Characteristics of patients in the analysis
| Total | Colorectal | Stomach | Lung | Breast | Prostate | Cervical | Liver | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 128 353 | 28 108 | 25 116 | 23 150 | 19 674 | 16 677 | 8116 | 7512 |
| Age, mean (SD) | 67.2 (13.3) | 69.5 (11.6) | 70.7 (10.9) | 71.6 (7.9) | 59.1 (13.7) | 71.8 (7.9) | 46.2 (15.2) | 71.0 (10.7) |
| Sex, male (%) | 71 872 (56.0) | 16 540 (58.8) | 17 461 (69.5) | 15 956 (68.9) | 103 (0.5) | 16 677 (100) | 0 (0) | 5135 (68.4) |
| Stage | ||||||||
| 0 (%) | 13 760 (10.7) | 6195 (22.0) | 5 (0.02) | 45 (0.2) | 2537 (12.9) | 0 (0) | 4978 (61.3) | 0 (0) |
| I (%) | 40 001 (31.2) | 5124 (18.2) | 15 692 (62.5) | 8419 (36.4) | 7433 (37.8) | 199 (1.2) | 1401 (17.3) | 1733 (23.1) |
| II (%) | 30 539 (23.8) | 6089 (21.7) | 1828 (7.3) | 1452 (6.3) | 6552 (33.3) | 11 363 (68.2) | 503 (6.2) | 2752 (36.6) |
| III (%) | 19 902 (15.5) | 5870 (20.9) | 1716 (6.8) | 5353 (23.1) | 2114 (10.8) | 2409 (14.5) | 741 (9.1) | 1699 (22.6) |
| IV (%) | 22 526 (17.6) | 4553 (16.2) | 5540 (22.1) | 7497 (32.4) | 960 (4.9) | 2408 (14.4) | 431 (5.3) | 1137 (15.1) |
| Unknown (%) | 1625 (1.3) | 277 (1.0) | 335 (1.3) | 384 (1.7) | 78 (0.4) | 298 (1.8) | 62 (0.8) | 191 (2.5) |
Quality indicator scores
Quality indicator scores for pre‐treatment testing were high across most hospitals (Table 3). Among 16 332 invasive breast cancer patients and 2094 liver cancer patients, 83.3% (95% CI 82.7–83.8) received testing for HER2 (breast cancer) and 91.4% (90.1–92.6) received ICG testing before liver surgery.
Quality indicator scores for standard first‐course treatments were also high. We found that 85.4% (84.7–86.1) of 10 026 stage I–II NSCLC patients received resection or SBRT, and 80.9% (79.1–82.7) of 1973 locally advanced prostate cancer patients (cT3‐4cN1‐3 and M0) received CAB therapy (Table 3). The centipede plots depicting performance of these four QIs (QI3, QI6, QI8 and QI10) showed that most hospitals scored high for these QIs (Fig. 1).
Figure 1.
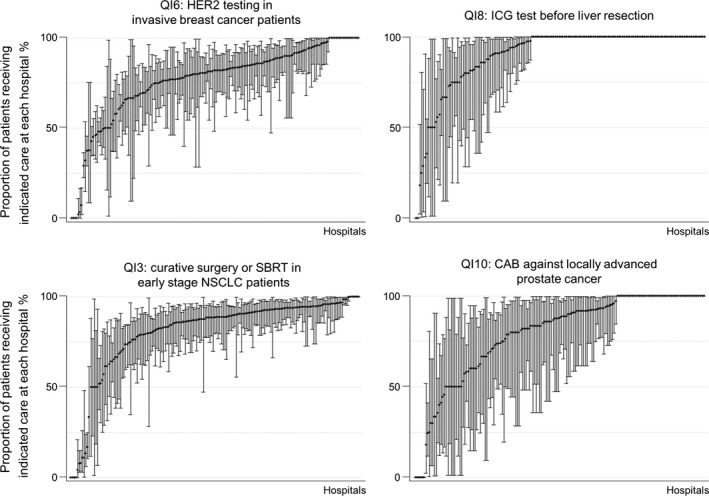
Distribution of quality indicator (QI) scores on pre‐treatment testing (QI6 and QI8), surgery and medication (QI3 and QI10) by hospital. CAB, combined androgen blockade; HER2, human epidermal growth receptor 2; ICG, indocyanine green; NSCLC, non‐small cell lung cancer; SBRT, stereotactic body radiotherapy.
There was greater variability across hospitals for patients receiving adjuvant chemotherapy and radiotherapies. Among 2258 patients with stage II or III (except pT1, pT3N0) stomach cancer who had curative surgery, 62.8% (60.8–64.8) received postoperative chemotherapy within 8 weeks of surgery. QI scores for adjuvant chemotherapy were low for colorectal and lung cancer patients: only 44.8% of 5127 pStage III colorectal cancer patients who had curative surgery received adjuvant chemotherapy within 8 weeks of surgery, and 49.0% (46.6–51.4) of 1691 stage II–IIIA NSCLC patients who had resection surgeries received postoperative chemotherapy within 6 months of surgery. The centipede plots for the three QI scores on adjuvant chemotherapy (QI1, QI2 and QI4) showed large variations across hospitals (Fig. 2).
Figure 2.
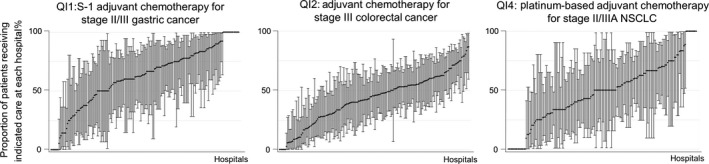
Distribution of quality indicator (QI) scores on adjuvant chemotherapy by hospital. NSCLC, non‐small cell lung cancer.
Quality indicator scores on adjuvant radiotherapy were generally lower compared to adjuvant chemotherapy. Although 73.6% (72.7–74.6) of 8258 breast cancer patients who had breast‐conserving surgery received adjuvant radiotherapy within 20 weeks of surgery, only 26.5% (23.7–29.4) of 966 stage pT3‐4 (but not N0) or pN2‐3 breast cancer patients who had resection received adjuvant radiotherapy within 20 weeks of surgery. QI scores were lower for prostate cancer, where only 19.6% (16.8–22.5) of 772 of pT3N0M0 prostate cancer patients who had resection surgeries received adjuvant radiation or hormone therapy within a year of surgery. For cervical cancer, 37.1% (30.3–44.2) of 197 cT1‐2pN1‐3pM0 patients received postoperative chemoradiation therapy within 6 months of surgery. The centipede plots in Figure 3 show that most hospitals received low scores for QI7, QI9 and QI11, but scores were higher for radiation after breast‐conserving surgery (QI5).
Figure 3.
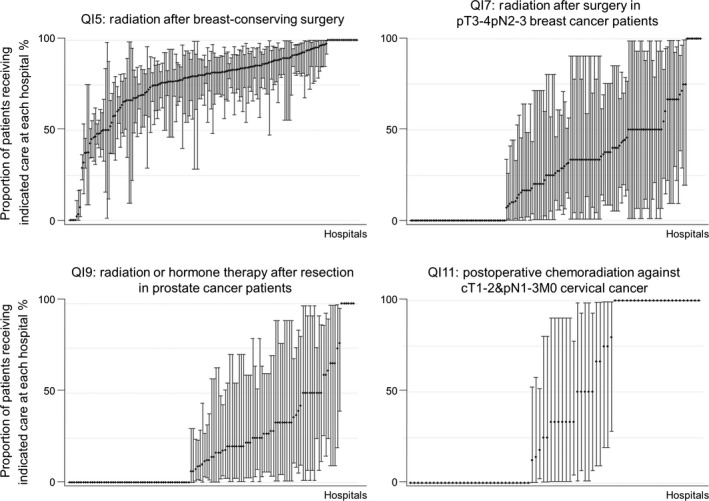
Distribution of quality indicator (QI) scores on adjuvant radiotherapy by hospital.
Similar to QIs for adjuvant chemotherapy, we observed variations in scores among hospitals for the two QI on supportive care. Among patients who received highly‐emetic chemotherapy, 68.1% (68.7–70.5) of 10 104 cancer patients received appropriate antiemetics (NK1 antagonist, 5HT3 antagonist and corticosteroids) prior to receiving chemotherapy. Among 2610 patients who received narcotics in an outpatient setting for the first time, 64.9% (63.1–66.8) received laxatives prior to or at the same time as receiving narcotics. Scores for supportive care QI (QI12 and QI13) ranged widely across hospitals, ranging from 0 to 100% (Fig. 4).
Figure 4.
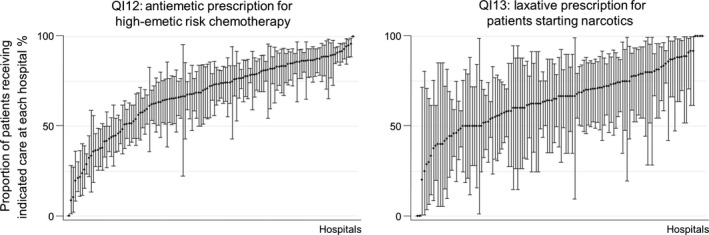
Distribution of quality indicator (QI) scores on supportive care by hospital
Reasons for non‐adherence to quality indicators
The frequencies and reasons for non‐adherence to the specified care are shown in Table 4 for the 2252 patients that were reported from 26 hospitals. For HER2 testing (QI6), reports from hospitals showed that the majority of these patients (90.7%) had actually received HER2 testing, but were erroneously disregarded from the claims data and counted as not having received the test. For QI8 (ICG testing), errors in data accounted for 45.5% of non‐adherence, while another 45.5% did not have valid reasons for not receiving the test. For all other indicators, errors in claims data were not a major cause of non‐adherence.
Table 4.
Reasons for non‐adherence to care specified in the quality indicators (QI)
| Type of QI | Pre‐treatment testing | Surgery or medication | Adjuvant chemotherapy | Adjuvant radiotherapy | Supportive care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality Indicator (QI) | QI 6 | QI 8 | QI 3 | QI 10 | QI 1 | QI 2 | QI 4 | QI 5 | QI 7 | QI 9 | QI 11 | QI 12 | QI 13 |
| Cancer types | Breast | Liver | Lung | Prostate | Stomach | Colorectal | Lung | Breast | Breast | Prostate | Cervical | All | All |
| Number of patients | N = 451 | N = 22 | N = 197 | N = 41 | N = 120 | N = 412 | N = 135 | N = 340 | N = 96 | N = 73 | N = 19 | N = 260 | N = 86 |
| QI score (%) | 83 | 91 | 85 | 81 | 63 | 45 | 49 | 74 | 27 | 20 | 37 | 68 | 65 |
| Sufficient reasons (%) | |||||||||||||
| Comorbidities | 0.4 | — | 33.5 | 2.4 | 14.2 | 14.1 | 20.7 | 3.2 | 6.3 | 1.4 | 10.5 | 1.2 | 2.3 |
| Frailty and cognitive decline from ageing | — | — | 12.2 | 7.3 | 20.8 | 11.4 | 15.6 | 0.6 | 8.3 | 1.4 | 5.3 | — | — |
| Patient preference | 0.4 | — | 25.4 | 9.8 | 17.5 | 19.2 | 24.4 | 14.7 | 14.6 | 16.4 | 5.3 | 0 | 2.3 |
| Referral | 7.3 | 9.1 | 4.6 | 9.8 | 14.2 | 8.5 | 13.3 | 37.9 | 11.5 | 9.6 | 5.3 | — | — |
| Out‐of‐hospital prescription before October 2012 | — | — | — | 9.8 | 19.2 | 13.8 | 1.5 | — | — | — | 0 | 1.5 | 7 |
| Delayed treatment to treat concurrent cancer | — | — | 2.5 | 2.4 | 2.5 | 3.2 | 5.2 | 2.1 | 0 | 0 | 0 | — | — |
| Complications from previous treatment | — | — | — | — | 4.2 | 1.5 | 0 | 0 | 0 | 0 | 0 | — | — |
| Death | 0 | — | 0.5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | — | — |
| Errors in data (patients received care) | 90.7 | 45.5 | 9.1 | 2.4 | 1.7 | 2.7 | 4.4 | 6.2 | 6.3 | 1.4 | 5.3 | 15.8 | 0 |
| Insufficient reasons (%) | |||||||||||||
| Received other treatments | 0.2 | — | 9.1 | 22 | 0 | 11.2 | 3.7 | 1.2 | 9.4 | 1.4 | 21.1 | 46.9 | — |
| Treatment delayed for no reason | — | — | — | — | 0.8 | 8.3 | 0 | 10 | 9.4 | 8.2 | 15.8 | 0 | 14 |
| Unknown | 0.9 | 45.5 | 3 | 34.1 | 5 | 6.1 | 11.1 | 24.1 | 33.3 | 60.3 | 31.6 | 34.6 | 74.4 |
For QIs on adjuvant chemotherapy (QI1, QI2 and QI4) and curative surgery or SBRT for NSCLC patients (QI3), reviews of medical charts showed that most of these patients had clinically valid reasons for not receiving the specified care, such as the presence of comorbidities, frailty from advanced age, poor physical status and cognitive decline, and approximately one‐fifth of these patients did not receive the indicated care due to patient preference (17.5, 19.2, 25.4 and 24.4% for QI1, QI2, QI3 and QI4, respectively). There were also a large number of patients who had actually received adjuvant chemotherapy, but were not reflected in the QI scores because they received their treatment at other institutions upon referral (14.2, 8.5 and 13.3% for QI1, QI2 and QI4). Part of the out‐of‐hospital prescription data before October 2012 were not required to be entered into the claims database. This resulted in 19.2 and 13.8% of non‐adherence for QI1 and QI2, respectively. Contrary to adjuvant chemotherapy, many patients did not have clinically valid reasons for non‐adherence to QIs on adjuvant radiation and chemoradiation therapies (52.1, 69.9 and 68.5% for QI7, QI9 and QI11), except for breast cancer (QI5), where patient referrals to other hospitals for radiotherapy were observed in 38% of the 340 reported patients.
For the two QIs on supportive care (QI12 and QI13), the majority of patients (81.5 and 88.4%, respectively) were identified as not having sufficient reasons for non‐adherence, which included receiving other sub‐optimal treatments (47%) or not making the benchmarked timeline for treatment initiation (14%).
Discussion
We measured 13 process‐of‐care QIs for patients who received initial treatment for cancer in 178 hospitals, and found that QI scores for pretreatment testing were high in most hospitals (above 80%), but scores on adjuvant radiation and chemoradiation therapies were low (20 to 37%), except for breast cancer (74%). We also observed substantial variability in QI scores for adjuvant chemotherapy and supportive care across hospitals, with mean scores ranging from 45 to 63%, but scores for each hospital ranging from 0 to 100%.
Further review of medical charts of patients who did not received the specified care in 26 hospitals showed that contraindications due to patient's poor physical status from comorbidities and frailty from ageing were major reasons for non‐adherence for adjuvant chemotherapy and curative surgery/SBRT for early stage NSCLC patients. Patient preference was also one of the important reasons for non‐adherence across many indicators. However, the majority of patients who did not receive the specified care for QIs on adjuvant radiotherapy and supportive care did not have valid reasons for non‐adherence.
Comparing our results to previous studies, we found similar reasons for non‐adherence in patients who did not fulfill the QI, but in different proportions. In a study by Ryoo et al. that evaluated the quality of cancer care among lung patients at Veterans Health Administration (VHA) hospitals in the USA, 65% of patients received curative resections for stage I/II NSCLC, while 6% did not receive surgery due to patient preference and 27% due to contraindications.14 Our study showed a much higher proportion of patients receiving curative treatment (85%), as we also counted patients receiving SBRT, but analysis of 197 patients who did not receive curative treatment in 26 hospitals showed that patient preference (25%) and contraindications (48%) were also the major reasons for non‐adherence. Similar results were found for resected stage II/III NSCLC patients: 47% received platinum‐based chemotherapy in the VHA hospitals, while 49% received the same treatment in our study. The ratio between the proportion of patients not receiving chemotherapy due to patient preference and contraindications among non‐adherent cases were also very similar: 54% in the VHA hospitals compared to 59% in our study. Reports for adjuvant chemotherapy among stage III colorectal cancer patients receiving first course cancer treatment in VHA hospitals by Landrum et al.15 showed a higher proportion of patients refusing chemotherapy due to patient preference (58%) compared to our study (19%). Further research of the factors related to non‐adherence, incorporating both patient and hospital factors, may help us to understand the mechanisms and, hopefully, the solution to the deficits in care.
One of the major limitations of using administrative data for process measurement was the inability to capture care that is continued and provided at other hospitals. Chart reviews of non‐adherent patients revealed a non‐negligible proportion of patients who received treatments at other institutions. These patients constituted a major proportion of non‐adherent patients for QIs on adjuvant chemotherapy for stomach, lung and colorectal cancer patients as well as adjuvant radiotherapy for breast cancer patients. This meant that hospitals that have an active care coordination program across hospitals, such that patients receive surgery at their own hospital but are referred to an affiliated hospital for adjuvant chemotherapy, would appear to have a low QI score even if their patients are receiving appropriate care.
Another limitation is that our study findings are not generalizable to all cancer patients in Japan. This initiative was voluntary, and invitations to participate were only sent to DCCHs, which cover roughly half of the care given to all cancer patients in Japan.16 Findings in our study are only reflective, but not generalizable to all DCCHs, as the patients in the analyses were not randomly sampled from HBCR. DCCHs that did not participate in the study had similar mean hospital volume, patient age, sex, cancer type and stage distributions as those that participated in the initiative, and, therefore, may have similar QI scores. Unlike the SEER‐Medicare linked database in the USA,17 our database contains information on all patients of all ages who were treated in the participating hospital regardless of their health insurance, and thus may be more representative of the total cancer patient population.
The use of administrative claims data for quality measurement is commonly criticized in the USA as its use is often limited to measuring process‐of‐care indicators.18 However, in countries where the importance of monitoring and evaluating the quality of care is not yet widely recognized by policy‐makers and health professionals to the extent that adequate resources are allocated, the use of existing administrative data allows hospitals to more easily participate in the program and begin their first step towards making quality improvements. Our resources were severely limited as hospitals could not afford to allocate any financial resources to infrastructures for a new data system or to human resources to conduct chart reviews. By utilizing the claims database, which is standardized in Japan across hospitals for patients of all insurers, hospitals were able to participate in the program by simply re‐submitting data that had already been prepared for other purposes in the past.
The aforementioned limitations of the administrative data prohibited us from obtaining an accurate measurement of some of the QI that were specified to take into account patient preference, patient referrals and clinical reasons for not providing the indicated care, such as comorbidities. However, the purpose of launching this quality‐of‐care monitoring program is to assist hospitals to take their first step towards making quality improvements by providing them with data on care quality that can be compared across other institutions. Hospitals can use the results to review the care that they have given to patients in the past, and to apply the lessons learned to future patients so that their patients do not receive suboptimal treatments.
To help hospitals use the results of our analyses for quality improvement, we developed an interactive website where hospitals were able to compare their results to other hospitals anonymously, which also added to the value of participating in the program. These efforts led to the strength of this study, which was a nation‐wide implementation of the program at a very low cost, without having to make the system compulsory.
Despite the limitations associated with our data source, we were able to obtain baseline estimates of the quality of cancer care in Japan. Participation in the quality measurement program allows providers to improve the quality of services they provide by understanding the reasons for non‐adherence through review of medical charts, thereby improving the services provided in the future, and potentially enhancing the clinical outcomes of patients in the long term. Future studies should examine hospital or regional factors that may be associated with differences in performance of QI scores across hospitals, and how performance may be associated with survival outcomes for cancer patients in Japan.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This study was supported by the National Cancer Center Research and Development Grant.
Cancer Sci 107 (2016) 68–75
Funding Information
This study was supported by the National Cancer Center Research and Development Grant.
References
- 1. Committee on Quality of Health Care in America . Institute of Medicine: Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press, 2001. [Google Scholar]
- 2. Japan Ministry of Internal Affairs and Communications . Cancer Control Act, 2006. [Cited 5 June 2015]. Available from URL: http://law.e-gov.go.jp/htmldata/H18/H18HO098.html.
- 3. Japan Ministry of Health, Labour, and Welfare, Japan: Outline of the Cancer Control Act . Annual Health, Labour and Welfare Report 2008–2009, 2014. [Cited 5 June 2015.] Available from URL: http://www.mhlw.go.jp/english/wp/wp-hw8/dl/02e.pdf
- 4. Higashi T, Nakamura F, Saruki N, Sobue T. Establishing a quality measurement system for cancer care in Japan. Jpn J Clin Oncol 2013; 43: 225–32. [DOI] [PubMed] [Google Scholar]
- 5. Higashi T, Nakamura F, Mukai H et al Assessing the quality of breast cancer care in cancer center hospitals in Japan. J Clin Oncol 2011; 29(Suppl.): e16566. [Google Scholar]
- 6. Higashi T, Nakamura F, Sugihara K et al Quality of colorectal cancer care in Japan: a nationwide multicenter study. J Clin Oncol 2010; 28(Suppl.): e16542. [Google Scholar]
- 7. Nakamura F, Higashi T, Asamura H et al Assessing the quality of lung cancer care in community cancer center hospitals in Japan. J Clin Oncol 2011; 29(Suppl.): e16570. [Google Scholar]
- 8. Japan Ministry of Health, Labor, and Welfare . The List of Designated of Cancer Care Hospitals, 2014. [Cited 5 June 2015.] Available from URL: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000063314.pdf.
- 9. Japan Ministry of Health, Labor, and Welfare, Designation of Cancer Care Hospitals , 2014. [Cited 5 June 2015.] Available from URL: http://www.mhlw.go.jp/bunya/kenkou/dl/gan_byoin_03.pdf.
- 10. Higashi T, Nakamura F, Shibata A, Emori Y, Nishimoto H. The national database of hospital‐based cancer registries: a nationwide infrastructure to support evidence‐based cancer care and cancer control policy in Japan. Jpn J Clin Oncol 2014; 44: 2–8. [DOI] [PubMed] [Google Scholar]
- 11. Matsuda S, Ishikawa K, Kuwabara K, Fujimori K, Fushimi K, Hashimoto H. Development and use of the Japanese case‐mix system. Eurohealth 2008; 14: 25–30. [Google Scholar]
- 12. Research Group on the Development of Quality Indicators and the Measurement System for Cancer Care: Quality Indicator, 2009. [Cited 5 June 2015.] Available from URL: http://qi.ncc.go.jp.
- 13. Brook RH, McGlynn EA, Shekelle PG. Defining and measuring quality of care: a perspective from US researchers. Int J Qual Health Care 2000; 12: 281–95. [DOI] [PubMed] [Google Scholar]
- 14. Ryoo JJ, Ordin DL, Antonio AL et al Patient preference and contraindications in measuring quality of care: what do administrative data miss? J Clin Oncol 2013; 31: 2716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer 2012; 118: 3345–55. [DOI] [PubMed] [Google Scholar]
- 16. Japan. Ministry of Health, Labour, and Welfare . Scale of DPC hospitals, 2015. [cited 5 June 2015.] Available from URL: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000084609.pdf
- 17. Surveillance, Epidemiology, and End Results (SEER) Program . SEER‐Medicare: Brief Description of the SEER‐Medicare Database [Internet]. National Cancer Institute. [updated 2 March 2015; cited 5 June 2015.] Available from URL: http://healthcaredelivery.cancer.gov/seermedicare/overview/.
- 18. Iezzoni L. Assessing quality using administrative data. Ann Intern Med 1997; 127: 666–74. [DOI] [PubMed] [Google Scholar]


